Primary Cell Culture as a Model System for Evolutionary Molecular Physiology
Abstract
1. Introduction
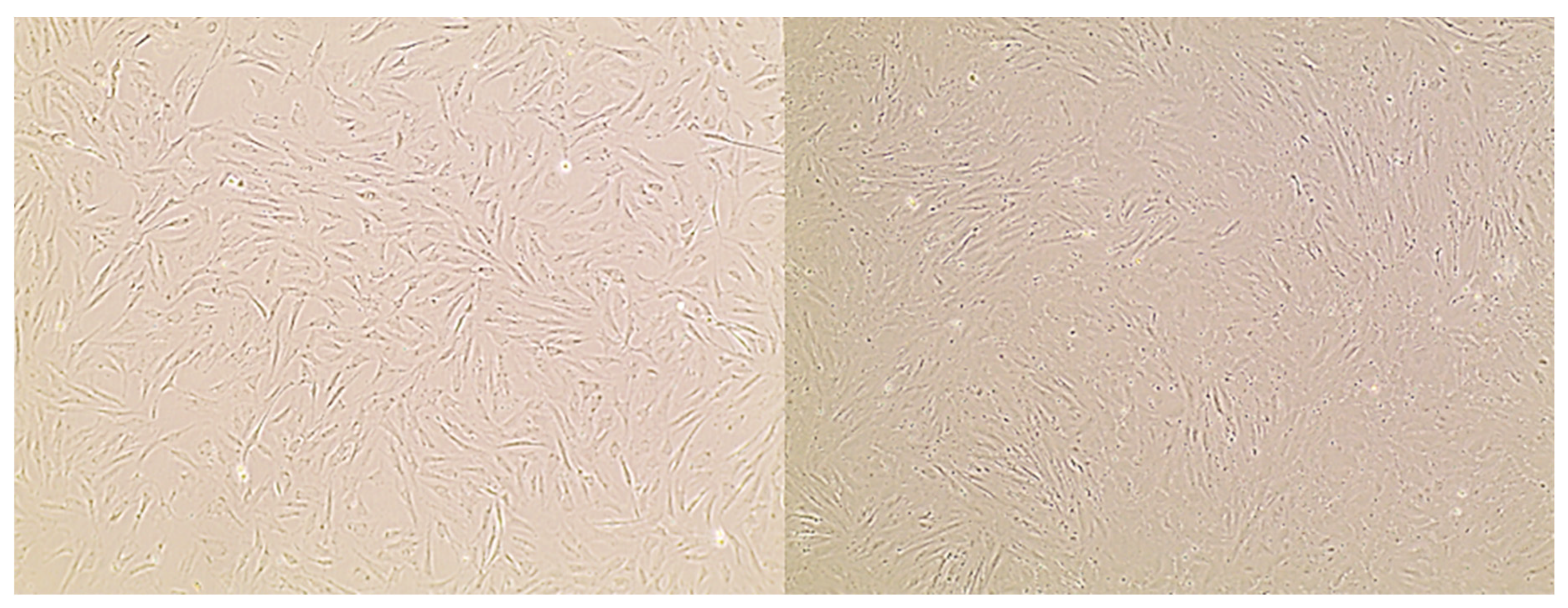
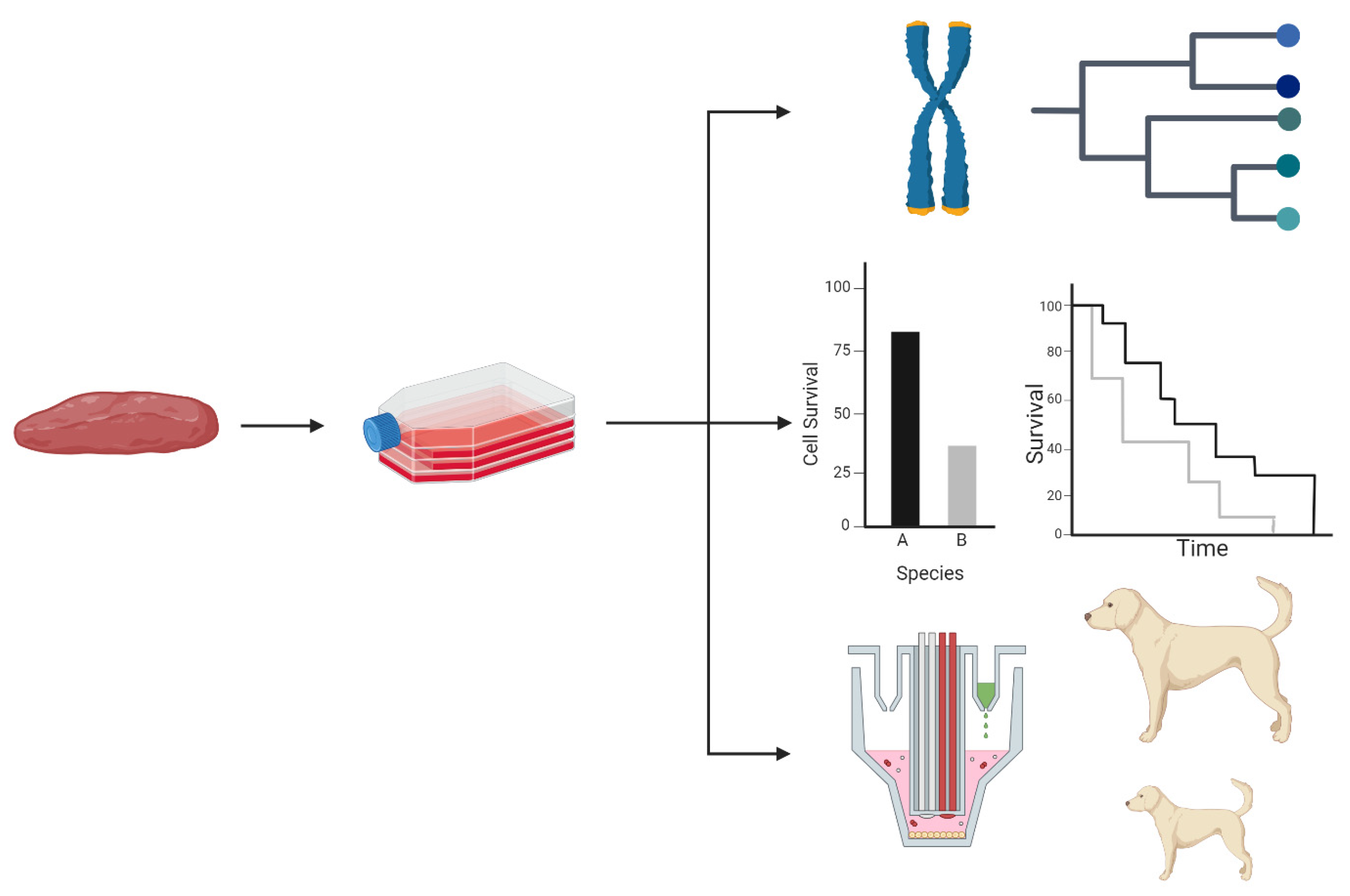
2. Cell Culture Models
3. Genetic Profiling, Genome Evolution, and Phylogenetic Reconstruction
4. Longevity Associated Gene Expression and Cellular Function
5. Life History Evolution
6. Future Directions
6.1. CRISPR/Cas9
6.2. Induced Pluripotent Stem Cells (iPSC)
6.3. Transformed Human Cell Lines
7. Conclusions
8. Perspective and Significance
Funding
Conflicts of Interest
References
- Steensma, D.P.; Robert, K.A.; Shampo, M.A. Abbie Lathrop, the “Mouse Woman of Granby”: Rodent Fancier and Accidental Genetics Pioneer. Mayo Clin. Proc. 2010, 85, e83. [Google Scholar] [CrossRef]
- Baker, H.J.; Lindsey, J.R.; Wesibroth, S.H. (Eds.) The Laboratory Rat: Biology and Diseases; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1. [Google Scholar]
- Carrel, A.; Burrows, M.T. Cultivation of Tissues In Vitro and Its Technique. J. Exp. Med. 1911, 13, 387–405. [Google Scholar] [CrossRef]
- Jiménez, A.G.; Harper, J.M. Exploring the Role of Primary Fibroblast Cells in Comparative Physiology: A Historical and Contemporary Overview. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R45–R54. [Google Scholar] [CrossRef] [PubMed]
- Dewyse, L.; Reynaert, H.; Grunsven, L.A. Best Practices and Progress in Precision-Cut Liver Slice Cultures. Int. J. Mol. Sci. 2021, 22, 7137. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Gow, A. Precision Cut Lung Slices as a Model for 2R Application in Toxicology. Appl. In Vitro Toxicol. 2020, 6, 47–48. [Google Scholar] [CrossRef]
- Corrò, C.; Novellasdemunt, L.; Li, V.S.W. A Brief History of Organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Lodewijk, G.A.; de Geus, M.; Guimarães, R.L.F.P.; Jacobs, F.M.J. Emergence of the ZNF675 Gene during Primate Evolution-Influenced Human Neurodevelopment through Changing HES1 Autoregulation. J. Comp. Neurol. 2024, 532, e25648. [Google Scholar] [CrossRef] [PubMed]
- Pollen, A.A.; Bhaduri, A.; Andrews, M.G.; Nowakowski, T.J.; Meyerson, O.S.; Mostajo-Radji, M.A.; Di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M.L.; et al. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 2019, 176, 743–756.e17. [Google Scholar] [CrossRef]
- Rankin, S.A.; Steimle, J.D.; Yang, X.H.; Rydeen, A.B.; Agarwal, K.; Chaturvedi, P.; Ikegami, K.; Herriges, M.J.; Moskowitz, I.P.; Zorn, A.M. Tbx5 drives Aldh1a2 expression to regulate a RA-Hedgehog-Wnt gene regulatory network coordinating cardiopulmonary development. Elife 2021, 10, e69288. [Google Scholar] [CrossRef]
- Post, Y.; Puschhof, J.; Beumer, J.; Kerkkamp, H.M.; de Bakker, M.A.G.; Slagboom, J.; de Barbanson, B.; Wevers, N.R.; Spijkers, X.M.; Olivier, T.; et al. Snake Venom Gland Organoids. Cell 2020, 180, 233–247.e21. [Google Scholar] [CrossRef]
- Kawasaki, M.; Goyama, T.; Tachibana, Y.; Nagao, I.; Ambrosini, Y.M. Farm and Companion Animal Organoid Models in Translational Research: A Powerful Tool to Bridge the Gap Between Mice and Humans. Front. Med. Technol. 2022, 4, 895379. [Google Scholar] [CrossRef]
- Baglole, C.J.; Reddy, S.Y.; Pollock, S.J.; Feldon, S.E.; Sime, P.J.; Smith, T.J.; Phipps, R.P. Isolation and Phenotypic Characterization of Lung Fibroblasts. Methods Mol. Med. 2005, 117, 115–127. [Google Scholar] [PubMed]
- Randall, K.J.; Turton, J.; Foster, J.R. Explant Culture of Gastrointestinal Tissue: A Review of Methods and Applications. Cell Biol. Toxicol. 2011, 27, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Perillo, M.; Punzo, A.; Caliceti, C.; Sell, C.; Lorenzini, A. The Spontaneous Immortalization Probability of Mammaliam Cell Culture Strains, as their Proliferative Capacity, Correlates with Species Body Mass, Not Longevity. Biomed. J. 2023, 46, 100596. [Google Scholar] [CrossRef] [PubMed]
- Hasche, D.; Stephan, S.; Savelyeva, L.; Westermann, F.; Rösl, F.; Vinzón, S.E. Establishment of an Immortalized Cell Line Derived from the Animal Model Mastomys coucha. PLoS ONE 2016, 11, e0161283. [Google Scholar] [CrossRef] [PubMed]
- Lewerentz, J.; Johansson, A.-M.; Stenberg, P. The Path to Immortalization of Cells Starts by Managing Stress through Gene Duplications. Exp. Cell Res. 2023, 422, 113431. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhang, L.; Wang, X.; Zheng, J.; Lin, S. Establishment Methods and Research Progress of Livestock and Poultry Immortalized Cell Lines: A Review. Front. Vet. Sci. 2022, 9, 956357. [Google Scholar] [CrossRef] [PubMed]
- Bosque, A.; Dietz, L.; Gallego-Lleyda, A.; Sanclemente, M.; Iturralde, M.; Naval, J.; Alava, M.A.; Martínez-Lostao, L.; Thierse, H.J.; Anel, A. Comparative Proteomics of Exosomes Secreted by Tumoral Jurkat T cells and Normal Human T cell Blasts Unravels a Potential Tumorigenic Role for Valosin-Containing Protein. Oncotarget 2016, 7, 29287–29305. [Google Scholar] [CrossRef] [PubMed]
- De Vries, G.H.; Boullerne, A.I. Glial Cell Lines: An Overview. Neurochem. Res. 2010, 35, 1978–2000. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Siranosian, B.; Ha, G.; Tang, H.; Oren, Y.; Hinohara, K.; Strathdee, C.A.; Dempster, J.; Lyons, N.J.; Burns, R.; et al. Genetic and Transcriptional Evolution alters Cancer Cell Line Drug Response. Nature 2018, 560, 325–330. [Google Scholar] [CrossRef]
- Gutbier, S.; May, P.; Berthelot, S.; Krishna, A.; Trefzer, T.; Behbehani, M.; Efremova, L.; Delp, J.; Gastraunthaler, G.; Waldmann, T.; et al. Major Changes of Cell Function and Toxicant Sensitivity in Cultured Cells Undergoing Mild, Quasi-Natural Genetic Drift. Arch. Toxicol. 2018, 92, 3487–3503. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, P.J.; Yang, L.; Gunter, L.E. Demethylation of Satellite I DNA during Senescence of Bovine Adrenocortical Cells in Culture. Mutat. Res. 1992, 275, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nestor, C.E.; Ottaviano, R.; Reinhardt, D.; Cruickshanks, H.A.; Mjoseng, H.K.; McPherson, R.C.; Lentini, A.; Thomson, J.P.; Dunican, D.S.; Pennings, S.; et al. Rapid Reprogramming of Epigenetic and Transcriptional Profiles in Mammalian Culture Systems. Genome Biol. 2015, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Franzen, J.; Georgomanolis, T.; Selich, A.; Kuo, C.C.; Stöger, R.; Brant, L.; Mulabdić, M.S.; Fernandez-Rebollo, E.; Grezella, C.; Ostrowska, A.; et al. DNA Methylation Changes During Long-Term In Vitro Cell Culture are Caused by Epigenetic Drift. Commun. Biol. 2021, 4, 598. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Dias, G.B.; Basting, P.J.; Nelson, M.G.; Patel, S.; Marzo, M.; Bergman, C.M. Ongoing Transposition in Cell Culture Reveals the Phylogeny of Diverse Drosophila S2 Sublines. Genetics 2022, 221, iyac077. [Google Scholar] [CrossRef] [PubMed]
- Ramboer, E.; De Craene, B.; De Kick, J.; Vanhaecke, T.; Berx, G.; Rogiers, V.; Vinken, M. Strategies for Immortalization of Primary Hepatocytes. J. Hepatol. 2014, 61, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of Antioxidants in the Fibroblast Replicative Lifespan In Vitro. Oxid. Med. Cell Longev. 2020, 2020, 6423783. [Google Scholar] [CrossRef] [PubMed]
- Van Gansen, P.; Van Lerberghe, N. Potential and Limitations of Cultivated Fibroblasts in the Study of Senescence in Animals. A Review on the Murine Skin Fibroblasts System. Arch. Gerontol. Geriatr. 1988, 7, 31–74. [Google Scholar] [CrossRef]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmueller, U.; Mann, M. Comparative Proteomic Phenotyping of Cell Lines and Primary Cells to Assess Preservation of Cell Type-specific Functions. Mol. Cell. Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef]
- Madelaire, C.B.; Klink, A.C.; Israelsen, W.J.; Hindle, A.G. Fibroblasts as an Experimental Model System for the Study of Comparative Physiology. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 260, 110375. [Google Scholar] [CrossRef]
- Pathak, J.; Singh, S.P.; Kharche, S.D.; Goel, A.; Soni, Y.K.; Kaushik, R.; Kose, M.; Kumar, A. Cell Culture Media Dependent In Vitro Dynamics and Culture Characteristics of Adult Caprine Dermal Fibroblast Cells. Sci. Rep. 2023, 13, 13716. [Google Scholar] [CrossRef]
- Luo, J.; Liang, M.-M.; Yang, X.-G.; Xu, H.-Y.; Shi, D.-S.; Lu, S.-S. Establishment and Biological Characteristics Comparison of Chinese Swamp Buffalo (Bubalus bubalis) Fibroblast Cell Lines. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.A.; Gollin, S.M. Rapid Cell Culture Procedure for Tissue Samples. Am. J. Med. Genet. 1987, 28, 521–526. [Google Scholar] [CrossRef]
- Biederman, B.M.; Lin, C.C. A Leukocyte Culture and Chromosome Preparation Technique for Avian Species. In Vitro 1982, 18, 415–418. [Google Scholar] [CrossRef]
- Ishijima, J.; Uno, Y.; Nunome, M.; Nishida, C.; Kuraku, S.; Matsuda, Y. Molecular Cytogenetic Characterization of Chromosome Site-specific Repetitive Sequences in the Arctic lamprey (Lethenteron camtschaticum, Petromyzontidae). DNA Res. 2017, 24, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Pristyazhnyuk, I.E.; Malinovskaya, L.P.; Borodin, P.M. Establishment of the Primary Avian Gonadal Somatic Cell Lines for Cytogenetic Studies. Animals 2022, 12, 1724. [Google Scholar] [CrossRef]
- Paim, F.G.; Maia, L.; da Cruz Landim-Alverenga, F.; Foresti, F.; Oliveira, C. New Protocol for Cell Culture to Obtain Mitotic Chromosomes in Fishes. Methods Protoc. 2018, 1, 47. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.B.; Paim, F.G.; Ramos, L.P.; Foresti, F.; Oliveira, C. Molecular Cytogenetic Analysis and the Establishment of a Cell Culture in the Fish Species Hollandichthys multifasciatus (Eigenmann & Norris, 1900) (Characiformes, Characidae). Genet. Mol. Biol. 2021, 44, e20200260. [Google Scholar]
- He, L.; Zhao, C.; Xiao, Q.; Zhao, J.; Liu, H.; Jiang, J.; Cao, Q. Profiling the Physiological Roles in Fish Primary Cell Culture. Biology 2023, 12, 1454. [Google Scholar] [CrossRef]
- Jin, W.; Jia, K.; Yang, L.; Chen, J.; Wu, Y.; Yi, M. Derivation and Characterization of Cell Cultures from the Skin of the Indo-Pacific Humpback Dolphin Sousa chinensis. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 449–457. [Google Scholar] [CrossRef]
- Pavlova, S.V.; Biltueva, L.S.; Romanenko, S.A.; Lemskaya, N.A.; Shchinov, A.V.; Abramov, A.V.; Rozhnov, V.V. First Cytogenetic Analysis of Lesser Gymnures (Mammalia, Galericidae, Hylomys) from Vietnam. Comp. Cytogenet. 2018, 12, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Nozu, R.; Kiyatake, I.; Higashiguchi, N.; Sodeyama, S.; Murakumo, K.; Sato, K.; Kuraku, S. Cell Culture-based Karyotyping of Orectolobiform Sharks for Chromosome-scale Genome Analysis. Commun. Biol. 2020, 3, 652. [Google Scholar] [CrossRef] [PubMed]
- Nash, W.G.; O’Brien, S.J. A Comparative Chromosome Banding Analysis of the Ursidae and Their Relationship to Other Carnivores. Cytogenet. Cell. Genet. 1987, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Vassart, M.; Seguela, A.; Hayes, H. Chromosomal Evolution in Gazelle. J. Hered. 1995, 86, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, J.; Su, W.; Nie, W.; Yang, F. Karyotypic Evolution of the Family Sciuridae: Inferences from the Genome Organizations of Ground Squirrels. Cytogenet. Genome Res. 2006, 112, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, J.; Su, W.; Yang, F. Chromosomal Mechanisms Underlying the Karyotype Evolution of the Oriental Voles (Muridae, Eothenomys). Cytogenet. Genome Res. 2006, 114, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kacprzyk, J.; Teeling, E.C.; Kelleher, C.; Volleth, M. Wing Membrane Biopsies for Bat Cytogenetics: Finding of 2n = 54 in Irish Rhinolophus hipposideros (Rhinolophidae, Chiroptera, Mammalia) Supports Two Geographically Separated Chromosomal Variants in Europe. Cytogenet. Genome Res. 2016, 148, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Oliveira, I.H.; Penteado, P.R.; Pasa, R.; Menegídio, F.B.; Kavalco, K.F. Phylogeography and Karyotypic Evolution of Some Deuterodon Species from Southeastern Brazil (Characiformes, Characidae, Stethaprioninae). Genet. Mol. Biol. 2023, 46, e20230044. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Martínková, N.; Galindo, D.J.; Bernegossi, A.M.; Cernohorska, H.; Kadlcikova, D.; Musilová, P.; Duarte, J.M.; Rubes, J. Satellite DNA in Neotropical Deer Species. Genes 2021, 12, 123. [Google Scholar] [CrossRef]
- de Sousa, R.P.C.; Furo, I.O.; Silva-Oliveira, G.C.; de Sousa-Felix, R.C.; Bessa-Brito, C.D.; Mello, R.C.; Sampaio, I.; Artoni, R.F.; de Oliveira, E.H.C.; Vallinoto, M. Comparative Cytogenetics of Microsatellite Distribution in Two Tetra Fishes Astyanax bimaculatus (Linnaeus, 1758) and Psalidodon scabripinnis (Jenyns, 1842). PeerJ 2024, 12, e16924. [Google Scholar] [CrossRef]
- Gu, J.; Gu, X. Further Statistical Analysis for Genome-wide Expression Evolution in Primate Brain/Liver/Fibroblast Tissues. Hum. Genom. 2004, 1, 247–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uno, Y.; Asada, Y.; Nishida, C.; Takehana, Y.; Sakaizumi, M.; Matsuda, Y. Divergence of Repetitive DNA Sequences in the Heterochromatin of Medaka Fishes: Molecular Cytogenetic Characterization of Constitutive Heterochromatin in Two Medaka Species: Oryzias hubbsi and O. celebensis (Adrianichthyidae, Beloniformes). Cytogenet. Genome Res. 2013, 141, 212–226. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Qin, Y.-J.; Wu, D.; Bai, L.-W.; Pei, A.-J. Establishment and Characterization of Fin Cell Lines from Diploid, Triploid and Tetraploid Oriental Weatherfish (Misgurnus anguillicaudatus). Fish Physiol. Biochem. 2015, 41, 661–672. [Google Scholar] [CrossRef]
- Santagostino, M.; Piras, F.M.; Cappelletti, E.; Del Giudice, S.; Semino, O.; Nergadze, S.G.; Giulotto, E. Insertion of Telomeric Repeats in the Human and Horse Genomes: An Evolutionary Perspective. Int. J. Mol. Sci. 2020, 21, 2838. [Google Scholar] [CrossRef] [PubMed]
- Goodier, J.L.; Mandall, P.K.; Zhang, L.; Kazazian, H.H., Jr. Discrete Subcellular Partitioning of Human Retrotransposon RNAs Despite a Common Mechanism of Genome Insertion. Hum. Mol. Genet. 2010, 19, 1712–1725. [Google Scholar] [CrossRef]
- Sands, W.; Page, M.M.; Selman, C. Proteostasis and Ageing: Insights from Long-Lived Mutant Mice. J. Physiol. 2017, 595, 6383–6390. [Google Scholar] [CrossRef]
- Bartke, A.; Quainoo, N. Impact of Growth Hormone-Related Mutations on Mammalian Aging. Front. Genet. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.G. A Revisiting of “the Hallmarks of Aging: In Domestic Dogs: Current Status of the Literature. GeroScience 2024, 46, 241–255. [Google Scholar] [CrossRef]
- Alper, S.J.; Bronikowski, A.M.; Harper, J.M. Comparative Cellular Biogerontology: Where Do We Stand? Exp. Geron. 2015, 71, 109–117. [Google Scholar] [CrossRef]
- Stier, A.; Reichert, S.; Criscuolo, F.; Bize, P. Red Blood Cells Open Promising Avenues for Longitudinal Studies of Ageing in Laboratory, Non-model and Wild animals. Exp. Geron. 2015, 71, 118–134. [Google Scholar] [CrossRef]
- DiLoret, R.; Murphy, C.T. The Cell Biology of Aging. Mol. Biol. Cell. 2015, 26, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, P.; Boulton, M.E.; Kirkwood, T.B.L. Positive Correlation Between Mammalian Life Span and Cellular Resistance to Stress. Free Rad. Biol. Med. 1999, 26, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.M.; Salmon, A.B.; Leiser, S.F.; Galecki, A.T.; Miller, R.A. Skin-derived Fibroblasts from Long-lived Species are Resistant to Some, But Not All, Lethal Stresses and to the Mitochondrial Inhibitor Rotenone. Aging Cell. 2007, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.B.; Sadighi Akha, A.A.; Buffenstein, R.; Miller, R.A. Fibroblasts from Naked Mole-Rats Are Resistant to Multiple Forms of Cell Injury, but Sensitive to Peroxide, UV Light, and ER Stress. J. Geron. A Biol. Sci. Med. Sci. 2008, 63, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Podlutsky, A.; Podlutskaya, N.; Sonntag, W.E.; Merlin, S.Z.; Philipp, E.E.; Doyle, K.; Davila, A.; Recchia, F.A.; Ballabh, P.; et al. Testing the Oxidative Stress Hypothesis of Aging in Primate Fibroblasts: Is There a Correlation Between Species Longevity and Cellular ROS Production? J. Geron. A Biol. Sci. Med. Sci. 2012, 67, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Ogburn, C.E.; Carlberg, K.; Ottinger, M.A.; Holmes, D.J.; Martin, G.M.; Austad, S.N. Exceptional Cellular Resistance to Oxidative Damage in Long-lived Birds Requires Active Gene Expression. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B468–B474. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.M.; Wang, M.; Galecki, A.T.; Ro, J.; Williams, J.B.; Miller, R.A. Fibroblasts from Long-lived Bird Species Are Resistant to Multiple Forms of Stress. J. Exp. Biol. 2011, 214, 1902–1910. [Google Scholar] [CrossRef]
- Jimenez, A.G.; Harper, J.M.; Queenborough, S.A.; Williams, J.B. Linkages Between the Life-history Evolution of Tropical and Temperate Birds and the Resistance of Cultured Skin Fibroblasts to Oxidative and Non-oxidative Chemical Injury. J. Exp. Biol. 2013, 216, 1373–1380. [Google Scholar] [PubMed]
- Dostál, L.; Kohler, W.M.; Penner-Hahn, J.E.; Miller, R.A.; Fierke, C.A. Fibroblasts from Long-lived Rodent Species Exclude Cadmium. J. Geron. A Biol. Sci. Med. Sci. 2015, 70, 10–19. [Google Scholar] [CrossRef][Green Version]
- Ma, S.; Upneja, A.; Galecki, A.; Tsai, Y.M.; Burant, C.F.; Raskind, S.; Zhang, Q.; Zhang, Z.D.; Seluanov, A.; Gorbunova, V.; et al. Cell Culture-based Profiling Across Mammals Reveals DNA Repair and Metabolism as Determinants of Species Longevity. Elife 2016, 5, e19130. [Google Scholar] [CrossRef]
- Brown, M.E.; Stuart, J.A. Correlation of Mitochondrial Superoxide Dismutase and DNA Polymerase β in Mammalian Dermal Fibroblasts with Species Maximal Lifespan. Mech. Ageing Dev. 2007, 128, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Jimenez, A.G. Metabolomics of Aging in Primary Fibroblasts from Small and Large Breed Dogs. GeroScience 2021, 43, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Elbourkadi, N.; Austad, S.N.; Miller, R.A. Fibroblasts from Long-lived Species of Mammals and Birds Show Delayed, but Prolonged, Phosphorylation of ERK. Aging Cell. 2014, 13, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.P.; Wise, S.S.; Kraus, S.; Shaffiey, F.; Grau, M.; Chen, T.L.; Perkins, C.; Thompson, W.D.; Zheng, T.; Zhang, Y.; et al. Hexavalent Chromium is Cytotoxic and Genotoxic to the North Atlantic Right Whale (Eubalaena glacialis) Lung and Testes Fibroblasts. Mutat. Res. 2008, 650, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.J.; Zychowski, G.V.; Bauman, S.W.; Higgins, B.M.; Raudsepp, T.; Gollahon, L.S.; Wooten, K.J.; Cole, J.M.; Godard-Codding, C. Establishment, Characterization, and Toxicological Application of Loggerhead Sea Turtle (Caretta caretta) Primary Skin Fibroblast Cell Cultures. Environ. Sci. Technol. 2014, 48, 14728–14737. [Google Scholar] [CrossRef] [PubMed]
- Boroda, A.V.; Kipryushina, Y.O.; Golochvastova, R.V.; Shevchenko, O.G.; Shulgina, M.A.; Efimova, K.V.; Katin, I.O.; Maiorova, M.A. Isolation, Characterization, and Ecotoxicological Application of Marine Mammal Skin Fibroblast Cultures. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Součková, K.; Jasík, M.; Sovadinová, I.; Sember, A.; Sychrová, E.; Konieczna, A.; Bystrý, V.; Dyková, I.; Blažek, R.; Lukšíková, K.; et al. From Fish to Cells: Establishment of Continuous Cell Lines from Embryos of Annual Killifish Nothobranchius furzeri and N. kadleci. Aquat. Toxicol. 2023, 259, 106517. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.M.; Lehr, M.; Kohler, W.J.; Han, M.L.; Miller, R.A. Fibroblasts from Longer-Lived Species of Primates, Rodents, Bats Carnivores, and Birds Resist Protein Damage. J. Geron. A Biol. Sci. Med. Sci. 2015, 70, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.I.; Buffenstein, R.; Masamsetti, V.; Leonard, S.; Salmon, A.B.; Mele, J.; Andziak, B.; Yang, T.; Edrey, Y.; Friguet, B.; et al. Protein Stability and Resistance to Oxidative Stress are Determinants of Longevity in the Longest-Living Rodent, the Naked Mole-Rat. Proc. Natl. Acad. Sci. USA 2009, 106, 3059–3064. [Google Scholar] [CrossRef]
- Azpurua, J.; Ke, Z.; Chen, I.X.; Zhang, Q.; Ermolenk, D.N.; Zhang, Z.D.; Gorbunova, V.; Seluanov, A. Naked Mole-Rat Has Increased Translational Fidelity Compared With the Mouse, As Well As Unique 28S Ribosomal RNA Cleavage. Proc. Natl. Acad. Sci. USA 2013, 110, 17350–17355. [Google Scholar] [CrossRef]
- Pickering, A.M.; Lehr, M.; Miller, R.A. Lifespan of Mice and Primates Correlated with Immunoproteasome Expression. J. Clin. Investig. 2015, 125, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Pride, H.; Yu, Z.; Sunchu, B.; Mochnick, J.; Coles, A.; Zhang, Y.; Buffenstein, R.; Hornsby, P.J.; Austad, S.N.; Perez, V.I. Long-Lived Species Have Improved Proteostasis Compared to Phylogenetically-Related Shorter-Lived Species. Biochem. Biophys. Res. Commun. 2015, 457, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Swovick, K.; Firsanov, D.; Welle, K.A.; Hyyhorenko, J.R.; Wise, J.P., Sr.; George, C.; Sformo, T.L.; Selianov, A.; Gorbunova, V.; Ghaemmaghami, S. Interspecies Differences in Proteome Turnover Kinetics Are Correlated with Life Spans and Energetic Demands. Mol. Cell. Proteom. 2021, 20, 100041. [Google Scholar] [CrossRef] [PubMed]
- Kacprzyk, J.; Locatelli, A.G.; Hughes, G.M.; Huang, Z.; Clarke, M.; Gorbunova, V.; Sacchi, C.; Stewart, G.S.; Teeling, E.C. Evolution of Mammalian Longevity: Age-related Increase in Autophagy in Bats Compared to Other Mammals. Aging 2021, 13, 7998–8025. [Google Scholar] [CrossRef]
- Seluanov, A.; Hine, C.; Azpurua, J.; Feigenson, M.; Bozzella, M.; Mao, Z.; Catania, K.C.; Gorbunova, V. Hypersensitivity to Contact Inhibition Provides a Clue to Cancer Resistance of Naked Mole-Rat. Proc. Natl. Acad. Sci. USA 2009, 106, 19352–19357. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-Molecular-Mass Hyaluronan Mediates the Cancer Resistance of the Naked Mole Rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Doerig, K.; Park, R.; Can Ran Qin, A.; Hwang, C.; Neary, A.; Gilbert, M.; Seluanov, A.; Gorbunova, V. Evolution of telomere maintenance and tumour suppressor mechanisms across mammals. Phil. Trans. R. Soc. 2018, B373, 20160443. [Google Scholar] [CrossRef] [PubMed]
- Deuker, M.M.; Lewis, K.N.; Ingaramo, M.; Kimmel, J.; Buffenstein, R.; Settleman, J. Unprovoked Stabilization and Nuclear Accumulation of the Naked Mole-Rat p53 Protein. Sci. Rep. 2020, 10, 6966. [Google Scholar] [CrossRef] [PubMed]
- Manov, I.; Hirsh, M.; Iancu, T.C.; Malik, A.; Sotnichenko, N.; Band, M.; Avivi, A.; Shams, I. Pronounced Cancer Resistance in a Subterranean Rodent, the Blind Mole-Rat, Spalax: In Vivo and In Vitro Evidence. BMC Biol. 2013, 11, 91. [Google Scholar] [CrossRef]
- Patrick, A.; Seluanov, M.; Hwang, C.; Tam, J.; Khan, T.; Morgenstern, A.; Wiener, L.; Vazquez, J.M.; Zafar, H.; Wen, R.; et al. Sensitivity of Primary Fibroblasts in Culture to Atmospheric Oxygen Does Not Correlate with Species Lifespan. Aging 2016, 8, 841–847. [Google Scholar] [CrossRef][Green Version]
- Adekunbi, D.A.; Huber, H.F.; Li, C.; Nathanelsz, P.W.; Cox, L.A.; Salmon, A.B. Differential Mitochondrial Bioenergetics and Cellular Resilience in Astrocytes, Hepatocytes, and Fibroblasts from Aging Baboons. GeroScience 2024. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S.C. Life History Evolution: Successes, Limitations, and Prospects. Naturwissenschaften 2000, 87, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.F.; Gratton, T.P.; Stuart, J.A. Metabolic Rate Does Not Scale with Body Mass in Cultured Mammalian Cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2115–R2121. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.G.; Williams, J.B. Cellular Metabolic Rates from Primary Dermal Fibroblast Cells Isolated from Birds of Different Body Masses. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 176, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.G.; Cooper-Mullin, C.; Anthony, N.B.; Williams, J.B. Cellular Metabolic Rates in Cultured Primary Dermal Fibroblasts and Myoblasts from Fast-Growing and Control Coturnix Quail. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 171, 23–30. [Google Scholar] [CrossRef]
- Cooper-Mullin, C.; Jimenez, A.G.; Anthony, N.B.; Wortman, M.; Williams, J.B. The Metabolic Rate of Cultured Muscle Cells from Hybrid Coturnix Quail is Intermediate to that of Muscle Cells from Fast-growing and Slow-growing Coturnix Quail. J. Comp. Physiol. B. 2015, 185, 547–557. [Google Scholar] [CrossRef]
- Calhoon, E.A.; Jimenez, A.G.; Harper, J.M.; Jurkowitz, M.S.; Williams, J.B. Linkages Between Mitochondrial Lipids and Life History in Temperate and Tropical Birds. Physiol. Biochem. Zool. 2014, 87, 265–275. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, H.; Liu, L.; Kong, M.; Yin, T.; Zhao, X. Mitochondrial Haplotypes Influence Metabolic Traits Across Bovine Inter- and Intra-Species Cybrids. Sci. Rep. 2017, 7, 4179. [Google Scholar] [CrossRef] [PubMed]
- Hogan, H.R.H.; Hutzenbiler, B.D.E.; Robbins, C.T.; Jansen, H.T. Changing Lanes: Seasonal Differences in Cellular Metabolism of Adipocytes in Grizzly Bears (Ursus arctos horribilis). J. Comp. Physiol. B. 2022, 192, 397–410. [Google Scholar] [CrossRef]
- Lu, W.; Meng, Q.-J.; Tyler, N.J.C.; Stokkan, K.-A.; Loudon, A.S.I. A Circadian Clock in not Requires in an Arctic Mammal. Curr. Biol. 2010, 20, 533–537. [Google Scholar] [CrossRef]
- Sulak, M.; Fong, L.; Mika, K.; Chigurupati, S.; Yon, L.; Mongan, N.P.; Emes, R.D.; Lynch, V.J. TP53 Copy Number Expansion is Associated with the Evolution of Increased Body Size and an Enhanced DNA Damage Response in Elephants. Elife 2016, 5, e11994. [Google Scholar] [CrossRef] [PubMed]
- Winward, J.D.; Ragan, C.M.; Jimenez, A.G. Cellular Metabolic Rates and Oxidative Stress Profiles in Primary Fibroblast Cells Isolated from Virgin Females, Reproductively Experienced Females, and Male Sprague-Dawley Rats. Physiol. Rep. 2018, 20, e13909. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.N.; Yamada, K.; Zikeli, S.; Kiaris, H.; Hood, W.R. Evaluating Endoplasmic Reticulum Stress and Unfolded Protein Response Through the Lens of Ecology and Evolution. Biol. Rev. Camb. Philos. Soc. 2021, 96, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Glaberman, S.; Bulls, S.E.; Vazquez, J.M.; Chiari, Y.; Lynch, V.J. Concurrent Evolution of Antiaging Gene Duplications and Cellular Phenotypes in Long-Lived Turtles. Genome Biol. Evol. 2021, 13, evab244. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.K.; Allen, K.N.; Torres-Velarde, J.M.; Vázquez-Medina, J.P. Functional Studies with Primary Cells Provide a System for Genome-to-Phenome Investigations in Marine Mammals. Integr. Comp. Biol. 2020, 60, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Boroda, A.V. Marine Mammal Cell Cultures: To Obtain, to Apply, and to Preserve. Mar. Environ. Res. 2017, 129, 316–328. [Google Scholar] [CrossRef]
- Tovar, H.; Navarrete, F.; Rodriguez, L.; Skewes, O.; Castro, F.O. Cold Storage of Biopsies from Wild Endangered Chilean Species in Field Conditions and Subsequent Isolation of Primary Cell Culture Cell Lines. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 309–320. [Google Scholar] [CrossRef]
- Golkar-Narenji, A.; Dziegel, P.; Kempisty, B.; Petitte, J.; Mozdziak, P.E.; Bryja, A. In Vitro Culture of Reptile PGCS to Preserve Endangered Species. Cell Biol. Int. 2023, 47, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Seridi, N.; Hamidouche, M.; Belmessabih, N.; El Kennani, S.; Gagnon, J.; Martine, G.; Coutton, C.; Marchal, T.; Chebloune, Y. Immortalization of Primary Sheep Embryo Kidney Cells. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 76–85. [Google Scholar] [CrossRef]
- Hopperstad, K.; Truschel, T.; Wahlicht, T.; Stewart, W.; Eicher, A.; May, T.; Deisenroth, C. Characterization of Novel Human Immortalized Thyroid Follicular Epithelial Cell Lines. Appl. In Vitro Toxicol. 2021, 7, 39–49. [Google Scholar] [CrossRef]
- Fus-Kujawa, A.; Prus, P.; Bajdak-Rusinek, K.; Teper, P.; Gawron, K.; Kowalczuk, A.; Sieron, A.L. An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro. Front. Bioeng. Biotechnol. 2021, 9, 701031. [Google Scholar] [CrossRef] [PubMed]
- Tyumentseva, M.; Tyumentsev, A.; Akimkin, V. CRISPR/Cas9 Landscape: Current State and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 16077. [Google Scholar] [CrossRef] [PubMed]
- Hryhorowicz, M.; Grześkowiak, B.; Mazurkiewicz, N.; Śledziński, P.; Lipiński, D.; Słomski, R. Improved Delivery of CRISPR/Cas9 System Using Magnetic Nanoparticles into Porcine Fibroblast. Mol. Biotechnol. 2019, 61, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, K.K.; Punetha, M.; Kumar, D.; Yadav, P.S.; Long, C.R.; Selokar, N.L. Electroporation-based CRISPR gene editing in adult buffalo fibroblast cells. Anim. Biotech. 2023, 34, 5055–5066. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.C.; Gomes Mariano Junior, C.; Belizário, J.E.; Krieger, J.E.; Fernandes Bressan, F.; Roballo, K.C.S.; Fantinato-Neto, P.; Meirelles, F.V.; Chiaratti, M.R.; Concordet, J.P.; et al. Characterization of Post-Edited Cells Modified in the TFAM Gene by CRISPR/Cas9 Technology in the Bovine Model. PLoS ONE 2020, 15, e0235856. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, S.; Miyake, M.; Tsugawa, K.; Oyadomari, M.; Oyadomari, S. Integrated Stress Response of Vertebrates is Regulated by Four eIF2α Kinases. Sci. Rep. 2016, 6, 32886. [Google Scholar] [CrossRef] [PubMed]
- Square, T.; Romášek, M.; Jandzik, D.; Cattell, M.V.; Klymkowsky, M.; Medeiros, D.M. CRISPR/Cas9-Mediated Mutagenesis in the Sea Lamprey Petromyzon marinus: A Powerful Tool for Understanding Ancestral Gene Functions in Vertebrates. Development 2015, 142, 4180–4187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, H.; Lian, Z. Bioinformatics Analysis of Evolutionary Characteristics and Biochemical Structure of FGF5 Gene in Sheep. Gene 2019, 720, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Aguillon, R.; Rinsky, M.; Simon-Blecher, N.; Doniger, T.; Appelbaum, L.; Levy, O. CLOCK evolved in cnidaria to synchronize internal rhythms with diel environmental cues. Elife 2024, 12, RP89499. [Google Scholar] [CrossRef]
- Jacobus, A.P.; Cavassana, S.D.; de Oliveira, I.I.; Barreto, J.A.; Rohwedder, E.; Frazzon, J.; Basso, T.P.; Basso, L.C.; Gross, J. Optimal trade-off between boosted tolerance and growth fitness during adaptive evolution of yeast to ethanol shocks. Biotechnol. Biofuels Bioprod. 2024, 17, 63. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, I.P.M.; Costa, G.M.J.; Lacerda, S.M.D.S.N. Avian iPSC Derivation to Recover Threatened Wild Species: A Comprehensive Review in Light of Well-Established Protocols. Animals 2024, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Pessôa, L.V.; Bressan, F.F.; Freude, K.K. Induced Pluripotent Stem Cells Throughout the Animal Kingdom: Availability and Applications. World J. Stem Cells. 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nun, I.F.; Montague, S.C.; Houck, M.L.; Ryder, O.; Loring, J.F. Generation of Induced Pluripotent Stem Cells from Mammalian Endangered Species. Methods Mol. Biol. 2015, 1330, 101–109. [Google Scholar] [PubMed]
- Hirata, M.; Ichiyanagi, T.; Katoh, H.; Hashimoto, T.; Suzuki, H.; Nitta, H.; Kawase, M.; Nakai, R.; Imamura, M.; Ichiyanagi, K. Sequence divergence and retrotransposon insertion underlie interspecific epigenetic differences in primates. Mol. Biol. Evol. 2022, 39, msac208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, L.; Fu, Y.; Liu, F.; Wang, Z.; Li, Y.; Zhao, G.; Sun, W.; Wu, B.; Song, Y.; et al. Reprogramming efficiency and pluripotency of mule iPSCs over its parents. Biol. Reprod. 2023, 108, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Choijookhuu, N.; Izu, H.; Kawano, Y.; Inokuchi, M.; Honsho, K.; Lee, A.R.; Nabekura, H.; Ohta, H.; Tsukiyama, T.; et al. Flexible adaptation of male germ cells from female iPSCs of endangered Tokudaia osimensis. Sci. Adv. 2017, 12, e1602179. [Google Scholar] [CrossRef] [PubMed]
- Honda, A. Applying iPSCs for Preserving Endangered Species and Elucidating the Evolution of Mammalian Sex Determination. Bioessays 2018, 40, e1700152. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, D.J.; Limnios, I.J.; Gauthier, M.E.; Weeratunga, P.; Ovchinnikov, D.A.; Baillie, G.; Grimmond, S.M.; Graves, J.A.M.; Wolvetang, E.J. Platypus Induced Pluripotent Stem Cells: The Unique Pluripotency Signature of a Monotreme. Stem Cells Dev. 2019, 28, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Chin, C.S.H.; Lim, Z.F.S.; Ng, S.K. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front. Bioeng. Biotechnol. 2021, 9, 796991. [Google Scholar] [CrossRef]
- Toomey, M.B.; Marques, C.I.; Araújo, P.M.; Huang, D.; Zhong, S.; Liu, Y.; Schreiner, G.D.; Myers, C.A.; Pereira, P.; Afonso, S.; et al. A Mechanism for Red Coloration in Vertebrates. Curr. Biol. 2022, 32, 4201–4214.e12. [Google Scholar] [CrossRef] [PubMed]
- Bendernou, N.; Tacher, S.; Robin, S.; Rakotomanga, M.; Senger, F.; Galibert, F. Functional Analysis of a Subset of Olfactory Receptor Genes. J. Hered. 2007, 98, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Poliakov, E.; Gubin, A.N.; Stearn, O.; Li, Y.; Campos, M.M.; Gentleman, S.; Rogozin, I.B.; Redmond, T.M. Origin and Evolution of Retinoid Isomerization Machinery in Vertebrate Visual Cycle: Hint from Jawless Vertebrates. PLoS ONE 2012, 7, e49975. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Chen, S.L.; Gao, F.T.; Li, H.L.; Liu, X.F.; Wang, N.; Sha, Z.X. Establishment and Characterization of a Gonad Cell Line from Half-Smooth Tongue Sole Cynoglossus semilaevis Pseudomale. Fish Physiol. Biochem. 2015, 41, 673–683. [Google Scholar] [CrossRef]
- Callol, A.; Roher, N.; Amaro, C.; MacKenzie, S. Characterization of PAMP/PRR Interactions in European Eel (Anguilla anguilla) Macrophage-like Primary Cell Cultures. Fish Shellfish Immunol. 2013, 35, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Briscoe, E.; Gilad, Y. Evolutionary Insights into Primate Skeletal Gene Regulation Using a Comparative Cell Culture Model. PLoS Genet. 2022, 18, e1010073. [Google Scholar] [CrossRef] [PubMed]
- Roboon, J.; Hattori, T.; Nguyen, D.T.; Ishii, H.; Takarada-Iemata, M.; Kannon, T.; Hosomichi, K.; Maejima, T.; Saito, K.; Shinmyo, Y.; et al. Isolation of Ferret Astrocytes Reveals Their Morphological, Transcriptional, and Functional Differences from Mouse Astrocytes. Front. Cell. Neurosci. 2022, 16, 877131. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.S.; Bai, M.; Lee, E.M.; Luo, S.; Cook, K.R.; Ma, D.K. Cytoprotection by a Naturally Occurring Variant of ATP5G1 in Arctic Ground Squirrel Neural Progenitor Cells. eLife 2020, 9, e55578. [Google Scholar] [CrossRef]
- Vara, C.; Paytuví-Gallart, A.; Cuartero, Y.; Álvarez-González, L.; Marín-Gual, L.; Garcia, F.; Florit-Sabater, B.; Capilla, L.; Sanchéz-Guillén, R.A.; Sarrate, Z.; et al. The Impact of Chromosomal Fusions on 3D Genome Folding and Recombination in the Germ Line. Nat. Commun. 2021, 12, 2981. [Google Scholar] [CrossRef]
- Erkenbrack, E.M.; Maziarz, J.D.; Griffith, O.W.; Liang, C.; Chavan, A.R.; Nnamani, M.C.; Wagner, G.P. The mammalian Decidual Cell Evolved from a Cellular Stress Response. PLoS Biol. 2018, 16, e2005594. [Google Scholar] [CrossRef]
- Karim, N.; Lin, L.W.; Van Eenennaam, J.P.; Fangue, N.A.; Schreier, A.D.; Phillips, M.A.; Rice, R.H. Epidermal Cell Cultures from White and Green Sturgeon (Acipenser transmontanus and medirostris): Expression of TGM1-like Transglutaminases and CYP4501A. PLoS ONE 2022, 17, e0265218. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Jung, Y.; Char, K.; Jang, Y. Advances in cell coculture membranes recapitulating in vivo microenvironments. Trends Biotechnol. 2023, 41, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.L. Neural-immune interactions--an evolutionary perspective. Neuroimmunomodulation 1998, 5, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.X.; Youhanna, S.; Zandi Shafagh, R.; Kele, J.; Lauschke, V.M. Organotypic and Microphysiological Models of Liver, Gut, and Kidney for Studies of Drug Metabolism, Pharmacokinetics, and Toxicity. Chem. Res. Toxicol. 2020, 33, 38–60. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harper, J.M. Primary Cell Culture as a Model System for Evolutionary Molecular Physiology. Int. J. Mol. Sci. 2024, 25, 7905. https://doi.org/10.3390/ijms25147905
Harper JM. Primary Cell Culture as a Model System for Evolutionary Molecular Physiology. International Journal of Molecular Sciences. 2024; 25(14):7905. https://doi.org/10.3390/ijms25147905
Chicago/Turabian StyleHarper, James M. 2024. "Primary Cell Culture as a Model System for Evolutionary Molecular Physiology" International Journal of Molecular Sciences 25, no. 14: 7905. https://doi.org/10.3390/ijms25147905
APA StyleHarper, J. M. (2024). Primary Cell Culture as a Model System for Evolutionary Molecular Physiology. International Journal of Molecular Sciences, 25(14), 7905. https://doi.org/10.3390/ijms25147905





