Applications of Modified Mesenchymal Stem Cells as Targeted Systems against Tumor Cells
Abstract
1. Introduction
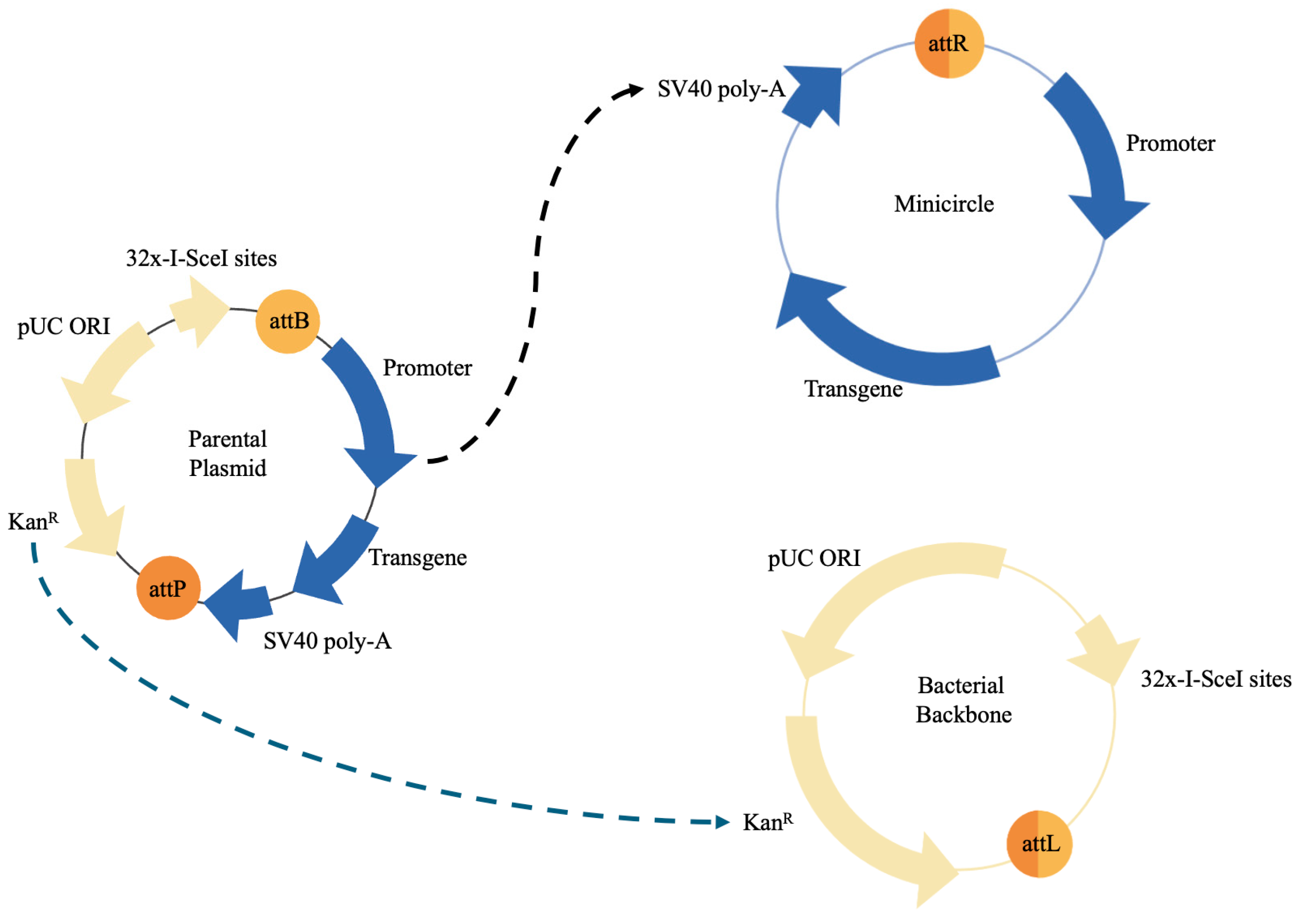
2. Plasmid-Based Genetic Modification of Mesenchymal Stem Cells
3. Exosomes
4. Use and Applications of Viral Vectors by Modifying MSCs against Tumor Cells
5. Clinical Trials and Combination of Treatments
6. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tonk, C.H.; Witzler, M.; Schulze, M.; Tobiasch, E. Mesenchymal Stem Cells. In Essential Current Concepts in Stem Cell Biology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 21–39. [Google Scholar] [CrossRef]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 2021, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.; Zhang, W.; Wang, S.; Song, J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res. Ther. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Mashal, M.; Puras, G.; Pedraz, J.L. Mesenchymal Stem Cells as a Gene Delivery Tool: Promise, Problems, and Prospects. Pharmaceutics 2021, 13, 843. [Google Scholar] [CrossRef]
- Almeida-Porada, G.; Atala, A.J.; Porada, C.D. Therapeutic Mesenchymal Stromal Cells for Immunotherapy and for Gene and Drug Delivery. Mol. Ther.—Methods Clin. Dev. 2020, 16, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells Mesenchymal Stem/Stromal Cells—An update. Stem. Cell Res. Ther. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kusamori, K.; Tsukimori, C.; Shimizu, Y.; Hayashi, M.; Kiyama, I.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Nishikawa, M. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J. Control. Release 2020, 329, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P. Breast Cancer Therapy: The Potential Role of Mesenchymal Stem Cells in Translational Biomedical Research. Biomedicines 2022, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. The Role of Mesenchymal Stem Cells in Modulating the Breast Cancer Microenvironment. Cell Transplant. 2023, 32, 9636897231220073. [Google Scholar] [CrossRef]
- Castell, A.; Yan, Q.; Fawkner, K.; Bazzar, W.; Zhang, F.; Wickström, M.; Alzrigat, M.; Franco, M.; Krona, C.; Cameron, D.P.; et al. MYCMI-7: A Small MYC-Binding Compound that Inhibits MYC: MAX Interaction and Tumor Growth in a MYC-Dependent Manner. Cancer Res. Commun. 2022, 2, 182–201. [Google Scholar] [CrossRef]
- Jaradat, S.K.; Ayoub, N.M.; Al Sharie, A.H.; Aldaod, J.M. Targeting Receptor Tyrosine Kinases as a Novel Strategy for the Treatment of Triple-Negative Breast Cancer. Technol. Cancer Res. Treat. 2024, 23, 15330338241234780. [Google Scholar] [CrossRef]
- Cai, Y.; Xi, Y.; Cao, Z.; Xiang, G.; Ni, Q.; Zhang, R.; Chang, J.; Du, X.; Yang, A.; Yan, B.; et al. Dual targeting and enhanced cytotoxicity to HER2-overexpressing tumors by immunoapoptotin-armored mesenchymal stem cells. Cancer Lett. 2016, 381, 104–112. [Google Scholar] [CrossRef]
- Yoshioka, T.; Shien, K.; Namba, K.; Torigoe, H.; Sato, H.; Tomida, S.; Yamamoto, H.; Asano, H.; Soh, J.; Tsukuda, K.; et al. Antitumor activity of pan-HER inhibitors in HER2-positive gastric cancer. Cancer Sci. 2018, 109, 1166–1176. [Google Scholar] [CrossRef]
- Martorana, F.; Motta, G.; Pavone, G.; Motta, L.; Stella, S.; Vitale, S.R.; Manzella, L.; Vigneri, P. AKT Inhibitors: New Weapons in the Fight Against Breast Cancer? Front. Pharmacol. 2021, 12, 662232. [Google Scholar] [CrossRef]
- Domagala, P.; Huzarski, T.; Lubinski, J.; Gugala, K.; Domagala, W. PARP-1 expression in breast cancer including BRCA1-associated, triple negative and basal-like tumors: Possible implications for PARP-1 inhibitor therapy. Breast Cancer Res. Treat. 2011, 127, 861–869. [Google Scholar] [CrossRef]
- Han, S.; Wei, R.; Zhang, X.; Jiang, N.; Fan, M.; Huang, J.H.; Xie, B.; Zhang, L.; Miao, W.; Butler, A.C.-P.; et al. CPT1A/2-Mediated FAO Enhancement—A Metabolic Target in Radioresistant Breast Cancer. Front. Oncol. 2019, 9, 1201. [Google Scholar] [CrossRef]
- Amara, I.; Pramil, E.; Senamaud-Beaufort, C.; Devillers, A.; Macedo, R.; Lescaille, G.; Seguin, J.; Tartour, E.; Lemoine, F.M.; Beaune, P.; et al. Engineered mesenchymal stem cells as vectors in a suicide gene therapy against preclinical murine models for solid tumors. J. Control. Release 2016, 239, 82–91. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, X.; Liang, L.; Xin, H.; Dong, X.; Li, W.; Li, J.; Guo, X.; Li, Y.; He, J.; et al. Elevated expression of CXCL3 in colon cancer promotes malignant behaviors of tumor cells in an ERK-dependent manner. BMC Cancer 2023, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Matsumoto, K.; Taniura, N.; Tomioka, D.; Nakamura, T. Inhibition of colon cancer growth and metastasis by NK4 gene repetitive delivery in mice. Biochem. Biophys. Res. Commun. 2007, 358, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Yang, E.J.; Tao, S.; Mou, P.K.; Pu, Y.; Chen, L.-J.; Shim, J.S. MDM2 inhibition is synthetic lethal with PTEN loss in colorectal cancer cells via the p53-dependent mechanism. Int. J. Biol. Sci. 2023, 19, 3544–3557. [Google Scholar] [CrossRef] [PubMed]
- Luetzkendorf, J.; Mueller, L.P.; Mueller, T.; Caysa, H.; Nerger, K.; Schmoll, H. Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. J. Cell. Mol. Med. 2009, 14, 2292–2304. [Google Scholar] [CrossRef]
- Davies, D.M.J. PD-1/PD-L1 Inhibitors for Non–Small Cell Lung Cancer: Incorporating Care Step Pathways for Effective Side-Effect Management. J. Adv. Pr. Oncol. 2019, 10, 21–35. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, H.-J.; Jung, C.-W.; Lee, T.S.; Kim, E.H.; Park, M.-J. CXCR4 uses STAT3-mediated slug expression to maintain radioresistance of non-small cell lung cancer cells: Emerges as a potential prognostic biomarker for lung cancer. Cell Death Dis. 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, S. CXCR4/CXCL12 in Non-Small-Cell Lung Cancer Metastasis to the Brain. Int. J. Mol. Sci. 2013, 14, 1713–1727. [Google Scholar] [CrossRef]
- Kolluri, K.K.; Laurent, G.J.; Janes, S.M. Mesenchymal Stem Cells as Vectors for Lung Cancer Therapy. Respiration 2013, 85, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.H.; Cecchini, M. Targeted Therapies in Advanced Gastric Cancer. Curr. Treat. Options Oncol. 2020, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, X.; Xiao, D.; Jia, Y.; Wang, Y. Efficacy and safety of targeting VEGFR drugs in treatment for advanced or metastatic gastric cancer: A systemic review and meta-analysis. Oncotarget 2018, 9, 8120–8132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- An, J.Y.; Kim, K.M.; Choi, M.G.; Noh, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Prognostic role of p-mTOR expression in cancer tissues and metastatic lymph nodes in pT2b gastric cancer. Int. J. Cancer 2010, 126, 2904–2913. [Google Scholar] [CrossRef]
- Endo, K.; Kohnoe, S.; Tsujita, E.; Watanabe, A.; Nakashima, H.; Baba, H.; Maehara, Y. Modulation of Anti-Apoptosis by Endogenous IAP Expression in MKN45 Human Gastric Cancer Cells. Anticancer Res. 2005, 25, 2713–2717. [Google Scholar]
- Tesiye, M.R.; Kia, Z.A.; Rajabi-Maham, H. Mesenchymal stem cells and prostate cancer: A concise review of therapeutic potentials and biological aspects. Stem Cell Res. 2022, 63, 102864. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, E.G.; Ali, H.Y.; Khan, M.; Pockley, G.A.; McArdle, S.E. Novel Combinatorial Approaches to Tackle the Immunosuppressive Microenvironment of Prostate Cancer. Cancers 2021, 13, 1145. [Google Scholar] [CrossRef] [PubMed]
- Tisseverasinghe, S.; Bahoric, B.; Anidjar, M.; Probst, S.; Niazi, T. Advances in PARP Inhibitors for Prostate Cancer. Cancers 2023, 15, 1849. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kumar, S.; Chanda, D.; Kallman, L.; Chen, J.; Mountz, J.D.; Ponnazhagan, S. Cancer gene therapy using mesenchymal stem cells expressing interferon-β in a mouse prostate cancer lung metastasis model. Gene Ther. 2008, 15, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Chen, S.; Kmieciak, M.; Leng, Y.; Lin, H.; A Rizzo, K.; I Dumur, C.; Ferreira-Gonzalez, A.; Dai, Y.; et al. A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations. Leukemia 2015, 29, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.S.; Dantonio, P.M.; Guimarães, T.; de Oliveira, M.B.; Alves, V.L.F.; Sandes, A.F.; Fernando, R.C.; Colleoni, G.W. Sequential combination of bortezomib and WEE1 inhibitor, MK-1775, induced apoptosis in multiple myeloma cell lines. Biochem. Biophys. Res. Commun. 2019, 519, 597–604. [Google Scholar] [CrossRef]
- Di Rorà, A.G.L.; Beeharry, N.; Imbrogno, E.; Ferrari, A.; Robustelli, V.; Righi, S.; Sabattini, E.; Falzacappa, M.V.V.; Ronchini, C.; Testoni, N.; et al. Targeting WEE1 to enhance conventional therapies for acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 11, 99. [Google Scholar] [CrossRef]
- de Jong, M.R.W.; Langendonk, M.; Reitsma, B.; Herbers, P.; Nijland, M.; Huls, G.; Berg, A.v.D.; Ammatuna, E.; Visser, L.; van Meerten, T. WEE1 Inhibition Enhances Anti-Apoptotic Dependency as a Result of Premature Mitotic Entry and DNA Damage. Cancers 2019, 11, 1743. [Google Scholar] [CrossRef]
- Weisberg, E.; Nonami, A.; Chen, Z.; Liu, F.; Zhang, J.; Sattler, M.; Nelson, E.; Cowens, K.; Christie, A.L.; Mitsiades, C.; et al. Identification of Wee1 as a novel therapeutic target for mutant RAS-driven acute leukemia and other malignancies. Leukemia 2015, 29, 27–37. [Google Scholar] [CrossRef]
- Leroux, C.; Konstantinidou, G. Targeted Therapies for Pancreatic Cancer: Overview of Current Treatments and New Opportunities for Personalized Oncology. Cancers 2021, 13, 799. [Google Scholar] [CrossRef]
- Brown, T.J.; Reiss, K.A. PARP Inhibitors in Pancreatic Cancer. Cancer J. 2021, 27, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Green, M.D.; Lang, X.; Lazarus, J.; Parsels, J.D.; Wei, S.; Parsels, L.A.; Shi, J.; Ramnath, N.; Wahl, D.R.; et al. Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer Res. 2019, 79, 3940–3951. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.; Bin Hong, Y.; Kim, J.S.; Lee, H.; Yi, Y.W.; Kim, Y.J.; Wang, A.; Zhao, W.; Cho, C.H.; Seong, Y.; et al. Inhibition of checkpoint kinase 2 (CHK 2) enhances sensitivity of pancreatic adenocarcinoma cells to gemcitabine. J. Cell. Mol. Med. 2013, 17, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, M.; Pan, L.-H.; Qian, Q.; Yao, D.-F. Glypican-3 is a biomarker and a therapeutic target of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Shimada, M.; Okano, S.; Suehiro, T.; Soejima, Y.; Tomita, Y.; Maehara, Y. IL-12 Gene Therapy Is an Effective Therapeutic Strategy for Hepatocellular Carcinoma in Immunosuppressed Mice. J. Immunol. 2004, 173, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, X.; Tang, Q.; Zhang, F.; Li, Y.; Feng, Z.; Zhu, J. Prognostic significance and potential therapeutic target of VEGFR2 in hepatocellular carcinoma. J. Clin. Pathol. 2011, 64, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiu, S.-J.; Ye, S.-L.; Tang, Z.-Y.; Xiao, X. Combined IL-12 and GM-CSF gene therapy for murine hepatocellular carcinoma. Cancer Gene Ther. 2001, 8, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, B.; Qin, J.-J.; Cheng, J.-W.; Li, X.; Rajaei, M.; Fan, J.; Yang, X.-R.; Zhang, R. A novel inhibitor of MDM2 oncogene blocks metastasis of hepatocellular carcinoma and overcomes chemoresistance. Genes Dis. 2019, 6, 419–430. [Google Scholar] [CrossRef]
- Deng, L.; Wang, C.; He, C.; Chen, L. Bone mesenchymal stem cells derived extracellular vesicles promote TRAIL-related apoptosis of hepatocellular carcinoma cells via the delivery of microRNA-20a-3p. Cancer Biomark. 2021, 30, 223–235. [Google Scholar] [CrossRef]
- Heidari, R.; Dehkordi, N.G.; Mohseni, R.; Safaei, M. Engineering mesenchymal stem cells: A novel therapeutic approach in breast cancer. J. Drug Target. 2020, 28, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Vahedi, G.; Biglari, A.; Esmaeilzadeh, A.; Athari, S.S. Mesenchymal stromal/stem cells: A new era in the cell-based targeted gene therapy of cancer. Front. Immunol. 2017, 8, 1770. [Google Scholar] [CrossRef]
- Shams, F.; Pourjabbar, B.; Hashemi, N.; Farahmandian, N.; Golchin, A.; Nuoroozi, G.; Rahimpour, A. Current progress in engineered and nano-engineered mesenchymal stem cells for cancer: From mechanisms to therapy. Biomed. Pharmacother. 2023, 167, 115505. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.; Krebsbach, P. Gene Therapy: Design and Prospects for Craniofacial Regeneration. J. Dent. Res. 2009, 88, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puente, D.H.; Pérez-Trujillo, J.J.; Zavala-Flores, L.M.; García-García, A.; Villanueva-Olivo, A.; Rodríguez-Rocha, H.; Valdés, J.; Saucedo-Cárdenas, O.; de Oca-Luna, R.M.; Loera-Arias, M.d.J. Plasmid DNA for Therapeutic Applications in Cancer. Pharmaceutics 2022, 14, 1861. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.C.; Uppalapati, M.; Ray, A. Small Circular DNAs in Human Pathology. Malays. J. Med. Sci. 2014, 21, 4–18. [Google Scholar] [PubMed]
- Gonzalez-Villarreal, C.; Said-Fernandez, S.; Soto-Dominguez, A.; Padilla-Rivas, G.; Garza-Trevino, E.; Rocha, H.R.; Martinez-Rodriguez, H. Bone marrow mesenchymal stem cells: Improving transgene expression level, transfection efficiency and cell viability. J. BUON 2018, 23, 1893–1903. [Google Scholar] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Queiroz, J.A.; Sousa, F.; Sousa, Â. Minicircle DNA: The Future for DNA-Based Vectors? Trends Biotechnol. 2020, 38, 1047–1051. [Google Scholar] [CrossRef]
- Gaspar, V.M.; Maia, C.J.; Queiroz, J.A.; Pichon, C.; Correia, I.J.; Sousa, F. Improved minicircle DNA biosynthesis for gene therapy applications. Hum. Gene Ther. Methods 2014, 25, 93–105. [Google Scholar] [CrossRef]
- Florian, M.; Wang, J.-P.; Deng, Y.; Souza-Moreira, L.; Stewart, D.J.; Mei, S.H.J. Gene engineered mesenchymal stem cells: Greater transgene expression and efficacy with minicircle vs. plasmid DNA vectors in a mouse model of acute lung injury. Stem Cell Res. Ther. 2021, 12, 184. [Google Scholar] [CrossRef]
- Ho, Y.K.; Woo, J.Y.; Tu, G.X.E.; Deng, L.-W.; Too, H.-P. A highly efficient non-viral process for programming mesenchymal stem cells for gene directed enzyme prodrug cancer therapy. Sci. Rep. 2020, 10, 14257. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Childs, J.; Salazar, L.; Disis, M. Abstract OT1-01-01: A phase I trial of the safety and immunogenicity of a multiple antigen vaccine (STEMVAC) in HER2 negative advanced stage breast cancer patients. Cancer Res. 2016, 76, OT1-01-01. [Google Scholar] [CrossRef]
- Zhuang, M.; Chen, X.; Du, D.; Shi, J.; Deng, M.; Long, Q.; Yin, X.; Wang, Y.; Rao, L. SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale 2019, 12, 173–188. [Google Scholar] [CrossRef]
- Mangraviti, A.; Tzeng, S.Y.; Gullotti, D.; Kozielski, K.L.; Kim, J.E.; Seng, M.; Abbadi, S.; Schiapparelli, P.; Sarabia-Estrada, R.; Vescovi, A.; et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials 2016, 100, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Henkle, S.L.; Betancourt, A.M. Mesenchymal Stem Cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS ONE 2012, 7, e45590. [Google Scholar] [CrossRef] [PubMed]
- Hombach, A.A.; Geumann, U.; Günther, C.; Hermann, F.G.; Abken, H. IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells. Cells 2020, 9, 873. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Du, C.; Zhang, Y.; Li, Y.; Chu, L.; Han, X.; Galons, H.; Zhang, Y.; Sun, H.; et al. Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 2020, 207, 112784. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal—Redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, X.; Song, H.; Bie, Q.; Zhang, B. Dual Role of MSC-Derived Exosomes in Tumor Development. Stem Cells Int. 2020, 2020, 8844730. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, X.; Tan, Y.; Li, Q.; Ma, J.; Wang, G. Mesenchymal Stem Cell Derived Exosomes in Cancer Progression, Metastasis and Drug Delivery: A Comprehensive Review. J. Cancer 2018, 9, 3129–3137. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, K.C.; Penfornis, P.; Dhule, S.; Guillonneau, F.; Adams, K.V.; Mo, Y.Y.; Xu, R.; Liu, Y.; Watabe, K.; Vemuri, M.C.; et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget 2014, 6, 4953–4967. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, C.; Shi, H.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, S.; Wu, X.; Yang, T.; Huang, F.; et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: Novel biomarkers and a mechanism for gastric cancer. Br. J. Cancer 2014, 110, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.-T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, T.; Huang, T.; Gao, J. Current advances and challenges of mesenchymal stem cells-based drug delivery system and their improvements. Int. J. Pharm. 2021, 600, 120477. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, X.; Wang, M.; Li, Z.; Qiao, M.; Hu, H.; Chen, D. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: Recent advances and challenges. J. Mater. Chem. B 2019, 7, 2421–2433. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; von der Ohe, J.; Hass, R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell. Mol. Biol. Lett. 2021, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liao, R.; Bai, L.; Guo, M.; Zhang, Y.; Zhang, Y.; Yang, Q.; Song, Y.; Li, Z.; Meng, Q.; et al. Anticancer effect of hUC-MSC-derived exosome-mediated delivery of PMO-miR-146b-5p in colorectal cancer. Drug Deliv. Transl. Res. 2023, 14, 1352–1369. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.C.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Jin, C.; Zhang, J.; Xu, M.; Xu, W.; Sun, Z.; Qian, H. MSC-Derived Extracellular Vesicle-Delivered L-PGDS Inhibit Gastric Cancer Progression by Suppressing Cancer Cell Stemness and STAT3 Phosphorylation. Stem Cells Int. 2022, 2022, 9668239. [Google Scholar] [CrossRef]
- Cavarretta, I.T.; Altanerova, V.; Matuskova, M.; Kucerova, L.; Culig, Z.; Altaner, C. Adipose Tissue–derived Mesenchymal Stem Cells Expressing Prodrug-converting Enzyme Inhibit Human Prostate Tumor Growth. Mol. Ther. 2010, 18, 223–231. [Google Scholar] [CrossRef]
- Matuskova, M.; Hlubinova, K.; Pastorakova, A.; Hunakova, L.; Altanerova, V.; Altaner, C.; Kucerova, L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010, 290, 58–67. [Google Scholar] [CrossRef]
- Gomari, H.; Moghadam, M.F.; Soleimani, M. Targeted cancer therapy using engineered exosome as a natura drug delivery vehicle. OncoTargets Ther. 2018, 11, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Kurniawati, I.; Liu, M.-C.; Hsieh, C.-L.; Do, A.D.; Sung, S.-Y. Targeting Castration-Resistant Prostate Cancer Using Mesenchymal Stem Cell Exosomes for Therapeutic MicroRNA-let-7c Delivery. Front. Biosci. 2022, 27, 256. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, Y.; Liao, C.; Ma, X.; Wang, M.; Li, Q.; Wang, D.; Li, Y.; Zhang, X.; Li, L.; et al. Engineered mesenchymal stem cell exosomes loaded with miR-34c-5p selectively promote eradication of acute myeloid leukemia stem cells. Cancer Lett. 2023, 575, 216407. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Sun, J.; Qiu, J.; Chen, G.; Wang, X.; Mu, Y.; Li, K.; Wang, W. Antitumor Activity of Cabazitaxel and MSC-TRAIL Derived Extracellular Vesicles in Drug-Resistant Oral Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 10809–10820. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, F.; Chen, J.; Lin, H.; Wang, S.; Wang, Y.; Wu, C.; Lin, J.; Zhong, G. Mesenchymal Stem Cell Derived Exosomes as Nanodrug Carrier of Doxorubicin for Targeted Osteosarcoma Therapy via SDF1-CXCR4 Axis. Int. J. Nanomed. 2022, 17, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Study Details|iExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation|ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/study/NCT03608631?cond=cancer&term=msc%20exosome&rank=1 (accessed on 31 March 2024).
- Study Details|UCMSC-Exo for Chemotherapy-induced Myelosuppression in Acute Myeloid Leukemia ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/study/NCT06245746?cond=cancer&term=stem%20cell%20exosome&rank=6 (accessed on 31 March 2024).
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mese, K.; Bunz, O.; Ehrhardt, A. State-of-the-art human adenovirus vectorology for therapeutic approaches. FEBS Lett. 2019, 593, 3609–3622. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Ferreira, M.V.; Fernandes, S.; Almeida, A.I.; Neto, S.; Mendes, J.P.; Silva, R.J.S.; Peixoto, C.; Coroadinha, A.S. Extending AAV Packaging Cargo through Dual Co-Transduction: Efficient Protein Trans-Splicing at Low Vector Doses. Int. J. Mol. Sci. 2023, 24, 10524. [Google Scholar] [CrossRef]
- Hammer, K.; Kazcorowski, A.; Liu, L.; Behr, M.; Schemmer, P.; Herr, I.; Nettelbeck, D.M. Engineered adenoviruses combine enhanced oncolysis with improved virus production by mesenchymal stromal carrier cells. Int. J. Cancer 2015, 137, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Chira, S.; Jackson, C.S.; Oprea, I.; Ozturk, F.; Pepper, M.S.; Diaconu, I.; Braicu, C.; Raduly, L.-Z.; Calin, G.A.; Berindan-Neagoe, I. Progresses towards safe and efficient gene therapy vectors. Oncotarget 2015, 6, 30675–30703. [Google Scholar] [CrossRef]
- So, P.-W.; Parkes, H.G.; Bell, J.D. Application of magnetic resonance methods to studies of gene therapy. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 51, 49–62. [Google Scholar] [CrossRef]
- Terskikh, A.V.; Ershler, M.A.; Drize, N.J.; Nifontova, I.N.; Chertkov, J.L. Long-term persistence of a nonintegrated lentiviral vector in mouse hematopoietic stem cells. Exp. Hematol. 2005, 33, 873–882. [Google Scholar] [CrossRef]
- Shaw, A.; Cornetta, K. Design and Potential of Non-Integrating Lentiviral Vectors. Biomedicines 2014, 2, 14–35. [Google Scholar] [CrossRef]
- Golinelli, G.; Mastrolia, I.; Aramini, B.; Masciale, V.; Pinelli, M.; Pacchioni, L.; Casari, G.; Dall’ora, M.; Soares, M.B.P.; Damasceno, P.K.F.; et al. Arming Mesenchymal Stromal/Stem Cells Against Cancer: Has the Time Come? Front. Pharmacol. 2020, 11, 529921. [Google Scholar] [CrossRef]
- Darestani, N.G.; Gilmanova, A.I.; Al-Gazally, M.E.; Zekiy, A.O.; Ansari, M.J.; Zabibah, R.S.; Jawad, M.A.; Al-Shalah, S.A.J.; Rizaev, J.A.; Alnassar, Y.S.; et al. Mesenchymal stem cell-released oncolytic virus: An innovative strategy for cancer treatment. Cell Commun. Signal. 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, A.; Hammer, K.; Liu, L.; Villhauer, S.; Nwaeburu, C.; Fan, P.; Zhao, Z.; Gladkich, J.; Groß, W.; Nettelbeck, D.M.; et al. Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget 2016, 7, 9046–9059. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hong, J.A.; Choi, H.J.; Song, J.J. Enhanced tumor targeting and timely viral release of mesenchymal stem cells/oncolytic virus complex due to GRP78 and inducible E1B55K expressions greatly increase the antitumor effect of systemic treatment. Mol. Ther.—Oncolytics 2022, 27, 26–47. [Google Scholar] [CrossRef]
- Stoff-Khalili, M.A.; Rivera, A.A.; Mathis, J.M.; Banerjee, N.S.; Moon, A.S.; Hess, A.; Rocconi, R.P.; Numnum, T.M.; Everts, M.; Chow, L.T.; et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res. Treat. 2007, 105, 157–167. [Google Scholar] [CrossRef]
- Xin, H.; Kanehira, M.; Mizuguchi, H.; Hayakawa, T.; Kikuchi, T.; Nukiwa, T.; Saijo, Y. Targeted Delivery of CX3CL1 to Multiple Lung Tumors by Mesenchymal Stem Cells. Stem Cells 2007, 25, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cheng, P.; Yin, T.; He, H.; Yang, L.; Wei, Y.; Chen, X. Therapeutic potential of bone marrow-derived mesenchymal stem cells producing pigment epithelium-derived factor in lung carcinoma. Int. J. Mol. Med. 2012, 30, 527–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hakkarainen, T.; Särkioja, M.; Lehenkari, P.; Miettinen, S.; Ylikomi, T.; Suuronen, R.; Desmond, R.A.; Kanerva, A.; Hemminki, A. Human Mesenchymal Stem Cells Lack Tumor Tropism but Enhance the Antitumor Activity of Oncolytic Adenoviruses in Orthotopic Lung and Breast Tumors. Hum. Gene Ther. 2007, 18, 627–641. [Google Scholar] [CrossRef]
- Mohr, A.; Lyons, M.; Deedigan, L.; Harte, T.; Shaw, G.; Howard, L.; Barry, F.; O’Brien, T.; Zwacka, R. Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J. Cell. Mol. Med. 2008, 12, 2628–2643. [Google Scholar] [CrossRef] [PubMed]
- Studeny, M.; Marini, F.C.; Dembinski, J.L.; Zompetta, C.; Cabreira-Hansen, M.; Bekele, B.N.; Champlin, R.E.; Andreeff, M. Mesenchymal Stem Cells: Potential Precursors for Tumor Stroma and Targeted-Delivery Vehicles for Anticancer Agents. JNCI J. Natl. Cancer Inst. 2004, 96, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.P.; Luetzkendorf, J.; Widder, M.; Nerger, K.; Caysa, H.; Mueller, T. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2010, 18, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Reyes, A.G.; Delgado-González, P.; Islas, J.F.; Soto-Domínguez, A.; González-Villarreal, C.A.; Padilla-Rivas, G.R.; Garza-Treviño, E.N. Oxaliplatin Enhances the Apoptotic Effect of Mesenchymal Stem Cells, Delivering Soluble TRAIL in Chemoresistant Colorectal Cancer. Pharmaceuticals 2023, 16, 1448. [Google Scholar] [CrossRef] [PubMed]
- Fakiruddin, K.S.; Ghazalli, N.; Lim, M.N.; Zakaria, Z.; Abdullah, S. Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. Int. J. Mol. Sci. 2018, 19, 2188. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, S.; Daneshmandi, S.; Menaa, F. Tumor Necrosis Factor-α/CD40 Ligand-Engineered Mesenchymal Stem Cells Greatly Enhanced the Antitumor Immune Response and Lifespan in Mice. Hum. Gene Ther. 2014, 25, 240–253. [Google Scholar] [CrossRef]
- Yan, C.; Song, X.; Yu, W.; Wei, F.; Li, H.; Lv, M.; Zhang, X.; Ren, X. Human umbilical cord mesenchymal stem cells delivering sTRAIL home to lung cancer mediated by MCP-1/CCR2 axis and exhibit antitumor effects. Tumor Biol. 2016, 37, 8425–8435. [Google Scholar] [CrossRef]
- Harati, M.D.; Amiri, F.; Jaleh, F.; Mehdipour, A.; Molaee, S.; Bahadori, M.; Shokrgozar, M.A.; Jalili, M.A.; Roudkenar, M.H. Targeting delivery of lipocalin 2-engineered mesenchymal stem cells to colon cancer in order to inhibit liver metastasis in nude mice. Tumor Biol. 2015, 36, 6011–6018. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Xu, C.; Xu, X. Apoptin-modified human mesenchymal stem cells inhibit growth of lung carcinoma in nude mice. Mol. Med. Rep. 2015, 12, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Gui, L.; Wang, C.; Yan, J.; Liu, M.; Ji, L.; Wang, Y.; Ma, B.; Gao, W.-Q. Targeted Delivery of CXCL9 and OX40L by Mesenchymal Stem Cells Elicits Potent Antitumor Immunity. Mol. Ther. 2020, 28, 2553–2563. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Marini, F.; Konopleva, M.; Schober, W.; Shi, Y.; Burks, J.; Clise-Dwyer, K.; Wang, R.-Y.; Zhang, W.; Yuan, X.; et al. Mesenchymal Stem Cells Overexpressing IFN-β Inhibit Breast Cancer Growth and Metastases through Stat3 Signaling in a Syngeneic Tumor Model. Cancer Microenviron. 2010, 3, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, J.; Xu, X.; Xu, C.; Song, W. IFN-γ-Secreting-Mesenchymal Stem Cells Exert an Antitumor Effect In Vivo via the TRAIL Pathway. J. Immunol. Res. 2014, 2014, 318098. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.H.; Mikkelsen, J.G. Delivering genes with human immunodeficiency virus-derived vehicles: Still state-of-the-art after 25 years. J. Biomed. Sci. 2022, 29, 79. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, L.; Altanerova, V.; Matuskova, M.; Tyciakova, S.; Altaner, C. Adipose Tissue–Derived Human Mesenchymal Stem Cells Mediated Prodrug Cancer Gene Therapy. Cancer Res. 2007, 67, 6304–6313. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, P.; Liu, X.; Lv, P. Expression of interleukin-12 by adipose-derived mesenchymal stem cells for treatment of lung adenocarcinoma. Thorac. Cancer 2015, 6, 80–84. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xu, W.; Qian, H.; Ye, S.; Zhu, W.; Cao, H.; Yan, Y.; Li, W.; Wang, M.; et al. Experimental Therapy for Lung Cancer: Umbilical Cord-Derived Mesenchymal Stem Cell-Mediated Interleukin-24 Delivery. Curr. Cancer Drug Targets 2012, 13, 92–102. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawamura, K.; Li, Q.; Okamoto, S.; Tada, Y.; Tatsumi, K.; Shimada, H.; Hiroshima, K.; Yamaguchi, N.; Tagawa, M. Mesenchymal stem cells are efficiently transduced with adenoviruses bearing type 35-derived fibers and the transduced cells with the IL-28A gene produces cytotoxicity to lung carcinoma cells co-cultured. BMC Cancer 2014, 14, 713. [Google Scholar] [CrossRef]
- Loebinger, M.R.; Eddaoudi, A.; Davies, D.; Janes, S.M. Mesenchymal Stem Cell Delivery of TRAIL Can Eliminate Metastatic Cancer. Cancer Res. 2009, 69, 4134–4142. [Google Scholar] [CrossRef]
- Hoyos, V.; Del Bufalo, F.; Yagyu, S.; Ando, M.; Dotti, G.; Suzuki, M.; Bouchier-Hayes, L.; Alemany, R.; Brenner, M.K. Mesenchymal Stromal Cells for Linked Delivery of Oncolytic and Apoptotic Adenoviruses to Non-small-cell Lung Cancers. Mol. Ther. 2015, 23, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Z.; Xu, X.; Xu, Z.; Wang, S.; Huang, D.; Li, Y.; Mou, X.; Liu, F.; Xiang, C. Menstrual Blood-Derived Stem Cells as Delivery Vehicles for Oncolytic Adenovirus Virotherapy for Colorectal Cancer. Stem Cells Dev. 2019, 28, 882–896. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Sun, H.; Hua, Y.; Zhang, G.; Jiang, J.; Wang, X. Adipose Mesenchymal Stem Cell-Derived Exosomal microRNA-1236 Reduces Resistance of Breast Cancer Cells to Cisplatin by Suppressing SLC9A1 and the Wnt/β-Catenin Signaling. Cancer Manag. Res. 2020, 12, 8733–8744. [Google Scholar] [CrossRef]
- Kucerova, L.; Skolekova, S.; Matuskova, M.; Bohac, M.; Kozovska, Z. Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer 2013, 13, 535. [Google Scholar] [CrossRef]
- Cao, W.; Liu, B.; Xia, F.; Duan, M.; Hong, Y.; Niu, J.; Wang, L.; Liu, Y.; Li, C.; Cui, D. MnO2@Ce6-loaded mesenchymal stem cells as an “oxygen-laden guided-missile” for the enhanced photodynamic therapy on lung cancer. Nanoscale 2020, 12, 3090–3102. [Google Scholar] [CrossRef] [PubMed]
- Jahedi, M.; Meshkini, A. Tumor tropic delivery of FU.FA@NSs using mesenchymal stem cells for synergistic chemo-photodynamic therapy of colorectal cancer. Colloids Surf. B Biointerfaces 2023, 226, 113333. [Google Scholar] [CrossRef]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Wang, J.; Bin, L.; Hu, X. Delivery of sFIT-1 engineered MSCs in combination with a continuous low-dose doxorubicin treatment prevents growth of liver cancer. Aging 2016, 8, 3520–3534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimizu, Y.; Gumin, J.; Gao, F.; Hossain, A.; Shpall, E.J.; Kondo, A.; Kerrigan, B.C.P.; Yang, J.; Ledbetter, D.; Fueyo, J.; et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J. Neurosurg. 2022, 136, 757–767. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hwang, K.-A.; Go, R.-E.; Kim, C.-W.; Choi, K.-C. Gene therapy strategies using engineered stem cells for treating gynecologic and breast cancer patients (Review). Oncol. Rep. 2015, 33, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Ruano, D.; López-Martín, J.A.; Moreno, L.; Lassaletta, Á.; Bautista, F.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020, 28, 1033–1042. [Google Scholar] [CrossRef]
- Han, A.R.; Shin, H.R.; Kwon, J.; Lee, S.B.; Lee, S.E.; Kim, E.Y.; Kweon, J.; Chang, E.J.; Kim, Y.; Kim, S.W. Highly efficient ge-nome editing via CRISPR-Cas9 ribonucleoprotein (RNP) delivery in mesenchymal stem cells. BMB Rep. 2024, 57, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, A.; Malekpour, K.; Soudi, S.; Hashemi, S.M. CRISPR/Cas9-engineered mesenchymal stromal/stem cells and their extracellular vesicles: A new approach to overcoming cell therapy limitations. Biomed. Pharmacother. 2022, 156, 113943. [Google Scholar] [CrossRef]
- Bui, Q.T.; Lee, K.D.; Fan, Y.C.; Lewis, B.S.; Deng, L.W.; Tsai, Y.C. Disruption of CCL2 in Mesenchymal Stem Cells as an Anti-Tumor Approach against Prostate Cancer. Cancers 2023, 10, 441. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Ma, Y.; Hou, Y.-C.; Pan, J.; Chen, S.-Y.; Chien, M.-H.; Zhang, Z.-X.; Hsu, W.-H.; Wang, X.; Zhang, J.; et al. Dual targeted extracellular vesicles regulate oncogenic genes in advanced pancreatic cancer. Nat. Commun. 2023, 14, 6692. [Google Scholar] [CrossRef]
- Takahara, K.; Ii, M.; Inamoto, T.; Nakagawa, T.; Ibuki, N.; Yoshikawa, Y.; Tsujino, T.; Uchimoto, T.; Saito, K.; Takai, T.; et al. microRNA-145 Mediates the Inhibitory Effect of Adipose Tissue-Derived Stromal Cells on Prostate Cancer. Stem Cells Dev. 2016, 25, 1290–1298. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, S.-R.; Jung, B.-K.; Jeon, Y.-K.; Lee, Y.-S.; Kim, M.-K.; Kim, Y.-G.; Jang, J.-Y.; Kim, C.-W. Exosomes Derived from Mesenchymal Stem Cells Suppress Angiogenesis by Down-Regulating VEGF Expression in Breast Cancer Cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, Y.; Zeng, X.; He, M.; Gong, Y.; Liu, Y. Extracellular vesicles-encapsulated let-7i shed from bone mesenchymal stem cells suppress lung cancer via KDM3A/DCLK1/FXYD3 axis. J. Cell. Mol. Med. 2021, 25, 1911–1926. [Google Scholar] [CrossRef]
- Li, S.; Yan, G.; Yue, M.; Wang, L. Extracellular vesicles-derived microRNA-222 promotes immune escape via interacting with ATF3 to regulate AKT1 transcription in colorectal cancer. BMC Cancer 2021, 21, 349. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, C. Exosomes miR-22-3p Derived from Mesenchymal Stem Cells Suppress Colorectal Cancer Cell Proliferation and Invasion by Regulating RAP2B and PI3K/AKT Pathway. J. Oncol. 2021, 2021, 3874478. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.L.; Yao, J.L.; Wang, K.; Ai, H. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int. 2019, 2019, 8108576. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Mao, Y.; Wu, D.; Zhu, Y.; Lu, J.; Huang, Y.; Guo, Y.; Wang, Z.; Zhu, S.; Li, X.; et al. Exosomal circ_0030167 derived from BM-MSCs inhibits the invasion, migration, proliferation and stemness of pancreatic cancer cells by sponging miR-338-5p and targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 2021, 512, 38–50. [Google Scholar] [CrossRef] [PubMed]

| Function | Target Therapy | Therapeutic Approach | References | |
|---|---|---|---|---|
| Cancer Type | ||||
| Breast cancer | TNF-α, IL-1β, IL-6, IL-8, IFN-γ | Cytokines regulate immune system | [11] | |
| PI3k/AKT MYC-Max * inhibitors RTK inhibitors Anti-HER2 Anti-EGFR | Signaling pathways | [12] [13] [14] [15] [16] | ||
| Anti-PARP | DNA repair pathway | [17] | ||
| CPT1A/2 CYP2B6TM-RED Genes CDK4/6, | Suicide gene | [18] [19] | ||
| Colon cancer | BMP4, IL7-IL12 CX3CL NK4 Inhibitor MDM2 | Immune regulatory networks | [20] [21] [22] | |
| TRAIL | Apoptotic proteins | [23] | ||
| MDM2 | Negative regulator of p53 | [22] | ||
| Lung cancer | PD1/PDL-1 CXCL12 CXCR4 | Immune regulatory networks | [24] [25,26] | |
| Oncolytic virus | Elimination directly | [27] | ||
| Gastric cancer | Anti-HER2 Anti-EGFR Anti-VEGF TKIs Anti-mTOR Anti-HFG/MET | Key signaling pathways | [28] [29] [30] [28] | |
| Anti-PARP | DNA repair pathway | [31] | ||
| Prostate |
Anti-VEGFR PI3K ERK | Key signaling pathways | [32] | |
| Anti-CTLA-4 | Immune regulatory networks | [33] | ||
| Anti-PARP | DNA repair pathway | [34,35] | ||
| Pancreatic |
HDAC inhibitors TKIs RAS-RAF-MEK-ERK PI3K-AKT-mTOR TP53 | Key signaling pathways | [36] [37] [38] [39] [40] | |
| PARP inhibitors ATM inhibitors Checkpoint kinase 1 (CHK1) and CHK2 | DNA repair pathway | [41,42] [43] [44] | ||
| Enhance dependency on BCL-2 and/or MCL-1 inhibition) | Anti-apoptosis | [39] | ||
| Hepatocellular | Anti-HER2 GPC-3 IL-12 VEGFR GM-CSF | Key signaling pathways | [45] [46] [47] [48] [49] | |
| MDM2 | Negative regulator of p53 | [50] | ||
| TRAIL | Apoptosis protein | [51] | ||
| Source | Tumor Type | Approach | Reference |
|---|---|---|---|
| Umbilical cord MSC | Colorectal cancer | Exosomes loaded with Anti-miR—146b-5p ASO (PMO-146b) | [87] |
| Non-specified MSC | Cancer cell lines (lung, renal, breast and neuroblastoma) | Exosomes loaded with TRAIL (TNFa-Related Apoptosis Inducing Ligand). | [88] |
| Non-specified MSC | Gastric cancer | Exosomes loaded with lipocalin-type prostaglandin D2 synthetase (L-PGDS). | [89] |
| Adipose tissue MSC | Prostate cancer | Exosomes loaded with cytosine deaminase:uracil phosphoribosyl transferase along with 5-flucytosine treatment (enzyme and substrate-prodrug to synthesize 5-FU) | [90] |
| Adipose tissue MSC | Glioblastoma | Exosomes loaded with herpes simplex virus thymidine kinase (HSV-TK) along with ganciclovir treatment (enzyme and substrate prodrug to synthesize GCV-triphosphate) | [91] |
| Umbilical cord MSC | Breast cancer | Exosomes loaded with taxol. | [83] |
| Non-specified MSC | Breast cancer | Exosomes carrying DARPins (Designed Ankyrin Repeated Proteins) to enhance HER2+ cell uptake. Exosomes loaded with doxorubicin. | [92] |
| Bone marrow MSC | Castration-resistant prostate cancer | Exosomes loaded with miR-let-7c | [93] |
| Umbilical cord MSC | Acute myeloid leukemia | Exosomes overexpressing Lamp2b-IL3 to improve their targeting system against leukemia stem cells. Exosomes loaded with miR-34c-5p to eliminate malignant cells. | [94] |
| Non-specified MSC | Oral squamous cell carcinoma | Exosomes loaded with TRAIL and cabazitaxel. | [95] |
| Bone marrow MSC | Osteosarcoma | Exosomes loaded with doxorubicin. | [96] |
| Virus | Ad | AVV | Lentivirus |
|---|---|---|---|
| Advantages | Low pathogenicity Safety Well-tolerated Large transgene-carrying capacity (8–36 kb) Transduce-dividing and non-dividing cells Do not integrate their genome into the host genome and remain extrachromosomal. The most common viral vectors for MSC transduction | High efficiency, safety, and lowest risk (non-inflammatory and non-pathogenic) Transgene-carrying capacity 5 kb Transduce-dividing and non-dividing cells Genome episomal (>90%) site-specific integration (<10%) | Low pathogenicity Safety Well-tolerated transgene-carrying capacity (8 kb) Transduce-dividing and non-dividing cells Integration genome High infectivity Capability of stable gene transferring |
| Disadvantages | Inflammatory effect | Small packaging capacity Requiring helper AdV for replication-associated difficulty producing pure viral stocks Application of these vectors has been limited due to their low aptitude for MSC transduction. Improve the efficiency of transgene delivery of Ad vectors in MSC modifications done on the viral capsid and fibers. | Transgene integration might result in oncogenesis. Next-generation lentivirus block integration into the host cell genome, and a few mutations in viral integrase coding sequence are enough to inactivate the integrase function while preserving its role in transgene expression. |
| References | [99,100,101,102] | [103,104,105,106] | [106,107,108] |
| Author | Vector | Transgene | Cancer Model | Results’ Relevance | Reference |
|---|---|---|---|---|---|
| Proteins | |||||
| Michael R. Loebinger, 2009 | Lentivirus | TRAIL | Breast cancer Lung cancer | TRAIL-MSCs reduce tumor and metastasis. | [134] |
| Quiroz-Reyes, 2023 | Lentivirus | TRAIL | Colorectal cancer | Oxaliplatin increases the sensibility of cancer cells to soluble TRAIL apoptosis. | [120] |
| Shahrokhi, S., 2014 | Lentivirus | TNF-α and CD40L | Breast cancer | Increased mouse survival, optimized antitumor immunity response | [122] |
| Yan, C, 2016. | Lentivirus | ISZ-sTRAIL | Lung cancer | Apoptosis induction and tumor growth reduction in xenograft murine model | [123] |
| Harati, M.D, 2015 | Lentivirus | Lipocalin 2 | Colon cancer | Reduction of liver metastasis by downregulation of VEGF | [124] |
| Du, J., 2015. | Lentivirus | Apoptin | Lung cancer | Apoptosis via caspase-3 activation | [125] |
| Studeny, M., 2004 | Adenovirus | IFN-β | Breast cancer | In situ inhibition of proliferation | [118] |
| Ling, X, 2010. | Lentivirus | IFN-β | Breast cancer | Inactivation of Stat3, Src, and Akt; downregulation of cMyc and MMP2 expression | [127] |
| Yang, X, 2014 | Lentivirus | IFN-γ | Lung cancerBreast cancer | Activation of apoptosis by TRAIL-mediated caspase-3. Suppress tumor growth on a lung carcinoma xenograft. | [128]. |
| Li, X., 2015 | Lentivirus | IL-12 | Lung cancer | Prevent tumor growth and invasion of A549 carcinoma cells | [131] |
| Zhang, X, 2012. | Lentivirus | IL-24 | Lung cancer | Inhibit A549 cell growth in vitro and in vivo tumor xenograft. | [132]. |
| Suzuki, T., 2014. | Adenovirus AdF35 | IL-28A | Lung cancer | Reduction of OBA-LK1 viability. | [133]. |
| Yin, P. et al., 2020 | Lentivirus | CXCL9/OX40L | Colon cancer | Increase CD8+ T and NK cells in tumors and improve PD-1 response. | [126] |
| Oncolytic Virus | |||||
| Hoyos, V. et al., 2015 | Oncolytic adenovirus | ICOVIR15 and Ad.iC9 | Lung cancer | Increase overall survival and tumor control | [135] |
| Stoff-Khalili, M.A., 2007 | Oncolytic adenovirus Ad5/3 | CXCR4 | Breast cancer | Oncolysis in MDA-MB-231 cells and reduction of lung metastasis | [113]. |
| Guo, Y. et al., 2019 | Oncolytic adenovirus | ICOVIR5 | Lung cancer | Activation of T cell immunity and migration | [136] |
| Modification | MSC Delivering | Conventional Therapy | Model | Reference |
|---|---|---|---|---|
| Unmodified MSC | microRNA-1236 | Cisplatin | In vitro | [137] |
| SDF-1α/CXCR4 | 5-FU and doxorubicin | In vitro | [138] | |
| Nanoparticles | Manganese oxide (MnO2) nanoparticles | Ce6 | In vivo | [139] |
| Nanoparticles | 5-Fluorouracil (FU) and folinic acid (FA) | In vitro | [140] | |
| Nanoparticles | Paclitaxel | In vitro and in vivo | [141] | |
| Lentiviral | TRAIL | Oxaliplatin | In vitro | [120] |
| Adenoviral | sFlt-1 | Doxorubicin | In vitro and in vivo | [142] |
| Oncolytic virus | Delta-24-RGD | Chemotherapy and radiotherapy | In vivo | [143] |
| AF2.CD-TK | 5-FC and GCV | In vitro and in vivo | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garza Treviño, E.N.; Quiroz Reyes, A.G.; Delgado Gonzalez, P.; Rojas Murillo, J.A.; Islas, J.F.; Alonso, S.S.; Gonzalez Villarreal, C.A. Applications of Modified Mesenchymal Stem Cells as Targeted Systems against Tumor Cells. Int. J. Mol. Sci. 2024, 25, 7791. https://doi.org/10.3390/ijms25147791
Garza Treviño EN, Quiroz Reyes AG, Delgado Gonzalez P, Rojas Murillo JA, Islas JF, Alonso SS, Gonzalez Villarreal CA. Applications of Modified Mesenchymal Stem Cells as Targeted Systems against Tumor Cells. International Journal of Molecular Sciences. 2024; 25(14):7791. https://doi.org/10.3390/ijms25147791
Chicago/Turabian StyleGarza Treviño, Elsa N., Adriana G. Quiroz Reyes, Paulina Delgado Gonzalez, Juan Antonio Rojas Murillo, Jose Francisco Islas, Santiago Saavedra Alonso, and Carlos A. Gonzalez Villarreal. 2024. "Applications of Modified Mesenchymal Stem Cells as Targeted Systems against Tumor Cells" International Journal of Molecular Sciences 25, no. 14: 7791. https://doi.org/10.3390/ijms25147791
APA StyleGarza Treviño, E. N., Quiroz Reyes, A. G., Delgado Gonzalez, P., Rojas Murillo, J. A., Islas, J. F., Alonso, S. S., & Gonzalez Villarreal, C. A. (2024). Applications of Modified Mesenchymal Stem Cells as Targeted Systems against Tumor Cells. International Journal of Molecular Sciences, 25(14), 7791. https://doi.org/10.3390/ijms25147791






