Single-Molecule Mixture: A Concept in Polymer Science
Abstract
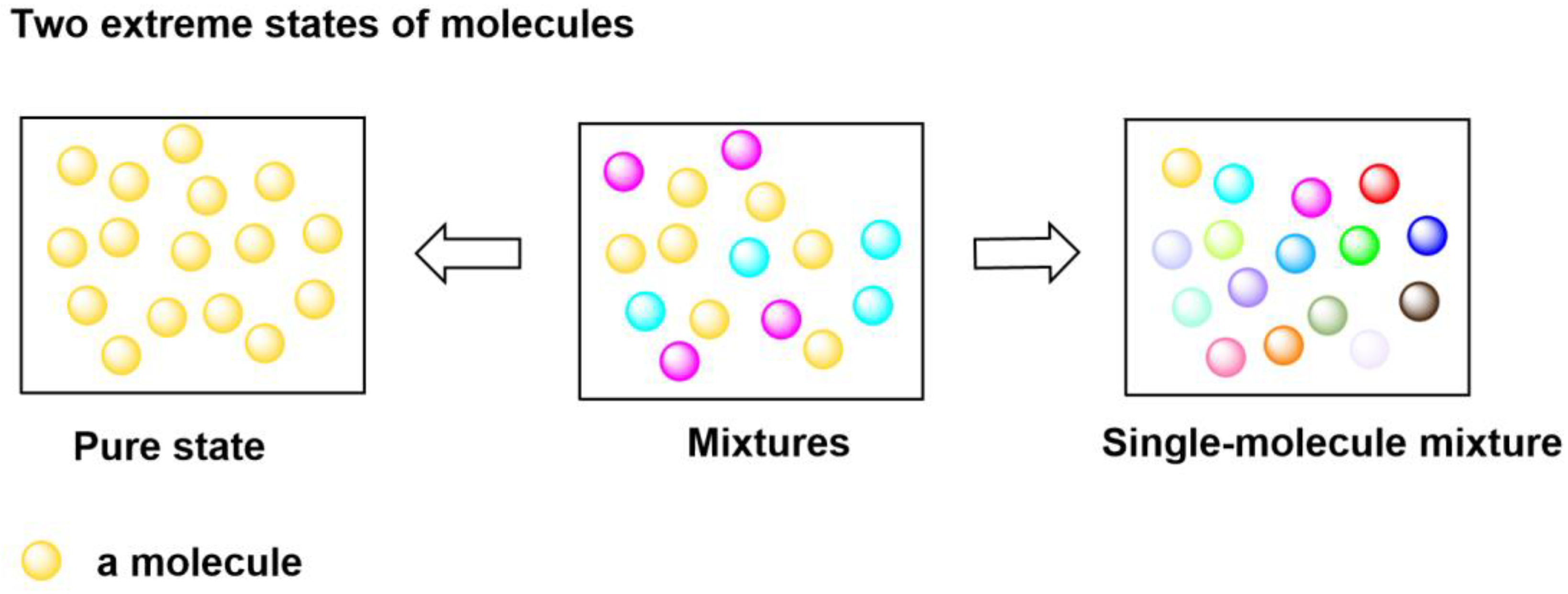
1. Introduction
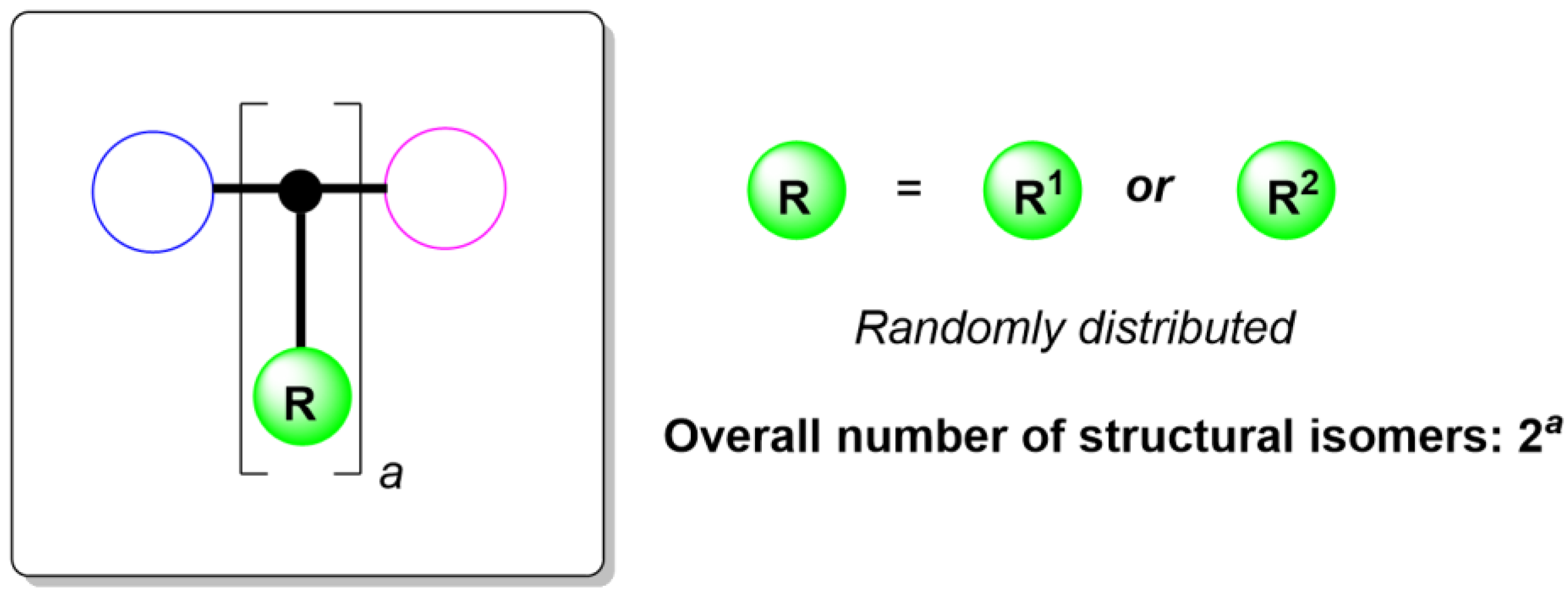
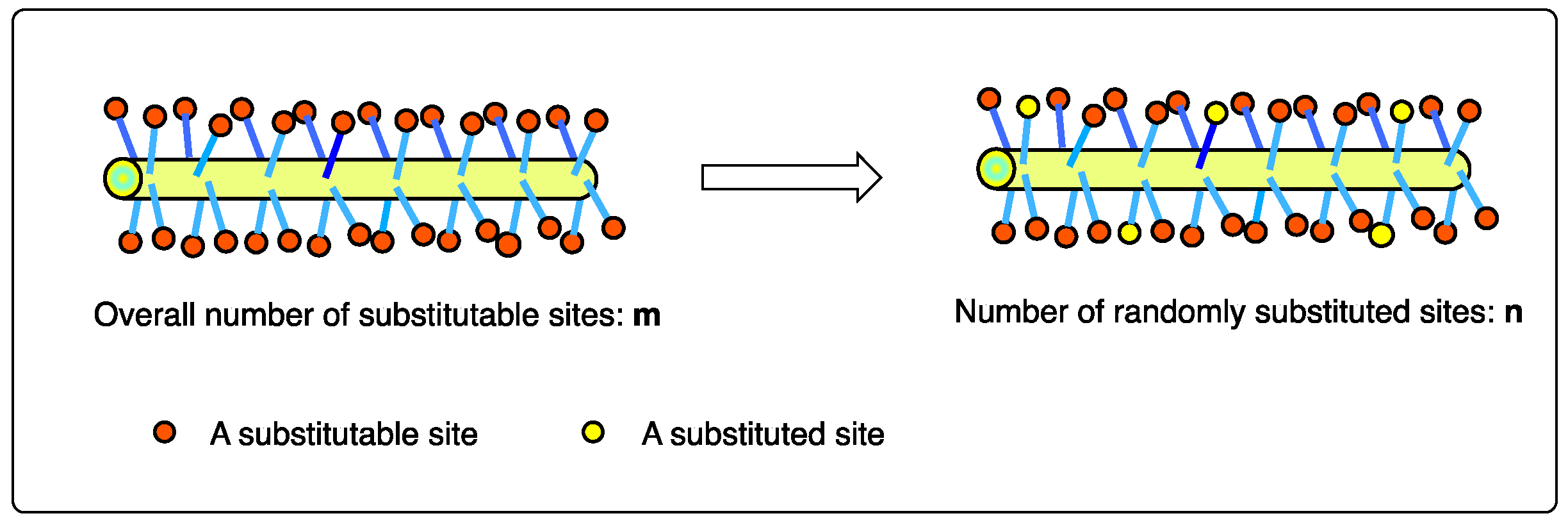
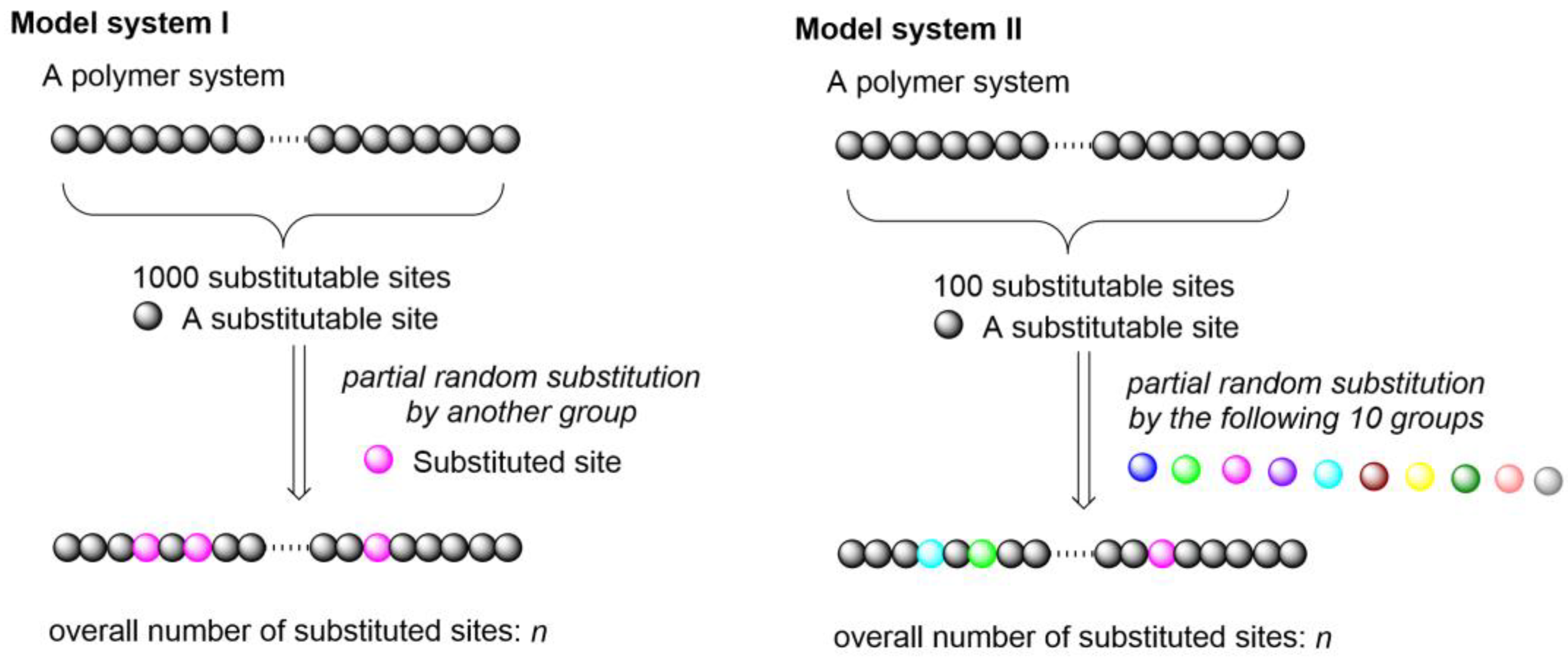
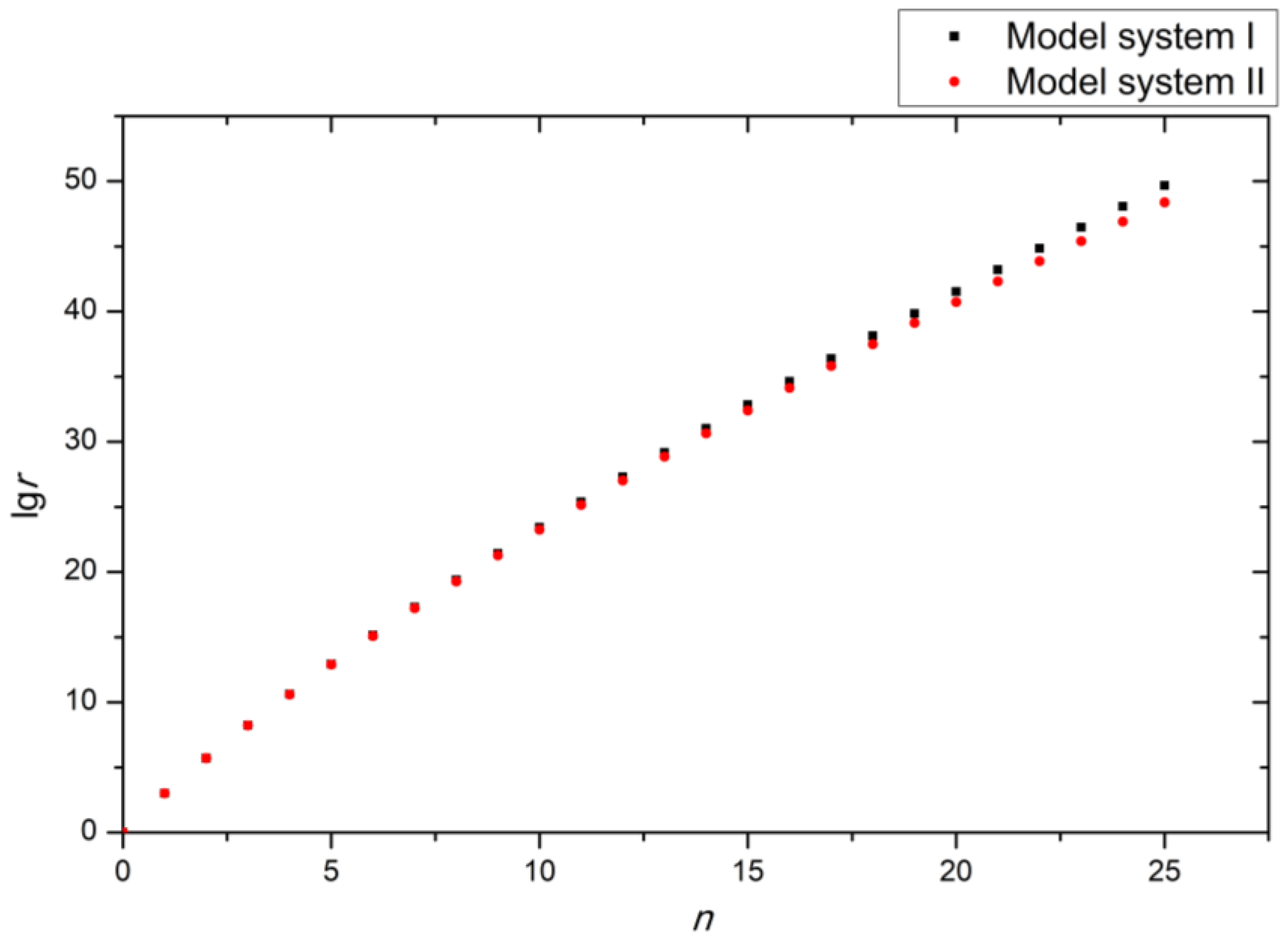
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, C.M. Chemical space and biology. Nature 2004, 432, 824–828. [Google Scholar] [CrossRef]
- Reymond, J.-L. The Chemical Space Project. Acc. Chem. Res. 2015, 48, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Llanos, E.J.; Leal, W.; Luu, D.H.; Jost, J.; Stadler, P.F.; Restrepo, G. Exploration of the chemical space and its three historical regimes. Proc. Natl. Acad. Sci. USA 2019, 116, 12660–12665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mathis, C.; Bajczyk, M.D.; Marshall, S.M.; Wilbraham, L.; Cronin, L. Exploring and mapping chemical space with molecular assembly trees. Sci. Adv. 2021, 7, eabj2465. [Google Scholar] [CrossRef] [PubMed]
- Semler, J.J.; Jhon, K.; Tonelli, A.; Beevers, M.; Krishnamoorti, R.; Genzer, J. Facile Method of Controlling Monomer Sequence Distributions in Random Copolymers. Adv. Mater. 2007, 19, 2877–2883. [Google Scholar] [CrossRef]
- Noble, K.F.; Noble, A.M.; Talley, S.J.; Moore, R.B. Blocky bromination of syndiotactic polystyrene via post-polymerization functionalization in the heterogeneous gel state. Polym. Chem. 2018, 9, 5095–5106. [Google Scholar] [CrossRef]
- Boaen, N.K.; Hillmyer, M.A. Post-Polymerization Functionalization of Polyolefins. Chem. Soc. Rev. 2005, 34, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Gibson, M.I.; Klok, H. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Zinck, P.; Bonnet, F.; Mortreux, A.; Visseaux, M. Functionalization of Syndiotactic Polystyrene. Prog. Polym. Sci. 2009, 34, 369–392. [Google Scholar] [CrossRef]
- Jo, T.S.; Kim, S.H.; Shin, J.; Bae, C. Highly Efficient Incorporation of Functional Groups into Aromatic Main-Chain Polymer Using Iridium-Catalyzed C−H Activation and Suzuki−Miyaura Reaction. J. Am. Chem. Soc. 2009, 131, 1656–1657. [Google Scholar] [CrossRef]
- Dizman, C.; Tasdelen, M.A.; Yagci, Y. Recent Advances in the Preparation of Functionalized Polysulfones. Polym. Int. 2013, 62, 991–1007. [Google Scholar] [CrossRef]
- Williamson, J.B.; Lewis, S.E.; Johnson, R.R.; Manning, I.M.; Leibfarth, F.A. C-H Functionalization of Commodity Polymers. Angew. Chem. Int. Ed. 2019, 58, 8654–8668. [Google Scholar] [CrossRef]
- Abatti, G.P.; Gross, I.P.; da Conceição, T.F. Tuning the Thermal and Mechanical Properties of PSU by Post-Polymerization Friedel-Crafts Acylation. Eur. Polym. J. 2021, 142, 110111. [Google Scholar] [CrossRef]
- King, E.R.; Hunt, S.B.; Hamernik, L.J.; Gonce, L.E.; Wiggins, J.S.; Azoulay, J.D. Gold-Catalyzed Post-Polymerization Modification of Commodity Aromatic Polymers. JACS Au 2021, 1, 1342–1347. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Lyko, F. The DNA Methyltransferase Family: A Versatile Toolkit for Epigenetic Regulation. Nat. Rev. Genet. 2017, 19, 81–92. [Google Scholar] [CrossRef]
- Luo, M. Chemical and Biochemical Perspectives of Protein Lysine Methylation. Chem. Rev. 2018, 118, 6656–6705. [Google Scholar] [CrossRef]
- Han, S.; Brunet, A. Histone Methylation Makes its Mark on Longevity. Trends Cell Biol. 2012, 22, 42–49. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond. Application of Results Obtained from the Quantum Mechanics and from a Theory of Paramagnetic Susceptibility to the Structure of Molecules. J. Am. Chem. Soc. 1931, 53, 1367–1400. [Google Scholar] [CrossRef]
- Slater, J.C. Directed Valence in Polyatomic Molecules. Phys. Rev. 1931, 37, 481–489. [Google Scholar] [CrossRef]
- Barbier, C.; Berthier, G. Half A Century of Hybridization. Adv. Quantum Chem. 2000, 36, 1–25. [Google Scholar]
- WBingel, A.; Lu, W. Hybrid Orbitals and Their Applications in Structural Chemistry. Angew. Chem. Int. Ed. 1981, 20, 899–911. [Google Scholar] [CrossRef]
- Gentili, P.L.; Perez-Mercader, J. Quantitative estimation of chemical microheterogeneity through the determination of fuzzy entropy. Front. Chem. 2022, 10, 950769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. Beyond snowflakes: Heterogeneity in nanomaterials. Nano Lett. 2022, 22, 3–5. [Google Scholar] [CrossRef]
- Zacchi, L.F.; Schulz, B.L. N-glycoprotein macroheterogeneity: Biological implications and proteomic characterization. Glycoconj. J. 2016, 33, 359–376. [Google Scholar] [CrossRef]
- Huang, Y. Novel Crosslinking Reagents, Macromolecules, Therapeutic Conjugates, and Synthetic Methods Thereof. WO2013012961A2, 24 January 2013. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y. Single-Molecule Mixture: A Concept in Polymer Science. Int. J. Mol. Sci. 2024, 25, 7571. https://doi.org/10.3390/ijms25147571
Tang Y. Single-Molecule Mixture: A Concept in Polymer Science. International Journal of Molecular Sciences. 2024; 25(14):7571. https://doi.org/10.3390/ijms25147571
Chicago/Turabian StyleTang, Yu. 2024. "Single-Molecule Mixture: A Concept in Polymer Science" International Journal of Molecular Sciences 25, no. 14: 7571. https://doi.org/10.3390/ijms25147571
APA StyleTang, Y. (2024). Single-Molecule Mixture: A Concept in Polymer Science. International Journal of Molecular Sciences, 25(14), 7571. https://doi.org/10.3390/ijms25147571




