Disulfidptosis: A Novel Prognostic Criterion and Potential Treatment Strategy for Diffuse Large B-Cell Lymphoma (DLBCL)
Abstract
1. Introduction
2. Results
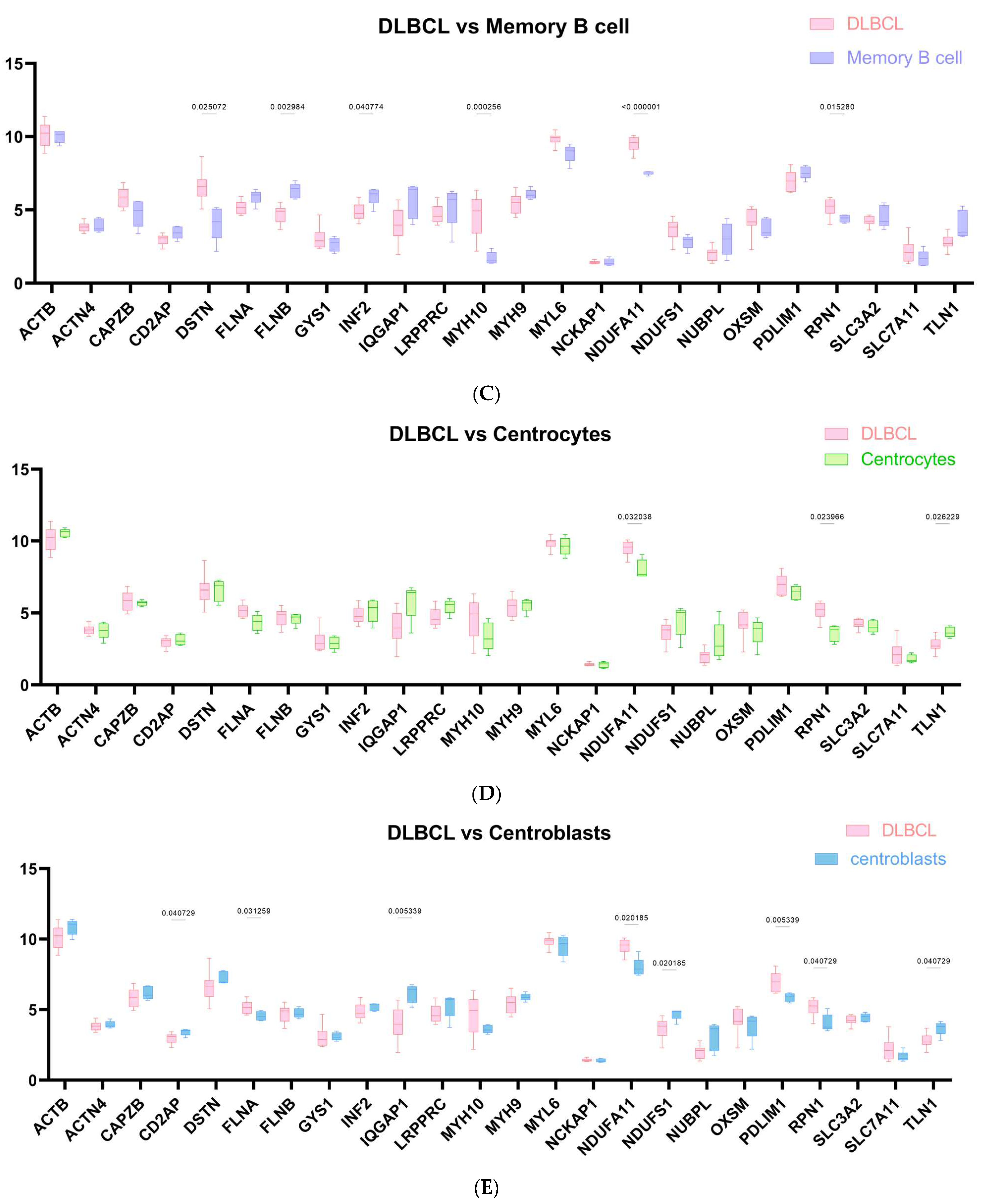
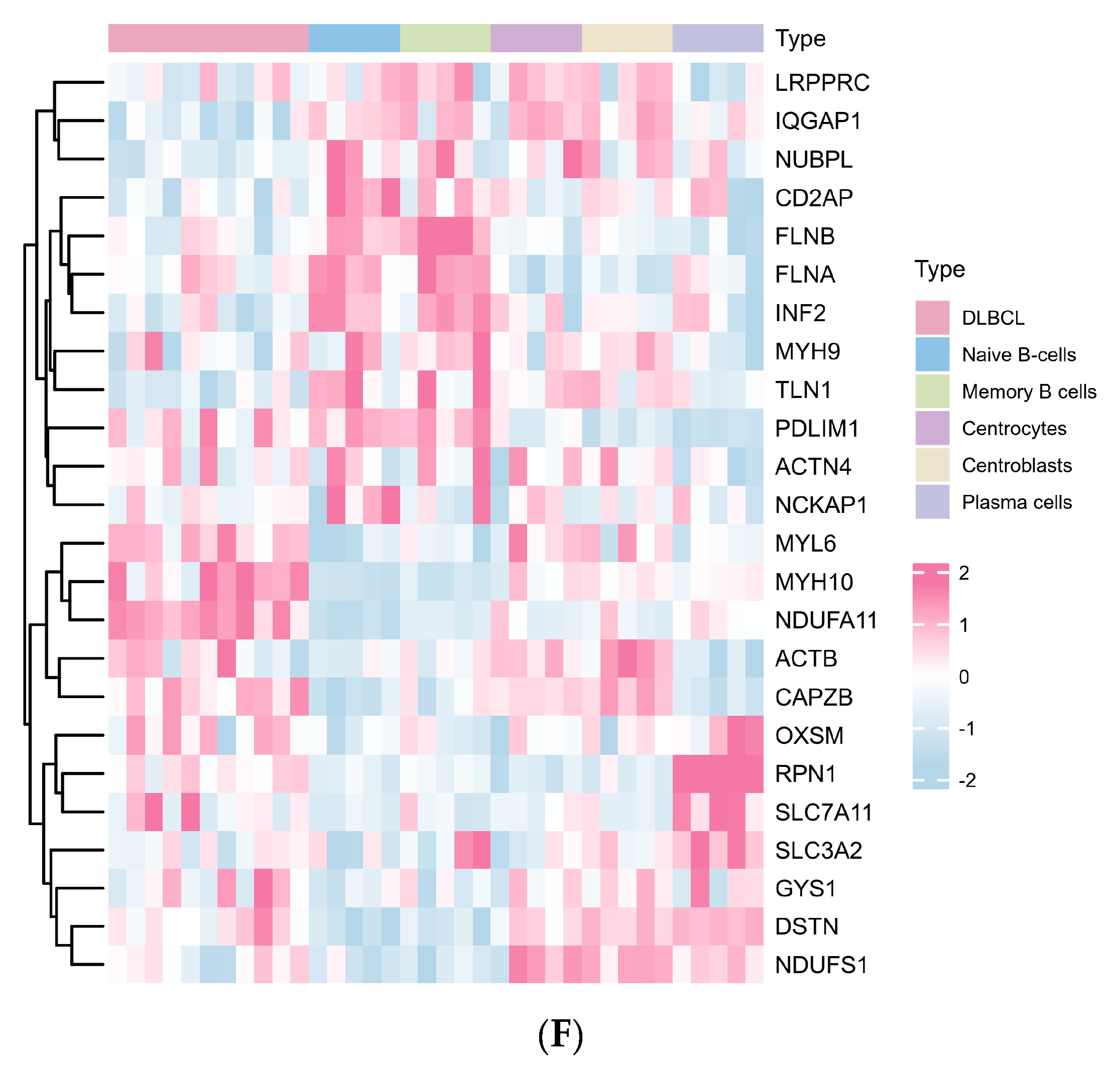
2.1. The Landscape of Disulfidptosis-Related Genes (DRGs) between DLBCL and Normal B Cell Subtypes
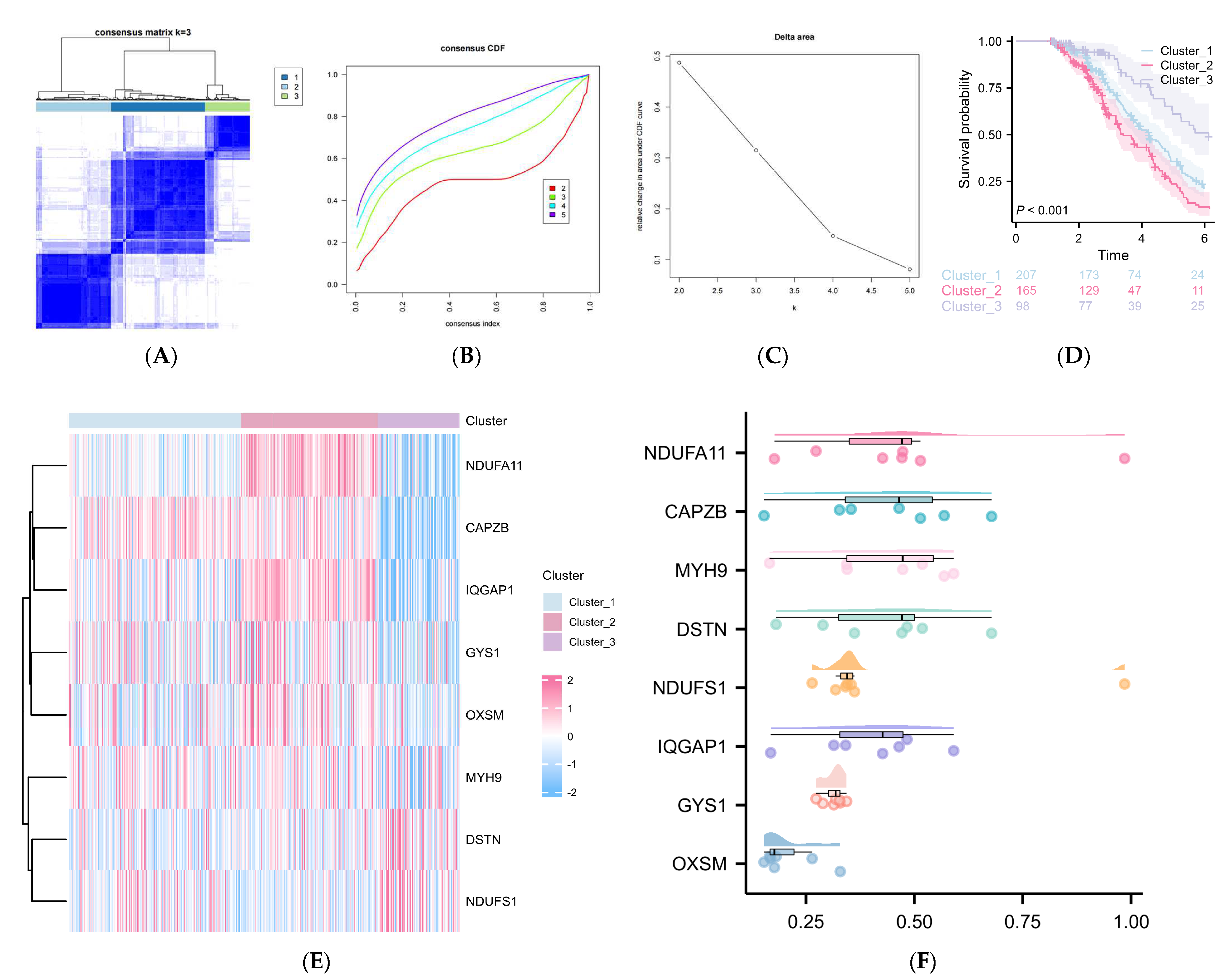
2.2. DRGs Were Found to Influence the Prognosis Prediction of DLBCL
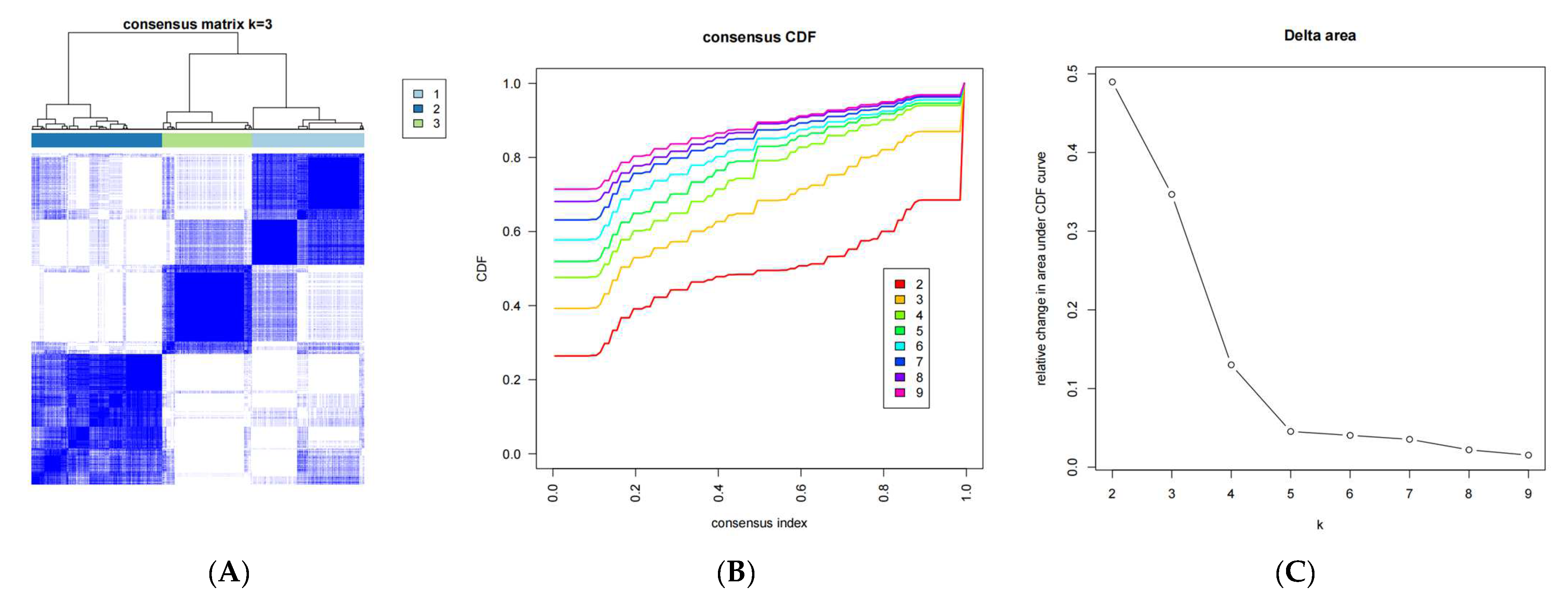
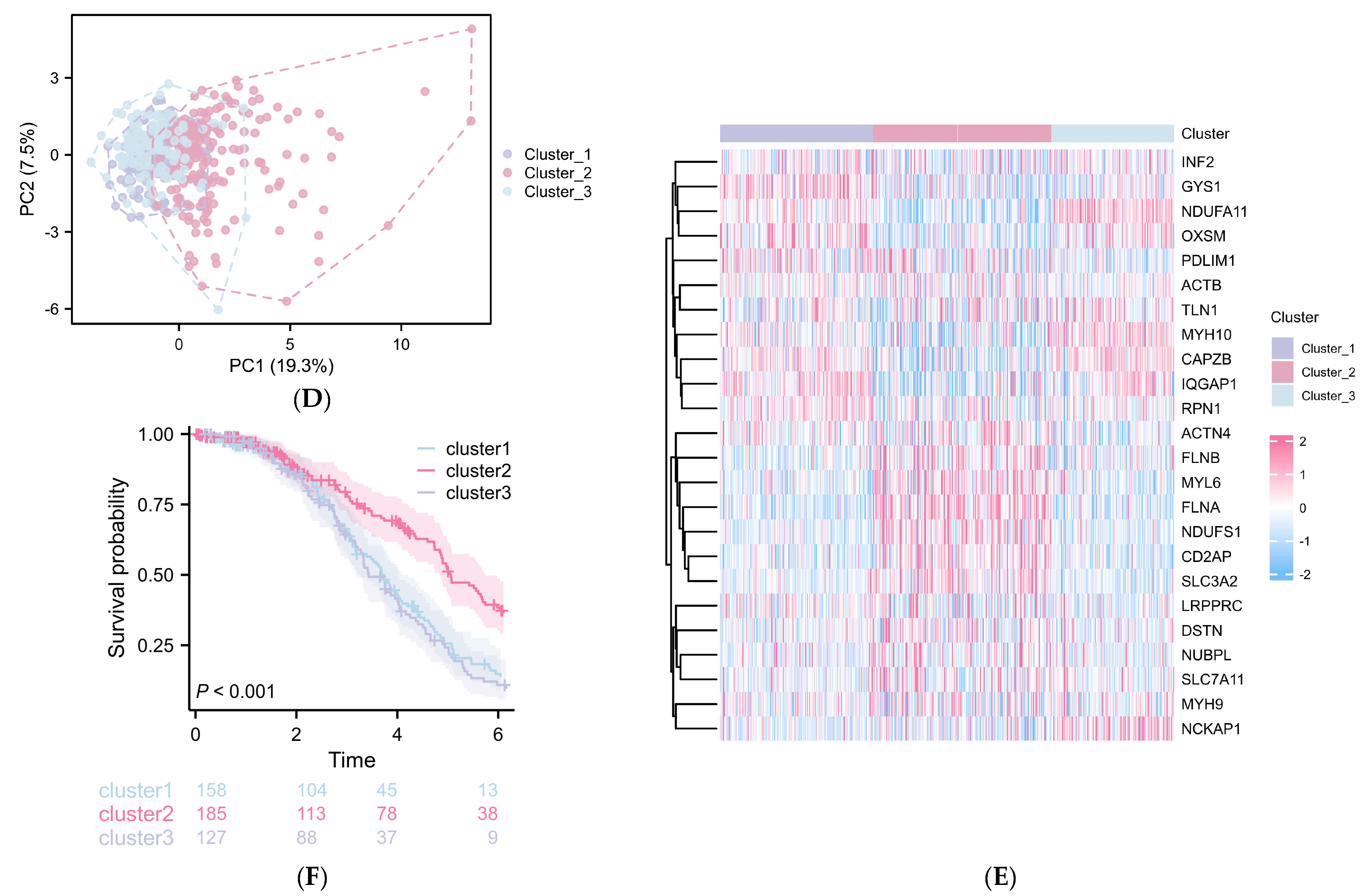
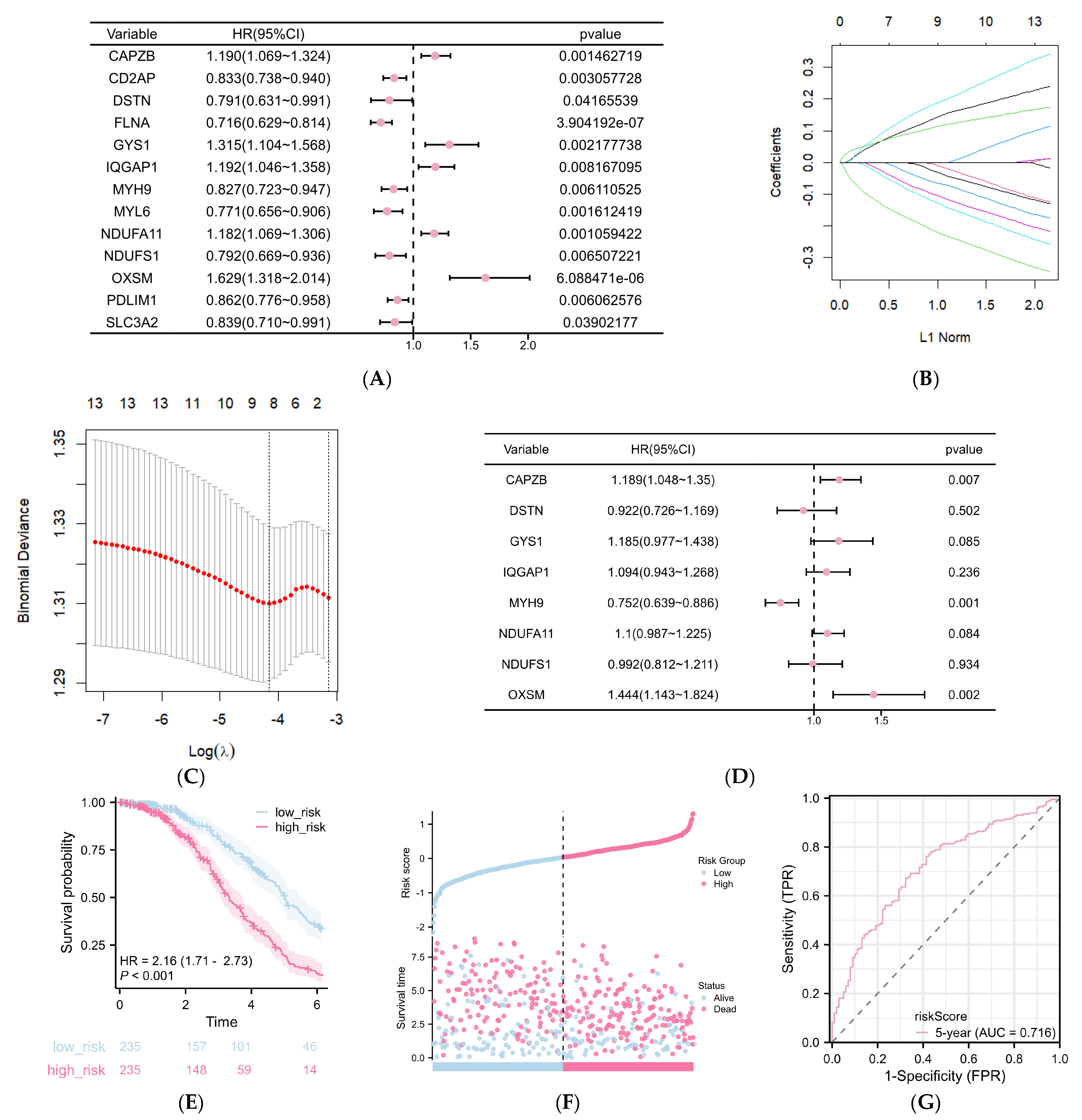
2.3. Construction of a DRG-Related Risk Signature
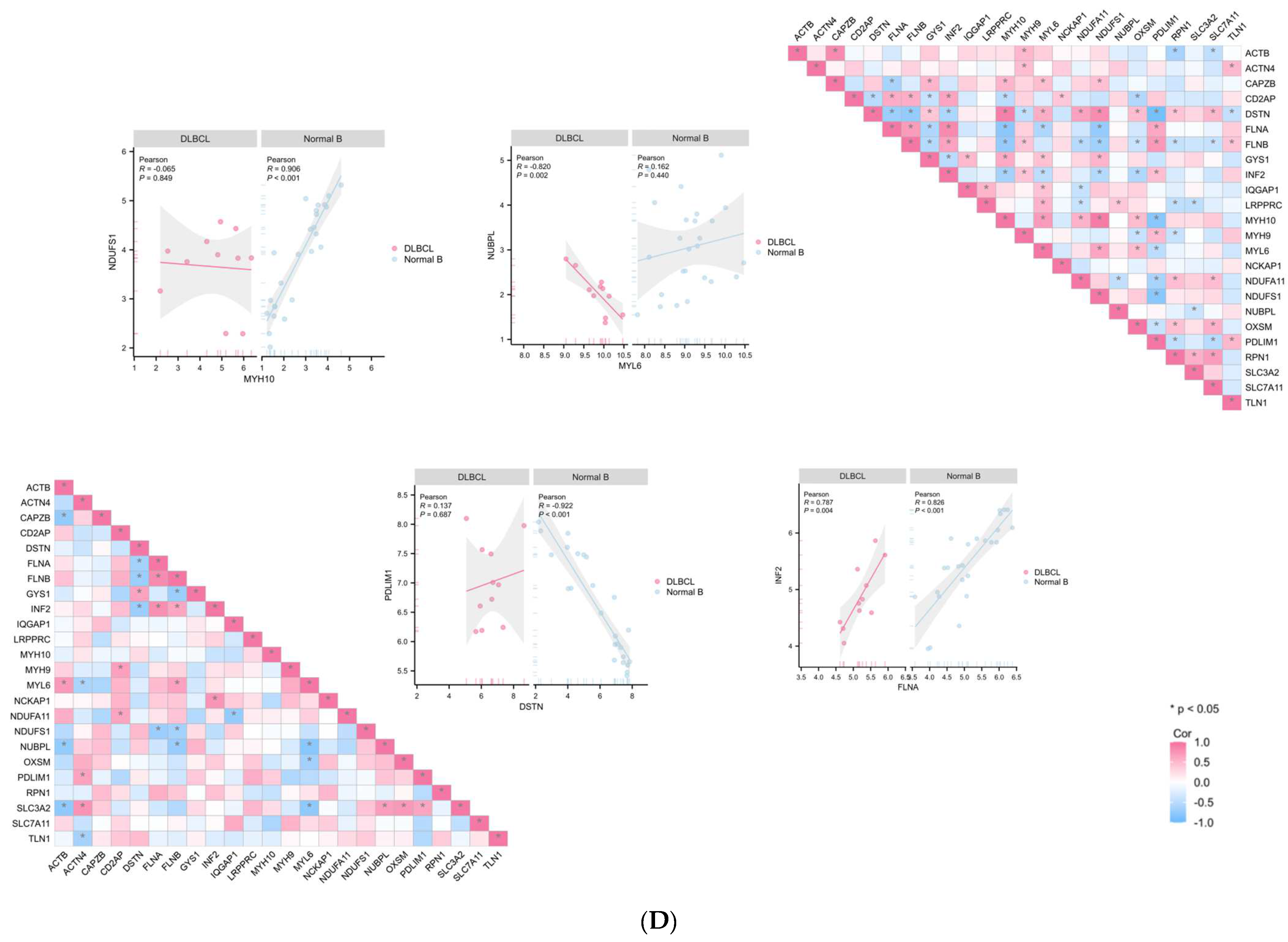
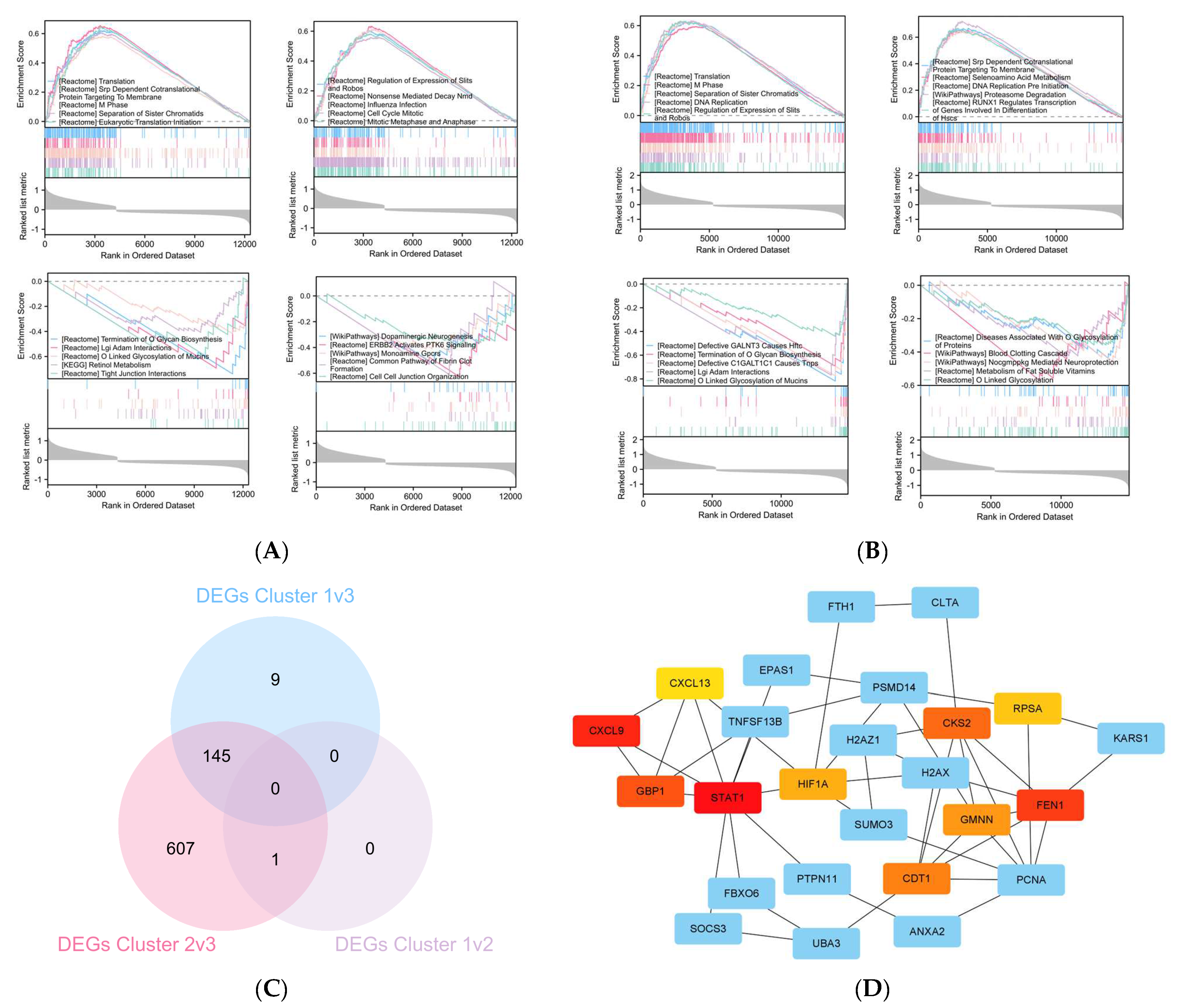
2.4. The Differentially Expressed Genes (DEGs) between Cluster 3 Compared with Cluster 1/2
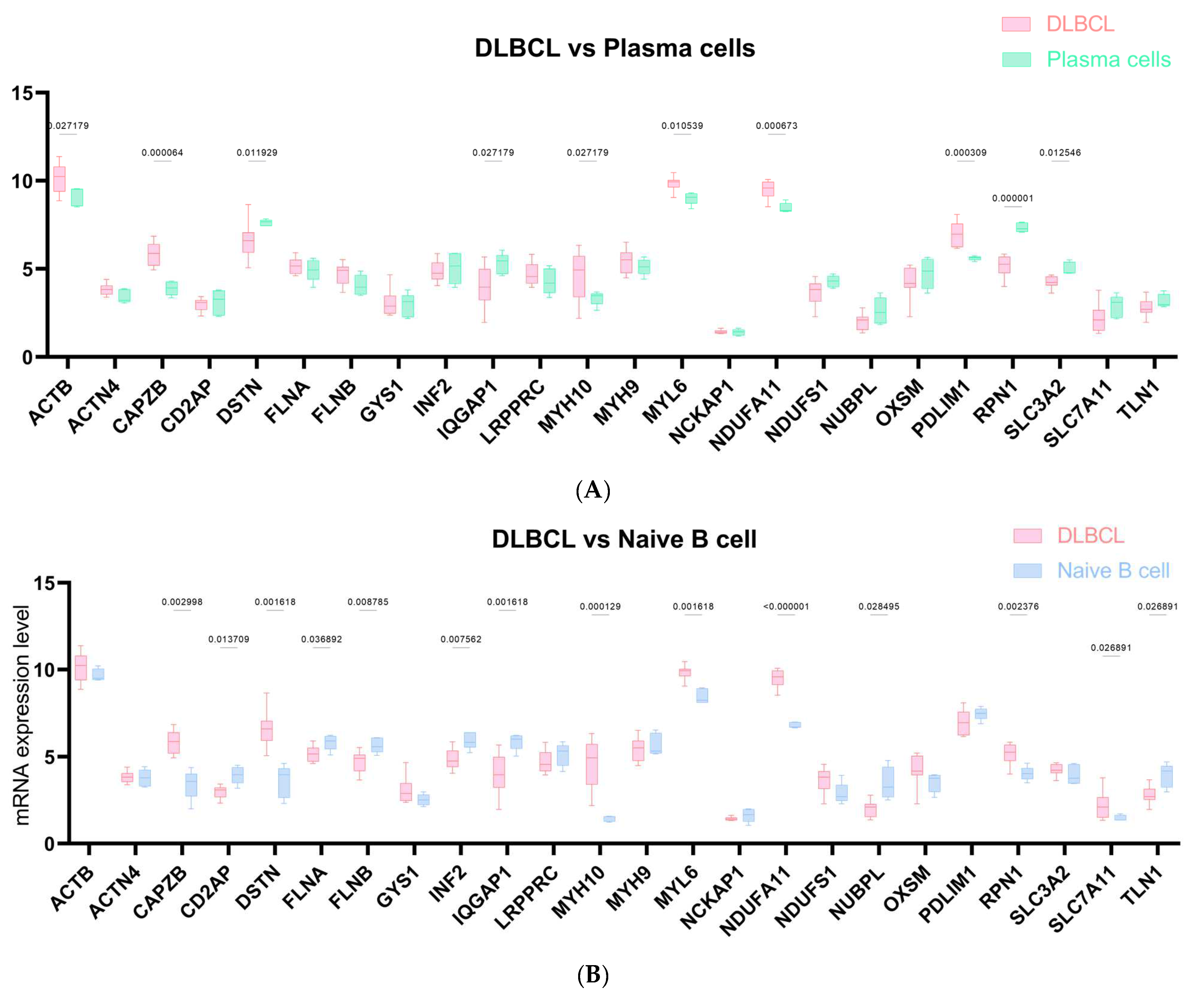
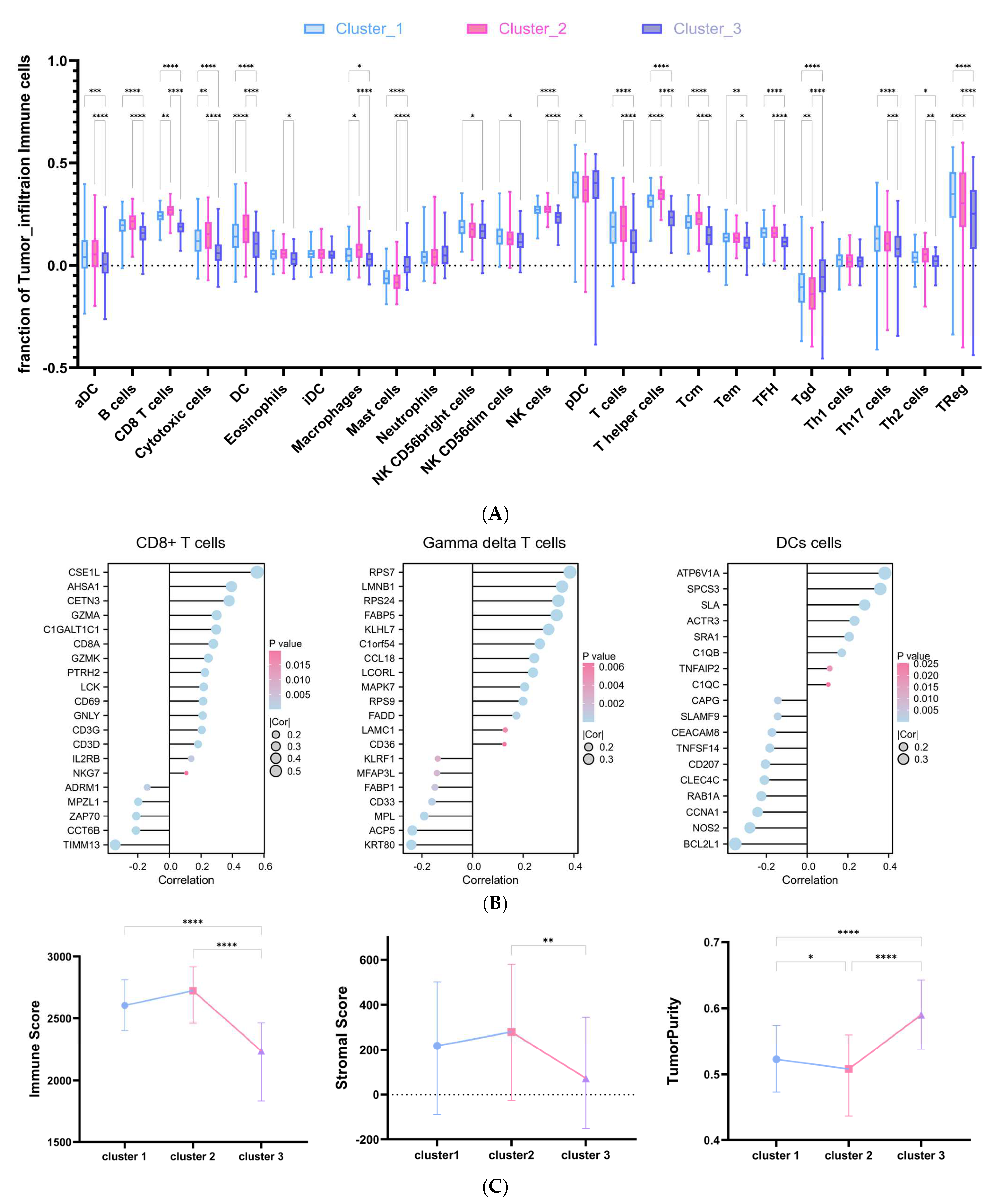
2.5. DRG-Related Risk Score Was Associated with Tumor Microenvironment (TME) Signature in DLBCL
3. Discussion
4. Materials and Methods
4.1. Data Acquisition and Preprocessing
4.2. Collecting Disulfidptosis-Related Genes (DRGs) via Systematic Review
4.3. Screening Prognosis-Associated DRGs in DLBCL
4.4. Unsupervised Clustering for Eight Prognosis-Associated DRGs
4.5. Cluster Differentially Expressed Genes (DEGs) and Enrichment Analysis
4.6. Potential Pharmacological
4.7. Tumor Microenvironment and Immune Cell Infiltration
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef]
- Susanibar-Adaniya, S.; Barta, S.K. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am. J. Hematol. 2021, 96, 617–629. [Google Scholar] [CrossRef]
- Melchardt, T.; Egle, A.; Greil, R. How I treat diffuse large B-cell lymphoma. ESMO Open 2023, 8, 100750. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Younes, A. Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin: Seeing the Forest and the Trees. J. Clin. Oncol. 2015, 33, 2835–2836. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska-Iwanicka, A.P.; Nowakowski, G.S. DLBCL: Who is high risk and how should treatment be optimized? Blood 2023. [Google Scholar] [CrossRef]
- Wright, G.; Tan, B.; Rosenwald, A.; Hurt, E.H.; Wiestner, A.; Staudt, L.M. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 2003, 100, 9991–9996. [Google Scholar] [CrossRef]
- Cai, Y.D.; Huang, T.; Feng, K.Y.; Hu, L.; Xie, L. A unified 35-gene signature for both subtype classification and survival prediction in diffuse large B-cell lymphomas. PLoS ONE 2010, 5, e12726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Liu, X.; Nie, L.; Zhang, Y.; Yan, Y.; Wang, C.; Colic, M.; Olszewski, K.; Horbath, A.; Chen, X.; Lei, G.; et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol. 2023, 25, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.C.; Andon, N.L.; Haynes, P.A.; Park, M.; Fischer, W.H.; Schubert, D. Protein disulfide bond formation in the cytoplasm during oxidative stress. J. Biol. Chem. 2004, 279, 21749–21758. [Google Scholar] [CrossRef] [PubMed]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Bannai, S. The synergistic action of the copper chelator bathocuproine sulphonate and cysteine in enhancing growth of L1210 cells in vitro. J. Cell Physiol. 1985, 125, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine metabolic circuitries: Druggable targets in cancer. Br. J. Cancer 2021, 124, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Teng, H.; Hang, Q.; Kondiparthi, L.; Lei, G.; Horbath, A.; Liu, X.; Mao, C.; Wu, S.; Zhuang, L.; et al. SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat. Commun. 2023, 14, 3673. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Zhuang, L.; Olszewski, K.; Gan, B. NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes Dis. 2021, 8, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Olszewski, K.; Zhang, Y.; Lim, E.W.; Shi, J.; Zhang, X.; Zhang, J.; Lee, H.; Koppula, P.; Lei, G.; et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020, 22, 476–486. [Google Scholar] [CrossRef]
- Joly, J.H.; Delfarah, A.; Phung, P.S.; Parrish, S.; Graham, N.A. A synthetic lethal drug combination mimics glucose deprivation-induced cancer cell death in the presence of glucose. J. Biol. Chem. 2020, 295, 1350–1365. [Google Scholar] [CrossRef]
- Michi, Y.; Harada, H.; Oikawa, Y.; Okuyama, K.; Kugimoto, T.; Kuroshima, T.; Hirai, H.; Mochizuki, Y.; Shimamoto, H.; Tomioka, H.; et al. Clinical manifestations of diffuse large B-cell lymphoma that exhibits initial symptoms in the maxilla and mandible: A single-center retrospective study. BMC Oral Health 2022, 22, 20. [Google Scholar] [CrossRef]
- Dulik, K.; Kaminska-Winciorek, G.; Swoboda, R.; Kwiatkowska-Pamula, A.; Giebel, S. Clinical manifestations of diffuse large B-cell lymphoma in the skin and subcutaneous tissue a case series study. Postepy Dermatol. Alergol. 2020, 37, 812–816. [Google Scholar] [CrossRef]
- Ziepert, M.; Hasenclever, D.; Kuhnt, E.; Glass, B.; Schmitz, N.; Pfreundschuh, M.; Loeffler, M. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010, 28, 2373–2380. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, Q.; Xing, F.; Zeng, C.; Wang, W. Disulfidptosis: A new form of programmed cell death. J. Exp. Clin. Cancer Res. 2023, 42, 137. [Google Scholar] [CrossRef]
- Xue, W.; Qiu, K.; Dong, B.; Guo, D.; Fu, J.; Zhu, C.; Niu, Z. Disulfidptosis-associated long non-coding RNA signature predicts the prognosis, tumor microenvironment, and immunotherapy and chemotherapy options in colon adenocarcinoma. Cancer Cell Int. 2023, 23, 218. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, T. Pan-cancer genetic analysis of disulfidptosis-related gene set. Cancer Genet. 2023, 278, 91–103. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, C.; Ding, Y.; Duan, S. Disulfidptosis: A new target for metabolic cancer therapy. J. Exp. Clin. Cancer Res. 2023, 42, 103. [Google Scholar] [CrossRef]
- Hatahet, F.; Ruddock, L.W. Protein disulfide isomerase: A critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal 2009, 11, 2807–2850. [Google Scholar] [CrossRef]
- Rietsch, A.; Beckwith, J. The genetics of disulfide bond metabolism. Annu. Rev. Genet. 1998, 32, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gao, J.; Liang, Z.; Yang, D. PDI-Regulated Disulfide Bond Formation in Protein Folding and Biomolecular Assembly. Molecules 2020, 26, 171. [Google Scholar] [CrossRef] [PubMed]
- Ogura, J. Association of Abnormal Disulfide Bond Formation with Disease Development and Progression. Yakugaku Zasshi 2022, 142, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.A.; Gannon, S.A.; Thorpe, C. Oxidative protein folding: From thiol-disulfide exchange reactions to the redox poise of the endoplasmic reticulum. Free Radic. Biol. Med. 2015, 80, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Inaba, K.; Kadokura, H. Methods to identify the substrates of thiol-disulfide oxidoreductases. Protein Sci. 2019, 28, 30–40. [Google Scholar] [CrossRef] [PubMed]
- West, J.D. Experimental Approaches for Investigating Disulfide-Based Redox Relays in Cells. Chem. Res. Toxicol. 2022, 35, 1676–1689. [Google Scholar] [CrossRef]
- Hogg, P.J. Biological regulation through protein disulfide bond cleavage. Redox Rep. 2002, 7, 71–77. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Z.; Chen, Y.; Qin, S.; Zhou, L.; Gao, W.; Shen, Z. Metabolic Adaptation-Mediated Cancer Survival and Progression in Oxidative Stress. Antioxidants 2022, 11, 1324. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, Z.; Li, B.; Zhu, H. Modulation of redox homeostasis: A strategy to overcome cancer drug resistance. Front. Pharmacol. 2023, 14, 1156538. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, Y.; Huang, C.; Wei, X. Redox signaling in drug-tolerant persister cells as an emerging therapeutic target. EBioMedicine 2023, 89, 104483. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B.; Zhou, M.; Liu, D.; Chen, F.; Zhao, X.; Lu, Y. Redox Dysregulation in the Tumor Microenvironment Contributes to Cancer Metastasis. Antioxid. Redox Signal 2023, 39, 472–490. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zuo, S.; Li, L.; Liu, T.; Dong, F.; Wang, X.; Zhang, X.; He, Z.; Zhai, Y.; Sun, B.; et al. The length of disulfide bond-containing linkages impacts the oral absorption and antitumor activity of paclitaxel prodrug-loaded nanoemulsions. Nanoscale 2021, 13, 10536–10543. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, J.; Wan, J.; Li, Z. Disulfide based prodrugs for cancer therapy. RSC Adv. 2020, 10, 24397–24409. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Xiao, F.; Zhao, D.; Luan, Y. Disulfide bond based polymeric drug carriers for cancer chemotherapy and relevant redox environments in mammals. Med. Res. Rev. 2018, 38, 1485–1510. [Google Scholar] [CrossRef]
- Liu, L.; He, J.; Sun, G.; Huang, N.; Bian, Z.; Xu, C.; Zhang, Y.; Cui, Z.; Xu, W.; Sun, F.; et al. The N6-methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin. Transl. Med. 2022, 12, e778. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jiang, L.; Tavana, O.; Gu, W. The Deubiquitylase OTUB1 Mediates Ferroptosis via Stabilization of SLC7A11. Cancer Res. 2019, 79, 1913–1924. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, J.; Cui, M.; Jing, R. SLC7A11 promotes the progression of gastric cancer and regulates ferroptosis through PI3K/AKT pathway. Pathol. Res. Pract. 2023, 248, 154646. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, J.; Huang, F.; Zhu, Y.Y.; Chen, D.D.; Zhang, Z.X.; Xie, Z.C.; Liu, Z.Y.; Hou, Q.; Xie, N.; et al. SLC7A11-associated ferroptosis in acute injury diseases: Mechanisms and strategies. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, Y.H.; Wang, J.; Quan, J.K.; Li, X.D.; Guan, K.P. The role of CSE1L silencing in the regulation of proliferation and apoptosis via the AMPK/mTOR signaling pathway in chronic myeloid leukemia. Hematology 2023, 28, 1–9. [Google Scholar] [CrossRef]
- Haas, W.; Pereira, P.; Tonegawa, S. Gamma/delta cells. Annu. Rev. Immunol. 1993, 11, 637–685. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Darcy, P.W.; Khan, I.Z.; Moon, C.S.; Kornberg, A.E.; Schneider, V.S.; Alvarez, Y.; Eleso, O.; Zhu, C.; Schernthanner, M.; et al. TCR-Vgammadelta usage distinguishes protumor from antitumor intestinal gammadelta T cell subsets. Science 2022, 377, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lafont, V.; Sanchez, F.; Laprevotte, E.; Michaud, H.A.; Gros, L.; Eliaou, J.F.; Bonnefoy, N. Plasticity of gammadelta T Cells: Impact on the Anti-Tumor Response. Front. Immunol. 2014, 5, 622. [Google Scholar] [CrossRef]
- O’Brien, R.L.; Born, W.K. Two functionally distinct subsets of IL-17 producing gammadelta T cells. Immunol. Rev. 2020, 298, 10–24. [Google Scholar] [CrossRef]
- Knight, A.; Piskacek, M.; Jurajda, M.; Prochazkova, J.; Racil, Z.; Zackova, D.; Mayer, J. Expansions of tumor-reactive Vdelta1 gamma-delta T cells in newly diagnosed patients with chronic myeloid leukemia. Cancer Immunol. Immunother. 2023, 72, 1209–1224. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, Y.; Lin, A.; Jiang, A.; Zhou, C.; Cheng, Q.; Liu, Z.; Chen, X.; Zhang, J.; Luo, P. Gamma delta T-cell-based immune checkpoint therapy: Attractive candidate for antitumor treatment. Mol. Cancer 2023, 22, 31. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Miyashita, M.; Shimizu, T.; Ashihara, E.; Ukimura, O. Strategies to Improve the Antitumor Effect of gammadelta T Cell Immunotherapy for Clinical Application. Int. J. Mol. Sci. 2021, 22, 8910. [Google Scholar] [CrossRef]
- Wesch, D.; Kabelitz, D.; Oberg, H.H. Tumor resistance mechanisms and their consequences on gammadelta T cell activation. Immunol. Rev. 2020, 298, 84–98. [Google Scholar] [CrossRef]
- Mao, Y.; Yin, S.; Zhang, J.; Hu, Y.; Huang, B.; Cui, L.; Kang, N.; He, W. A new effect of IL-4 on human gammadelta T cells: Promoting regulatory Vdelta1 T cells via IL-10 production and inhibiting function of Vdelta2 T cells. Cell Mol. Immunol. 2016, 13, 217–228. [Google Scholar] [CrossRef]
- Stroud, D.A.; Surgenor, E.E.; Formosa, L.E.; Reljic, B.; Frazier, A.E.; Dibley, M.G.; Osellame, L.D.; Stait, T.; Beilharz, T.H.; Thorburn, D.R.; et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 2016, 538, 123–126. [Google Scholar] [CrossRef]
- Berger, I.; Hershkovitz, E.; Shaag, A.; Edvardson, S.; Saada, A.; Elpeleg, O. Mitochondrial complex I deficiency caused by a deleterious NDUFA11 mutation. Ann. Neurol. 2008, 63, 405–408. [Google Scholar] [CrossRef]
- Peverelli, L.; Legati, A.; Lamantea, E.; Nasca, A.; Lerario, A.; Galimberti, V.; Ghezzi, D.; Lamperti, C. New missense variants of NDUFA11 associated with late-onset myopathy. Muscle Nerve 2019, 60, E11–E14. [Google Scholar] [CrossRef]
- Chugh, P.; Clark, A.G.; Smith, M.B.; Cassani, D.A.D.; Dierkes, K.; Ragab, A.; Roux, P.P.; Charras, G.; Salbreux, G.; Paluch, E.K. Actin cortex architecture regulates cell surface tension. Nat. Cell Biol. 2017, 19, 689–697. [Google Scholar] [CrossRef]
- Betapudi, V. Myosin II motor proteins with different functions determine the fate of lamellipodia extension during cell spreading. PLoS ONE 2010, 5, e8560. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Kong, S.J.; Wang, L.; Choi, B.K.; Lee, H.; Kim, C.; Kim, J.M.; Park, K. Targeting Actomyosin Contractility Suppresses Malignant Phenotypes of Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2020, 21, 3460. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, S.; Pope, B.; Mannherz, H.G.; Weeds, A. Determining the differences in actin binding by human ADF and cofilin. J. Mol. Biol. 2002, 315, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Estornes, Y.; Gay, F.; Gevrey, J.C.; Navoizat, S.; Nejjari, M.; Scoazec, J.Y.; Chayvialle, J.A.; Saurin, J.C.; Abello, J. Differential involvement of destrin and cofilin-1 in the control of invasive properties of Isreco1 human colon cancer cells. Int. J. Cancer 2007, 121, 2162–2171. [Google Scholar] [CrossRef]
- Rassner, G.; Steinert, M.; Bercher, M.; Rodermund, O.E.; Henning, D.; Mey, T.; Heinzel, M. Chronic systemic toxicity of oral photochemotherapy using 8-methoxypsoralen and UVA. Hautarzt 1987, 38, 10–17. [Google Scholar] [PubMed]
- Elkholi, R.; Abraham-Enachescu, I.; Trotta, A.P.; Rubio-Patino, C.; Mohammed, J.N.; Luna-Vargas, M.P.A.; Gelles, J.D.; Kaminetsky, J.R.; Serasinghe, M.N.; Zou, C.; et al. MDM2 Integrates Cellular Respiration and Apoptotic Signaling through NDUFS1 and the Mitochondrial Network. Mol. Cell 2019, 74, 452–465.e457. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Sharma, M.; Brocardo, M.G.; Henderson, B.R. IQGAP1 translocates to the nucleus in early S-phase and contributes to cell cycle progression after DNA replication arrest. Int. J. Biochem. Cell Biol. 2011, 43, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; McNulty, D.E.; Marler, K.J.; Lim, L.; Hall, C.; Annan, R.S.; Sacks, D.B. IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J. Biol. Chem. 2005, 280, 13871–13878. [Google Scholar] [CrossRef] [PubMed]
- Falantes, J.F.; Trujillo, P.; Piruat, J.I.; Calderon, C.; Marquez-Malaver, F.J.; Martin-Antonio, B.; Millan, A.; Gomez, M.; Gonzalez, J.; Martino, M.L.; et al. Overexpression of GYS1, MIF, and MYC is associated with adverse outcome and poor response to azacitidine in myelodysplastic syndromes and acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, 236–244. [Google Scholar] [CrossRef]
- Zhang, L.; Joshi, A.K.; Hofmann, J.; Schweizer, E.; Smith, S. Cloning, expression, and characterization of the human mitochondrial beta-ketoacyl synthase. Complementation of the yeast CEM1 knock-out strain. J. Biol. Chem. 2005, 280, 12422–12429. [Google Scholar] [CrossRef]
- Kourtidis, A.; Lu, R.; Pence, L.J.; Anastasiadis, P.Z. A central role for cadherin signaling in cancer. Exp. Cell Res. 2017, 358, 78–85. [Google Scholar] [CrossRef]
- Yu, W.; Yang, L.; Li, T.; Zhang, Y. Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target. Front. Oncol. 2019, 9, 989. [Google Scholar] [CrossRef]
- Vieira, A.F.; Paredes, J. P-cadherin and the journey to cancer metastasis. Mol. Cancer 2015, 14, 178. [Google Scholar] [CrossRef]
- Wong, W.W.; Dimitroulakos, J.; Minden, M.D.; Penn, L.Z. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002, 16, 508–519. [Google Scholar] [CrossRef]
- Zeiser, R.; Youssef, S.; Baker, J.; Kambham, N.; Steinman, L.; Negrin, R.S. Preemptive HMG-CoA reductase inhibition provides graft-versus-host disease protection by Th-2 polarization while sparing graft-versus-leukemia activity. Blood 2007, 110, 4588–4598. [Google Scholar] [CrossRef]
- Fritz, G. HMG-CoA reductase inhibitors (statins) as anticancer drugs (review). Int. J. Oncol. 2005, 27, 1401–1409. [Google Scholar] [CrossRef]
- Charatan, F. Bayer decides to withdraw cholesterol lowering drug. BMJ 2001, 323, 359. [Google Scholar] [CrossRef]
- Backman, J.T.; Kyrklund, C.; Neuvonen, M.; Neuvonen, P.J. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin. Pharmacol. Ther. 2002, 72, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Clarkson, P.; Karas, R.H. Statin-associated myopathy. JAMA 2003, 289, 1681–1690. [Google Scholar] [CrossRef]
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol. 2022, 40, 282–293. [Google Scholar] [CrossRef]
- Yi, L.; Hu, L.; Huang, K.; Li, Q.; Wang, Y.; Wang, J.; Zhai, Z. Palbociclib enhances the effect of doxorubicin-induced apoptosis in activated B-cell-like diffuse large B-cell lymphoma cells. Anticancer Drugs 2023, 34, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, V.; Theruvath, J.; Pauck, D.; Picard, D.; Qin, N.; Blumel, L.; Maue, M.; Bartl, J.; Ahmadov, U.; Langini, M.; et al. Tacedinaline (CI-994), a class I HDAC inhibitor, targets intrinsic tumor growth and leptomeningeal dissemination in MYC-driven medulloblastoma while making them susceptible to anti-CD47-induced macrophage phagocytosis via NF-kB-TGM2 driven tumor inflammation. J. Immunother. Cancer 2023, 11, e005871. [Google Scholar] [CrossRef]
- Loprevite, M.; Tiseo, M.; Grossi, F.; Scolaro, T.; Semino, C.; Pandolfi, A.; Favoni, R.; Ardizzoni, A. In vitro study of CI-994, a histone deacetylase inhibitor, in non-small cell lung cancer cell lines. Oncol. Res. 2005, 15, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.L.; DeKorte, C.J. Valdecoxib: A review. Clin. Ther. 2003, 25, 817–851. [Google Scholar] [CrossRef]
- Tychhon, B.; Allen, J.C.; Gonzalez, M.A.; Olivas, I.M.; Solecki, J.P.; Keivan, M.; Velazquez, V.V.; McCall, E.B.; Tapia, D.N.; Rubio, A.J.; et al. The prognostic value of 19S ATPase proteasome subunits in acute myeloid leukemia and other forms of cancer. Front. Med. 2023, 10, 1209425. [Google Scholar] [CrossRef]
- Bartaula-Brevik, S.; Leitch, C.; Hernandez-Valladares, M.; Aasebo, E.; Berven, F.S.; Selheim, F.; Brenner, A.K.; Rye, K.P.; Hagen, M.; Reikvam, H.; et al. Vacuolar ATPase Is a Possible Therapeutic Target in Acute Myeloid Leukemia: Focus on Patient Heterogeneity and Treatment Toxicity. J. Clin. Med. 2023, 12, 5546. [Google Scholar] [CrossRef]
- Rago, F.; Rodrigues, L.U.; Bonney, M.; Sprouffske, K.; Kurth, E.; Elliott, G.; Ambrose, J.; Aspesi, P.; Oborski, J.; Chen, J.T.; et al. Exquisite Sensitivity to Dual BRG1/BRM ATPase Inhibitors Reveals Broad SWI/SNF Dependencies in Acute Myeloid Leukemia. Mol. Cancer Res. 2022, 20, 361–372. [Google Scholar] [CrossRef]
- Mirabilii, S.; Ricciardi, M.R.; Piedimonte, M.; Gianfelici, V.; Bianchi, M.P.; Tafuri, A. Biological Aspects of mTOR in Leukemia. Int. J. Mol. Sci. 2018, 19, 2396. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Cassady, K.; Zou, Z.; Yang, S.; Wang, Z.; Zhang, X. The Role of mTOR Inhibitors in Hematologic Disease: From Bench to Bedside. Front. Oncol. 2020, 10, 611690. [Google Scholar] [CrossRef] [PubMed]
- Tarumoto, Y.; Lin, S.; Wang, J.; Milazzo, J.P.; Xu, Y.; Lu, B.; Yang, Z.; Wei, Y.; Polyanskaya, S.; Wunderlich, M.; et al. Salt-inducible kinase inhibition suppresses acute myeloid leukemia progression in vivo. Blood 2020, 135, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Seipel, K.; Kohler, S.; Bacher, U.; Pabst, T. HSP90 Inhibitor PU-H71 in Combination with BH3-Mimetics in the Treatment of Acute Myeloid Leukemia. Curr. Issues Mol. Biol. 2023, 45, 7011–7026. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, E.; Shimosaki, S.; Hasegawa, H.; Iha, H.; Tsukamoto, Y.; Wang, Y.; Sasaki, D.; Imaizumi, Y.; Miyazaki, Y.; Yanagihara, K.; et al. TAS-116 (pimitespib), a heat shock protein 90 inhibitor, shows efficacy in preclinical models of adult T-cell leukemia. Cancer Sci. 2022, 113, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, E.; Kawaguchi, A.; Tezuka, K.; Taguchi, S.; Hirose, S.; Matsumoto, T.; Mitsui, T.; Senba, K.; Nishizono, A.; Hori, M.; et al. Oral administration of an HSP90 inhibitor, 17-DMAG, intervenes tumor-cell infiltration into multiple organs and improves survival period for ATL model mice. Blood Cancer J. 2013, 3, e132. [Google Scholar] [CrossRef] [PubMed]
- Trabolsi, A.; Arumov, A.; Schatz, J.H. Bispecific antibodies and CAR-T cells: Dueling immunotherapies for large B-cell lymphomas. Blood Cancer J. 2024, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Cassanello, G.; Luna de Abia, A.; Falchi, L. Trial watch: Bispecific antibodies for the treatment of relapsed or refractory large B-cell lymphoma. Oncoimmunology 2024, 13, 2321648. [Google Scholar] [CrossRef] [PubMed]
- Al Sbihi, A.; Alasfour, M.; Pongas, G. Innovations in Antibody-Drug Conjugate (ADC) in the Treatment of Lymphoma. Cancers 2024, 16, 827. [Google Scholar] [CrossRef]
- Visco, C.; Li, Y.; Xu-Monette, Z.Y.; Miranda, R.N.; Green, T.M.; Li, Y.; Tzankov, A.; Wen, W.; Liu, W.M.; Kahl, B.S.; et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: A report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia 2012, 26, 2103–2113. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Moller, M.B.; Tzankov, A.; Montes-Moreno, S.; Hu, W.; Manyam, G.C.; Kristensen, L.; Fan, L.; Visco, C.; Dybkaer, K.; et al. MDM2 phenotypic and genotypic profiling, respective to TP53 genetic status, in diffuse large B-cell lymphoma patients treated with rituximab-CHOP immunochemotherapy: A report from the International DLBCL Rituximab-CHOP Consortium Program. Blood 2013, 122, 2630–2640. [Google Scholar] [CrossRef]
- Brune, V.; Tiacci, E.; Pfeil, I.; Doring, C.; Eckerle, S.; van Noesel, C.J.; Klapper, W.; Falini, B.; von Heydebreck, A.; Metzler, D.; et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008, 205, 2251–2268. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martinez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Trevino, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
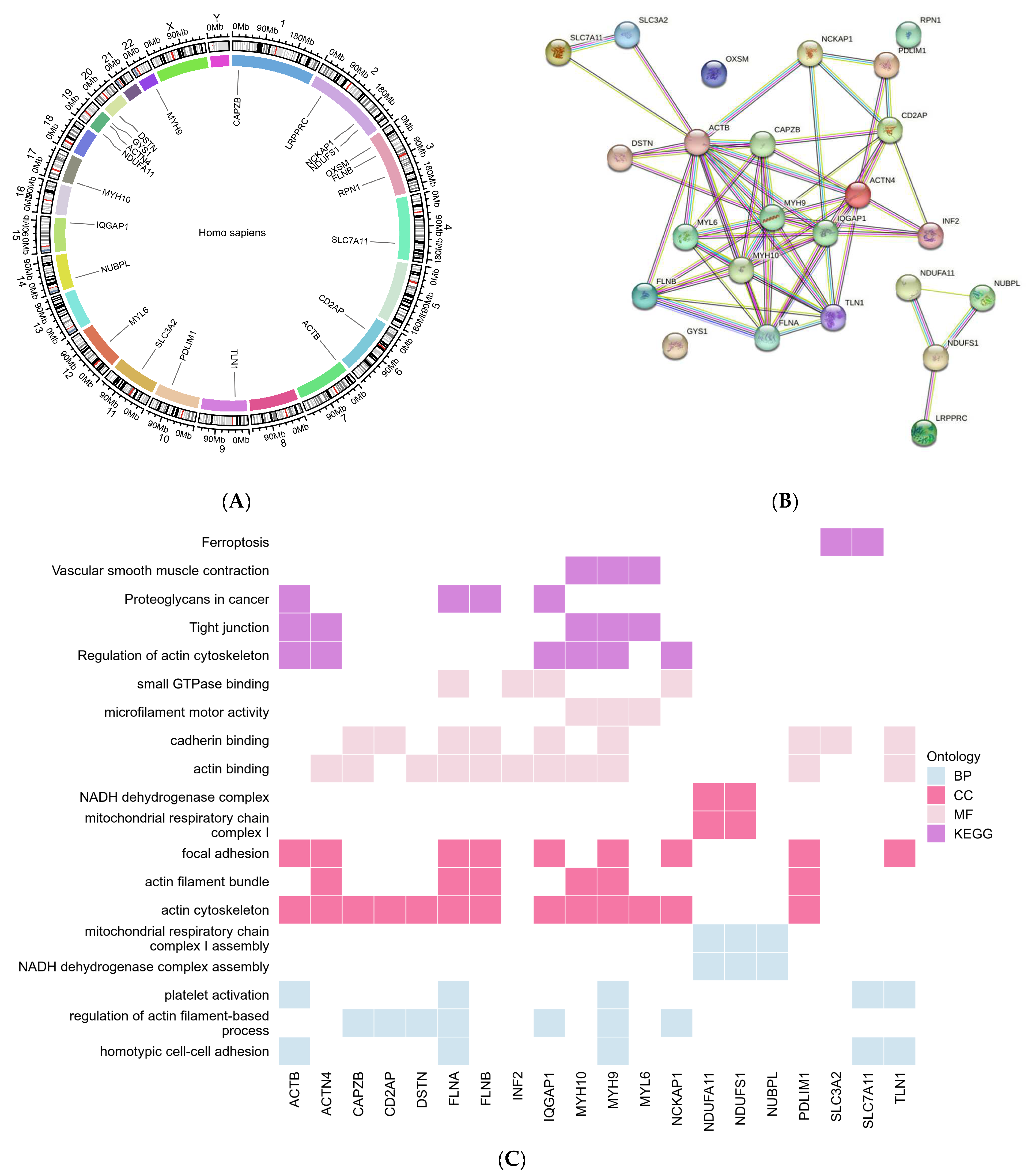
| Gene Symbol | Subcellular Location | Chromosomal Position |
|---|---|---|
| ACTB | intracellular | chr7:5,526,409−5,563,902(−) |
| ACTN4 | Actin filaments/Cytosol | chr19:38,647,649−38,731,589(+) |
| CAPZB | Nucleoplasm/Cytosol/Vesicles | chr1:19,338,775−19,485,539(−) |
| CD2AP | Plasma membrane/Centriolar satellite | chr6:47,477,789−47,627,263(+) |
| DSTN | Plasma membrane | chr20:17,570,075−17,609,919(+) |
| FLNA | Plasma membrane /Actin filaments/Cytosol | chrX:154,348,524−154,374,634(−) |
| FLNB | Plasma membrane/Golgi apparatus/Actin filament/Cytosol | chr3:58,008,398−58,172,251(+) |
| GYS1 | Microtubules/Cytosol | chr19:48,968,130−48,993,310(−) |
| INF2 | Endoplasmic reticulum/Nuclear bodies | chr14:104,681,146−104,722,535(+) |
| IQGAP1 | Plasma membrane/Cell Junctions | chr15:90,388,242−90,502,239(+) |
| LRPPRC | Mitochondria | chr2:43,886,224−43,996,265(−) |
| MYH10 | Actin filaments/Mitochondria/Cytosol | chr17:8,474,207−8,631,376(−) |
| MYH9 | Plasma membrane/Actin filaments/Nuclear bodies (uncertain)/Cytosol | chr22:36,281,280−36,388,010(−) |
| MYL6 | cytosol/cytoskeleton/Supramolecular fiber | chr12:56,158,346−56,163,496(+) |
| NCKAP1 | Cytosol | chr2:182,909,115−183,038,858(−) |
| NDUFA11 | Mitochondria | chr19:5,891,229−5,904,006(−) |
| NDUFS1 | Mitochondria | chr2:206,114,817−206,159,509(−) |
| NUBPL | Mitochondria | chr14:31,489,956−31,861,224(+) |
| OXSM | Mitochondria/Cytosol (uncertain) | chr3:25,782,917−25,794,534(+) |
| PDLIM1 | Actin filaments Plasma membrane (uncertain)/Cell Junctions (uncertain) | chr10:95,237,572−95,291,012(−) |
| RPN1 | Endoplasmic reticulum/Cytosol | chr3:128,619,969−128,681,075(−) |
| SLC3A2 | Plasma membrane/Nucleoplasm (uncertain) | chr11:62,856,004−62,888,880(+) |
| SLC7A11 | Vesicles | chr4:138,164,097−138,312,671(−) |
| TLN1 | Focal adhesion sites/Cytosol/Plasma membrane/Centriolar | chr9:35,696,948−35,732,195(−) |
| Gene Symbol | Full Name | Location | Function of the Encoded Protein |
|---|---|---|---|
| CAPZB | Capping Actin Protein of Muscle Z-Line Subunit Beta | Nucleoplasm/ Cytosol/ Vesicles | F-actin-capping proteins bind in a Ca(2+)-independent manner to the fast-growing ends of actin filaments (barbed end) thereby blocking the exchange of subunits at these ends. |
| DSTN | Destrin, Actin Depolymerizing Factor | Plasma membrane | Actin-depolymerizing protein. Severs actin filaments (F-actin) and binds to actin monomers (G-actin). |
| GYS1 | Glycogen Synthase 1 | Microtubules/ Cytosol | Transfers the glycosyl residue from UDP-Glc to the non-reducing end of alpha-1,4-glucan. |
| IQGAP1 | IQ Motif Containing GTPase Activating Protein 1 | Plasma membrane/ Cell Junctions | Could serve as an assembly scaffold for the organization of a multimolecular complex that would interface incoming signals to the reorganization of the actin cytoskeleton at the plasma membrane. |
| MYH9 | Myosin Heavy Chain 9 | Plasma membrane/ Actin filaments/ Nuclear bodies (uncertain)/Cytosol | Cellular myosin that appears to play a role in cytokinesis, cell shape, and specialized functions such as secretion and capping. |
| NDUFA11 | NADH:Ubiquinone Oxidoreductase Subunit A11 | Mitochondria | Accessory subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase (Complex I), that is believed not to be involved in catalysis. |
| NDUFS1 | NADH:Ubiquinone Oxidoreductase Core Subunit S1 | Mitochondria | Core subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase (Complex I) which catalyzes electron transfer from NADH through the respiratory chain, using ubiquinone as an electron acceptor. |
| OXSM | 3-Oxoacyl-ACP Synthase, Mitochondrial | Mitochondria/ Cytosol (uncertain) | May play a role in the biosynthesis of lipoic acid as well as longer chain fatty acids required for optimal mitochondrial function. |
| Score | ID | Name | Description |
|---|---|---|---|
| −99.89 | BRD-K81169441 | cerivastatin | HMGCR inhibitor |
| −99.82 | BRD-K51313569 | palbociclib | CDK inhibitor |
| −99.58 | BRD-K52313696 | tacedinaline | HDAC inhibitor |
| −99.58 | BRD-K12994359 | valdecoxib | Cyclooxygenase inhibitor |
| −99.51 | BRD-K05350981 | oligomycin-c | ATPase inhibitor |
| −99.47 | BRD-A45498368 | WYE-125132 | MTOR inhibitor |
| −99.47 | BRD-K63630713 | etacrynic-acid | Sodium/potassium/chloride transporter inhibitor |
| −99.37 | BRD-K65503129 | HSP90-inhibitor | HSP inhibitor |
| −99.37 | BRD-K53523901 | arctigenin | MEK inhibitor |
| −99.33 | BRD-K08417745 | SID-26681509 | Cathepsin inhibitor |
| −99.3 | BRD-A48261811 | argatroban | Thrombin inhibitor |
| −99.3 | BRD-K56429665 | calcipotriol | Vitamin D receptor agonist |
| −99.19 | BRD-K51805276 | temefos | Cholinesterase inhibitor |
| −99.19 | BRD-K32828673 | chelidonine | Tubulin inhibitor |
| −99.15 | BRD-A72703248 | SKF-96365 | Calcium channel blocker |
| −99.08 | BRD-K90382497 | GW-843682X | PLK inhibitor |
| −99.01 | BRD-K93201660 | ML-7 | Myosin light chain kinase inhibitor |
| −99.01 | BRD-A11678676 | wortmannin | PI3K inhibitor |
| −98.98 | BRD-K13049116 | BMS-754807 | IGF-1 inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tsukamoto, Y.; Hori, M.; Iha, H. Disulfidptosis: A Novel Prognostic Criterion and Potential Treatment Strategy for Diffuse Large B-Cell Lymphoma (DLBCL). Int. J. Mol. Sci. 2024, 25, 7156. https://doi.org/10.3390/ijms25137156
Wang Y, Tsukamoto Y, Hori M, Iha H. Disulfidptosis: A Novel Prognostic Criterion and Potential Treatment Strategy for Diffuse Large B-Cell Lymphoma (DLBCL). International Journal of Molecular Sciences. 2024; 25(13):7156. https://doi.org/10.3390/ijms25137156
Chicago/Turabian StyleWang, Yu, Yoshiyuki Tsukamoto, Mitsuo Hori, and Hidekatsu Iha. 2024. "Disulfidptosis: A Novel Prognostic Criterion and Potential Treatment Strategy for Diffuse Large B-Cell Lymphoma (DLBCL)" International Journal of Molecular Sciences 25, no. 13: 7156. https://doi.org/10.3390/ijms25137156
APA StyleWang, Y., Tsukamoto, Y., Hori, M., & Iha, H. (2024). Disulfidptosis: A Novel Prognostic Criterion and Potential Treatment Strategy for Diffuse Large B-Cell Lymphoma (DLBCL). International Journal of Molecular Sciences, 25(13), 7156. https://doi.org/10.3390/ijms25137156






