The Urinary Glycopeptide Profile Differentiates Early Cardiorenal Risk in Subjects Not Meeting Criteria for Chronic Kidney Disease
Abstract
1. Introduction
2. Results
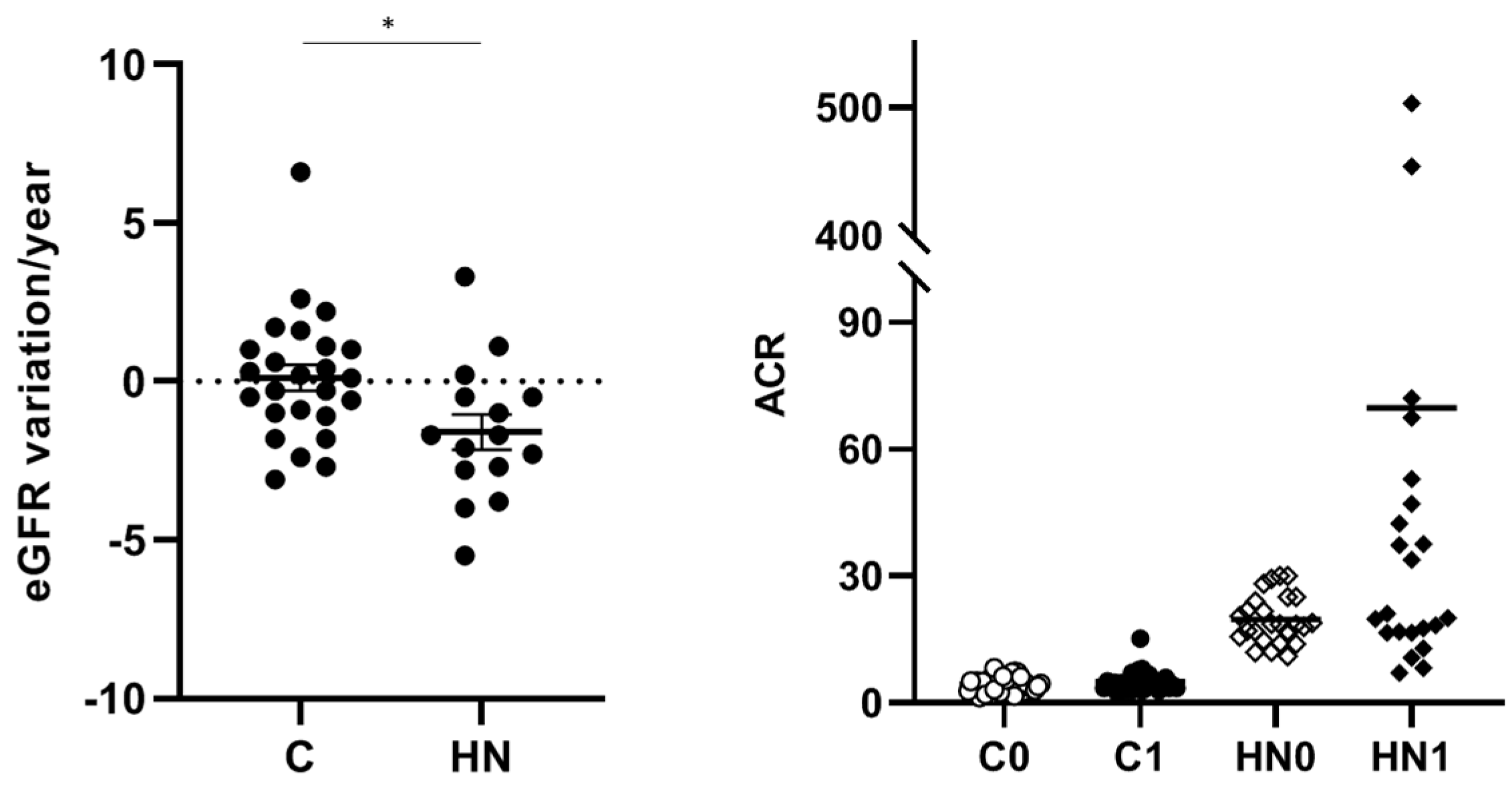
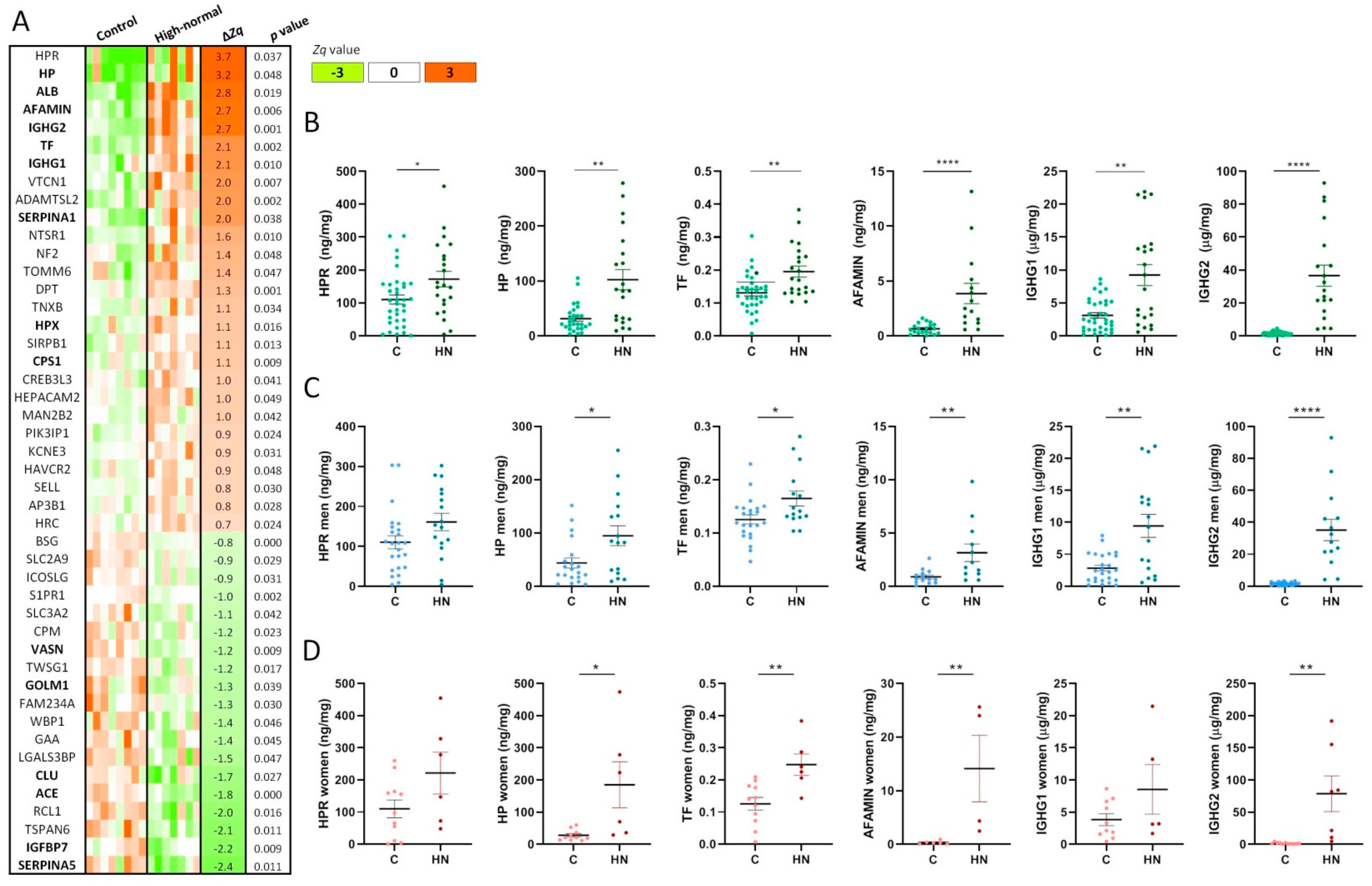
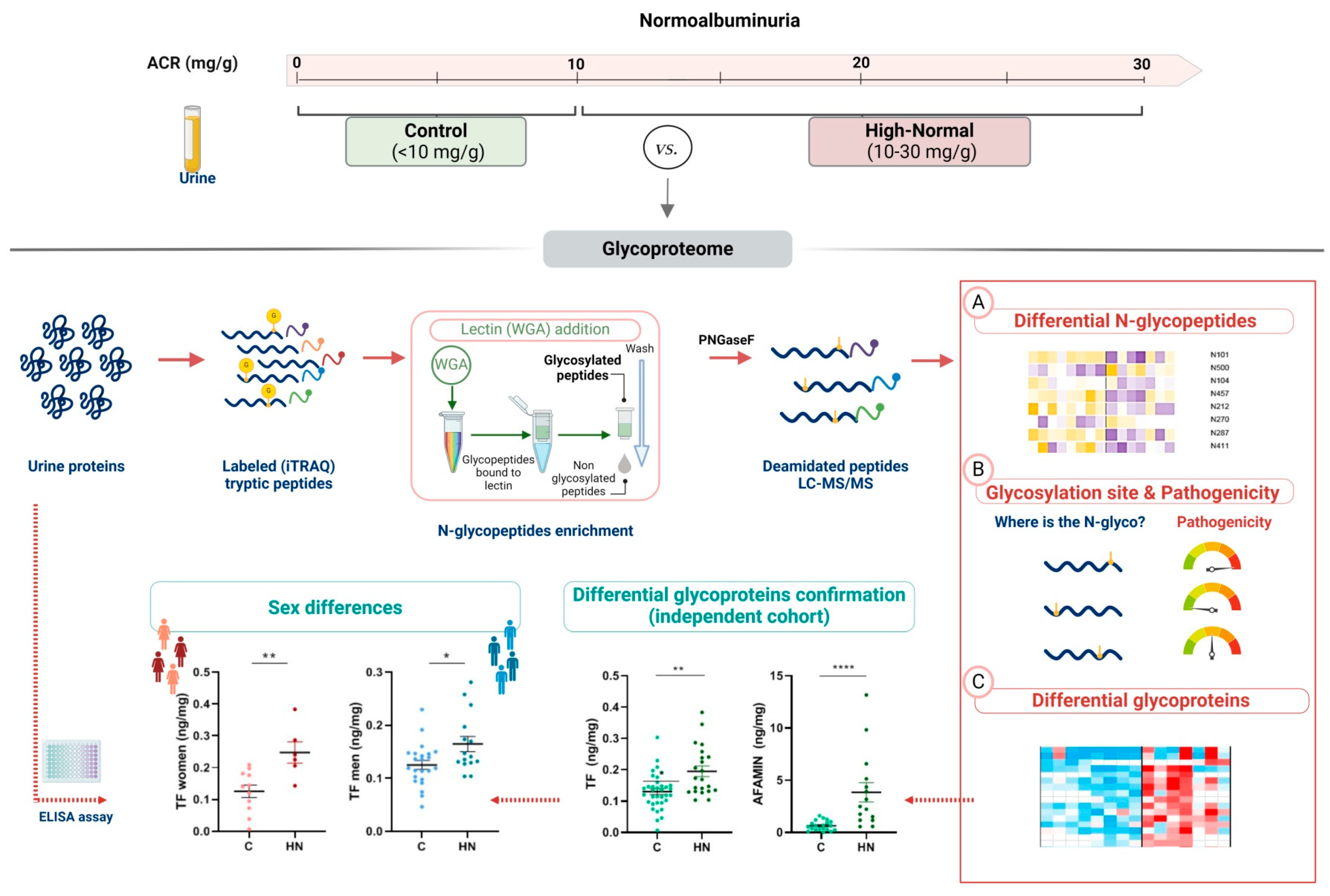
2.1. The Urinary Glycoproteome Differentiates Patients with Higher Cardiorenal Risk in Women and Men Not Meeting Criteria for CKD
2.2. Pathogenic N-Glycopeptides Differentiate Normoalbuminuric Patients with Higher Cardiorenal Risk
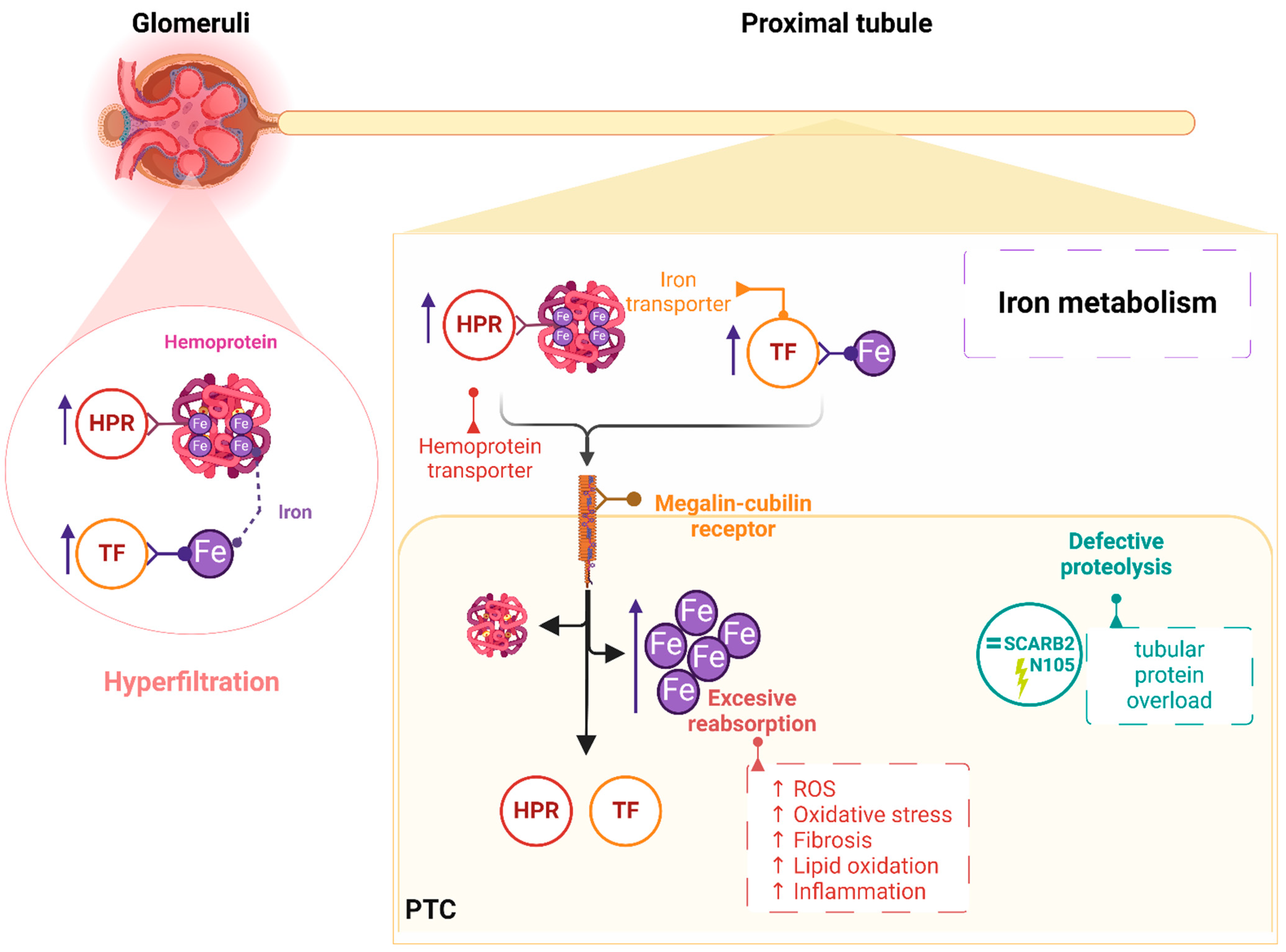
3. Discussion
4. Materials and Methods
4.1. Patients’ Selection and Urine Samples Collection
4.2. Glycoproteins Quantitation by Untargeted Mass Spectrometry
4.2.1. Protein Digestion and Peptide Isobaric Labelling
4.2.2. N-Glycopeptides Enrichment for LC-MS/MS Analysis
4.2.3. Enriched N-Glycopeptides Analysis by LC-MS/MS
4.2.4. Differential Quantitation between Clinical Groups
4.3. Targeted Glycoproteins Quantitation and Sex Differences
4.4. Pathogenicity Associated to Differential N-Glycosylation Sites
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-Cause and cause-Specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Ortiz, A.; Lucia, A.; Miranda, B.; Alvarez-Llamas, G.; Barderas, M.G.; Volpe, M.; Ruiz-Hurtado, G.; Pitt, B. Prevention of cardiorenal damage: Importance of albuminuria. Eur. Hear. J. 2022, 44, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Ärnlöv, J.; Evans, J.C.; Meigs, J.B.; Wang, T.J.; Fox, C.S.; Levy, D.; Benjamin, E.J.; D’agostino, R.B.; Vasan, R.S. Low-Grade Albuminuria and Incidence of Cardiovascular Disease Events in Nonhypertensive and Nondiabetic Individuals. Circulation 2005, 112, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.G.; Grams, M.E.; Ballew, S.H.; Sang, Y.; Azizi, F.; Chadban, S.J.; Chaker, L.; Dunning, S.C.; Fox, C.; Hirakawa, Y.; et al. Development of Risk Prediction Equations for Incident Chronic Kidney Disease. JAMA 2019, 322, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2012, 3, 1–150. [Google Scholar]
- Melsom, T.; Solbu, M.D.; Schei, J.; Stefansson, V.T.N.; Norvik, J.V.; Jenssen, T.G.; Wilsgaard, T.; Eriksen, B.O. Mild Albuminuria Is a Risk Factor for Faster GFR Decline in the Nondiabetic Population. Kidney Int. Rep. 2018, 3, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kwon, S.; Lee, J.; Shin, J.-I.; Kim, Y.C.; Park, J.Y.; Bae, E.; Kim, E.Y.; Kim, D.K.; Lim, C.S.; et al. Albuminuria within the Normal Range Can Predict All-Cause Mortality and Cardiovascular Mortality. Kidney360 2022, 3, 74–82. [Google Scholar] [CrossRef]
- Hallan, S.I.; Matsushita, K.; Sang, Y.; Mahmoodi, B.K.; Black, C.; Ishani, A.; Kleefstra, N.; Naimark, D.; Roderick, P.; Tonelli, M.; et al. Age and Association of Kidney Measures With Mortality and End-stage Renal Disease. JAMA 2012, 308, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Porrini, E.; Motterlini, N.; Perna, A.; Ilieva, A.P.; Iliev, I.P.; Dodesini, A.R.; Trevisan, R.; Bossi, A.; Sampietro, G.; et al. Measurable Urinary Albumin Predicts Cardiovascular Risk among Normoalbuminuric Patients with Type 2 Diabetes. J. Am. Soc. Nephrol. 2012, 23, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Blecker, S.; Matsushita, K.; Köttgen, A.; Loehr, L.R.; Bertoni, A.G.; Boulware, L.E.; Coresh, J. High-Normal Albuminuria and Risk of Heart Failure in the Community. Am. J. Kidney Dis. 2011, 58, 47–55. [Google Scholar] [CrossRef]
- Okubo, A.; Nakashima, A.; Doi, S.; Doi, T.; Ueno, T.; Maeda, K.; Tamura, R.; Yamane, K.; Masaki, T. High-normal albuminuria is strongly associated with incident chronic kidney disease in a nondiabetic population with normal range of albuminuria and normal kidney function. Clin. Exp. Nephrol. 2020, 24, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Klausen, K.; Borch-Johnsen, K.; Feldt-Rasmussen, B.; Jensen, G.; Clausen, P.; Scharling, H.; Appleyard, M.; Jensen, J.S. Very Low Levels of Microalbuminuria Are Associated With Increased Risk of Coronary Heart Disease and Death Independently of Renal Function, Hypertension, and Diabetes. Circulation 2004, 110, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Ihara, K.; Skupien, J.; Kobayashi, H.; Dom, Z.I.M.; Wilson, J.M.; O’neil, K.; Badger, H.S.; Bowsman, L.M.; Satake, E.; Breyer, M.D.; et al. Profibrotic Circulating Proteins and Risk of Early Progressive Renal Decline in Patients With Type 2 Diabetes With and Without Albuminuria. Diabetes Care 2020, 43, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Zürbig, P.; Jerums, G.; Hovind, P.; MacIsaac, R.J.; Mischak, H.; Nielsen, S.E.; Panagiotopoulos, S.; Persson, F.; Rossing, P. Urinary Proteomics for Early Diagnosis in Diabetic Nephropathy. Diabetes 2012, 61, 3304–3313. [Google Scholar] [CrossRef]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; Martínez, P.J.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Segura, J.; de la Cuesta, F.; Barderas, M.G.; Ruilope, L.M.; Vivanco, F.; et al. Hypertensive patients exhibit an altered metabolism. A specific metabolite signature in urine is able to predict albuminuria progression. Transl. Res. 2016, 178, 25–37.e7. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; de la Cuesta, F.; Maroto, A.S.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Pulido-Olmo, H.; Segura, J.; Barderas, M.G.; Ruilope, L.M.; et al. Urinary alpha-1 antitrypsin and CD59 glycoprotein predict albuminuria development in hypertensive patients under chronic renin-angiotensin system suppression. Cardiovasc. Diabetol. 2016, 15, 8. [Google Scholar] [CrossRef]
- Santiago-Hernandez, A.; Martin-Lorenzo, M.; Martin-Blazquez, A.; Ruiz-Hurtado, G.; Barderas, M.G.; Segura, J.; Ruilope, L.M.; Alvarez-Llamas, G. TCA Cycle and Fatty Acids Oxidation Reflect Early Cardiorenal Damage in Normoalbuminuric Subjects with Controlled Hypertension. Antioxidants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Santiago-Hernandez, A.; Martin-Lorenzo, M.; Martínez, P.J.; Gómez-Serrano, M.; Lopez, J.A.; Cannata, P.; Esteban, V.; Heredero, A.; Aldamiz-Echevarria, G.; Vázquez, J.; et al. Early renal and vascular damage within the normoalbuminuria condition. J. Hypertens. 2021, 39, 2220–2231. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Li, M.; Shao, C.; Tao, J.; Sun, W.; Li, M. Differential urinary glycoproteome analysis of type 2 diabetic nephropathy using 2D-LC–MS/MS and iTRAQ quantification. J. Transl. Med. 2015, 13, 371. [Google Scholar] [CrossRef]
- Kamiya, T.; Kadowaki, M.; Atobe, T.; Kunieda, K.; Morimoto, K.; Hara, H. Inhibition of N-glycosylation by glucosamine hydrochloride inhibits TGF-β1-induced LOXL2 secretion. J. Cell. Biochem. 2023, 124, 797–807. [Google Scholar] [CrossRef] [PubMed]
- van den Boogert, M.A.; Larsen, L.E.; Ali, L.; Kuil, S.D.; Chong, P.L.; Loregger, A.; Kroon, J.; Schnitzler, J.G.; Schimmel, A.W.; Peter, J.; et al. N-Glycosylation Defects in Humans Lower Low-Density Lipoprotein Cholesterol Through Increased Low-Density Lipoprotein Receptor Expression. Circulation 2019, 140, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Vellosillo, P.; Minguez, P. A global map of associations between types of protein posttranslational modifications and human genetic diseases. iScience 2021, 24, 102917. [Google Scholar] [CrossRef] [PubMed]
- Titan, S.M.; Pecoits-Filho, R.; Barreto, S.M.; Lopes, A.A.; Bensenor, I.J.; Lotufo, P.A. GlycA, a marker of protein glycosylation, is related to albuminuria and estimated glomerular filtration rate: The ELSA-Brasil study. BMC Nephrol. 2017, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Bhensdadia, N.M.; Hunt, K.J.; Lopes-Virella, M.F.; Tucker, J.M.; Mataria, M.R.; Alge, J.L.; Neely, B.A.; Janech, M.G.; Arthur, J.M. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int. 2013, 83, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Kaburagi, Y.; Takahashi, E.; Kajio, H.; Yamashita, S.; Yamamoto-Honda, R.; Shiga, T.; Okumura, A.; Goto, A.; Fukazawa, Y.; Seki, N.; et al. Urinary afamin levels are associated with the progression of diabetic nephropathy. Diabetes Res. Clin. Pr. 2018, 147, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Llamas, G.; Santiago-Hernandez, A.; Ruilope, L.M. Evidence of chronic kidney injury in patients not meeting KDIGO criteria for chronic kidney disease. Clin. Kidney J. 2022, 15, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; de Dios, O.; Lahoz, C.; Arribas, M.; Arroyo, A.P.; Salinero-Fort, M.A.; Laguna, F.; Estirado, E.; García-Iglesias, F.; Alegre, T.G.; et al. Fenotipo de la haptoglobina y presencia de enfermedad vascular subclínica: Estudio poblacional. Clin. Investig. Arter. 2019, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Sun, W.; Cleary, P.A.; Genuth, S.M.; Lachin, J.M.; McGee, P.; Paterson, A.D.; Raskin, P.; Anbinder, Y.; Levy, A.P.; et al. Haptoglobin Genotype and the Rate of Renal Function Decline in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes 2013, 62, 3218–3223. [Google Scholar] [CrossRef]
- Baldan-Martin, M.; Rodríguez-Sánchez, E.; González-Calero, L.; Ruilope, L.M.; Alvarez-Llamas, G.; Barderas, M.G.; Ruiz-Hurtado, G. Translational science in albuminuria: A new view of de novo albuminuria under chronic RAS suppression. Clin. Sci. 2018, 132, 739–758. [Google Scholar] [CrossRef]
- van Raaij, S.; van Swelm, R.; Bouman, K.; Cliteur, M.; Heuvel, M.C.v.D.; Pertijs, J.; Patel, D.; Bass, P.; van Goor, H.; Unwin, R.; et al. Tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Zhuo, W.-Q.; Wen, Y.; Luo, H.-J.; Luo, Z.-L.; Wang, L. Mechanisms of ferroptosis in chronic kidney disease. Front. Mol. Biosci. 2022, 9, 975582. [Google Scholar] [CrossRef] [PubMed]
- Sathe, G.; George, I.A.; Deb, B.; Jain, A.P.; Patel, K.; Nayak, B.; Karmakar, S.; Seth, A.; Pandey, A.; Kumar, P. Urinary glycoproteomic profiling of non-muscle invasive and muscle invasive bladder carcinoma patients reveals distinct N-glycosylation pattern of CD44, MGAM, and GINM1. Oncotarget 2020, 11, 3244–3255. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lih, T.-S.M.; Li, Q.K.; Zhang, H. Comparing Urinary Glycoproteins among Three Urogenital Cancers and Identifying Prostate Cancer-Specific Glycoproteins. ACS Omega 2022, 7, 9172–9180. [Google Scholar] [CrossRef]
- Li, Q.K.; Chen, L.; Ao, M.-H.; Chiu, J.H.; Zhang, Z.; Zhang, H.; Chan, D.W. Serum Fucosylated Prostate-specific Antigen (PSA) Improves the Differentiation of Aggressive from Non-aggressive Prostate Cancers. Theranostics 2015, 5, 267–276. [Google Scholar] [CrossRef] [PubMed]
- DeCoux, A.; Tian, Y.; DeLeon-Pennell, K.Y.; Nguyen, N.T.; Brás, L.E.d.C.; Flynn, E.R.; Cannon, P.L.; Griswold, M.E.; Jin, Y.-F.; Puskarich, M.A.; et al. Plasma Glycoproteomics Reveals Sepsis Outcomes Linked to Distinct Proteins in Common Pathways*. Crit. Care Med. 2015, 43, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Dotz, V.; Visconti, A.; Lomax-Browne, H.J.; Clerc, F.; Ederveen, A.L.H.; Medjeral-Thomas, N.R.; Cook, H.T.; Pickering, M.C.; Wuhrer, M.; Falchi, M. O- and N-Glycosylation of Serum Immunoglobulin A is Associated with IgA Nephropathy and Glomerular Function. J. Am. Soc. Nephrol. 2021, 32, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Budhraja, R.; Saraswat, M.; De Graef, D.; Ranatunga, W.; Ramarajan, M.G.; Mousa, J.; Kozicz, T.; Pandey, A.; Morava, E. N-glycoproteomics reveals distinct glycosylation alterations in NGLY1-deficient patient-derived dermal fibroblasts. J. Inherit. Metab. Dis. 2022, 46, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.J.; Lee, D.; Fraser, S.A.; Katerelos, M.; Gleich, K.; Martinello, P.; Li, Y.Q.; Thomas, M.C.; Michelucci, R.; Cole, A.J.; et al. Tubular proteinuria in mice and humans lacking the intrinsic lysosomal protein SCARB2/Limp-2. Am. J. Physiol. Physiol. 2011, 300, F1437–F1447. [Google Scholar] [CrossRef]
- Holmes, R.S. Vertebrate Scavenger Receptor Class B Member 2 (SCARB2): Comparative Studies of a Major Lysosomal Mem-brane Glycoprotein. J. Mol. Biochem. 2012, 1, 99–115. [Google Scholar]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Zhong, X.; Jagarlapudi, S.; Weng, Y.; Ly, M.; Rouse, J.C.; McClure, K.; Ishino, T.; Zhang, Y.; Sousa, E.; Cohen, J.; et al. Structure-function relationships of the soluble form of the antiaging protein Klotho have therapeutic implications for managing kidney disease. J. Biol. Chem. 2020, 295, 3115–3133. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Martinez, P.J.; Baldan-Martin, M.; Lopez, J.A.; Minguez, P.; Santiago-Hernandez, A.; Vazquez, J.; Segura, J.; Ruiz-Hurtado, G.; Vivanco, F.; et al. Urine Haptoglobin and Haptoglobin-Related Protein Predict Response to Spironolactone in Patients With Resistant Hypertension. Hypertension 2019, 73, 794–802. [Google Scholar] [CrossRef]
- Cardona, M.; López, J.A.; Serafín, A.; Rongvaux, A.; Inserte, J.; García-Dorado, D.; Flavell, R.; Llovera, M.; Cañas, X.; Vázquez, J.; et al. Executioner Caspase-3 and 7 Deficiency Reduces Myocyte Number in the Developing Mouse Heart. PLoS ONE 2015, 10, e0131411. [Google Scholar] [CrossRef]
- Zielinska, D.F.; Gnad, F.; Wiśniewski, J.R.; Mann, M. Precision Mapping of an In Vivo N-Glycoproteome Reveals Rigid Topological and Sequence Constraints. Cell 2010, 141, 897–907. [Google Scholar] [CrossRef]
- Martiánez-Bartolomé, S.; Navarro, P.; Martián-Maroto, F.; López-Ferrer, D.; Ramos-Fernández, A.; Villar, M.; Garciáa-Ruiz, J.P.; Vázquez, J. Properties of Average Score Distributions of SEQUEST. Mol. Cell. Proteom. 2008, 7, 1135–1145. [Google Scholar] [CrossRef]
- Navarro, P.; Vázquez, J. A Refined Method To Calculate False Discovery Rates for Peptide Identification Using Decoy Databases. J. Proteome Res. 2009, 8, 1792–1796. [Google Scholar] [CrossRef]
- Bonzon-Kulichenko, E.; Garcia-Marques, F.; Trevisan-Herraz, M.; Vázquez, J. Revisiting Peptide Identification by High-Accuracy Mass Spectrometry: Problems Associated with the Use of Narrow Mass Precursor Windows. J. Proteome Res. 2014, 14, 700–710. [Google Scholar] [CrossRef]
- Trevisan-Herraz, M.; Bagwan, N.; García-Marqués, F.; Rodriguez, J.M.; Jorge, I.; Ezkurdia, I.; Bonzon-Kulichenko, E.; Vázquez, J. SanXoT: A modular and versatile package for the quantitative analysis of high-throughput proteomics experiments. Bioinformatics 2018, 35, 1594–1596. [Google Scholar] [CrossRef]
- Navarro, P.; Trevisan-Herraz, M.; Bonzon-Kulichenko, E.; Núñez, E.; Martínez-Acedo, P.; Pérez-Hernández, D.; Jorge, I.; Mesa, R.; Calvo, E.; Carrascal, M.; et al. General Statistical Framework for Quantitative Proteomics by Stable Isotope Labeling. J. Proteome Res. 2014, 13, 1234–1247. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M.; et al. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2015, 44, D286–D293. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013. [Google Scholar] [CrossRef] [PubMed]
| Untargeted Mass Spectrometry (LC-MS/MS) (Discovery Cohort) | Targeted Quantitation (ELISA) (Confirmation Cohort) | |||||
|---|---|---|---|---|---|---|
| Control (C) (n = 8) | High-Normal (HN) (n = 7) | p Value | Control (C) (n = 38) | High-Normal (HN) (n = 25) | p Value | |
| Age (years) | 55 ± 8 | 63 ± 6 | 0.0535 | 58 ± 7 | 63 ± 6 | 0.0140 |
| Sex (% male) | 8 (100) | 7 (100) | >0.9999 | 25 (66) | 18 (72) | 0.7829 |
| Cholesterol total (mg/dL) | 161 ± 37 | 154 ± 34 | >0.9999 | 180 ± 32 | 171 ± 29 | 0.4158 |
| Triglycerides (mg/dL) | 104 ± 34 | 113 ± 44 | 0.6733 | 109 ± 39 | 118 ± 50 | 0.7754 |
| Cholesterol HDL (mg/dL) | 44 ± 8 | 48 ± 15 | 0.6794 | 54 ± 15 | 54 ± 16 | 0.7500 |
| Cholesterol LDL (mg/dL) | 95 ± 35 | 84 ± 30 | 0.8065 | 103 ± 28 | 93 ± 30 | 0.3628 |
| Glycemia (mg/dL) | 104 ± 9 | 103 ± 20 | 0.3792 | 103 ± 11 | 104 ± 17 | 0.7610 |
| Uric acid (mg/dL) | 6 ± 1 | 7 ± 2 | 0.2426 | 6 ± 1 | 6 ± 1 | 0.0925 |
| ACR (mg/g) | 4 ± 2 | 18 ± 4 | 0.0003 | 4 ± 2 | 20 ± 6 | <0.0001 |
| Diabetes mellitus type 2 (%) | 25 | 14 | >0.9999 | 11 | 16 | 0.7019 |
| SBP (mmHg) | 136 ± 15 | 142 ± 13 | 0.2645 | 139 ± 15 | 141 ± 13 | 0.6883 |
| DBP (mmHg) | 85 ± 8 | 82 ± 7 | 0.7167 | 83 ± 8 | 82 ± 8 | 0.3767 |
| Antihypertensive Treatment (%) | ||||||
| iECAs | 38 | 29 | >0.9999 | 24 | 20 | 0.7683 |
| ARA | 50 | 57 | >0.9999 | 68 | 64 | 0.7965 |
| Diuretic | 50 | 43 | >0.9999 | 34 | 52 | 0.3007 |
| Calcium channel blocker | 25 | 71 | 0.1319 | 42 | 76 | 0.0053 |
| α-blocker | 50 | 0 | 0.0769 | 26 | 0 | 0.1474 |
| β-blocker | 38 | 29 | >0.9999 | 13 | 36 | 0.5755 |
| Other Treatment (%) | ||||||
| Anticoagulant | 13 | 0 | >0.9999 | 5 | 4 | >0.9999 |
| Lipid lowering | 50 | 71 | 0.6084 | 63 | 56 | 0.5965 |
| Antidiabetic | 25 | 14 | >0.9999 | 8 | 8 | >0.9999 |
| Peptide Sequence Identified by MS/MS | Gene | Zp Values (Peptide Abundance) C - HN | N-Glycosylation Site | Pathogenicity Score | HN vs. C(Peptide) | HN vs. C(Protein) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-glycopeptidome specific signature | ||||||||||||||||||||||
| N$ITEGEAR | ACAN | N387 | 0.9158 | ↓ | - | |||||||||||||||||
| QEDLSVGSVLLTVN$ATDPDSLQHQTIR | CDH13 | N500 | 0.9970 | ↑ | - | |||||||||||||||||
| TALFPDLLAQGN$ASLR | CD276 | N104 | 0.9813 | ↓ | - | |||||||||||||||||
| GTEWLVN$SSR | CNTN1 | N457 | 0.9998 | ↓ | - | |||||||||||||||||
| N$FTAADWGQSR | COL6A1 | N212 | 0.9968 | ↓ | - | |||||||||||||||||
| QLDM_LDLSNN$SLASVPEGLWASLGQPNWDM_R | LRG1 | N270 | 0.9990 | ↑ | - | |||||||||||||||||
| STEGQSSLTVTNVTEEHYGN$YTC#VAANK@ | LSAMP | N287 | 0.9984 | ↓ | - | |||||||||||||||||
| LN$DTTLQVLNTWYTK@ | OLFM4 | N411 | 0.9935 | ↓ | - | |||||||||||||||||
| YGTALVHLYVN$ETLAN$R | PCDH1 | N813/N818 | 0.9998/0.9999 | ↑ | - | |||||||||||||||||
| ANIQFGDN$GTTISAVSNK@ | SCARB2 | N105 | 0.9077 | ↓ | - | |||||||||||||||||
| Other N-glycopeptides | ||||||||||||||||||||||
| ELPGVC#N$ETMMALWEEC#K@PC#LK@ | CLU | N103 | 0.9996 | ↓ | ↓ | |||||||||||||||||
| MLN$TSSLLEQLN$EQFNWVSR | CLU | N363 | 0.9242 | ↓ | ↓ | |||||||||||||||||
| AVLVNN$ITTGER | GOLM1 | N109 | 0.9983 | ↓ | ↓ | |||||||||||||||||
| LFN$VTPQDEQK@ | ICOSLG | N102 | 0.9674 | ↓ | ↓ | |||||||||||||||||
| TDNSLLDQALQN$DTVFLNMR | ICOSLG | N186 | 0.9896 | ↓ | ↓ | |||||||||||||||||
| TDN$SLLDQALQN$DTVFLNMR | ICOSLG | N186 | 0.9896 | ↓ | ↓ | |||||||||||||||||
| TDNSLLDQALQN$DTVFLNM_R | ICOSLG | N186 | 0.9896 | ↓ | ↓ | |||||||||||||||||
| EEQYN$STYR | IGHG1 | N180 | 0.9551 | ↑ | ↑ | |||||||||||||||||
| TK@PREEQFN$STFR | IGHG2 | N176 | 0.9916 | ↑ | ↑ | |||||||||||||||||
| EEQFN$STFR | IGHG2 | N176 | 0.9916 | ↑ | ↑ | |||||||||||||||||
| ALN$ATLHSN$LLC#RPGPGLGPDNQTEER | KCNE3 | N22 | 0.9872 | ↑ | ↑ | |||||||||||||||||
| AQAGLEEALLAPGFGN$ASGN$ASER | NTSR1 | N37 | 0.9399 | ↑ | ↑ | |||||||||||||||||
| VVGVPYQGN$ATALFILPSEGK@ | SERPINA5 | N262 | 0.9475 | ↓ | ↓ | |||||||||||||||||
| AFYN$ESWER | SLC2A9 | N90 | 0.9788 | ↓ | ↓ | |||||||||||||||||
| N$LSDIDLM_APQPGV | TOMM6 | N61 | 0.9951 | ↑ | ↑ | |||||||||||||||||
| LHEITN$ETFR | VASN | N117 | 0.9184 | ↓ | ↓ | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Hernandez, A.; Martin-Lorenzo, M.; Gómez-Serrano, M.; Lopez, J.A.; Martin-Blazquez, A.; Vellosillo, P.; Minguez, P.; Martinez, P.J.; Vázquez, J.; Ruiz-Hurtado, G.; et al. The Urinary Glycopeptide Profile Differentiates Early Cardiorenal Risk in Subjects Not Meeting Criteria for Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 7005. https://doi.org/10.3390/ijms25137005
Santiago-Hernandez A, Martin-Lorenzo M, Gómez-Serrano M, Lopez JA, Martin-Blazquez A, Vellosillo P, Minguez P, Martinez PJ, Vázquez J, Ruiz-Hurtado G, et al. The Urinary Glycopeptide Profile Differentiates Early Cardiorenal Risk in Subjects Not Meeting Criteria for Chronic Kidney Disease. International Journal of Molecular Sciences. 2024; 25(13):7005. https://doi.org/10.3390/ijms25137005
Chicago/Turabian StyleSantiago-Hernandez, Aranzazu, Marta Martin-Lorenzo, María Gómez-Serrano, Juan Antonio Lopez, Ariadna Martin-Blazquez, Perceval Vellosillo, Pablo Minguez, Paula J. Martinez, Jesús Vázquez, Gema Ruiz-Hurtado, and et al. 2024. "The Urinary Glycopeptide Profile Differentiates Early Cardiorenal Risk in Subjects Not Meeting Criteria for Chronic Kidney Disease" International Journal of Molecular Sciences 25, no. 13: 7005. https://doi.org/10.3390/ijms25137005
APA StyleSantiago-Hernandez, A., Martin-Lorenzo, M., Gómez-Serrano, M., Lopez, J. A., Martin-Blazquez, A., Vellosillo, P., Minguez, P., Martinez, P. J., Vázquez, J., Ruiz-Hurtado, G., Barderas, M. G., Sarafidis, P., Segura, J., Ruilope, L. M., & Alvarez-Llamas, G. (2024). The Urinary Glycopeptide Profile Differentiates Early Cardiorenal Risk in Subjects Not Meeting Criteria for Chronic Kidney Disease. International Journal of Molecular Sciences, 25(13), 7005. https://doi.org/10.3390/ijms25137005











