Plasma Proteins Associated with COVID-19 Severity in Puerto Rico
Abstract
1. Introduction
2. Results
2.1. Demographics
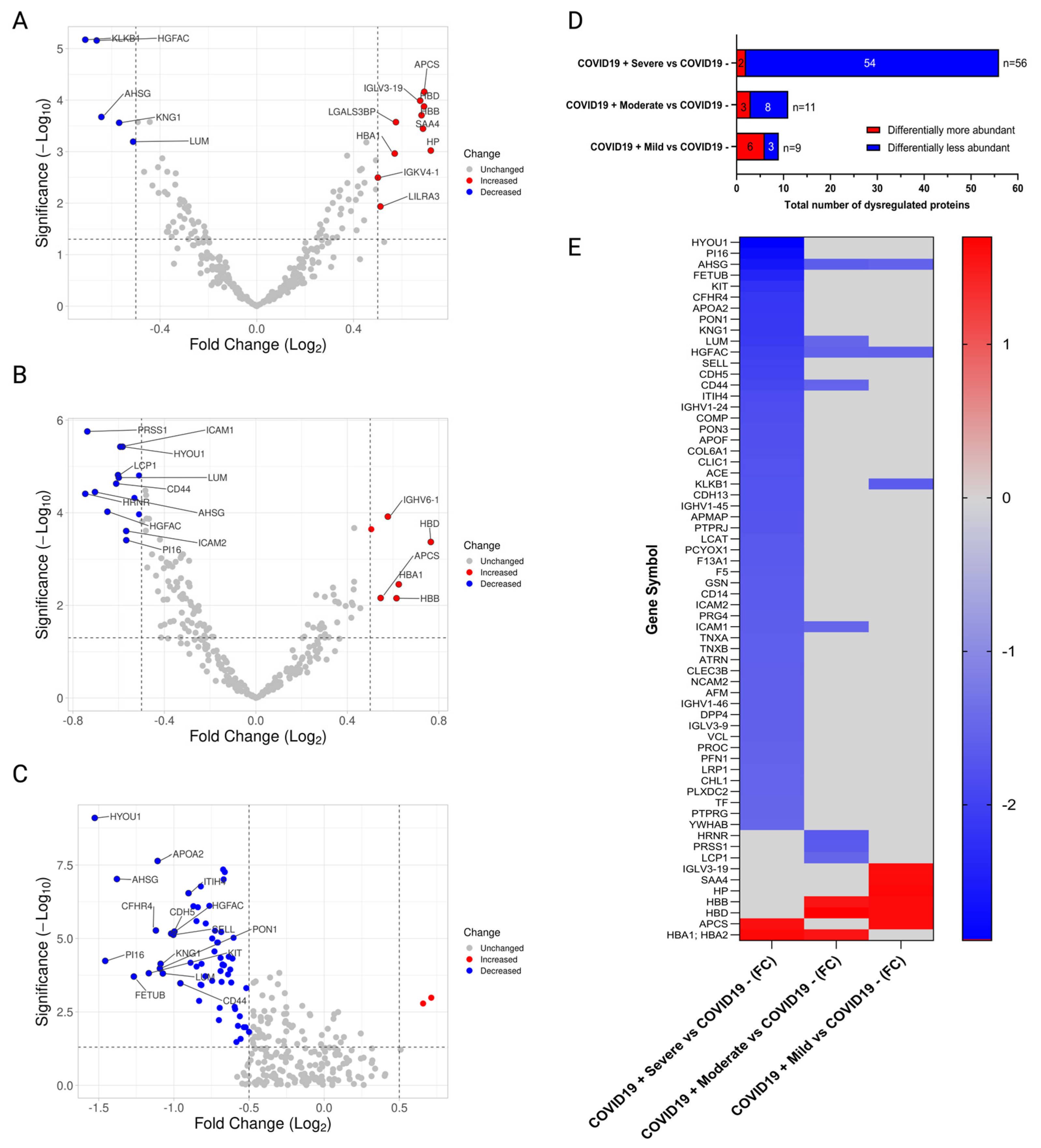
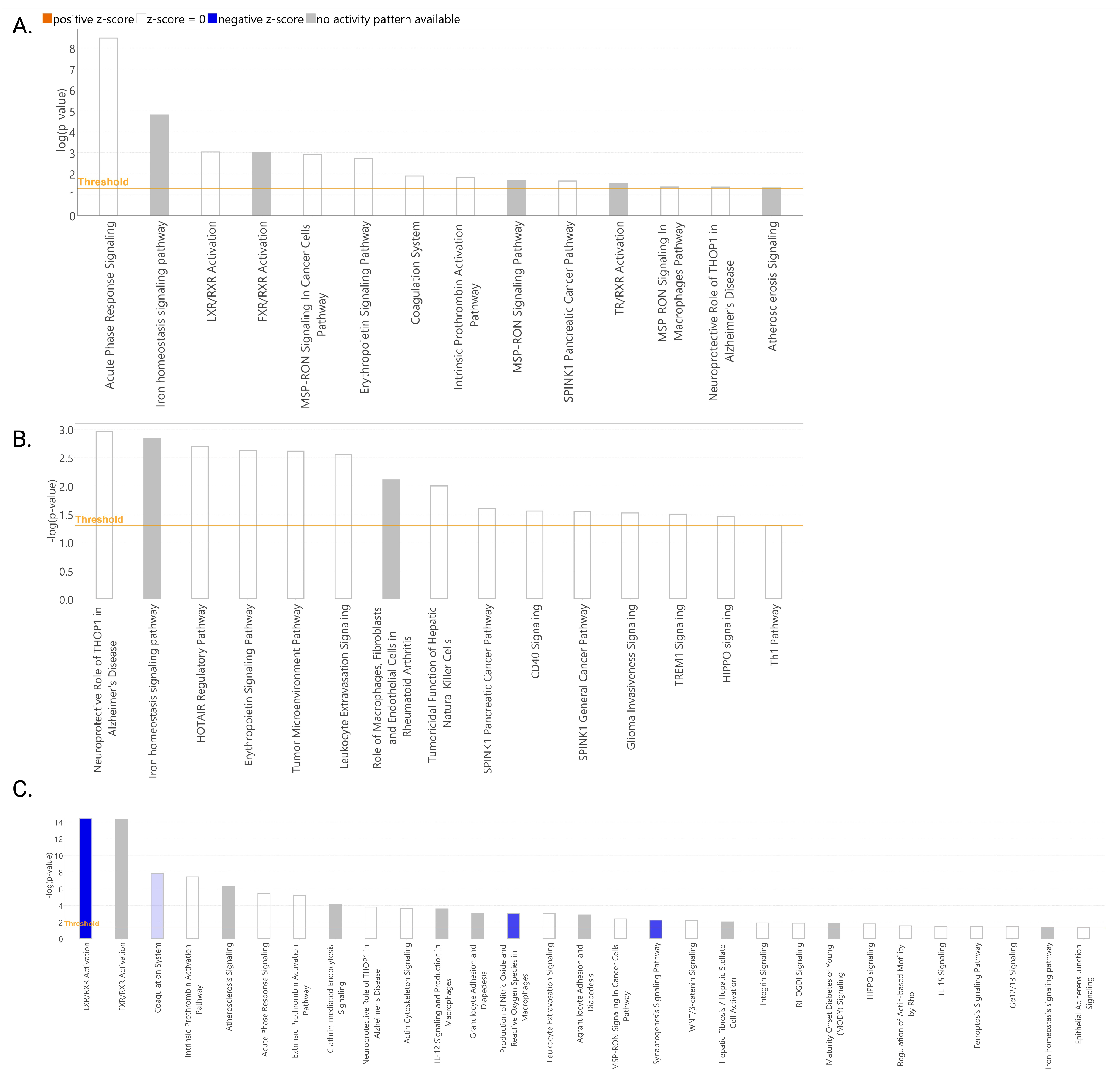
2.2. Proteomic Profile of Puerto Rican COVID-19 Patients
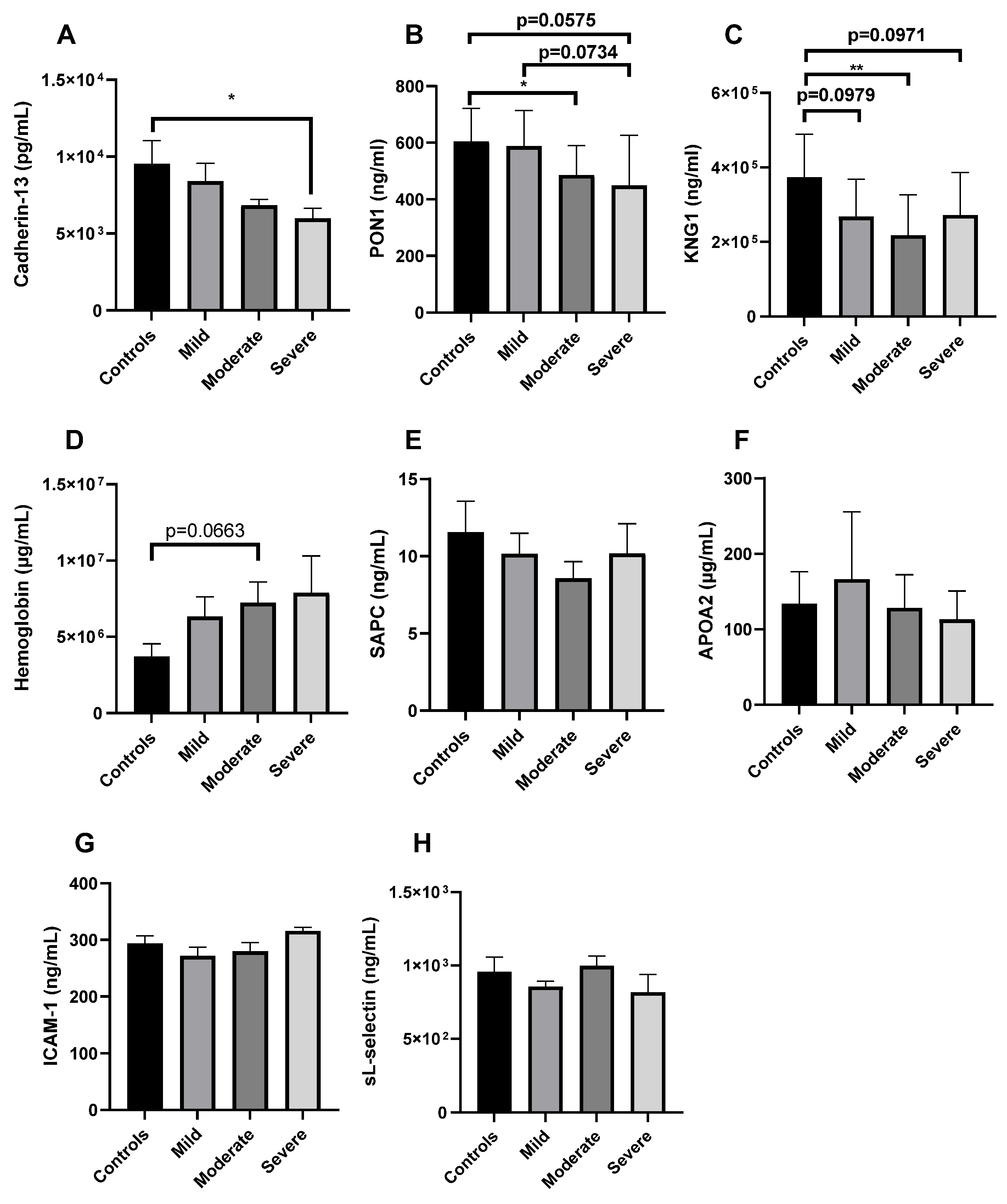
2.3. Validation of Cadherin-13 as Potential Biomarker of Severe COVID-19 in Puerto Ricans
2.4. Cytokine Profile of Puerto Rican COVID-19 Patients
3. Discussion
4. Materials and Methods
4.1. Study Participants, Ethics, and Sample Collection
4.2. Proteomics
4.2.1. Depletion of Most Abundant Proteins
4.2.2. TMT Labeling, Fractionation, and Mass Spectrometry Analyses
4.2.3. Protein Identification and Quantitation
4.3. Cytokines
4.4. Validation Using ELISA
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 3 April 2024).
- Mehta, P.; Fajgenbaum, D.C. Is Severe COVID-19 a Cytokine Storm Syndrome: A Hyperinflammatory Debate. Curr. Opin. Rheumatol. 2021, 33, 419–430. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; François, B.; Sève, P. Should We Stimulate or Suppress Immune Responses in COVID-19? Cytokine and Anti-Cytokine Interventions. Autoimmun. Rev. 2020, 19, 102567. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex Differences in Immune Responses That Underlie COVID-19 Disease Outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine Storm Intervention in the Early Stages of COVID-19 Pneumonia. Cytokine Growth Factor. Rev. 2020, 53, 38. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The Cytokine Storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhu, Y.; Mao, J.; Du, R.C. T Cell Immunobiology and Cytokine Storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989. [Google Scholar] [CrossRef]
- Dimitsaki, S.; Gavriilidis, G.I.; Dimitriadis, V.K.; Natsiavas, P. Benchmarking of Machine Learning Classifiers on Plasma Proteomic for COVID-19 Severity Prediction through Interpretable Artificial Intelligence. Artif. Intell. Med. 2023, 137, 102490. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Royston, L.; Mabanga, T.; Berini, C.A.; Tremblay, C.; Lebouché, B.; Cox, J.; Costiniuk, C.T.; Durand, M.; Isnard, S.; et al. Proteomics Validate Circulating GDF-15 as an Independent Biomarker for COVID-19 Severity. Front. Immunol. 2024, 15, 1377126. [Google Scholar] [CrossRef]
- Harriott, N.C.; Ryan, A.L. Proteomic Profiling Identifies Biomarkers of COVID-19 Severity. Heliyon 2024, 10, e23320. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; McIntyre, R.S.; Choo, F.N.; Tran, B.; Ho, R.; Sharma, V.K.; et al. A Longitudinal Study on the Mental Health of General Population during the COVID-19 Epidemic in China. Brain Behav. Immun. 2020, 87, 40. [Google Scholar] [CrossRef]
- Meister, T.; Pisarev, H.; Kolde, R.; Kalda, R.; Suija, K.; Milani, L.; Karo-Astover, L.; Piirsoo, M.; Uuskula, A. Clinical Characteristics and Risk Factors for COVID-19 Infection and Disease Severity: A Nationwide Observational Study in Estonia. PLoS ONE 2022, 17, e0270192. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, F.; Shi, Y.; Chen, Y.; Shi, B.; Yu, G. Causal Association of Epigenetic Aging and COVID-19 Severity and Susceptibility: A Bidirectional Mendelian Randomization Study. Front. Med. 2022, 9, 989950. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Falfán-Valencia, R. Genetics Insight for COVID-19 Susceptibility and Severity: A Review. Front. Immunol. 2021, 12, 622176. [Google Scholar] [CrossRef]
- Martono; Fatmawati, F.; Mulyanti, S. Risk Factors Associated with the Severity of COVID-19. Malays. J. Med. Sci. 2023, 30, 84. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, H.; Kim, S.Y.; Kim, Y.; Lee, J.S.; Dan, K.; Seong, M.W.; Han, D. In-Depth Blood Proteome Profiling Analysis Revealed Distinct Functional Characteristics of Plasma Proteins between Severe and Non-Severe COVID-19 Patients. Sci. Rep. 2020, 10, 22418. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59. [Google Scholar] [CrossRef]
- Messner, C.B.; Demichev, V.; Wendisch, D.; Michalick, L.; White, M.; Freiwald, A.; Textoris-Taube, K.; Vernardis, S.I.; Egger, A.S.; Kreidl, M.; et al. Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection. Cell Syst. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Ciccosanti, F.; Antonioli, M.; Sacchi, A.; Notari, S.; Farina, A.; Beccacece, A.; Fusto, M.; Vergori, A.; D’offizi, G.; Taglietti, F.; et al. Proteomic Analysis Identifies a Signature of Disease Severity in the Plasma of COVID-19 Pneumonia Patients Associated to Neutrophil, Platelet and Complement Activation Clinical Proteomics. Clin. Proteom. 2022, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Pagani, L.; Chinello, C.; Risca, G.; Capitoli, G.; Criscuolo, L.; Lombardi, A.; Ungaro, R.; Mangioni, D.; Piga, I.; Muscatello, A.; et al. Plasma Proteomic Variables Related to COVID-19 Severity: An Untargeted NLC-MS/MS Investigation. Int. J. Mol. Sci. 2023, 24, 3570. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.; Biswas, D.; Pai, M.G.J.; Acharjee, A.; Bankar, R.; Palanivel, V.; Salkar, A.; Verma, A.; Mukherjee, A.; Choudhury, M.; et al. Proteomics and Machine Learning Approaches Reveal a Set of Prognostic Markers for COVID-19 Severity With Drug Repurposing Potential. Front. Physiol. 2021, 12, 652799. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Tober-Lau, P.; Lemke, O.; Nazarenko, T.; Thibeault, C.; Whitwell, H.; Röhl, A.; Freiwald, A.; Szyrwiel, L.; Ludwig, D.; et al. A Time-Resolved Proteomic and Prognostic Map of COVID-19. Cell Syst. 2021, 12, 780–794.e7. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, E.; Orera, I.; Carmona-Rodríguez, L.; Paño, J.R.; Vázquez, J.; Corrales, F.J. Mapping the Serum Proteome of COVID-19 Patients; Guidance for Severity Assessment. Biomedicines 2022, 10, 1690. [Google Scholar] [CrossRef]
- Alaiya, A.; Alshukairi, A.; Shinwari, Z.; Al-Fares, M.; Alotaibi, J.; Alomaim, W.; Alsharif, I.; Bakheet, R.; Alharbi, L.; Allam, R.; et al. Alterations in the Plasma Proteome Induced by SARS-CoV-2 and MERS-CoV Reveal Biomarkers for Disease Outcomes for COVID-19 Patients. J. Inflamm. Res. 2021, 14, 4313–4328. [Google Scholar] [CrossRef] [PubMed]
- Faguer, S.; Del Bello, A.; Danet, C.; Renaudineau, Y.; Izopet, J.; Kamar, N. Apolipoprotein-A-I for Severe COVID-19-Induced Hyperinflammatory States: A Prospective Case Study. Front. Pharmacol. 2022, 13, 936659. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Western, D.; Timsina, J.; Repaci, C.; Song, W.M.; Norton, J.; Kohlfeld, P.; Budde, J.; Climer, S.; Butt, O.H.; et al. Plasma Proteomics of SARS-CoV-2 Infection and Severity Reveals Impact on Alzheimer’s and Coronary Disease Pathways. iScience 2023, 26, 106408. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Thomas, T.; Dzieciatkowska, M.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hod, E.A.; Spitalnik, S.L.; Hansen, K.C. Serum Proteomics in COVID-19 Patients: Altered Coagulation and Complement Status as a Function of IL-6 Level. J. Proteome Res. 2020, 19, 4417. [Google Scholar] [CrossRef]
- Asare-Werehene, M.; McGuinty, M.; Vranjkovic, A.; Galipeau, Y.; Cowan, J.; Cameron, B.; Cooper, C.L.; Langlois, M.A.; Crawley, A.M.; Tsang, B.K. Longitudinal Profiles of Plasma Gelsolin, Cytokines and Antibody Expression Predict COVID-19 Severity and Hospitalization Outcomes. Front. Immunol. 2022, 13, 1011084. [Google Scholar] [CrossRef] [PubMed]
- Messner, C.B.; Demichev, V.; Bloomfield, N.; Yu, J.S.L.; White, M.; Kreidl, M.; Egger, A.S.; Freiwald, A.; Ivosev, G.; Wasim, F.; et al. Ultra-Fast Proteomics with Scanning SWATH. Nat. Biotechnol. 2021, 39, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sun, R.; Ge, W.; Mao, T.; Qian, L.; Huang, C.; Kang, Z.; Xiao, Q.; Luo, M.; Zhang, Q.; et al. Enhanced Inflammation and Suppressed Adaptive Immunity in COVID-19 with Prolonged RNA Shedding. Cell Discov. 2022, 8, 70. [Google Scholar] [CrossRef]
- Kozlov, E.M.; Ivanova, E.; Grechko, A.V.; Wu, W.-K.; Starodubova, A.V.; Orekhov, A.N. Involvement of Oxidative Stress and the Innate Immune System in SARS-CoV-2 Infection. Diseases 2021, 9, 17. [Google Scholar] [CrossRef]
- Forcados, G.E.; Muhammad, A.; Oladipo, O.O.; Makama, S.; Meseko, C.A. Metabolic Implications of Oxidative Stress and Inflammatory Process in SARS-CoV-2 Pathogenesis: Therapeutic Potential of Natural Antioxidants. Front. Cell Infect. Microbiol. 2021, 11, 654813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883. [Google Scholar] [CrossRef] [PubMed]
- Mesci, P.; de Souza, J.S.; Martin-Sancho, L.; Macia, A.; Saleh, A.; Yin, X.; Snethlage, C.; Adams, J.W.; Avansini, S.H.; Herai, R.H.; et al. SARS-CoV-2 Infects Human Brain Organoids Causing Cell Death and Loss of Synapses That Can Be Rescued by Treatment with Sofosbuvir. PLoS Biol. 2022, 20, e3001845. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.A.; Semina, E.V.; Kalinina, N.I.; Sysoeva, V.Y.; Balatskiy, A.V.; Tkachuk, V.A. Revisiting the Multiple Roles of T-Cadherin in Health and Disease. Eur. J. Cell Biol. 2021, 100, 151183. [Google Scholar] [CrossRef] [PubMed]
- Mossink, B.; van Rhijn, J.R.; Wang, S.; Linda, K.; Vitale, M.R.; Zöller, J.E.M.; van Hugte, E.J.H.; Bak, J.; Verboven, A.H.A.; Selten, M.; et al. Cadherin-13 Is a Critical Regulator of GABAergic Modulation in Human Stem-Cell-Derived Neuronal Networks. Mol. Psychiatry 2022, 27, 1. [Google Scholar] [CrossRef]
- Paradis, S.; Harrar, D.B.; Lin, Y.; Koon, A.C.; Hauser, J.L.; Griffith, E.C.; Zhu, L.; Brass, L.F.; Chen, C.; Greenberg, M.E. An RNAi-Based Approach Identifies New Molecules Required for Glutamatergic and GABAergic Synapse Development. Neuron 2007, 53, 217. [Google Scholar] [CrossRef]
- Pfaff, D.; Schoenenberger, A.W.; Dasen, B.; Erne, P.; Resink, T.J.; Philippova, M. Plasma T-Cadherin Negatively Associates with Coronary Lesion Severity and Acute Coronary Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Minnai, F.; Biscarini, F.; Esposito, M.; Dragani, T.A.; Bujanda, L.; Rahmouni, S.; Alarcón-Riquelme, M.E.; Bernardo, D.; Carnero-Montoro, E.; Buti, M.; et al. A Genome-Wide Association Study for Survival from a Multi-Centre European Study Identified Variants Associated with COVID-19 Risk of Death. Sci. Rep. 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.; Zakharia, F.; Moreno-Estrada, A.; Byrnes, J.K.; Muzzio, M.; Rodriguez-Flores, J.L.; Kenny, E.E.; Gignoux, C.R.; Maples, B.K.; Guiblet, W.; et al. Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data. PLoS Genet. 2013, 9, 1004023. [Google Scholar] [CrossRef] [PubMed]
- Begue, F.; Tanaka, S.; Mouktadi, Z.; Rondeau, P.; Veeren, B.; Diotel, N.; Tran-Dinh, A.; Robert, T.; Vélia, E.; Mavingui, P.; et al. Altered High-Density Lipoprotein Composition and Functions during Severe COVID-19. Sci. Rep. 2021, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Alghanem, B.; Mansour, F.A.; Shaibah, H.; Almuhalhil, K.; Almourfi, F.; Alamri, H.S.; Alajmi, H.; Rashid, M.; Alroqi, F.; Jalouli, M.; et al. Quantitative Proteomics Analysis of COVID-19 Patients: Fetuin-A and Tetranectin as Potential Modulators of Innate Immune Responses. Heliyon 2023, 9, e15224. [Google Scholar] [CrossRef] [PubMed]
- Sarcia, N.; Thai, D.; Bodine, A.M. Hemoglobin As A Predictor For COVID-19 Disease Severity. Adv. Clin. Med. Healthc. Deliv. 2022, 2, 3. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Qiu, J.; Li, L.; Jia, X.; Kai, G. COVID-19: Pathogenesis, Cytokine Storm and Therapeutic Potential of Interferons. Cytokine Growth Factor. Rev. 2020, 53, 66. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Santos, G.P.; Schulte, H.L.; Kurizky, P.S.; Gomes, C.M.; Nóbrega, O.T.; de Gois, E.T.; de Carvalho, M.R.M.; Martins, F.P.; Nicola, A.M.; de Albuquerque, C.P.; et al. Unbalanced Networks and Disturbed Kinetics of Serum Soluble Mediators Associated with Distinct Disease Outcomes in Severe COVID-19 Patients. Front. Immunol. 2022, 13, 1004023. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Morales, D.; Díaz-Papapietro, C.; Valdés, C.; Fernandez, C.; Valls, N.; Lazo, M.; Espinoza, C.; González, R.; Gutiérrez, R.; et al. Relationship Between Endothelial and Angiogenesis Biomarkers Envisage Mortality in a Prospective Cohort of COVID-19 Patients Requiring Respiratory Support. Front. Med. 2022, 9, 826218. [Google Scholar] [CrossRef]
- Petrey, A.C.; Qeadan, F.; Middleton, E.A.; Pinchuk, I.V.; Campbell, R.A.; Beswick, E.J. Cytokine Release Syndrome in COVID-19: Innate Immune, Vascular, and Platelet Pathogenic Factors Differ in Severity of Disease and Sex. J. Leukoc. Biol. 2021, 109, 55. [Google Scholar] [CrossRef]
- Merza, M.Y.; Hwaiz, R.A.; Hamad, B.K.; Mohammad, K.A.; Hama, H.A.; Karim, A.Y. Analysis of Cytokines in SARS-CoV-2 or COVID-19 Patients in Erbil City, Kurdistan Region of Iraq. PLoS ONE 2021, 16, e0250330. [Google Scholar] [CrossRef] [PubMed]
- Halim, C.; Mirza, A.F.; Sari, M.I. The Association between TNF-α, IL-6, and Vitamin D Levels and COVID-19 Severity and Mortality: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Soni, B.; Kabra, R.; Singh, S. Quantitative Insight into Immunopathology of SARS-CoV-2 Infection. J. Interf. Cytokine Res. 2021, 41, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.K.; Madugundu, A.K.; Garapati, K.; Ramarajan, M.G.; Saraswat, M.; Kumar, M.P.; Hughes, T.; Shah, R.; Patnaik, M.M.; Chia, N.; et al. Development of a Multiomics Model for Identification of Predictive Biomarkers for COVID-19 Severity: A Retrospective Cohort Study. Lancet Digit. Health 2022, 4, e632. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 9 May 2024).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Borges-Vélez, G.; Arroyo, J.A.; Cantres-Rosario, Y.M.; Rodriguez de Jesus, A.; Roche-Lima, A.; Rosado-Philippi, J.; Rosario-Rodríguez, L.J.; Correa-Rivas, M.S.; Campos-Rivera, M.; Meléndez, L.M. Decreased CSTB, RAGE, and Axl Receptor Are Associated with Zika Infection in the Human Placenta. Cells 2022, 11, 3627. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Rodríguez, L.J.; Cantres-Rosario, Y.M.; Carrasquillo-Carrión, K.; Rodríguez-De Jesús, A.E.; Cartagena-Isern, L.J.; García-Requena, L.A.; Roche-Lima, A.; Meléndez, L.M. Quantitative Proteomics Reveal That CB2R Agonist JWH-133 Downregulates NF-ΚB Activation, Oxidative Stress, and Lysosomal Exocytosis from HIV-Infected Macrophages. Int. J. Mol. Sci. 2024, 25, 3246. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Song, J.; Zhao, G.; Li, H.; Yang, Y.; Yu, Y.; Hu, Y.; Li, Y.; Li, J.; Hu, Y. Tandem Mass Tag (TMT) Labeling-Based Quantitative Proteomic Analysis Reveals the Cellular Protein Characteristics of 16HBE Cells Infected with Coxsackievirus A10 and the Potential Effect of HMGB1 on Viral Replication. Arch. Virol. 2023, 168, 217. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Zhang, Y.; Sun, H.; Zhang, K.; Bai, Z.; Dong, H.; Liu, Y.; Li, C. Tandem Mass Tag-Based (TMT) Quantitative Proteomics Analysis Reveals the Response of Fine Roots to Drought Stress in Cotton (Gossypium Hirsutum L.). BMC Plant Biol. 2020, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Li, D.F.; Cui, Z.H.; Wang, L.Y.; Zhang, K.H.; Cao, L.T.; Zheng, S.J.; Zhang, L.X. Tandem Mass Tag (TMT)-Based Proteomic Analysis of Cryptosporidium Andersoni Oocysts before and after Excystation. Parasit. Vectors 2021, 14, 608. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.Y.; et al. Systems Biological Assessment of Immunity to Mild versus Severe COVID-19 Infection in Humans. Science 2020, 369, 1210. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Nakai, Y.; Shin, J.; Hara, M.; Takeda, Y.; Kubo, S.; Jeremiah, S.S.; Ino, Y.; Akiyama, T.; Moriyama, K.; et al. Identification of Serum Prognostic Biomarkers of Severe COVID-19 Using a Quantitative Proteomic Approach. Sci. Rep. 2021, 11, 20638. [Google Scholar] [CrossRef]
- Palma Medina, L.M.; Babačić, H.; Dzidic, M.; Parke, Å.; Garcia, M.; Maleki, K.T.; Unge, C.; Lourda, M.; Kvedaraite, E.; Chen, P.; et al. Targeted Plasma Proteomics Reveals Signatures Discriminating COVID-19 from Sepsis with Pneumonia. Respir. Res. 2023, 24, 62. [Google Scholar] [CrossRef]
- Roh, J.D.; Kitchen, R.R.; Guseh, J.S.; McNeill, J.N.; Aid, M.; Martinot, A.J.; Yu, A.; Platt, C.; Rhee, J.; Weber, B.; et al. Plasma Proteomics of COVID-19–Associated Cardiovascular Complications: Implications for Pathophysiology and Therapeutics. JACC Basic Transl. Sci. 2022, 7, 425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cryar, A.; Lemke, O.; Tober-Lau, P.; Ludwig, D.; Helbig, E.T.; Hippenstiel, S.; Sander, L.E.; Blake, D.; Lane, C.S.; et al. A Multiplex Protein Panel Assay for Severity Prediction and Outcome Prognosis in Patients with COVID-19: An Observational Multi-Cohort Study. EClinicalMedicine 2022, 49, 101495. [Google Scholar] [CrossRef]
- Zoodsma, M.; de Nooijer, A.H.; Grondman, I.; Gupta, M.K.; Bonifacius, A.; Koeken, V.A.C.M.; Kooistra, E.; Kilic, G.; Bulut, O.; Gödecke, N.; et al. Targeted Proteomics Identifies Circulating Biomarkers Associated with Active COVID-19 and Post-COVID-19. Front. Immunol. 2022, 13, 1027122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schneider, A.M.; Mehta, A.; Sade-Feldman, M.; Kays, K.R.; Gentili, M.; Charland, N.C.; Gonye, A.L.K.; Gushterova, I.; Khanna, H.K.; et al. SARS-CoV-2 Viremia Is Associated with Distinct Proteomic Pathways and Predicts COVID-19 Outcomes. J. Clin. Investig. 2021, 131, e148635. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.; Salkar, A.; Palanivel, V.; Bankar, R.; Banerjee, N.; Pai, M.G.J.; Srivastava, A.; Singh, A.; Khatri, H.; Agrawal, S.; et al. A Multi-Omics Longitudinal Study Reveals Alteration of the Leukocyte Activation Pathway in COVID-19 Patients. J. Proteome Res. 2021, 20, 4667–4680. [Google Scholar] [CrossRef]
- Duijvelaar, E.; Gisby, J.; Peters, J.E.; Bogaard, H.J.; Aman, J. Longitudinal Plasma Proteomics Reveals Biomarkers of Alveolar-Capillary Barrier Disruption in Critically Ill COVID-19 Patients. Nat. Commun. 2024, 15, 744. [Google Scholar] [CrossRef]
- Villar, M.; Urra, J.M.; Rodríguez-del-Río, F.J.; Artigas-Jerónimo, S.; Jiménez-Collados, N.; Ferreras-Colino, E.; Contreras, M.; de Mera, I.G.F.; Estrada-Peña, A.; Gortázar, C.; et al. Characterization by Quantitative Serum Proteomics of Immune-Related Prognostic Biomarkers for COVID-19 Symptomatology. Front. Immunol. 2021, 12, 730710. [Google Scholar] [CrossRef]
- Kukla, M.; Menżyk, T.; Dembiński, M.; Winiarski, M.; Garlicki, A.; Bociąga-Jasik, M.; Skonieczna, M.; Hudy, D.; Maziarz, B.; Kuśnierz-Cabala, B.; et al. Fetuin-a Deficiency but Not Pentraxin 3, FGF-21, or Irisin, Predisposes to More Serious COVID-19 Course. Biomolecules 2021, 11, 1422. [Google Scholar] [CrossRef]
- Kurt, N.; Ozgeris, F.B.; Kocak, O.F.; Yuce, N.; Bayraktutan, Z.; Parlak, E.; Coban, T.A.; Bakan, E. Evaluation of Fetuin-A, CRP, and CRP/Fetuin-A Values in COVID-19 Patients. Int. J. Med. Biochem. 2022, 5, 125–131. [Google Scholar] [CrossRef]
- Di Flora, D.C.; Dionizio, A.; Pereira, H.A.B.S.; Garbieri, T.F.; Grizzo, L.T.; Dionisio, T.J.; de Lima Leite, A.; Silva-Costa, L.C.; Buzalaf, N.R.; Reis, F.N.; et al. Analysis of Plasma Proteins Involved in Inflammation, Immune Response/Complement System, and Blood Coagulation upon Admission of COVID-19 Patients to Hospital May Help to Predict the Prognosis of the Disease. Cells 2023, 12, 1601. [Google Scholar] [CrossRef]
- Lipman, D.; Safo, S.E.; Chekouo, T. Integrative Multi-Omics Approach for Identifying Molecular Signatures and Pathways and Deriving and Validating Molecular Scores for COVID-19 Severity and Status. BMC Genom. 2023, 24, 319. [Google Scholar] [CrossRef] [PubMed]
- Souza Junior, D.R.; Silva, A.R.M.; Rosa-Fernandes, L.; Reis, L.R.; Alexandria, G.; Bhosale, S.D.; de Rose Ghilardi, F.; Dalçóquio, T.F.; Bertolin, A.J.; Nicolau, J.C.; et al. HDL Proteome Remodeling Associates with COVID-19 Severity. J. Clin. Lipidol. 2021, 15, 796. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, F.; Blair, R.; Wang, C.; Yang, H.; Mudd, J.; Currey, J.M.; Iwanaga, N.; He, J.; Mi, R.; et al. Endothelial Cell Infection and Dysfunction, Immune Activation in Severe COVID-19. Theranostics 2021, 11, 8076. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.; Tugarov, Y.; Dvorshchenko, K.; Grebinyk, D.; Savchuk, O.; Korotkyi, O.; Ostapchenko, L. TGFB1, FOXO1, and COMP Genes Expression in Blood of Patients with Osteoarthritis after SARS-CoV2 Infection. Cytol. Genet. 2023, 57, 128. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; García-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange Consortium in 2020: Enabling ‘Big Data’ Approaches in Proteomics. Nucleic Acids Res. 2020, 48, D1145. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442. [Google Scholar] [CrossRef] [PubMed]



| COVID-19 Negative Controls | COVID-19 (+) Mild | COVID-19 (+) Moderate | COVID-19 (+) Severe | Total (%) | p-Value | |
|---|---|---|---|---|---|---|
| Number of participants | 56 | 18 | 13 | 8 | 95 | N/A |
| Mean age (range) | 45 (21–71) | 44 (28–56) | 34 (23–53) | 51 (28–67) | 43 (21–71) | 0.0584 |
| Women | 33 | 11 | 8 | 4 | 56 (58.9%) | 0.9532 |
| Men | 23 | 7 | 5 | 4 | 39 (41.1%) | 0.9532 |
| Hispanics | 51 | 18 | 13 | 8 | 90 (94.7%) | 0.2987 |
| Non-Hispanics | 5 | 0 | 0 | 0 | 5 (2.6%) | 0.2987 |
| Autoimmune diseases | 7 | 3 | 2 | 2 | 14 (14.7%) | 0.8130 |
| Cancer | 2 | 1 | 0 | 0 | 3 (3.2%) | 0.7881 |
| Diabetes | 4 | 2 | 0 | 1 | 7 (7.4%) | 0.6333 |
| Cardiovascular diseases | 0 | 2 | 1 | 3 | 6 (6.3%) | 0.0005 |
| High blood pressure | 8 | 5 | 1 | 2 | 16 (16.8%) | 0.3985 |
| HIV/AIDS | 1 | 1 | 1 | 0 | 3 (3.2%) | 0.6110 |
| Lung disease | 1 | 0 | 1 | 0 | 2 (2.1%) | 0.4653 |
| Kidney disease | 1 | 2 | 1 | 0 | 4 (4.2%) | 0.2997 |
| Loss of smell | 0 | 6 | 10 | 4 | 20 (21.1%) | N/A |
| Loss of taste | 0 | 5 | 9 | 4 | 18 (18.9%) | N/A |
| Muscle aches | 0 | 9 | 11 | 5 | 25 (26.3%) | N/A |
| Cough | 0 | 14 | 8 | 6 | 28 (29.5%) | N/A |
| Shortness of breath | 0 | 2 | 4 | 5 | 15 (30%) | N/A |
| Fever | 0 | 6 | 9 | 6 | 21 (22.1%) | N/A |
| Headache | 0 | 12 | 9 | 6 | 27 (28.4%) | N/A |
| Chest pain | 0 | 0 | 4 | 3 | 7 (7.4%) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosario-Rodríguez, L.J.; Cantres-Rosario, Y.M.; Carrasquillo-Carrión, K.; Rosa-Díaz, A.; Rodríguez-De Jesús, A.E.; Rivera-Nieves, V.; Tosado-Rodríguez, E.L.; Méndez, L.B.; Roche-Lima, A.; Bertrán, J.; et al. Plasma Proteins Associated with COVID-19 Severity in Puerto Rico. Int. J. Mol. Sci. 2024, 25, 5426. https://doi.org/10.3390/ijms25105426
Rosario-Rodríguez LJ, Cantres-Rosario YM, Carrasquillo-Carrión K, Rosa-Díaz A, Rodríguez-De Jesús AE, Rivera-Nieves V, Tosado-Rodríguez EL, Méndez LB, Roche-Lima A, Bertrán J, et al. Plasma Proteins Associated with COVID-19 Severity in Puerto Rico. International Journal of Molecular Sciences. 2024; 25(10):5426. https://doi.org/10.3390/ijms25105426
Chicago/Turabian StyleRosario-Rodríguez, Lester J., Yadira M. Cantres-Rosario, Kelvin Carrasquillo-Carrión, Alexandra Rosa-Díaz, Ana E. Rodríguez-De Jesús, Verónica Rivera-Nieves, Eduardo L. Tosado-Rodríguez, Loyda B. Méndez, Abiel Roche-Lima, Jorge Bertrán, and et al. 2024. "Plasma Proteins Associated with COVID-19 Severity in Puerto Rico" International Journal of Molecular Sciences 25, no. 10: 5426. https://doi.org/10.3390/ijms25105426
APA StyleRosario-Rodríguez, L. J., Cantres-Rosario, Y. M., Carrasquillo-Carrión, K., Rosa-Díaz, A., Rodríguez-De Jesús, A. E., Rivera-Nieves, V., Tosado-Rodríguez, E. L., Méndez, L. B., Roche-Lima, A., Bertrán, J., & Meléndez, L. M. (2024). Plasma Proteins Associated with COVID-19 Severity in Puerto Rico. International Journal of Molecular Sciences, 25(10), 5426. https://doi.org/10.3390/ijms25105426








