Tissue Elasticity as a Diagnostic Marker of Molecular Mutations in Morphologically Heterogeneous Colorectal Cancer
Abstract
1. Introduction
2. Results and Discussion
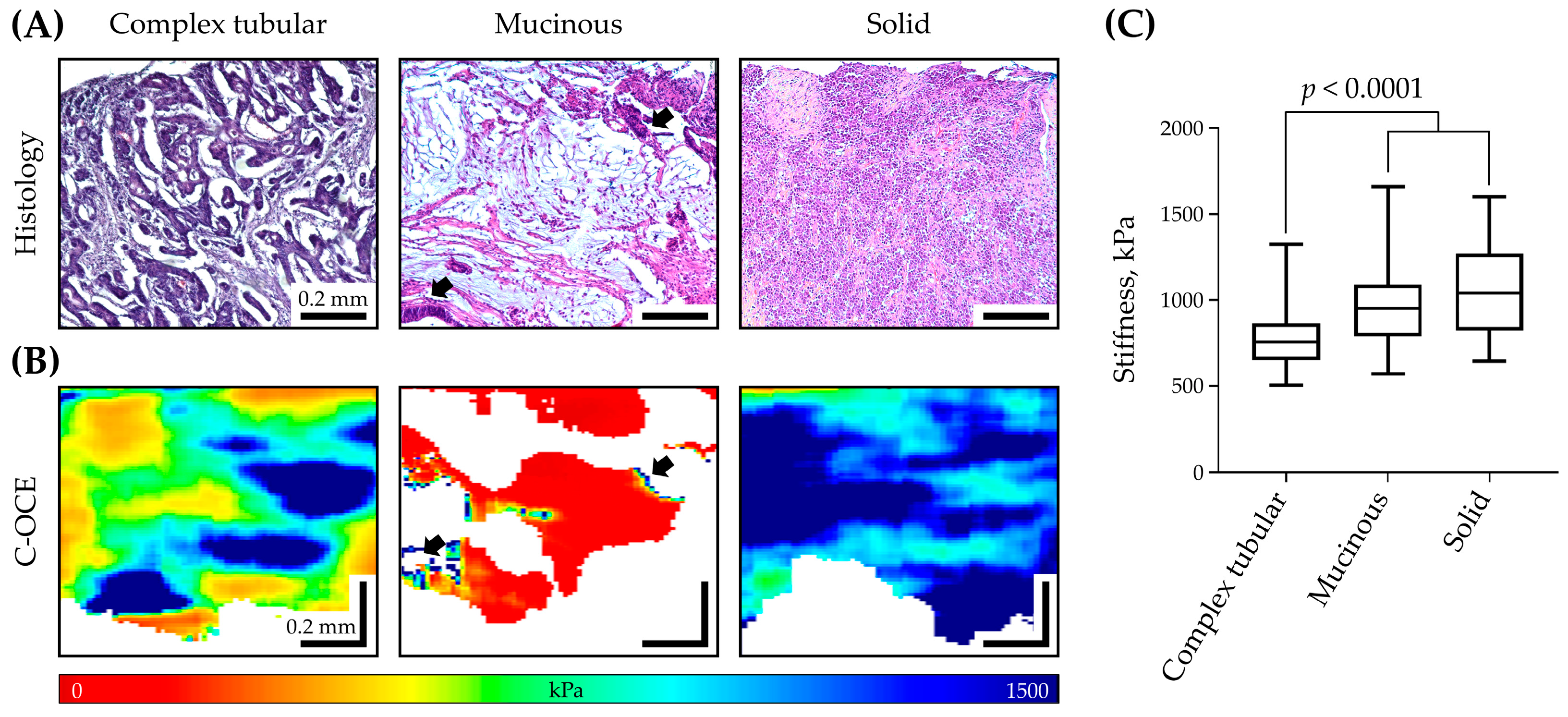
2.1. Stiffness Assessment of Colorectal Cancer Morphological Patterns
2.2. Molecular Analysis of Colorectal Cancer Tissues and Its Correlation with Morphological Patterns
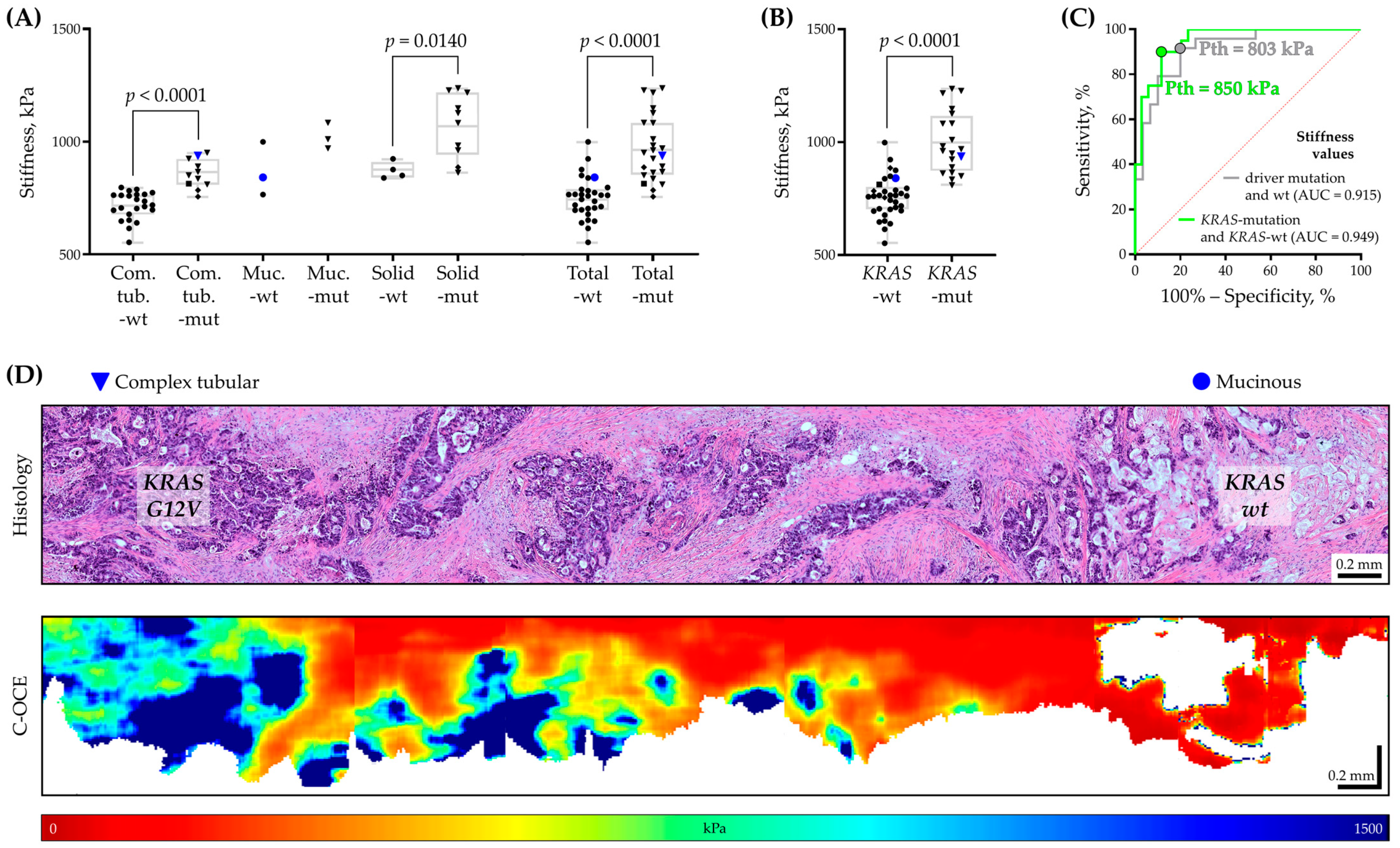
2.3. Relationship between Tissue Stiffness and Molecular Mutation in Colorectal Cancer
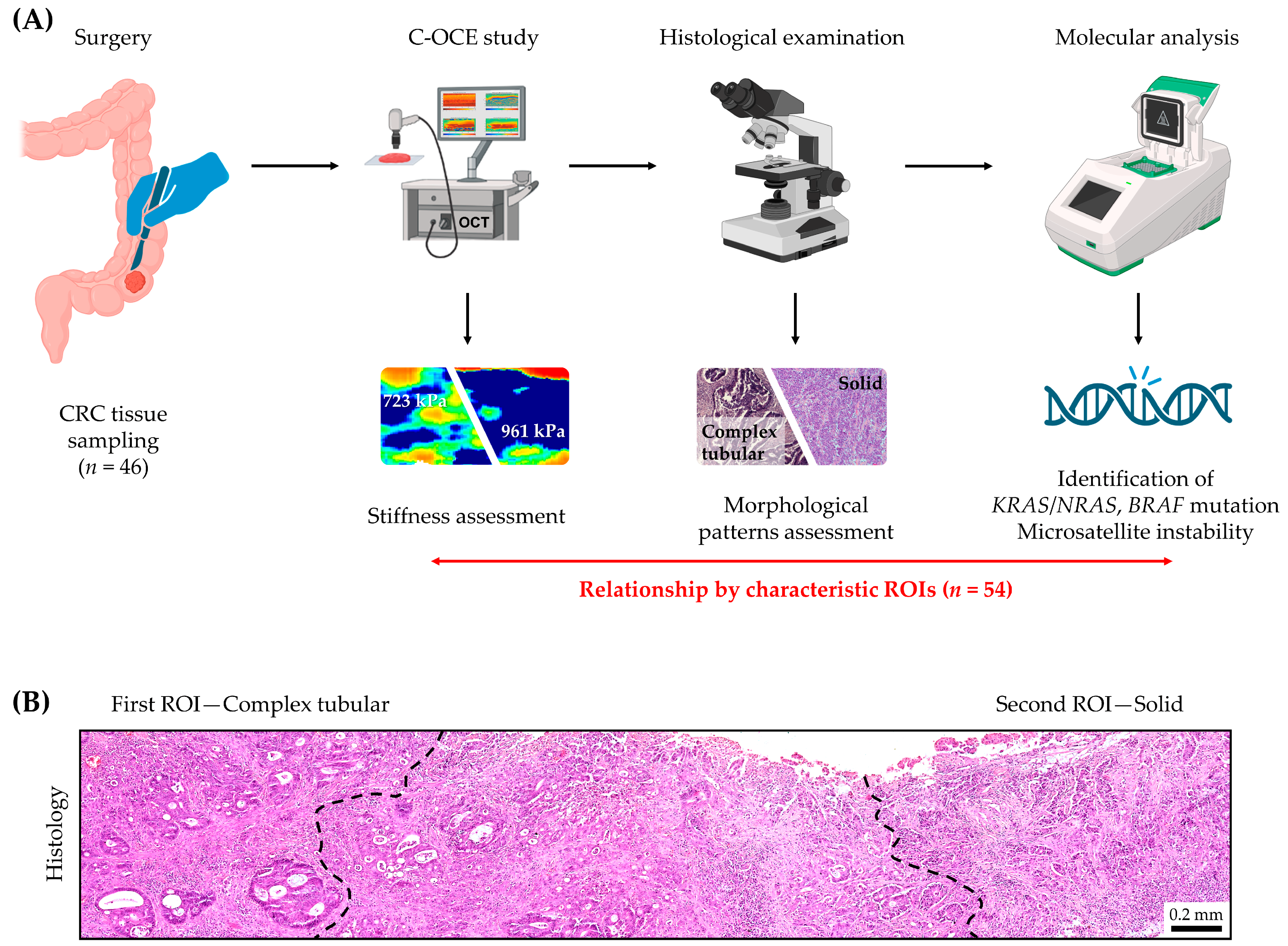
3. Materials and Methods
3.1. Colorectal Cancer Sample Characteristics
3.2. Compression Optical Coherence Elastography (C-OCE)
3.3. Histological Examination
3.4. Molecular Genetic Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Patient # | CRC Grade | Morphological Heterogeneity | Morphological Pattern | Average Stiffness (kPa) | Driver Mutation | MSR | ||
|---|---|---|---|---|---|---|---|---|
| KRAS | NRAS | BRAF | ||||||
| 1 | high | heterogeneous | Solid | 1217.8 | A146T | - | - | MSS |
| Mucinous | 1010.8 | A146T | - | - | MSS | |||
| 2 | low | homogeneous | Complex tubular | 866.4 | G13D | - | - | MSS |
| 3 | low | homogeneous | Complex tubular | 646.7 | - | - | - | MSS |
| 4 | high | heterogeneous | Solid | 1237 | G12D | - | - | MSS |
| Complex tubular | 810 | G12D | - | - | MSS | |||
| 5 | low | homogeneous | Complex tubular | 718.3 | - | - | - | MSS |
| 6 | low | homogeneous | Mucinous | 764.5 | - | - | - | MSS |
| 7 | low | homogeneous | Complex tubular | 711 | - | - | - | MSS |
| 8 | low | heterogeneous | Complex tubular | 695 | - | - | - | MSS |
| Solid | 838.7 | - | - | - | MSS | |||
| 9 | low | homogeneous | Complex tubular | 784.4 | - | - | V600E | MSS |
| 10 | low | homogeneous | Complex tubular | 796.3 | - | - | - | MSS |
| 11 | low | homogeneous | Complex tubular | 951 | G12D | - | - | MSS |
| 12 | low | homogeneous | Complex tubular | 835.3 | G12D | - | - | MSS |
| 13 | low | homogeneous | Mucinous | 998 | - | - | - | MSS |
| 14 | low | homogeneous | Complex tubular | 764.3 | - | - | - | MSS |
| 15 | low | heterogeneous | Complex tubular | 890.2 | G13C | - | - | MSS |
| Mucinous | 1084.1 | G13C | - | - | MSS | |||
| 16 | low | homogeneous | Complex tubular | 850.6 | G12D | - | - | MSS |
| 17 | low | homogeneous | Complex tubular | 553 | - | - | - | MSS |
| 18 | high | homogeneous | Solid | 849.6 | - | - | - | MSS |
| 19 | low | homogeneous | Complex tubular | 772,7 | - | - | - | MSS |
| 20 | high | heterogeneous | Solid | 923 | - | - | - | MSS |
| Complex tubular | 705.5 | - | - | - | MSS | |||
| 21 | low | heterogeneous | Complex tubular | 937.7 | G12V | - | - | MSS |
| Mucinous | 840.8 | - | - | - | MSS | |||
| 22 | high | homogeneous | Solid | 863 | G13D | - | - | MSS |
| 23 | low | homogeneous | Complex tubular | 787.5 | - | - | - | MSS |
| 24 | low | homogeneous | Complex tubular | 758.3 | - | - | - | MSS |
| 25 | high | heterogeneous | Solid | 875.5 | - | - | - | MSI |
| Complex tubular | 639.7 | - | - | - | MSI | |||
| 26 | low | homogeneous | Complex tubular | 755 | - | - | V600E | MSS |
| 27 | low | homogeneous | Complex tubular | 765.4 | - | - | - | MSS |
| 28 | high | homogeneous | Solid | 1148.4 | G12D | - | - | MSI |
| 29 | low | homogeneous | Complex tubular | 651 | - | - | - | MSS |
| 30 | high | homogeneous | Solid | 1082.8 | Q61L | - | - | MSS |
| 31 | high | homogeneous | Solid | 886 | - | - | V600E | MSI |
| 32 | low | homogeneous | Complex tubular | 677 | - | - | - | MSS |
| 33 | low | homogeneous | Complex tubular | 760.8 | - | - | - | MSS |
| 34 | high | homogeneous | Solid | 1227.6 | G12V | - | - | MSS |
| 35 | low | homogeneous | Complex tubular | 781.7 | - | - | - | MSS |
| 36 | low | homogeneous | Complex tubular | 812.8 | - | Q61X | - | MSS |
| 37 | high | homogeneous | Solid | 962.2 | G13D | - | - | MSS |
| 38 | low | homogeneous | Complex tubular | 614.7 | - | - | - | MSS |
| 39 | low | heterogeneous | Complex tubular | 924.4 | G13C | - | - | MSS |
| Mucinous | 970.2 | G13C | - | - | MSS | |||
| 40 | low | homogeneous | Complex tubular | 702.7 | - | - | - | MSS |
| 41 | high | homogeneous | Solid | 982.8 | G12V | - | - | MSS |
| 42 | low | homogeneous | Complex tubular | 743.4 | - | - | - | MSS |
| 43 | low | homogeneous | Complex tubular | 734.7 | - | - | - | MSS |
| 44 | low | homogeneous | Complex tubular | 697.2 | - | - | - | MSS |
| 45 | low | homogeneous | Complex tubular | 761.6 | - | - | - | MSS |
| 46 | high | homogeneous | Solid | 1127 | G12D | - | - | MSS |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Maurel, J.; Alonso, V.; Escudero, P.; Fernández-Martos, C.; Salud, A.; Méndez, M.; Gallego, J.; Rodriguez, J.R.; Martín-Richard, M.; Fernández-Plana, J.; et al. Clinical Impact of Circulating Tumor RAS and BRAF Mutation Dynamics in Patients With Metastatic Colorectal Cancer Treated With First-Line Chemotherapy Plus Anti-Epidermal Growth Factor Receptor Therapy. JCO Precis. Oncol. 2019, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, C.; Köhne, C.H.; Ciardiello, F.; Lenz, H.J.; Heinemann, V.; Klinkhardt, U.; Beier, F.; Duecker, K.; van Krieken, J.H.; Tejpar, S. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur. J. Cancer 2015, 51, 1243–1252. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Guler, I.; Askan, G.; Klostergaard, J.; Sahin, I.H. Precision medicine for metastatic colorectal cancer: An evolving era. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 919–931. [Google Scholar] [CrossRef]
- Losi, L.; Baisse, B.; Bouzourene, H.; Benhattar, J. Evolution of intratumoral genetic heterogeneity during colorectal cancer progression. Carcinogenesis 2005, 26, 916–922. [Google Scholar] [CrossRef]
- Compton, C.C.; Robb, J.A.; Anderson, M.W.; Berry, A.B.; Birdsong, G.G.; Bloom, K.J.; Branton, P.A.; Crothers, J.W.; Cushman-Vokoun, A.M.; Hicks, D.G.; et al. Preanalytics and Precision Pathology: Pathology Practices to Ensure Molecular Integrity of Cancer Patient Biospecimens for Precision Medicine. Arch. Pathol. Lab. Med. 2019, 143, 1346–1363. [Google Scholar] [CrossRef] [PubMed]
- Sedlackova, T.; Repiska, G.; Celec, P.; Szemes, T.; Minarik, G. Fragmentation of DNA affects the accuracy of the DNA quantitation by the commonly used methods. Biol. Proced. Online 2013, 15, 5. [Google Scholar] [CrossRef]
- De Las Casas, L.E.; Hicks, D.G. Pathologists at the Leading Edge of Optimizing the Tumor Tissue Journey for Diagnostic Accuracy and Molecular Testing. Am. J. Clin. Pathol. 2021, 155, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Gopalan, V.; Lam, A.K.; Shiddiky, M.J.A. Current advances in detecting genetic and epigenetic biomarkers of colorectal cancer. Biosens. Bioelectron. 2023, 239, 115611. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Lonardi, S. Is upfront full molecular profiling needed in all patients with colorectal cancer in daily practice? Lancet Oncol. 2022, 23, 1129–1131. [Google Scholar] [CrossRef]
- Kather, J.N.; Heij, L.R.; Grabsch, H.I.; Loeffler, C.; Echle, A.; Muti, H.S.; Krause, J.; Niehues, J.M.; Sommer, K.A.J.; Bankhead, P.; et al. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat. Cancer 2020, 1, 789–799. [Google Scholar] [CrossRef]
- Budinská, E.; Hrivňáková, M.; Ivkovic, T.C.; Madrzyk, M.; Nenutil, R.; Bencsiková, B.; Al Tukmachi, D.; Ručková, M.; Zdražilová Dubská, L.; Slabý, O.; et al. Molecular portraits of colorectal cancer morphological regions. eLife 2023, 12, RP86655. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Hong, M.; Shin, M.K.; Kim, B.C.; Shin, H.-S.; Yu, E.; Hong, S.-M.; Kim, J.; Chun, S.-M.; Kim, T.-I.; et al. KRAS and PIK3CA mutations in colorectal adenocarcinomas correlate with aggressive histological features and behavior. Hum. Pathol. 2017, 65, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Potocki, P.M.; Wójcik, P.; Chmura, Ł.; Goc, B.; Fedewicz, M.; Bielańska, Z.; Swadźba, J.; Konopka, K.; Kwinta, Ł.; Wysocki, P.J. Clinical Characterization of Targetable Mutations (BRAF V600E and KRAS G12C) in Advanced Colorectal Cancer-A Nation-Wide Study. Int. J. Mol. Sci. 2023, 24, 9073. [Google Scholar] [CrossRef] [PubMed]
- Hatthakarnkul, P.; Quinn, J.A.; Matly, A.A.M.; Ammar, A.; van Wyk, H.C.; McMillan, D.C.; Edwards, J. Systematic review of tumour budding and association with common mutations in patients with colorectal cancer. Crit. Rev. Oncol. /Hematol. 2021, 167, 103490. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Reggiani Bonetti, L.; Bettelli, S. KRAS, NRAS, BRAF mutations and high counts of poorly differentiated clusters of neoplastic cells in colorectal cancer: Observational analysis of 175 cases. Pathology 2015, 47, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Pearson, A.T.; Halama, N.; Jäger, D.; Krause, J.; Loosen, S.H.; Marx, A.; Boor, P.; Tacke, F.; Neumann, U.P.; et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 2019, 25, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Sampson, D.D. Optical coherence elastography-OCT at work in tissue biomechanics [Invited]. Biomed. Opt. Express 2017, 8, 1172–1202. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.F.; Wijesinghe, P.; Sampson, D.D. The emergence of optical elastography in biomedicine. Nat. Photonics 2017, 11, 215–221. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Hepburn, M.S.; Mowla, A.; Kennedy, B.F. Strain and elasticity imaging in compression optical coherence elastography: The two-decade perspective and recent advances. J. Biophotonics 2021, 14, e202000257. [Google Scholar] [CrossRef]
- Kennedy, B.F.; McLaughlin, R.A.; Kennedy, K.M.; Chin, L.; Wijesinghe, P.; Curatolo, A.; Tien, A.; Ronald, M.; Latham, B.; Saunders, C.M.; et al. Investigation of Optical Coherence Microelastography as a Method to Visualize Cancers in Human Breast Tissue. Cancer Res. 2015, 75, 3236–3245. [Google Scholar] [CrossRef]
- Li, C.; Guan, G.; Ling, Y.; Hsu, Y.T.; Song, S.; Huang, J.T.; Lang, S.; Wang, R.K.; Huang, Z.; Nabi, G. Detection and characterisation of biopsy tissue using quantitative optical coherence elastography (OCE) in men with suspected prostate cancer. Cancer Lett. 2015, 357, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gubarkova, E.V.; Sovetsky, A.A.; Zaitsev, V.Y.; Matveyev, A.L.; Vorontsov, D.A.; Sirotkina, M.A.; Matveev, L.A.; Plekhanov, A.A.; Pavlova, N.P.; Kuznetsov, S.S.; et al. OCT-elastography-based optical biopsy for breast cancer delineation and express assessment of morphological/molecular subtypes. Biomed. Opt. Express 2019, 10, 2244–2263. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Chin, S.L.; Allen, W.M.; Ballal, H.; Anstie, J.D.; Chin, L.; Ismail, H.M.; Zilkens, R.; Lakhiani, D.D.; McCarthy, M.; et al. Quantitative Micro-Elastography Enables In Vivo Detection of Residual Cancer in the Surgical Cavity during Breast-Conserving Surgery. Cancer Res. 2022, 82, 4093–4104. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Gelikonov, V.M.; Gelikonov, G.V.; Shilyagin, P.A. Optimization of Fizeau-based optical coherence tomography with a reference Michelson interferometer. Bull. Russ. Acad. Sci. Phys. 2008, 72, 93–97. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gelikonov, G.V.; Gelikonov, V.M.; Vitkin, A. Deformation-induced speckle-pattern evolution and feasibility of correlational speckle tracking in optical coherence elastography. J. Biomed. Opt. 2015, 20, 75006. [Google Scholar] [CrossRef] [PubMed]
- Plekhanov, A.A.; Gubarkova, E.V.; Sovietsky, A.A.; Zaitsev, V.Y.; Matveev, L.A.; Matveyev, A.L.; Timofeeva, L.B.; Kuznetsov, S.S.; Zagaynova, E.V.; Gladkova, N.D.; et al. Optical Coherence Elastography for Non-Invasive Monitoring of Tumor Elasticity under Chemotherapy: Pilot Study. Sovrem. Tehnol. V Med. 2018, 10, 9. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Sirotkina, M.A.; Sovetsky, A.A.; Gubarkova, E.V.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Zagaynova, E.V.; Gladkova, N.D.; Zaitsev, V.Y. Histological validation of in vivo assessment of cancer tissue inhomogeneity and automated morphological segmentation enabled by optical coherence elastography. Sci. Rep. 2020, 10, 11781. [Google Scholar] [CrossRef] [PubMed]
- Sirotkina, M.A.; Gubarkova, E.V.; Plekhanov, A.A.; Sovetsky, A.A.; Elagin, V.V.; Matveyev, A.L.; Matveev, L.A.; Kuznetsov, S.S.; Zagaynova, E.V.; Gladkova, N.D.; et al. In vivo assessment of functional and morphological alterations in tumors under treatment using OCT-angiography combined with OCT-elastography. Biomed. Opt. Express 2020, 11, 1365–1382. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Gubarkova, E.V.; Sirotkina, M.A.; Sovetsky, A.A.; Vorontsov, D.A.; Matveev, L.A.; Kuznetsov, S.S.; Bogomolova, A.Y.; Vorontsov, A.Y.; Matveyev, A.L.; et al. Compression OCT-elastography combined with speckle-contrast analysis as an approach to the morphological assessment of breast cancer tissue. Biomed. Opt. Express 2023, 14, 3037–3056. [Google Scholar] [CrossRef]
- Grechkanev, G.; Plekhanov, A.; Loginova, M.; Avetisyan, E.; Shepeleva, A.; Zaitseva, A.; Ushanova, A.; Gamayunov, S.; Sirotkina, M.; Zaitsev, V.; et al. First experience of using multimodal optical coherence tomography for diagnostics of hyperplastic processes in the endometrium. Russ. Bull. Obstet.-Gynecol. 2023, 23, 66–72. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Sirotkina, M.A.; Sovetsky, A.A.; Kuznetsov, S.S.; Matveev, L.A.; Zagainov, V.E.; Gubarkova, E.V.; Matveyev, A.L.; Zagaynova, E.V.; Zaitsev, V.Y.; et al. Optical coherence elastography to determine the high- and low-grade colon adenocarcinoma. In Optical Elastography and Tissue Biomechanics VIII; SPIE: Bellingham, DC, USA, 2021; Volume 11645, pp. 1–6. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Sirotkina, M.A.; Gubarkova, E.V.; Kiseleva, E.B.; Sovetsky, A.A.; Karabut, M.M.; Zagainov, V.E.; Kuznetsov, S.S.; Maslennikova, A.V.; Zagaynova, E.V.; et al. Towards targeted colorectal cancer biopsy based on tissue morphology assessment by compression optical coherence elastography. Front. Oncol. 2023, 13, 1121838. [Google Scholar] [CrossRef] [PubMed]
- Budinska, E.; Popovici, V.; Tejpar, S.; D’Ario, G.; Lapique, N.; Sikora, K.O.; Di Narzo, A.F.; Yan, P.; Hodgson, J.G.; Weinrich, S.; et al. Gene expression patterns unveil a new level of molecular heterogeneity in colorectal cancer. J. Pathol. 2013, 231, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.D.; McArt, D.G.; Bradley, C.A.; O’Reilly, P.G.; Barrett, H.L.; Cummins, R.; O’Grady, T.; Arthur, K.; Loughrey, M.B.; Allen, W.L.; et al. Challenging the Cancer Molecular Stratification Dogma: Intratumoral Heterogeneity Undermines Consensus Molecular Subtypes and Potential Diagnostic Value in Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Puppa, G.; Sonzogni, A.; Colombari, R.; Pelosi, G. TNM Staging System of Colorectal Carcinoma: A Critical Appraisal of Challenging Issues. Arch. Pathol. Lab. Med. 2010, 134, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Sagaert, X.; Vanstapel, A.; Verbeek, S. Tumor Heterogeneity in Colorectal Cancer: What Do We Know So Far? Pathobiology 2018, 85, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, C.; Wei, H.; He, Y.; Chai, X.; Ren, Q. Ex vivo determination of glucose permeability and optical attenuation coefficient in normal and adenomatous human colon tissues using spectral domain optical coherence tomography. J. Biomed. Opt. 2012, 17, 105004. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Wu, X.; Tang, T.; Liu, H.; Zhu, S.W.; Gao, B.Z.; Yuan, X.C. Quantitative Diagnosis of Colorectal Polyps by Spectral Domain Optical Coherence Tomography. BioMed Res. Int. 2014, 2014, 570629. [Google Scholar] [CrossRef]
- Waage, J.E.R.; Bach, S.P.; Pfeffer, F.; Leh, S.; Havre, R.F.; Ødegaard, S.; Baatrup, G. Combined endorectal ultrasonography and strain elastography for the staging of early rectal cancer. Color. Dis. 2015, 17, 50–56. [Google Scholar] [CrossRef]
- Zeng, Y.; Chapman, W.C., Jr.; Lin, Y.; Li, S.; Mutch, M.; Zhu, Q. Diagnosing colorectal abnormalities using scattering coefficient maps acquired from optical coherence tomography. J. Biophotonics 2021, 14, e202000276. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, S.; Chapman, W.C., Jr.; Li, S.; Alipour, Z.; Abdelal, H.; Chatterjee, D.; Mutch, M.; Zhu, Q. Real-time colorectal cancer diagnosis using PR-OCT with deep learning. Theranostics 2020, 10, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Yamamura, T.; Nakamura, M.; Maeda, K.; Sawada, T.; Mizutani, Y.; Ishikawa, E.; Suzuki, H.; Kuno, T.; Yamada, K.; et al. Endoscopic Ultrasound Elastography as a Novel Diagnostic Method for the Assessment of Hardness and Depth of Invasion in Colorectal Neoplasms. Digestion 2021, 102, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Worthley, D.L.; Leggett, B.A. Colorectal cancer: Molecular features and clinical opportunities. Clin. Biochem. Rev. 2010, 31, 31–38. [Google Scholar] [PubMed]
- Imai, K.; Yamamoto, H. Carcinogenesis and microsatellite instability: The interrelationship between genetics and epigenetics. Carcinogenesis 2008, 29, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Büttner, J.; Jöhrens, K.; Klauschen, F.; Hummel, M.; Lenze, D.; Saeger, W.; Lehmann, A. Intratumoral morphological heterogeneity can be an indicator of genetic heterogeneity in colorectal cancer. Exp. Mol. Pathol. 2018, 104, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.E.; Schaefer, K.L.; Engers, R.; Hartleb, D.; Stoecklein, N.H.; Gabbert, H.E. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Richman, S.D.; Chambers, P.; Seymour, M.T.; Daly, C.; Grant, S.; Hemmings, G.; Quirke, P. Intra-tumoral heterogeneity of KRAS and BRAF mutation status in patients with advanced colorectal cancer (aCRC) and cost-effectiveness of multiple sample testing. Anal. Cell. Pathol. 2011, 34, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.; Chiara, S.; Pfeffer, U. Molecular evolution of colorectal cancer: From multistep carcinogenesis to the big bang. Cancer Metastasis Rev. 2016, 35, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-x.; Yu, Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol. Sin. 2015, 36, 1219–1227. [Google Scholar] [CrossRef]
- Linthicum, W.; Thanh, M.H.; Vitolo, M.I.; Wen, Q. Effects of PTEN Loss and Activated KRAS Overexpression on Mechanical Properties of Breast Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 1613. [Google Scholar] [CrossRef]
- Lanzicher, T.; Martinelli, V.; Puzzi, L.; Del Favero, G.; Codan, B.; Long, C.S.; Mestroni, L.; Taylor, M.R.; Sbaizero, O. The Cardiomyopathy Lamin A/C D192G Mutation Disrupts Whole-Cell Biomechanics in Cardiomyocytes as Measured by Atomic Force Microscopy Loading-Unloading Curve Analysis. Sci. Rep. 2015, 5, 13388. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, E.; Varinelli, L.; Chighizola, M.; Brich, S.; Pisati, F.; Guaglio, M.; Baratti, D.; Deraco, M.; Gariboldi, M.; Podestà, A. Correlation between biological and mechanical properties of extracellular matrix from colorectal peritoneal metastases in human tissues. Sci. Rep. 2023, 13, 12175. [Google Scholar] [CrossRef] [PubMed]
- Plekhanov, A.A.; Potapov, A.L.; Pavlov, M.V.; Elagin, V.V.; Gubarkova, E.V.; Sovetsky, A.A.; Matveev, L.A.; Vorontsov, D.A.; Matveyev, A.L.; Vorontsov, A.Y.; et al. Side-by-Side OCE-Study of Elasticity and SHG-Characterization of Collagen Fibers in Breast Cancer Tissue before and after Chemotherapy. J. Biomed. Photonics Eng. 2023, 9, 020305. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, A.A.; Gelikonov, G.V.; Terpelov, D.A.; Shilyagin, P.A.; Gelikonov, V.M. Noniterative method of reconstruction optical coherence tomography images with improved lateral resolution in semitransparent media. Laser Phys. Lett. 2013, 10, 125601. [Google Scholar] [CrossRef]
- Gelikonov, V.M.; Romashov, V.N.; Shabanov, D.V.; Ksenofontov, S.Y.; Terpelov, D.A.; Shilyagin, P.A.; Gelikonov, G.V.; Vitkin, I.A. Cross-Polarization Optical Coherence Tomography with Active Maintenance of the Circular Polarization of a Sounding Wave in a Common Path System. Radiophys. Quantum Electron. 2018, 60, 897–911. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gelikonov, G.V.; Sovetsky, A.A.; Vitkin, A. Optimized phase gradient measurements and phase-amplitude interplay in optical coherence elastography. J. Biomed. Opt. 2016, 21, 116005. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gelikonov, G.V.; Gubarkova, E.V.; Gladkova, N.D.; Vitkin, A. Hybrid method of strain estimation in optical coherence elastography using combined sub-wavelength phase measurements and supra-pixel displacement tracking. J. Biophoton. 2016, 9, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Gelikonov, G.V.; Moiseev, A.A.; Zaitsev, V.Y. Vector method for strain estimation in phase-sensitive optical coherence elastography. Laser Phys. Lett. 2018, 15, 065603. [Google Scholar] [CrossRef]
- Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Gubarkova, E.V.; Plekhanov, A.A.; Sirotkina, M.A.; Gladkova, N.D.; Zaitsev, V.Y. Full-optical method of local stress standardization to exclude nonlinearity-related ambiguity of elasticity estimation in compressional optical coherence elastography. Laser Phys. Lett. 2020, 17, 065601. [Google Scholar] [CrossRef]
- Kang, H.; Salomon, M.P.; Sottoriva, A.; Zhao, J.; Toy, M.; Press, M.F.; Curtis, C.; Marjoram, P.; Siegmund, K.; Shibata, D. Many private mutations originate from the first few divisions of a human colorectal adenoma. J. Pathol. 2015, 237, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Feuerman, M.; Miller, A.R. Relationships between statistical measures of agreement: Sensitivity, specificity and kappa. J. Eval. Clin. Pract. 2008, 14, 930–933. [Google Scholar] [CrossRef] [PubMed]
| CRC Grade * | Morphological Patterns # | |||||||
|---|---|---|---|---|---|---|---|---|
| Low- Grade | High- Grade | All Cases | Complex Tubular | Mucinous | Solid | All ROIs | ||
| Driver mutations | KRAS | 21% (7/33) | 69% (9/13) | 35% (16/46) | 24% (8/34) | 50% (3/6) | 64% (9/14) | 37% (20/54) |
| NRAS | 3% (1/33) | - | 2% (1/46) | 3% (1/34) | - | - | 2% (1/54) | |
| BRAF | 6% (2/33) | 8% (1/13) | 7% (3/46) | 6% (2/34) | - | 7% (1/14) | 5% (3/54) | |
| Total | 30% (10/33) | 77% (10/13) | 44% (20/46) | 33% (11/34) | 50% (3/6) | 71% (10/14) | 44% (24/54) | |
| Wild- type | 70% (23/33) | 23% (3/13) | 56% (26/46) | 67% (23/34) | 50% (3/6) | 29% (4/14) | 56% (30/54) | |
| Microsatellite repeats | MSI | - | 23% (3/13) | 7% (3/46) | 3% (1/34) | - | 21% (3/14) | 7% (4/54) |
| MSS | 100% (33/33) | 77% (10/13) | 93% (43/46) | 97% (33/34) | 100% (6/6) | 79% (11/14) | 93% (50/54) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plekhanov, A.A.; Kozlov, D.S.; Shepeleva, A.A.; Kiseleva, E.B.; Shimolina, L.E.; Druzhkova, I.N.; Plekhanova, M.A.; Karabut, M.M.; Gubarkova, E.V.; Gavrina, A.I.; et al. Tissue Elasticity as a Diagnostic Marker of Molecular Mutations in Morphologically Heterogeneous Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 5337. https://doi.org/10.3390/ijms25105337
Plekhanov AA, Kozlov DS, Shepeleva AA, Kiseleva EB, Shimolina LE, Druzhkova IN, Plekhanova MA, Karabut MM, Gubarkova EV, Gavrina AI, et al. Tissue Elasticity as a Diagnostic Marker of Molecular Mutations in Morphologically Heterogeneous Colorectal Cancer. International Journal of Molecular Sciences. 2024; 25(10):5337. https://doi.org/10.3390/ijms25105337
Chicago/Turabian StylePlekhanov, Anton A., Dmitry S. Kozlov, Anastasia A. Shepeleva, Elena B. Kiseleva, Liubov E. Shimolina, Irina N. Druzhkova, Maria A. Plekhanova, Maria M. Karabut, Ekaterina V. Gubarkova, Alena I. Gavrina, and et al. 2024. "Tissue Elasticity as a Diagnostic Marker of Molecular Mutations in Morphologically Heterogeneous Colorectal Cancer" International Journal of Molecular Sciences 25, no. 10: 5337. https://doi.org/10.3390/ijms25105337
APA StylePlekhanov, A. A., Kozlov, D. S., Shepeleva, A. A., Kiseleva, E. B., Shimolina, L. E., Druzhkova, I. N., Plekhanova, M. A., Karabut, M. M., Gubarkova, E. V., Gavrina, A. I., Krylov, D. P., Sovetsky, A. A., Gamayunov, S. V., Kuznetsova, D. S., Zaitsev, V. Y., Sirotkina, M. A., & Gladkova, N. D. (2024). Tissue Elasticity as a Diagnostic Marker of Molecular Mutations in Morphologically Heterogeneous Colorectal Cancer. International Journal of Molecular Sciences, 25(10), 5337. https://doi.org/10.3390/ijms25105337











