The Synthesis and Analysis of the Cytotoxicity of Al2O3-Supported Silver Nanoparticles Prepared by the Plasma Chemical Process Initiated by Pulsed MW Radiation in the Al2O3–Ag Powder Mixtures
Abstract
1. Introduction
2. Results and Discussion
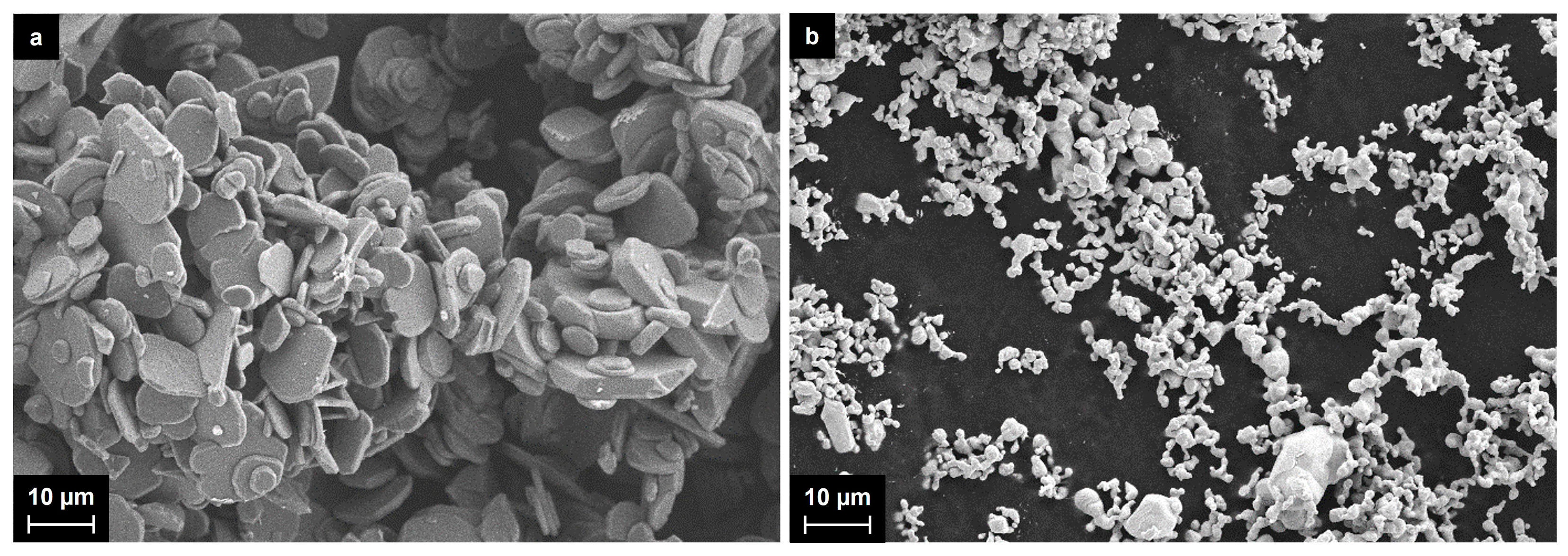
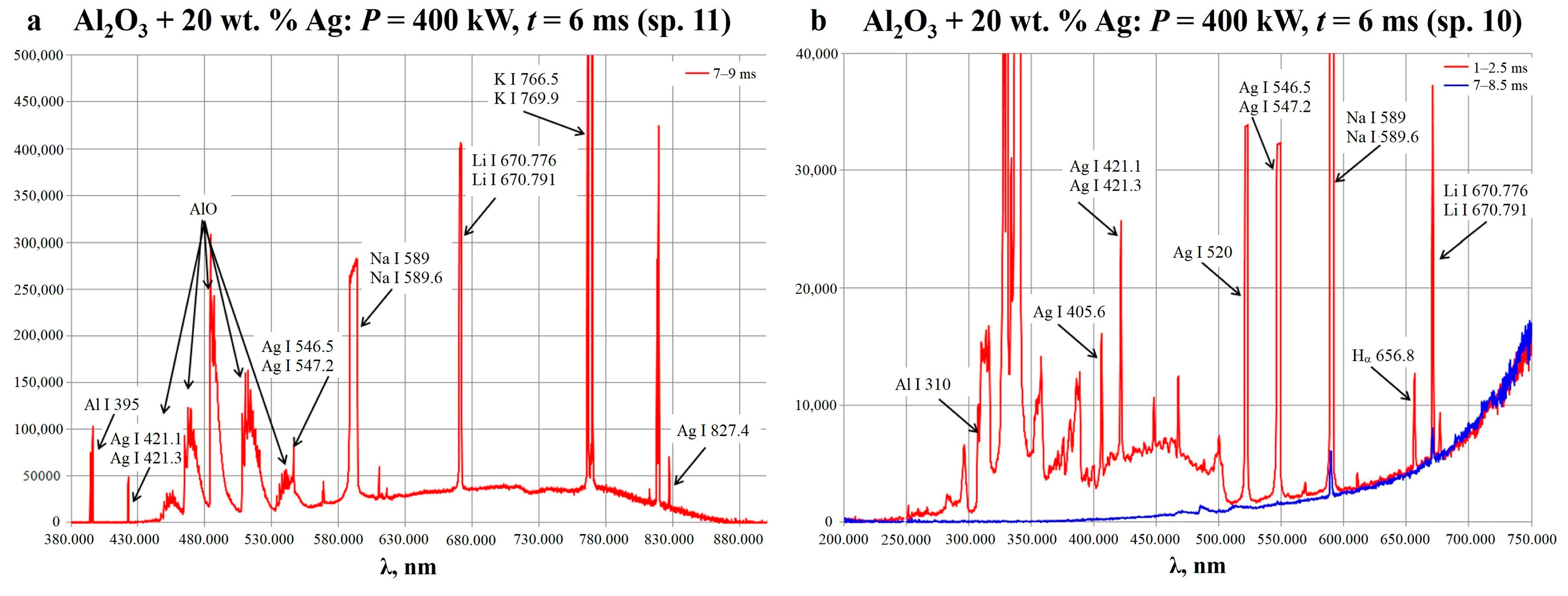
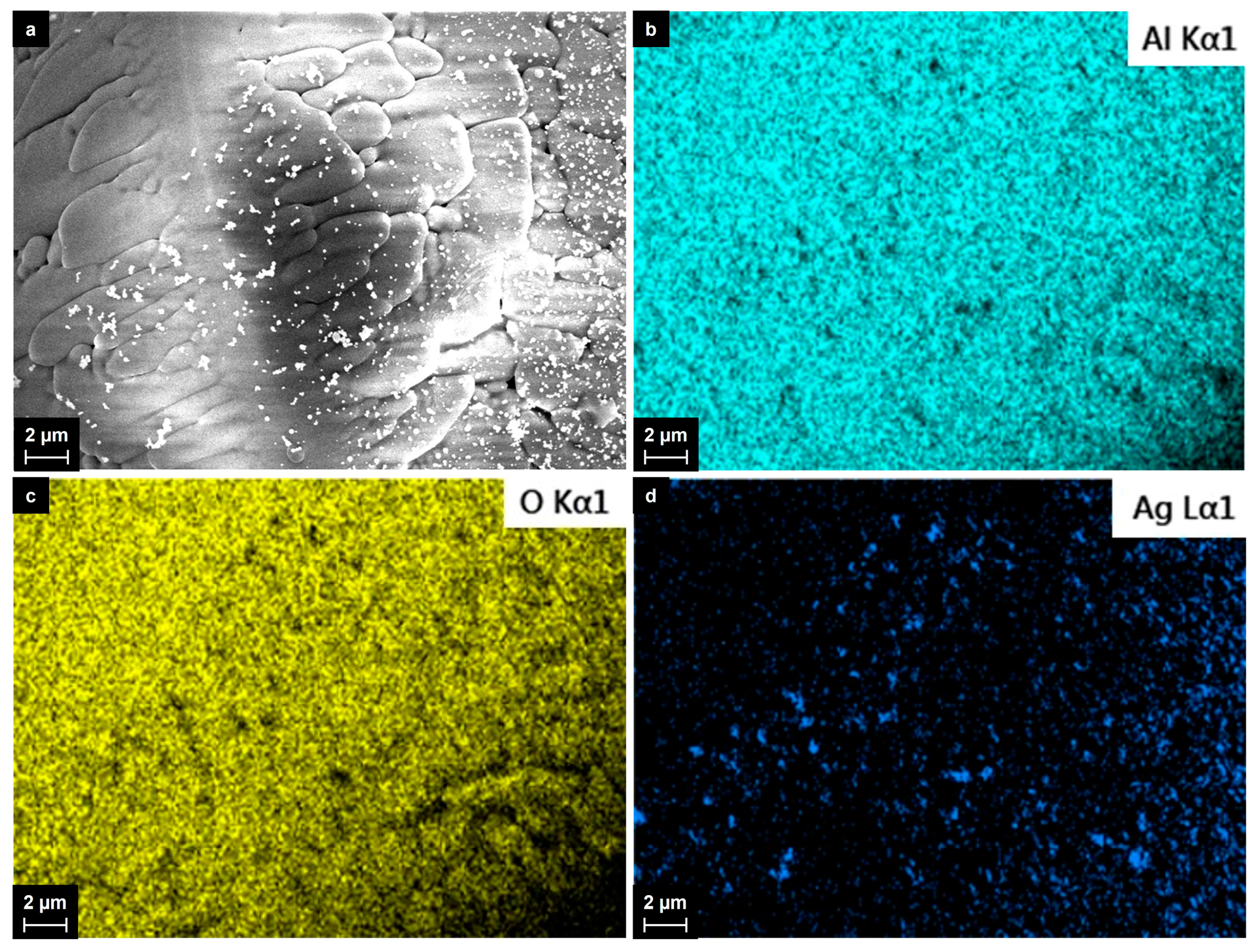
2.1. Synthesis of Al2O3-Supported Silver Nanoparticles
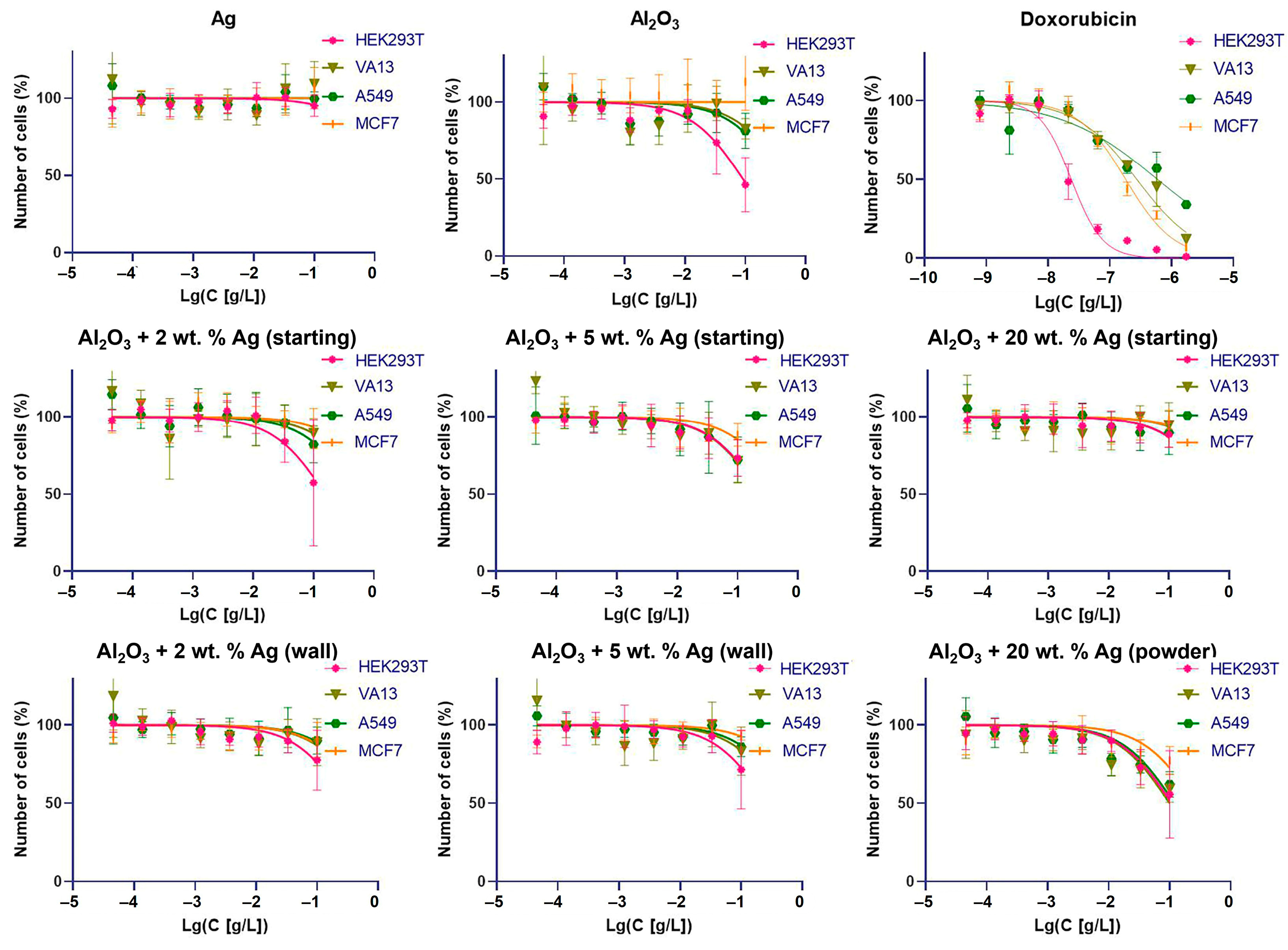
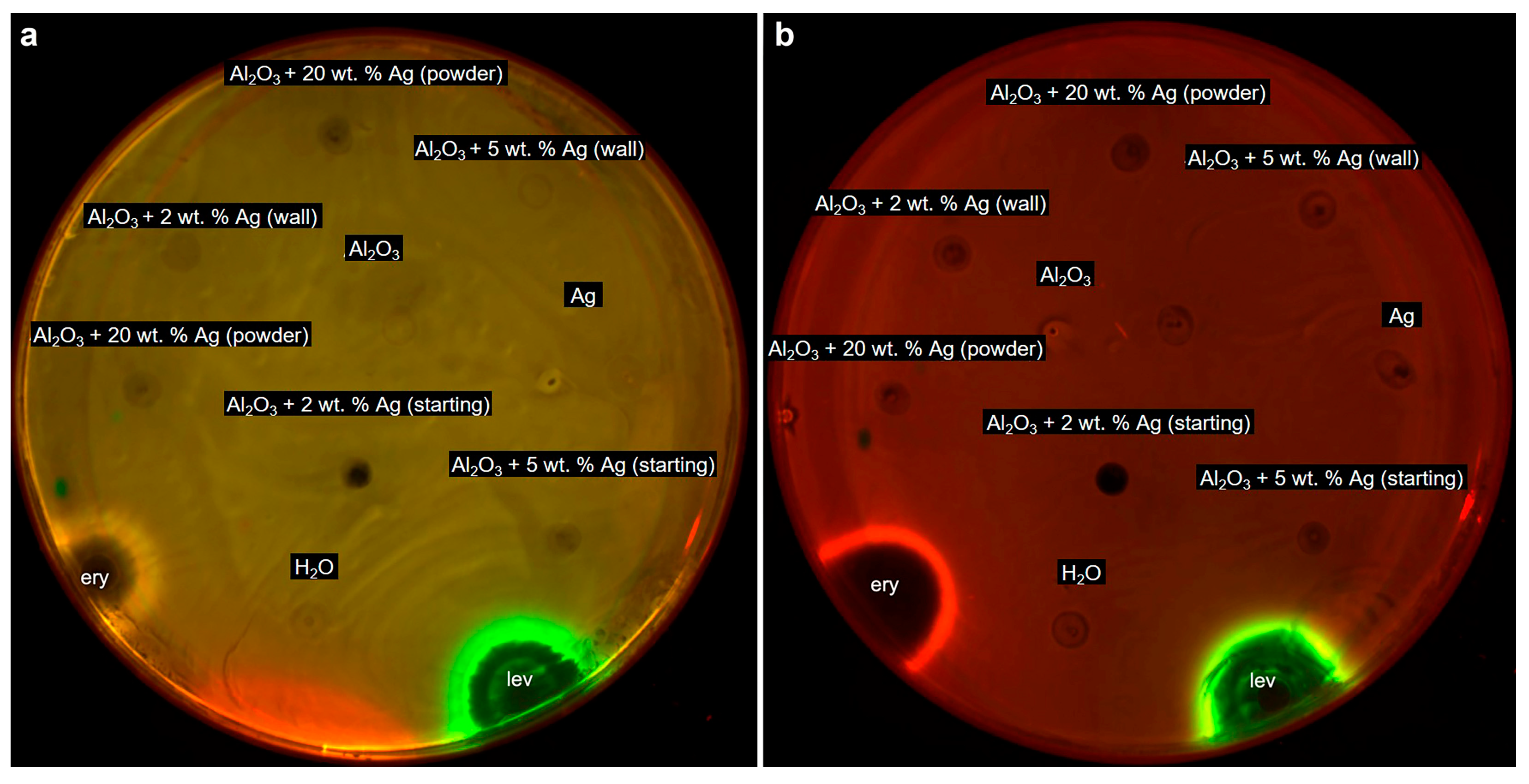
2.2. Cytotoxicity of Al2O3-Supported Silver Nanoparticles against Human Cells and Bacteria
3. Materials and Methods
3.1. Raw Materials
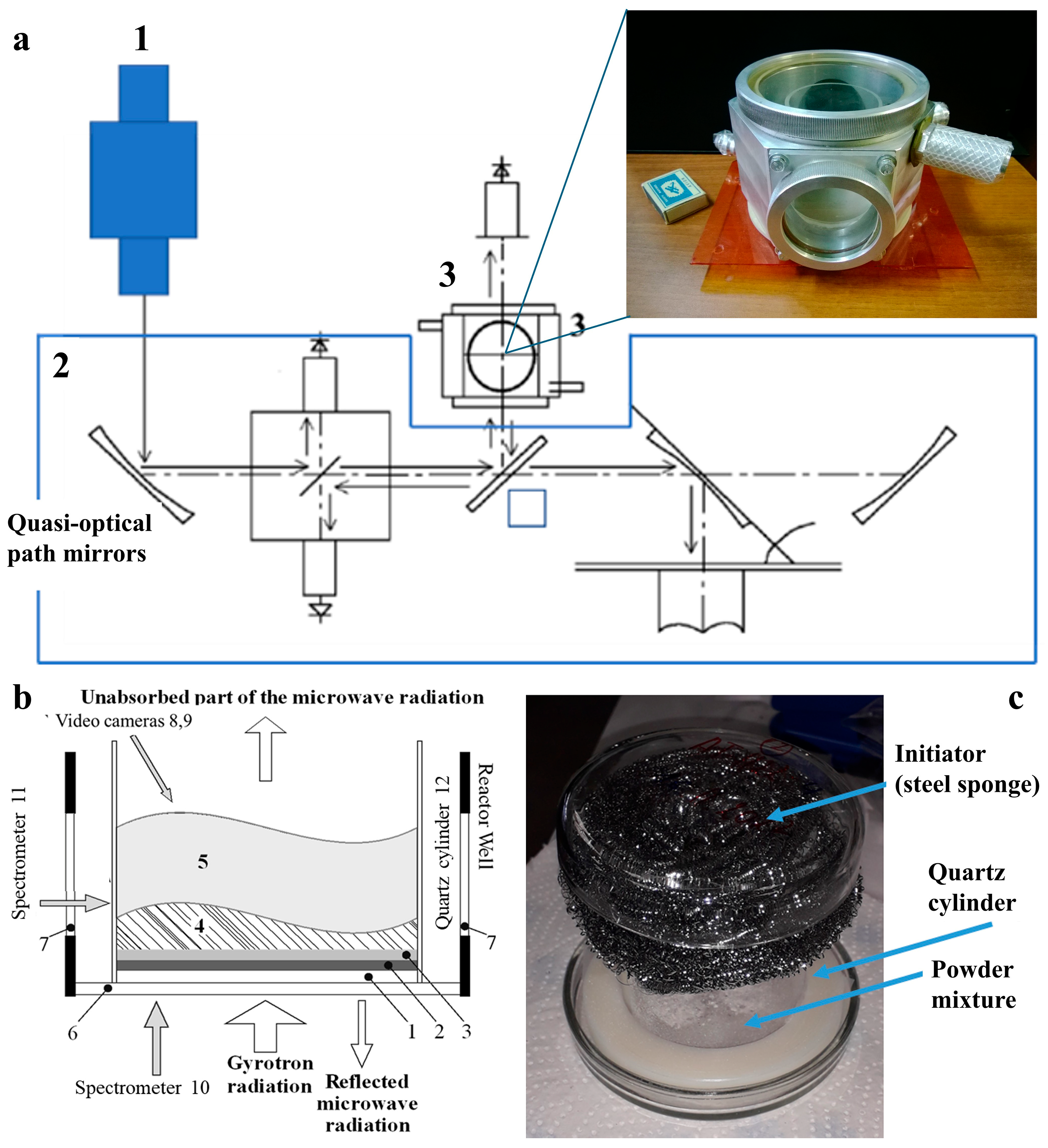
3.2. Plasma Chemical Synthesis
3.3. Composition and Morphological Analyses
3.4. Cytotoxicity Assay Protocols
3.4.1. Cytotoxicity Tests against Human Cells (MTT Test)
3.4.2. Cytotoxicity Tests against E. coli Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-Nano Approach: Enabling the Translation of Metal Nanomaterials to Clinics. Bioconjugate Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Pakhomy, S.S.; Bucharskaya, A.B.; Maslyakova, G.N.; Zlobina, O.V.; Bugaeva, I.O.; Navolokin, N.A.; Mudrak, D.A.; Khlebtsov, B.N.; Bogatyrev, V.A.; Khlebtsov, N.G. The Influence of Long-Term Peroral Administration of Gold Nanoparticles with Various Sizes on the Liver, Spleen, and Lymph Nodes of Laboratory Rats and Their Progeny. Opt. Spectrosc. 2019, 126, 681–689. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.M.; Uthaman, A.; Thomas, S. Silver Nanoparticle as an Effective Antiviral Agent. In Polymer Nanocomposites Based on Silver Nanoparticles, Synthesis, Characterization and Applications. Engineering Materials, 1st ed.; Lal, H.M., Thomas, S., Li, T., Maria, H.J., Eds.; Springer: Cham, Switzerland, 2021; pp. 247–265. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Chong, K.F.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Bhatt, M.; Rai, S.K.; Bhatt, A.; Dangwal, P.; Agrawal, P.K. Silver nanoparticles and its potential applications: A review. J. Pharmacogn. Phytochem. 2018, 7, 930–937. [Google Scholar]
- Pagliaro, M.; Pina, C.D.; Mauriello, F.; Ciriminna, R. Catalysis with Silver: From Complexes and Nanoparticles to MORALs and Single-Atom Catalysts. Catalysts 2020, 10, 1343. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Ardakani, L.S.; Surendar, A.; Thangavelu, L.; Mandal, T. Silver nanoparticles (Ag NPs) as catalyst in chemical reactions. Synth. Commun. 2021, 51, 1516–1536. [Google Scholar] [CrossRef]
- Le Hoang, T.T.T.; Insin, N.; Sukpirom, N. Catalytic activity of silver nanoparticles anchored on layered double hydroxides and hydroxyapatite. Inorg. Chem. Commun. 2020, 121, 108199. [Google Scholar] [CrossRef]
- Revathy, R.; Joseph, J.; Augustine, C.; Sajini, T.; Mathew, B. Synthesis and catalytic applications of silver nanoparticles: A sustainable chemical approach using indigenous reducing and capping agents from Hyptis capitata. Environ. Sci. Adv. 2022, 1, 491–505. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mishra, D.; Ramalingam, C. Thermal Co-reduction engineered silver nanoparticles induce oxidative cell damage in human colon cancer cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Chem. Biol. Interact. 2018, 295, 109–118. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Rajendran, B.; Manickam, V.; Ramalingam, C.; Avadhani, G.S.; Kumar, A. Thermal co-reduction approach to vary size of silver nanoparticle: Its microbial and cellular toxicology. Environ. Sci. Pollut. Res. 2016, 23, 4149–4163. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, M.; Park, H.-S.; Shin, U.S.; Gong, M.-S.; Kim, H.-W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. 2012, 100, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.; Ribeiro, D.; Proença, C.; Chisté, R.C.; Fernandes, E.; Freitas, M. Size-dependent cytotoxicity of silver nanoparticles in human neutrophils assessed by multiple analytical approaches. Life Sci. 2016, 145, 247–254. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Yan, J.; Ingle, T.; Jones, M.Y.; Mei, N.; Boudreau, M.D.; Cunningham, C.K.; Abbas, M.; Paredes, A.M.; et al. Size- and coating-dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays. Nanotoxicology 2016, 10, 1373–1384. [Google Scholar] [CrossRef]
- Sukdeb, P.; Yu, K.T.; Joon, M.S. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. Chapter One—A Review of Preparation Methods for Supported Metal Catalysts. Adv. Catal. 2017, 61, 1–35. [Google Scholar] [CrossRef]
- Vogt, C.; Meirer, F.; Monai, M.; Groeneveld, E.; Ferri, D.; van Santen, R.A.; Nachtegaal, M.; Unocic, R.R.; Frenkel, A.I.; Weckhuysen, B.M. Dynamic restructuring of supported metal nanoparticles and its implications for structure insensitive catalysis. Nat. Commun. 2021, 12, 7096. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- Vottero, E.; Groppo, E.; Piovano, A. Supported Metal Nanoparticles-Based Catalysts and their Interactions with H2: Insights from Inelastic Neutron Scattering Spectroscopy. ChemCatChem 2024, 16, e202301127. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Sun, B.; Mu, S.; Dang, F.; Guo, X.; Ma, D.; Shi, C. Engineering metal-support interaction to construct catalytic interfaces and redisperse metal nanoparticles. Chem Catal. 2023, 3, 100768. [Google Scholar] [CrossRef]
- Sokolov, A.S.; Borzosekov, V.D.; Voronova, E.V.; Malakhov, D.V.; Stepakhin, V.D.; Obraztsova, E.A.; Shishilov, O.N. Synthesis of Oxide, Nitride, and Oxynitride Materials of Micro- and Nano-Sizes Based on Al/AlN and Al/Si3N4 Powders. Dokl. Phys. 2022, 67, 132–137. [Google Scholar] [CrossRef]
- Shishilov, O.N.; Skvortsova, N.N.; Stepakhin, V.D.; Akhmadullina, N.S.; Gusein-zade, N.G. Synthesis of aluminum and silicon oxynitrides via microwave discharge in the mixtures of metal and dielectric powders. In Proceedings of the XII Kurnakov International Symposium on Physical and Chemical Analysis, Saint-Petersburg, Russia, 27–29 September 2021. (In Russian). [Google Scholar]
- Akhmadullina, N.S.; Batanov, G.M.; Borzosekov, V.D.; Voronova, E.V.; Gusein-zade, N.G.; Zakletsky, Z.A.; Kachmar, V.V.; Knyazev, A.V.; Kozak, A.K.; Kolik, L.V.; et al. A possibility of platinum catalyst synthesis in a microwave subthreshold discharge. In Proceedings of the XLIX International Conference on Plasma Physics and Controllable Thermonuclear Fusion, Zvenigorod, Russia, 14–18 March 2022. [Google Scholar] [CrossRef]
- Skvortsova, N.N.; Shishilov, O.N.; Akhmadullina, N.S.; Konchekov, E.M.; Letunov, A.A.; Malakhov, D.V.; Obraztsova, E.A.; Stepakhin, V.D. Synthesis of micro- and nanostructured materials via oscillating reactions initiated by high-power microwave pulses. Ceram. Int. 2021, 47, 3978–3987. [Google Scholar] [CrossRef]
- Skvortsova, N.N.; Stepakhin, V.D.; Malakhov, D.V.; Sorokin, A.A.; Batanov, G.M.; Borzosekov, V.D.; Glyavin, M.Y.; Kolik, L.V.; Konchekov, E.M.; Letunov, A.A.; et al. Relief Creation on Molybdenum Plates in Discharges Initiated by Gyrotron Radiation in Metal–Dielectric Powder Mixtures. Radiophys. Quantum Electron. 2016, 58, 701–709. [Google Scholar] [CrossRef]
- Batanov, G.M.; Berezhetskaya, N.K.; Borzosekov, V.D.; Iskhakova, L.D.; Kolik, L.V.; Konchekov, E.M.; Letunov, A.A.; Malakhov, D.V.; Milovich, F.O.; Obraztsova, E.A.; et al. Application of microwave discharge for the synthesis of TiB2 and BN nano- and microcrystals in a mixture of Ti-B powders in a nitrogen atmosphere. Plasma Phys. Rep. 2013, 39, 843–848. [Google Scholar] [CrossRef]
- Gayanova, T.E.; Stepakhin, V.D.; Obraztsova, E.A.; Voronova, E.V.; Skvortsova, N.N. Application of a Catalyst for Synthesis in Processes Initiated by Radiation of a Gyrotron in Mixtures of Titanium and Boron Nitride Powders. Phys. At. Nucl. 2022, 85, 2122–2126. [Google Scholar] [CrossRef]
- Skvortsova, N.N.; Obraztsova, E.A.; Stepakhin, V.D.; Konchekov, E.M.; Gayanova, T.E.; Vasilieva, L.A.; Lukianov, D.A.; Sybachin, A.V.; Skvortsov, D.A.; Gusein-Zade, N.G.; et al. Microdispersed Ti/B/N Materials Synthesized in Chain Reactions in Processes Initiated by Microwaves of a High-Power Gyrotron: Structure and Cytotoxicity. Fusion Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Artem’ev, K.V.; Batanov, G.M.; Berezhetskaya, N.K.; Borzosekov, V.D.; Davydov, A.M.; Kolik, L.V.; Konchekov, E.M.; Kossyi, I.A.; Petrov, A.E.; Sarksyan, K.A.; et al. Discharge in a Subthreshold Microwave Beam as an Unusual Type of Ionization Wave. Plasma Phys. Rep. 2018, 44, 1146–1154. [Google Scholar] [CrossRef]
- Akhmadullina, N.S.; Skvortsova, N.N.; Obraztsova, E.A.; Stepakhin, V.D.; Konchekov, E.M.; Letunov, A.A.; Konovalov, A.A.; Kargin, Y.F.; Shishilov, O.N. Plasma-chemical processes under high-power gyrotron’s discharge in the mixtures of metal and dielectric powders. Chem. Phys. 2019, 516, 63–70. [Google Scholar] [CrossRef]
- Magunov, A.N. Measuring the temperature of objects with unknown emissivity using spectral pyrometry. Nauchn. Priborostr. 2010, 20, 22–26. (In Russian) [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Osterman, I.A.; Komarova, E.S.; Shiryaev, D.I.; Korniltsev, I.A.; Khven, I.M.; Lukyanov, D.A.; Tashlitsky, V.N.; Serebryakova, M.V.; Efremenkova, O.V.; Ivanenkov, Y.A.; et al. Sorting Out Antibiotics’ Mechanisms of Action: A Double Fluorescent Protein Reporter for High-Throughput Screening of Ribosome and DNA Biosynthesis Inhibitors. Antimicrob. Agents Chemother. 2016, 60, 7481–7489. [Google Scholar] [CrossRef] [PubMed]
- Lukianov, D.A.; Buev, V.S.; Ivanenkov, Y.A.; Kartsev, V.G.; Skvortsov, D.A.; Osterman, I.A.; Sergiev, P.V. Imidazole Derivative As a Novel Translation Inhibitor. Acta Naturae 2022, 14, 71–77. [Google Scholar] [CrossRef] [PubMed]

| # | Silver Content, wt.% | Pulse Duration, ms | Pulse Power, kW | Specific Energy *, kJ/g | Initiator |
|---|---|---|---|---|---|
| 1 | 2.0 | 6 | 300 | 0.6 | Yes |
| 2 | 5.0 | 6 | 400 | 0.8 | Yes |
| 3 | 10.0 | 6 | 400 | 0.8 | No |
| 4 | 20.0 | 6 | 400 | 0.8 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skvortsova, N.N.; Akhmadullina, N.S.; Vafin, I.Y.; Obraztsova, E.A.; Hrytseniuk, Y.S.; Nikandrova, A.A.; A. Lukianov, D.; Gayanova, T.E.; Voronova, E.V.; Shishilov, O.N.; et al. The Synthesis and Analysis of the Cytotoxicity of Al2O3-Supported Silver Nanoparticles Prepared by the Plasma Chemical Process Initiated by Pulsed MW Radiation in the Al2O3–Ag Powder Mixtures. Int. J. Mol. Sci. 2024, 25, 5326. https://doi.org/10.3390/ijms25105326
Skvortsova NN, Akhmadullina NS, Vafin IY, Obraztsova EA, Hrytseniuk YS, Nikandrova AA, A. Lukianov D, Gayanova TE, Voronova EV, Shishilov ON, et al. The Synthesis and Analysis of the Cytotoxicity of Al2O3-Supported Silver Nanoparticles Prepared by the Plasma Chemical Process Initiated by Pulsed MW Radiation in the Al2O3–Ag Powder Mixtures. International Journal of Molecular Sciences. 2024; 25(10):5326. https://doi.org/10.3390/ijms25105326
Chicago/Turabian StyleSkvortsova, Nina N., Nailya S. Akhmadullina, Ildar Yu. Vafin, Ekaterina A. Obraztsova, Yanislav S. Hrytseniuk, Arina A. Nikandrova, Dmitrii A. Lukianov, Tatiana E. Gayanova, Elena V. Voronova, Oleg N. Shishilov, and et al. 2024. "The Synthesis and Analysis of the Cytotoxicity of Al2O3-Supported Silver Nanoparticles Prepared by the Plasma Chemical Process Initiated by Pulsed MW Radiation in the Al2O3–Ag Powder Mixtures" International Journal of Molecular Sciences 25, no. 10: 5326. https://doi.org/10.3390/ijms25105326
APA StyleSkvortsova, N. N., Akhmadullina, N. S., Vafin, I. Y., Obraztsova, E. A., Hrytseniuk, Y. S., Nikandrova, A. A., A. Lukianov, D., Gayanova, T. E., Voronova, E. V., Shishilov, O. N., & Stepakhin, V. D. (2024). The Synthesis and Analysis of the Cytotoxicity of Al2O3-Supported Silver Nanoparticles Prepared by the Plasma Chemical Process Initiated by Pulsed MW Radiation in the Al2O3–Ag Powder Mixtures. International Journal of Molecular Sciences, 25(10), 5326. https://doi.org/10.3390/ijms25105326






