Spontaneous Overactivation of Xenopus Frog Eggs Triggers Necrotic Cell Death
Abstract
1. Introduction
2. Results
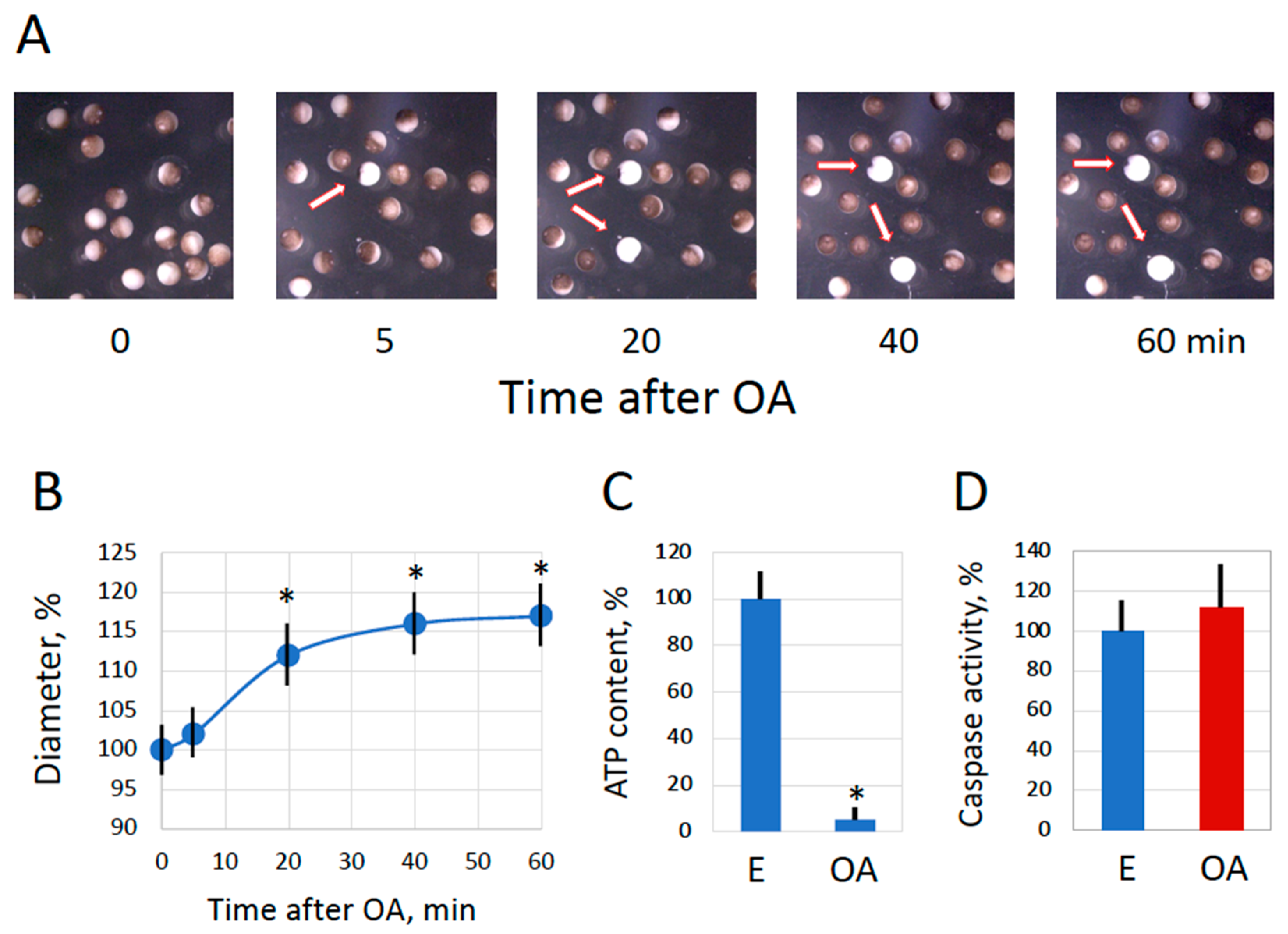
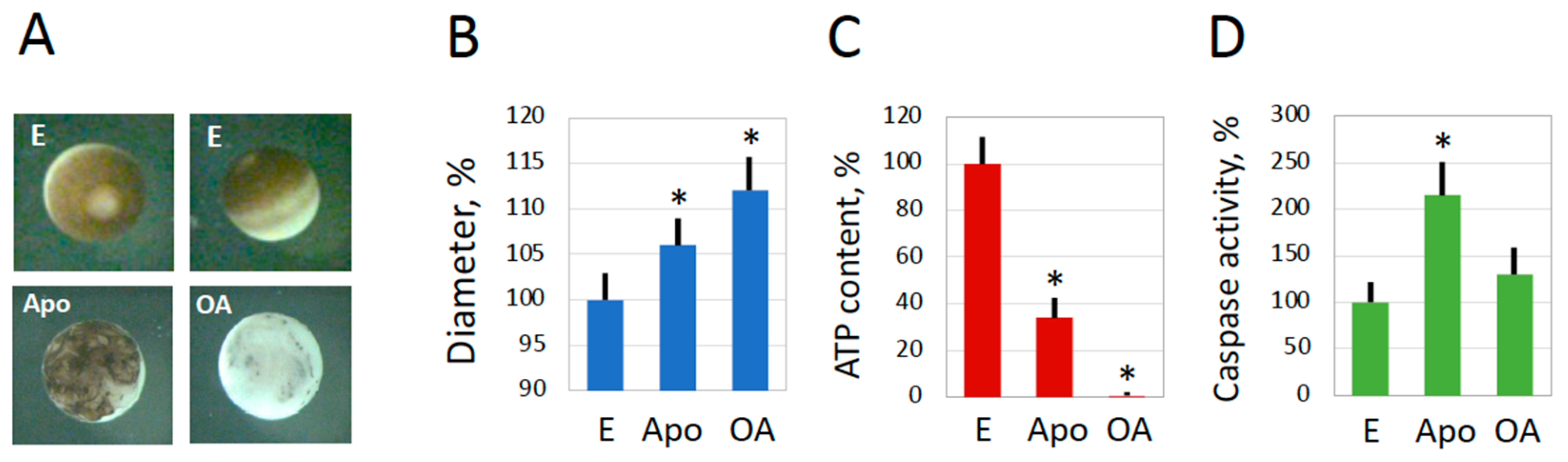
2.1. Spontaneous Overactivation of Spawned and In Vitro-Matured Xenopus Eggs
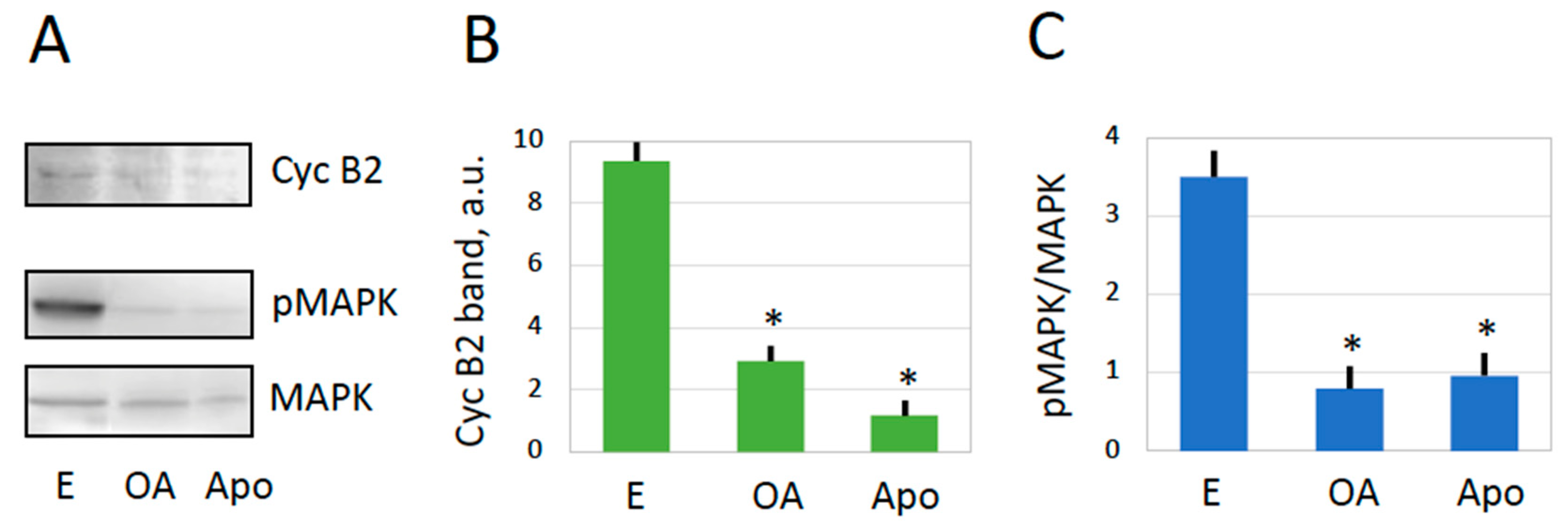
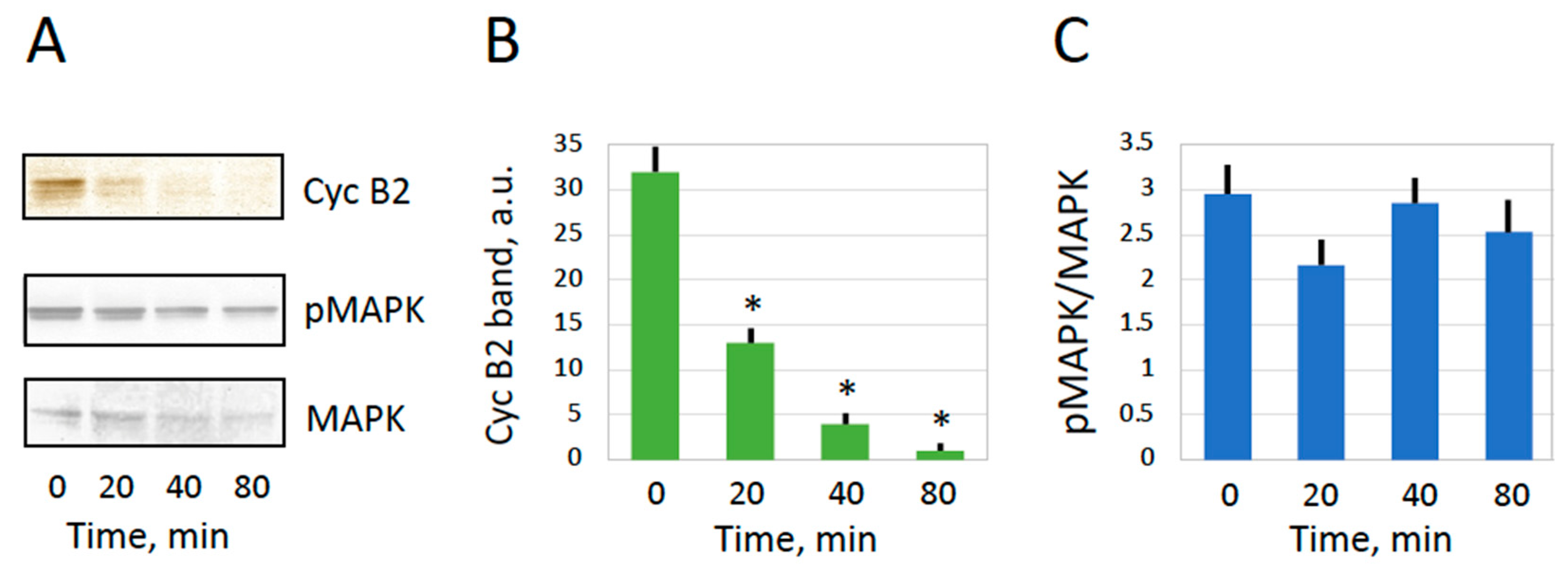
2.2. MPF and CSF in Overactivated Eggs
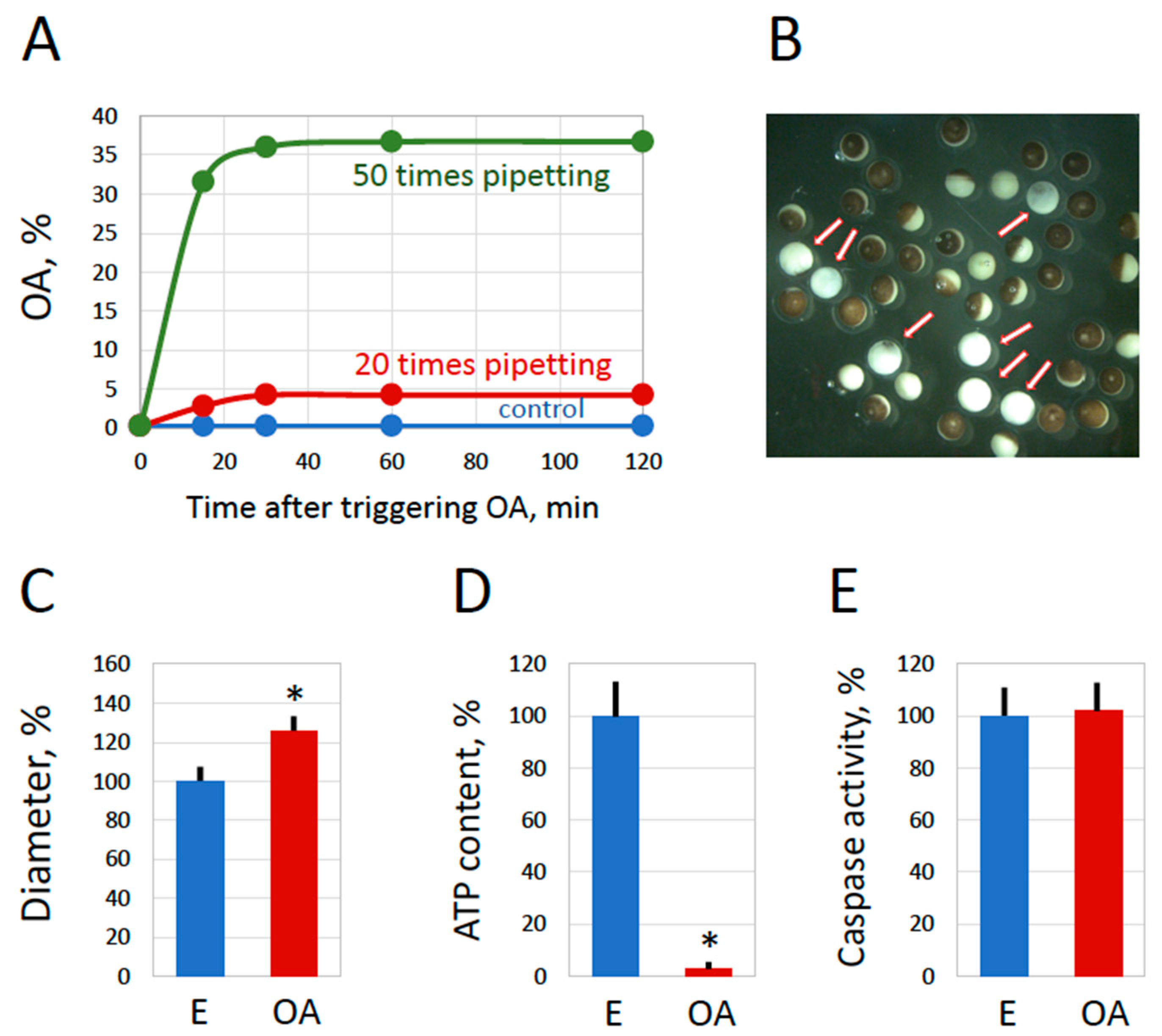
2.3. Inducing Egg Overactivation by Mechanical Stress
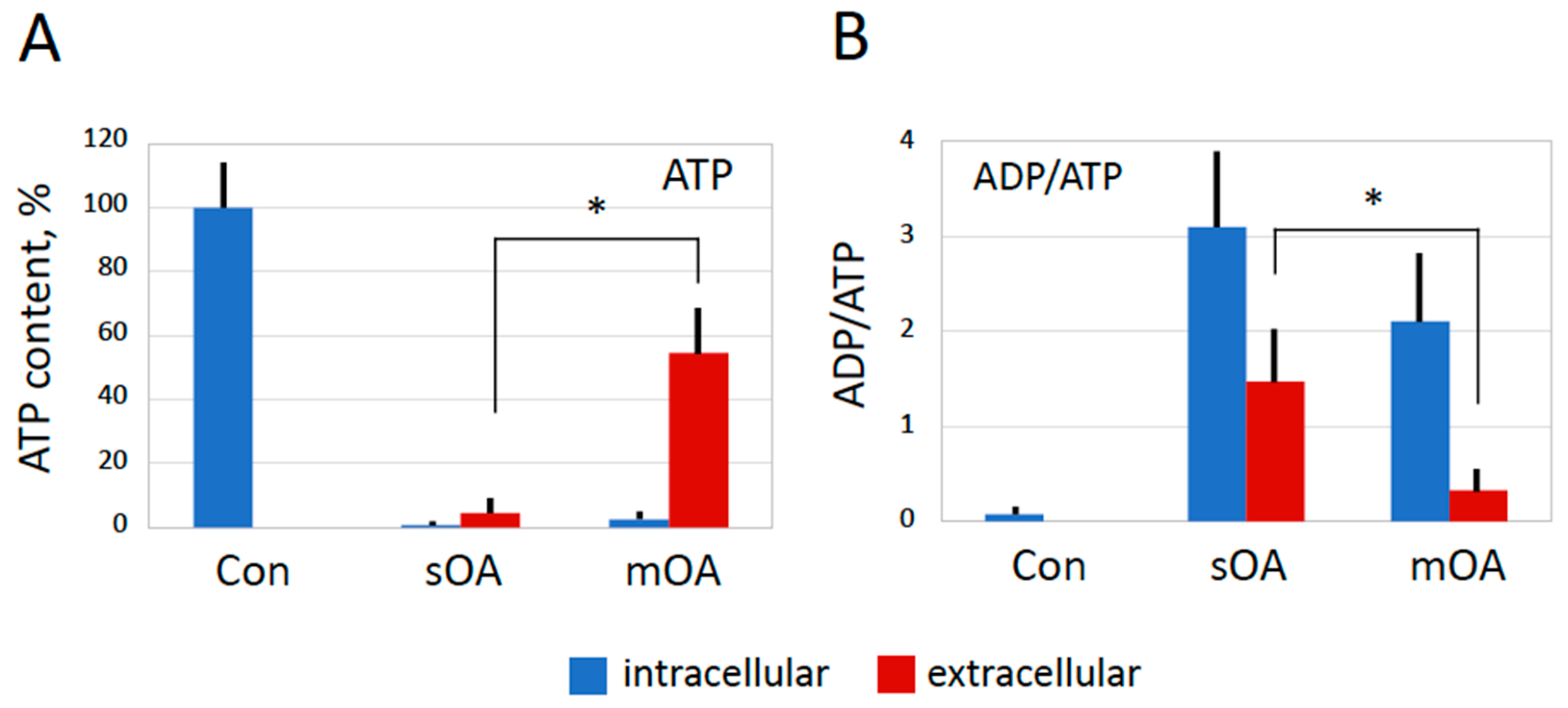
2.4. Changes in ATP and ADP Contents in Overactivated Eggs
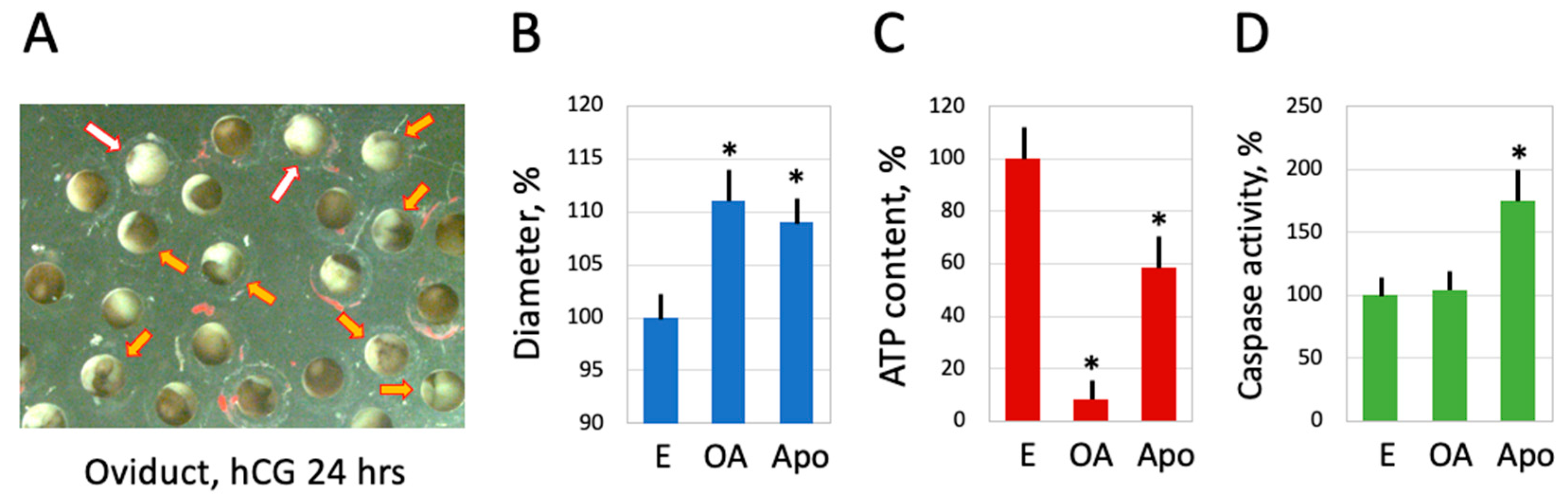
2.5. Egg Overactivation in the Frog Genital Tract
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and Cells
4.3. Microscopic Observations
4.4. Mechanical Stress
4.5. Measurement of Intracellular and Extracellular ATP and ATP/ADP Ratio
4.6. Immunoblotting
4.7. Other Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masui, Y.; Markert, C.L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971, 177, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-L.; Kikuchi, K.; Sun, Q.-Y.; Schatten, H. Oocyte aging: Cellular and molecular changes, developmental potential and reversal possibility. Hum. Reprod. Update 2009, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tiwari, M.; Koch, B.; Chaube, S.K. Morphological, cellular and molecular changes during postovulatory egg aging in mammals. J. Biomed. Sci. 2015, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.I.; Tao, X.-J.; Tilly, J.L. Fragmentation and death (also known as apoptosis) of ovulated oocytes. Mol. Hum. Reprod. 1999, 5, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Tilly, J.L. Oocyte apoptosis: Like sand through an hourglass. Dev. Biol. 1999, 213, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tokmakov, A.A.; Iguchi, S.; Iwasaki, T.; Fukami, Y. Unfertilized frog eggs die by apoptosis following meiotic exit. BMC Cell Biol. 2011, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, S.; Iwasaki, T.; Fukami, Y.; Tokmakov, A.A. Unlaid Xenopus eggs degrade by apoptosis in the genital tract. BMC Cell Biol. 2013, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, T.; Taylor, J.; Mullins, J.J.; Wilmut, I. Rat eggs cannot wait: Spontaneous exit from meiotic metaphase-II arrest. Mol. Reprod. Dev. 2011, 78, 795–807. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Sato, K.I.; Stefanov, V.E. Postovulatory cell death: Why eggs die via apoptosis in biological species with external fertilization. J. Reprod. Dev. 2018, 64, 1–6. [Google Scholar] [CrossRef]
- Premkumar, K.V.; Chaube, S.K. An insufficient increase of cytosolic free calcium level results postovulatory aging-induced abortive spontaneous egg activation in rat. J. Assist. Reprod. Genet. 2013, 30, 117–123. [Google Scholar] [CrossRef][Green Version]
- Premkumar, K.V.; Chaube, S.K. RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Abbott, A.; Kopf, G.S.; Schultz, R.M.; Ducibella, T. Spontaneous activation of ovulated mouse eggs: Time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol1,4,5-trisphosphate sensitivity. Biol. Reprod. 1997, 57, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, D.; Hou, Y.; Li, Y.-H.; Sun, Q.-Y.; Sun, X.-F.; Wang, W.-H. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol. Reprod. 2005, 72, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Houel-Renault, L.; Philippe, L.; Piquemal, M.; Ciapa, B. Autophagy is used as a survival program in unfertilized sea urchin eggs that are destined to die by apoptosis after inactivation of MAPK1/3 (ERK2/1). Autophagy 2013, 9, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Philippe, L.; Tosca, L.; Zhang, W.L.; Piquemal, M.; Ciapa, B. Different routes lead to apoptosis in unfertilized sea urchin eggs. Apoptosis 2014, 19, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, R217–R227. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ogawa, K.; Tokmakov, A.A.; Iwasaki, T.; Fukami, Y. Hydrogen peroxide induces Src family tyrosine kinase-dependent activation of Xenopus eggs. Dev. Growth Differ. 2001, 43, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Tokmakov, A.A.; Awamura, M.; Sato, K.I. Biochemical Hallmarks of Oxidative Stress-Induced Overactivation of Xenopus Eggs. BioMed Res. Int. 2019, 2019, 7180540. [Google Scholar] [CrossRef] [PubMed]
- Tokmakov, A.A.; Morichika, Y.; Teranishi, R.; Sato, K.I. Oxidative Stress-Induced Overactivation of Frog Eggs Triggers Calcium-Dependent Non-Apoptotic Cell Death. Antioxidants 2022, 11, 2433. [Google Scholar] [CrossRef]
- Bement, W.M.; Capco, D.G. Activators of protein kinase C trigger cortical granule exocytosis, cortical contraction, and cleavage furrow formation in Xenopus laevis oocytes and eggs. J. Cell Biol. 1989, 108, 885–892. [Google Scholar] [CrossRef]
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997, 57, 1835–1840. [Google Scholar] [PubMed]
- Miyoshi, N.; Watanabe, E.; Osawa, T.; Okuhira, M.; Murata, Y.; Ohshima, H.; Nakamura, Y. ATP depletion alters the mode of cell death induced by benzyl isothiocyanate. Biochim. Biophys. Acta 2008, 1782, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Vemuri, M.C.; Schneider, J.S. Modulation of ATP levels alters the mode of hydrogen peroxide-induced cell death in primary cortical cultures: Effects of putative neuroprotective agents. Brain Res. 2004, 997, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Polajzer, T.; Jarm, T.; Miklavcic, D. Analysis of damage-associated molecular pattern molecules due to electroporation of cells in vitro. Radiol. Oncol. 2020, 54, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Razakamanantsoa, L.; Rajagopalan, N.R.; Kimura, Y.; Sabbah, M.; Thomassin-Naggara, I.; Cornelis, F.H.; Srimathveeravalli, G. ATP loss during irreversible electroporation mediates caspase independent cell death. Bioelectrochemistry 2023, 150, 108355. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef]
- Kung, G.; Konstantinidis, K.; Kitsis, R.N. Programmed necrosis, not apoptosis, in the heart. Circ. Res. 2011, 108, 1017–1036. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Cuervo, A.M. Autophagy in the cellular energetic balance. Cell Metab. 2011, 13, 495–504. [Google Scholar] [CrossRef]
- Forrester, T.; Williams, C.A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J. Physiol. 1977, 268, 371–390. [Google Scholar] [CrossRef]
- Aleu, J.; Martín-Satué, M.; Navarro, P.; Pérez de Lara, I.; Bahima, L.; Marsal, J.; Solsona, C. Release of ATP induced by hypertonic solutions in Xenopus oocytes. J. Physiol. 2003, 547, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Martins, I.; Ma, Y.; Kepp, O.; Galluzzi, L.; Kroemer, G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 2013, 9, 1624–1625. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef] [PubMed]
- Covian-Nares, J.F.; Koushik, S.V.; Puhl, H.L., 3rd; Vogel, S.S. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J. Cell Sci. 2010, 123, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F.; Hermosilla, T.; Castro, J. Necrotic volume increase and the early physiology of necrosis. Comp. Biochem. Physiol. A Mol. Integr. Pphysiol. 2001, 130, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Karch, J.; Molkentin, J.D. Regulated necrotic cell death: The passive aggressive side of Bax and Bak. Circ. Res. 2015, 116, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza-Tesarik, R. Mitochondria in Human Fertility and Infertility. Int. J. Mol. Sci. 2023, 24, 8950. [Google Scholar] [CrossRef] [PubMed]
- Jurkowitz-Alexander, M.S.; Altschuld, R.A.; Hohl, C.M.; Johnson, J.D.; McDonald, J.S.; Simmons, T.D.; Horrocks, L.A. Cell swelling, blebbing, and death are dependent on ATP depletion and independent of calcium during chemical hypoxia in a glial cell line (ROC-1). J. Neurochem. 1992, 59, 344–352. [Google Scholar] [CrossRef]
- Carini, R.; Autelli, R.; Bellomo, G.; Albano, E. Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp. Cell Res. 1999, 248, 280–293. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar]
- King, P.E.; Rafai, J. A possible mechanism for initiating the parthenogenetic development of eggs in a parasitoid Hymenopteran, Nasonia vitripennis (Walker) (Pteromalidae). Entomologist 1970, 106, 118–120. [Google Scholar]
- Went, D.F.; Krause, G. Egg activation in Pimpla turionellae (Hym.). Naturwissenschaften 1974, 61, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, A.P.; Goralski, T.J.; Caulton, J.H. In vitro activation of Drosophila eggs. Dev. Biol. 1983, 98, 437–445. [Google Scholar] [CrossRef]
- Endow, S.A.; Komma, D.J. Spindle dynamics during meiosis in Drosophila oocytes. J. Cell Biol. 1997, 137, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Horner, V.L.; Wolfner, M.F. Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev. Dyn. 2008, 237, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Toro, E.; Michael, S.F. In vitro fertilization and artificial activation of eggs of the direct-developing anuran Eleutherodactylus coqui. Reprod. Biol. Endocrinol. 2004, 2, 60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kline, D.; Nuccitelli, R. The wave of activation current in the Xenopus egg. Dev. Biol. 1985, 111, 471–487. [Google Scholar] [CrossRef]
- Kubota, H.Y.; Yoshimoto, Y.; Yoneda, M.; Hiramoto, Y. Free calcium wave upon activation in Xenopus eggs. Dev. Biol. 1987, 119, 129–136. [Google Scholar] [CrossRef]
- Watanabe, N.; Hunt, T.; Ikawa, Y.; Sagata, N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature 1991, 352, 247–248. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Stefanov, V.E.; Iwasaki, T.; Sato, K.; Fukami, Y. Calcium signaling and meiotic exit at fertilization in Xenopus egg. Int. J. Mol. Sci. 2014, 15, 18659–18676. [Google Scholar] [CrossRef]
- Xiong, W.; Ferrell, J.E., Jr. A positive-feedback-based bistable memory module’ that governs a cell fate decision. Nature 2003, 426, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokmakov, A.A.; Teranishi, R.; Sato, K.-I. Spontaneous Overactivation of Xenopus Frog Eggs Triggers Necrotic Cell Death. Int. J. Mol. Sci. 2024, 25, 5321. https://doi.org/10.3390/ijms25105321
Tokmakov AA, Teranishi R, Sato K-I. Spontaneous Overactivation of Xenopus Frog Eggs Triggers Necrotic Cell Death. International Journal of Molecular Sciences. 2024; 25(10):5321. https://doi.org/10.3390/ijms25105321
Chicago/Turabian StyleTokmakov, Alexander A., Ryuga Teranishi, and Ken-Ichi Sato. 2024. "Spontaneous Overactivation of Xenopus Frog Eggs Triggers Necrotic Cell Death" International Journal of Molecular Sciences 25, no. 10: 5321. https://doi.org/10.3390/ijms25105321
APA StyleTokmakov, A. A., Teranishi, R., & Sato, K.-I. (2024). Spontaneous Overactivation of Xenopus Frog Eggs Triggers Necrotic Cell Death. International Journal of Molecular Sciences, 25(10), 5321. https://doi.org/10.3390/ijms25105321





