Evaluation of New Approaches to Depression Treatment Using an Animal Model of Pharmacoresistant Depression
Abstract
1. Introduction
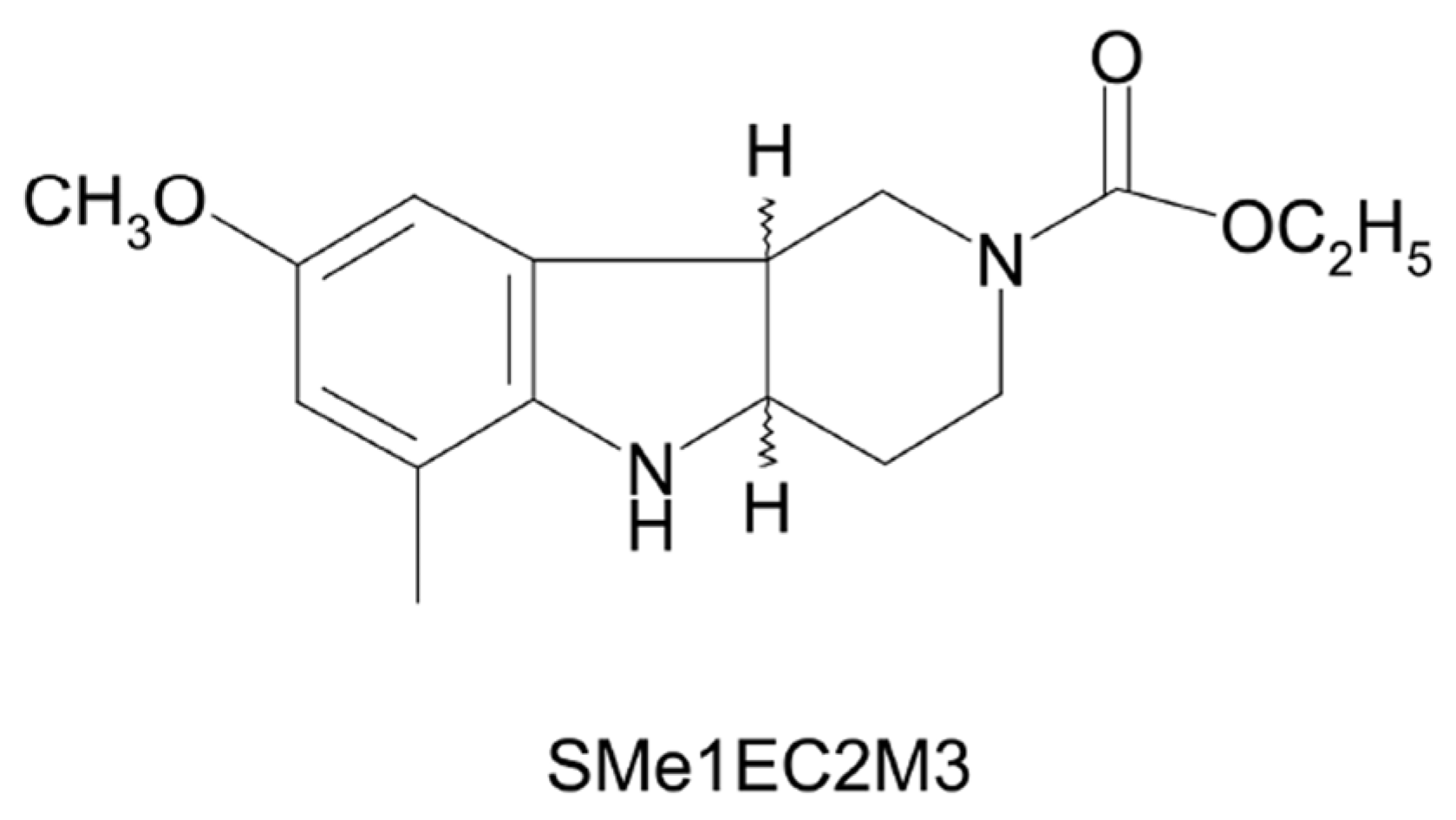
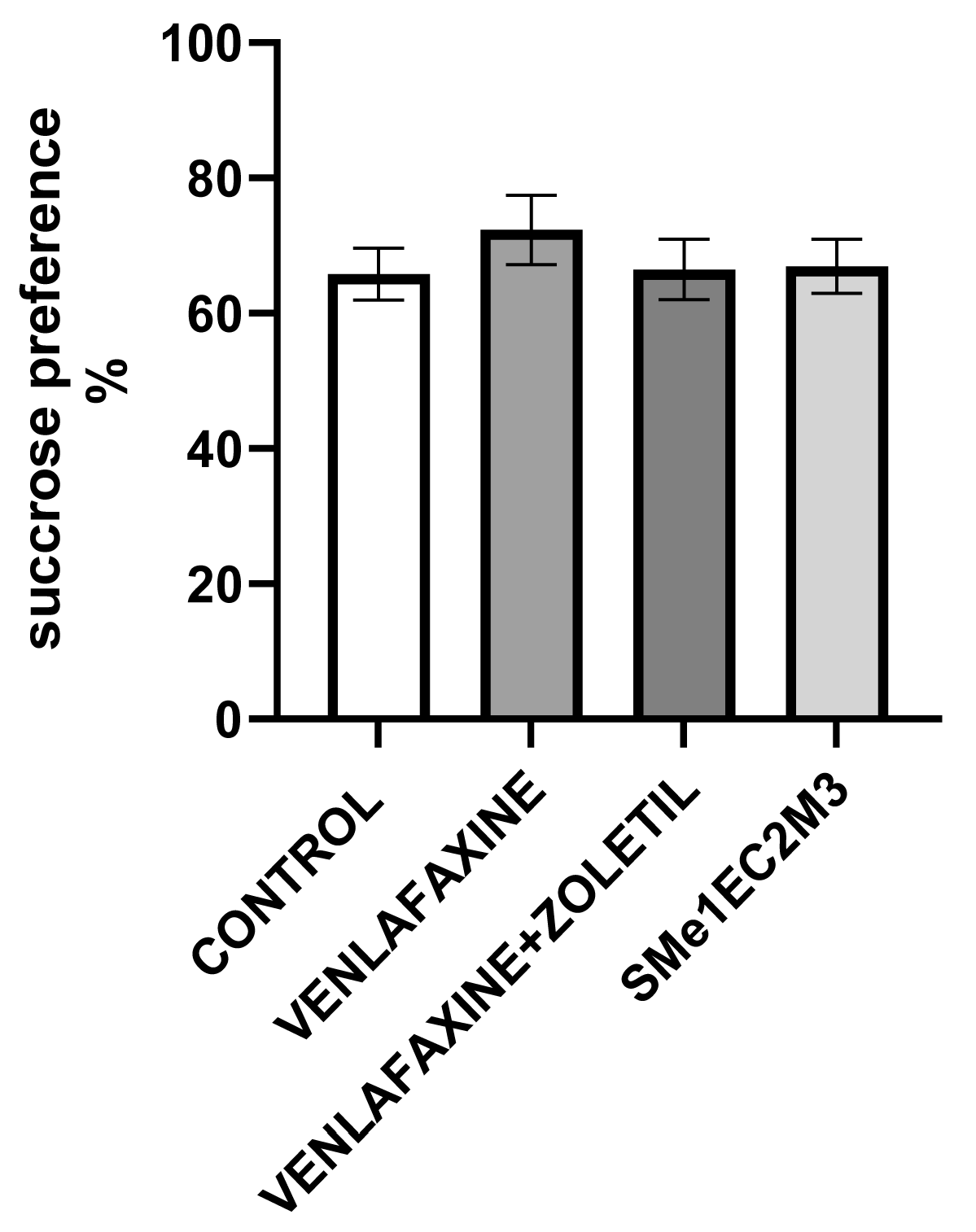
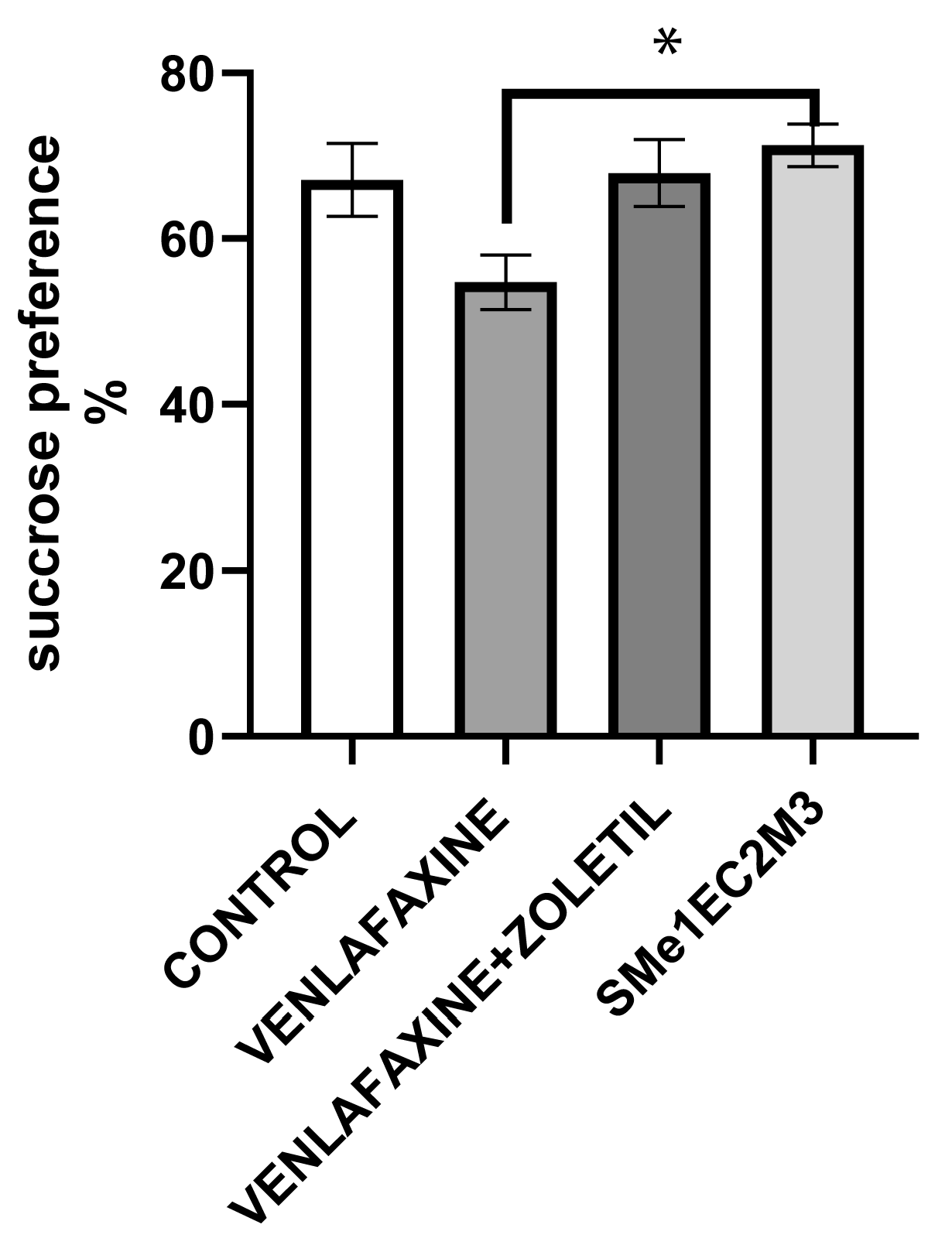
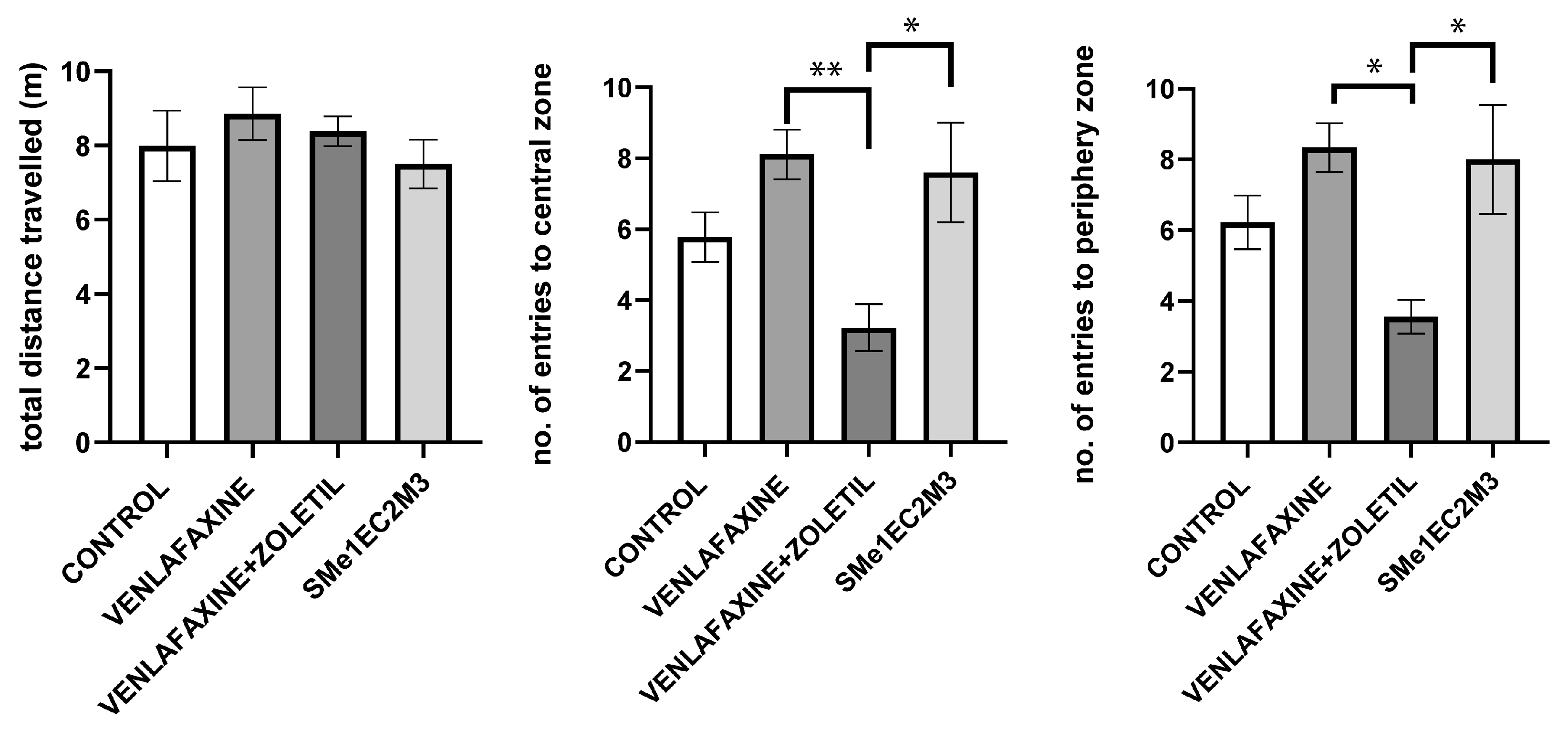
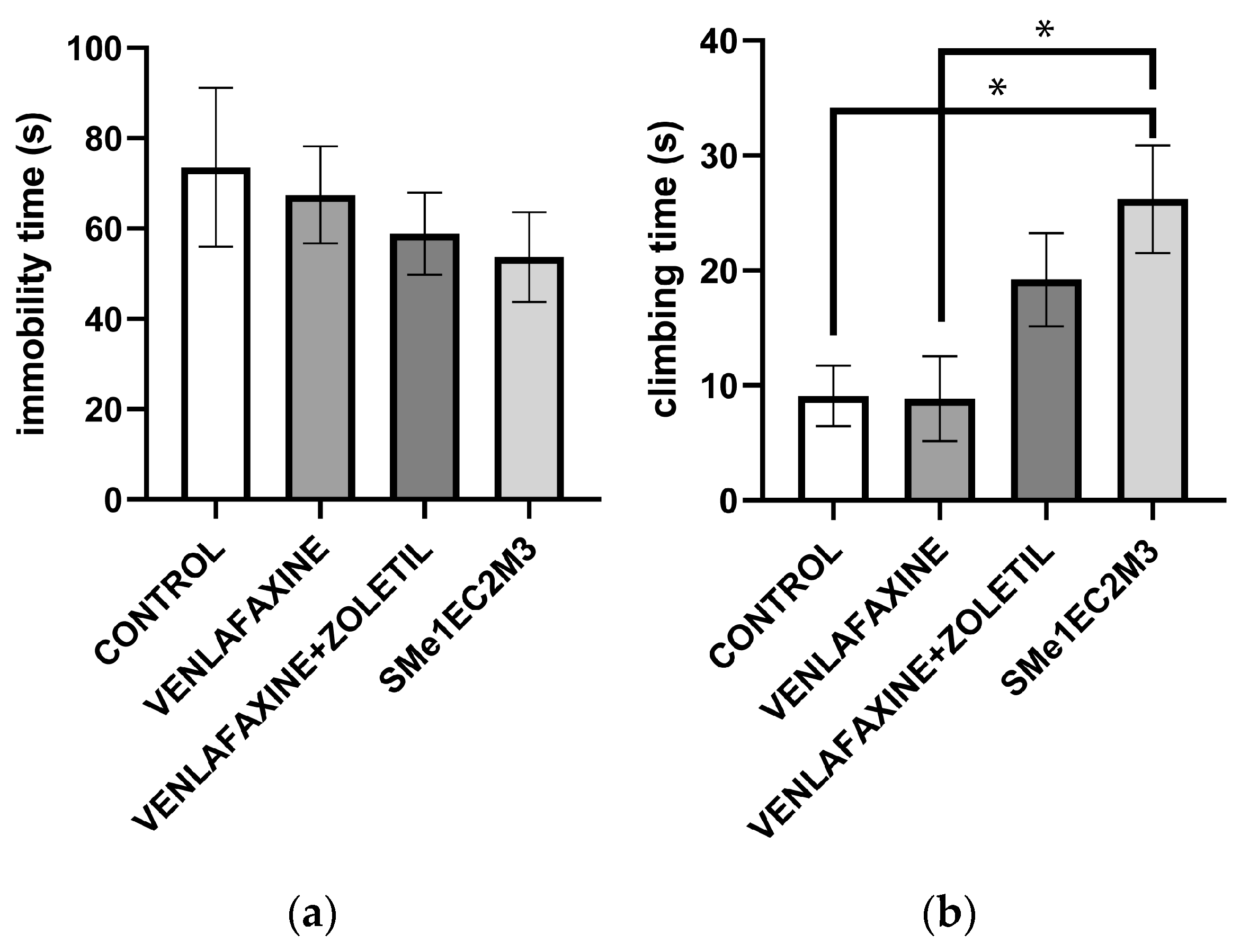
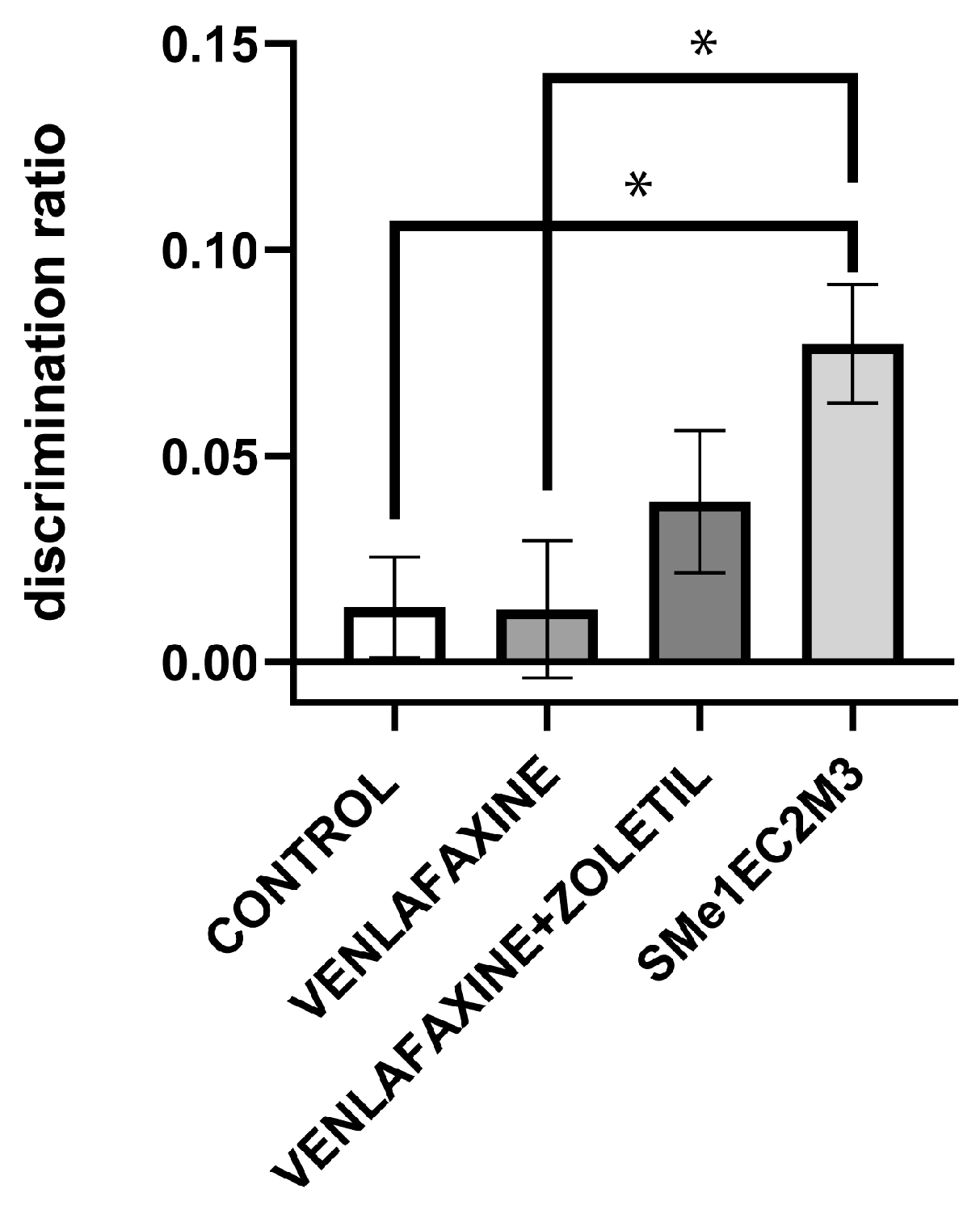
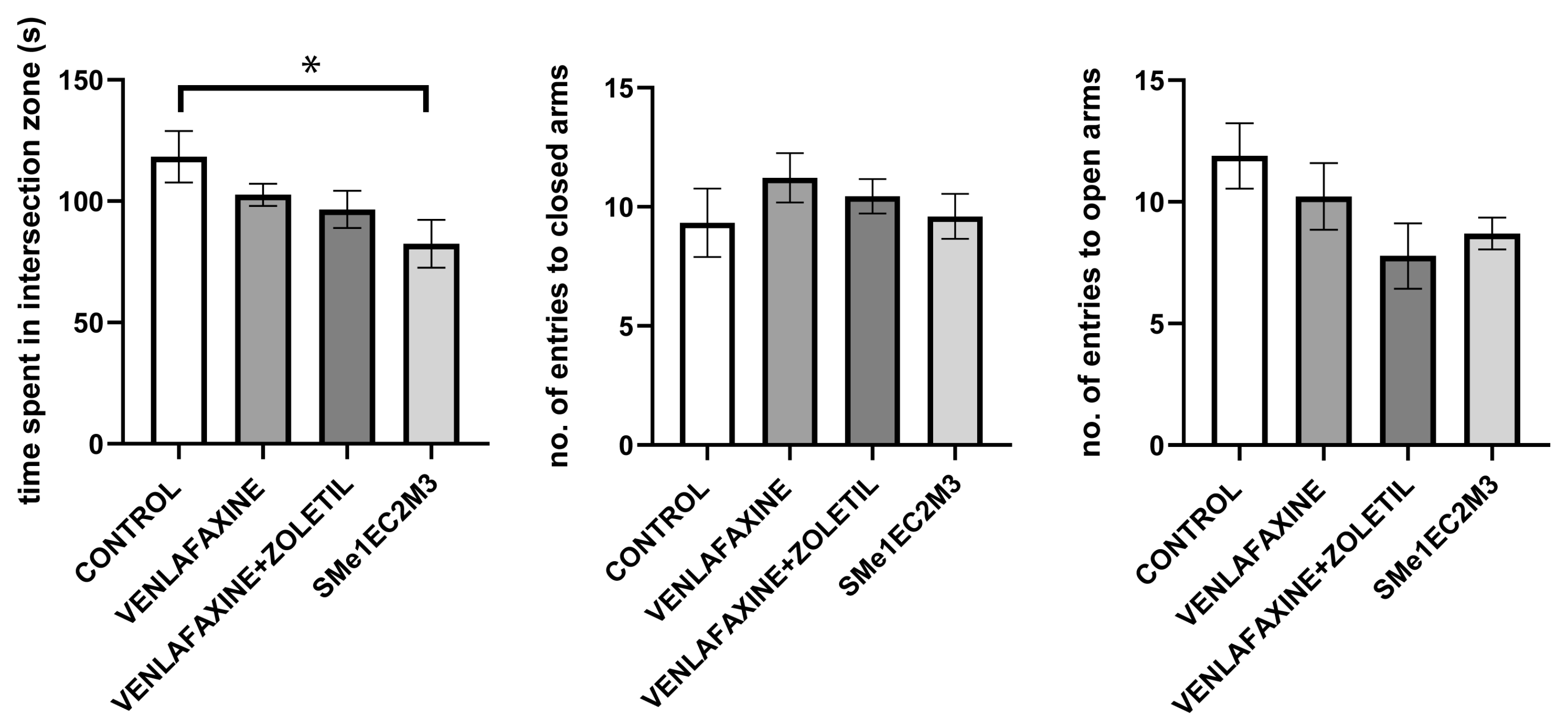
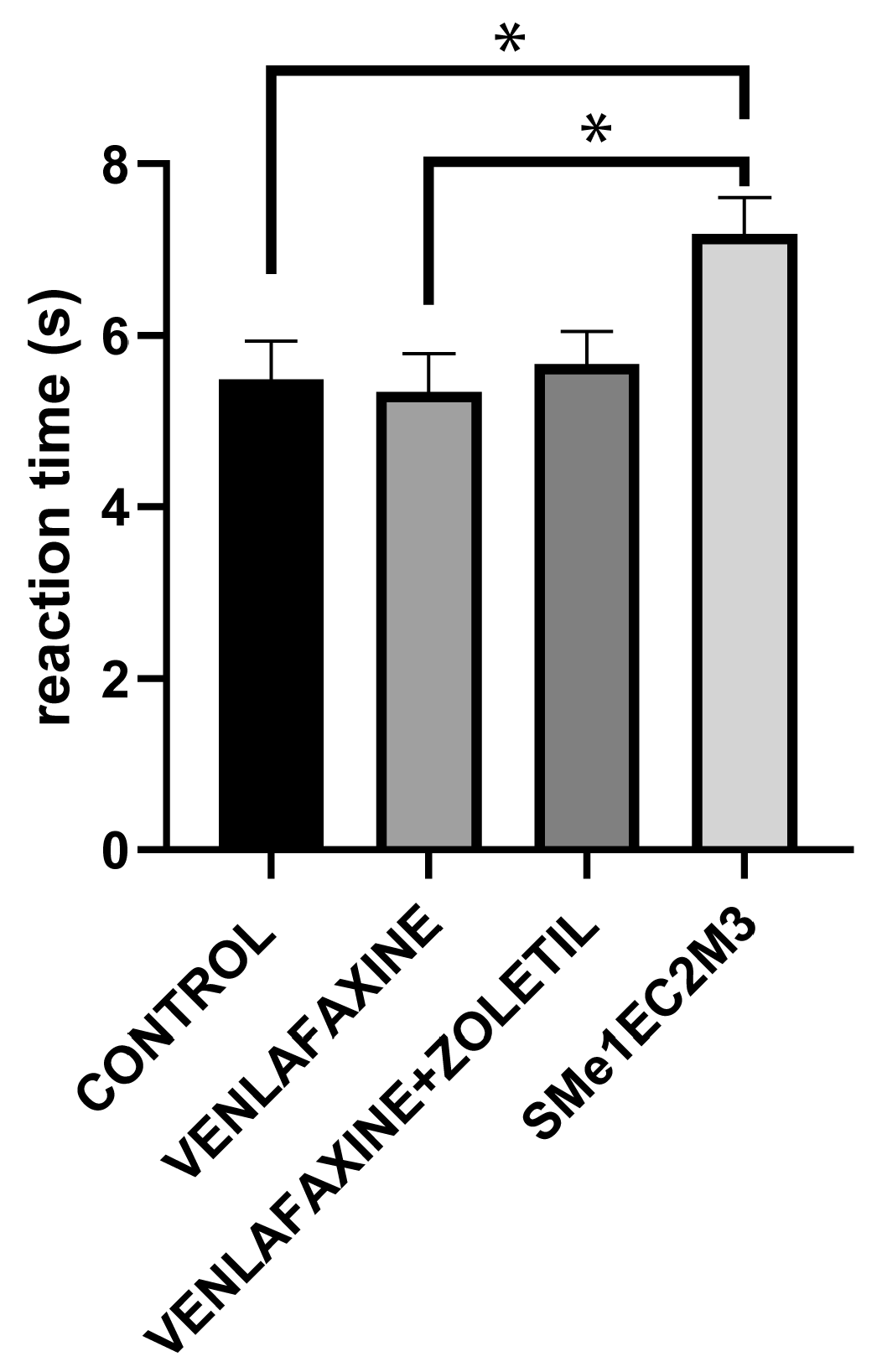
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental Design and Group Formation
4.4. Behavioral Analysis
4.4.1. Sucrose Preference Test (SPT)
4.4.2. Forced Swim Test (FST)
4.4.3. Open Field (OF)
4.4.4. Elevated plus Maze Test (EPM)
4.4.5. Novel Object Recognition Test (NOR)
4.4.6. Tail Flick Test
4.5. Statistical Analyses
5. Conclusions
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, I.; Thielen, K.; Bech, P.; Nygaard, E.; Diderichsen, F. Increasing prevalence of depression from 2000 to 2006. Scand. J. Public Health 2011, 39, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Lépine, J.P.; Briley, M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011, 7, 3–7. [Google Scholar]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61, 7–11. [Google Scholar] [PubMed]
- Thase, M.E.; Entsuah, A.R.; Rudolph, R.L. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br. J. Psychiatry 2001, 178, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Santra, S.; Dutta, A. Triple reuptake inhibitors as potential next-generation antidepressants: A new hope? Future Med. Chem. 2015, 7, 2385–2406. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H.; Young, A.H.; Blier, P. Strategies to achieve clinical effectiveness: Refining existing therapies and pursuing emerging targets. J. Affect. Disord. 2011, 132, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-López-Alberca, C.; González-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, K.S. Treatment resistantdepression: Therapeutictrends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Kessler, D.; Campbell, J.; Morrison, J.; Peters, T.J.; Williams, C.; Lewis, G.; Wiles, N. Prevalence of treatment-resistant depression in primary care: Cross-sectional data. Br. J. Gen. Pract. 2013, 63, 852–858. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Gratten, J.; Wray, N.R.; Keller, M.C.; Visscher, P.M. Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat. Neurosci. 2014, 17, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kellner, C.H.; Greenberg, R.M.; Murrough, J.W.; Bryson, E.O.; Briggs, M.C.; Pasculli, R.M. ECT in treatment-resistant depression. Am. J. Psychiatry 2012, 169, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Gruca, P.; Lason, M.; Tota-Glowczyk, K.; Litwa, E.; Niemczyk, M.; Papp, M. Validation of chronic mild stress in the Wistar-Kyoto rat as an animal model of treatment-resistant depression. Behav. Pharmacol. 2019, 30, 239–250. [Google Scholar] [CrossRef]
- Sattar, Y.; Wilson, J.; Khan, A.M.; Adnan, M.; Azzopardi-Larios, D.; Shrestha, S.; Rahman, Q.; Mansuri, Z.; Hassan, A.; Patel, N.B.; et al. A Review of the Mechanism of Antagonism of N-methyl-D-aspartate Receptor by Ketamine in Treatment-resistant Depression. Cureus 2018, 10, 2652. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.S.; Joca, S.R.L.; Wegener, G. Ketamine is effective in an animal model of treatment resistant depression. Int. J. Neuropsychopharmacol. 2016, 19, 48–49. [Google Scholar] [CrossRef]
- De la Peña, J.B.; Lee, H.C.; de la Peña, I.C.; Woo, T.S.; Yoon, S.Y.; Lee, H.L.; Han, J.S.; Lee, J.I.; Cho, Y.J.; Shin, C.Y.; et al. Rewarding and reinforcing effects of the NMDA receptor antagonist-benzodiazepine combination, Zoletil®: Difference between acute and repeated exposure. Behav. Brain Res. 2012, 233, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, H.; Kim, E.; Jin, W.; Lee, H.; Yoo, Y. A fatality due to injection of Tiletamine and Zolazepam. J. Anal. Toxicol. 2000, 24, 305–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stefek, M.; Milackova, I.; Juskova-Karasova, M.; Snirc, V. Antioxidant action of the hexahydropyridoindole SMe1EC2 in the cellular system of isolated red blood cells in vitro. Redox Rep. 2013, 18, 71–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koprdova, R.; Csatlosova, K.; Durisova, B.; Bogi, E.; Majekova, M.; Dremencov, E.; Mach, M. Electrophysiology and Behavioral Assessment of the New Molecule SMe1EC2M3 as a Representative of the Future Class of Triple Reuptake Inhibitors. Molecules 2019, 24, 4218. [Google Scholar] [CrossRef]
- Koprdova, R.; Osacka, J.; Mach, M.; Kiss, A. Acute Impact of Selected Pyridoindole Derivatives on Fos Expression in Different Structures of the Rat Brain. Cell Mol. Neurobiol. 2018, 138, 171–180. [Google Scholar] [CrossRef]
- Zvozilova, A.; Reichova, A.; Mach, M.; Bakos, J.; Koprdova, R. Effect of a New Substance with Pyridoindole Structure on Adult Neurogenesis, Shape of Neurons, and Behavioral Outcomes in a Chronic Mild Stress Model in Rats. Int. J. Mol. Sci. 2024, 25, 845. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, C.; Lichtsteiner, M.; Feer, H. Genetic and environmental influences on behavioral and neurochemical aspects of emotionality in rats. Experientia 1988, 44, 482–490. [Google Scholar] [CrossRef] [PubMed]
- López-Rubalcava, C.; Lucki, I. Strain differences in the behavioral Effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology 2000, 22, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Cunningham, J.I.; Eyerman, D.J.; Dean, R.L.; Deaver, D.R.; Sanchez, C. Opioid system modulators buprenorphine and samidorphan alter behavior and extracellular neurotransmitter concentrations in the Wistar Kyoto rat. Neuropharmacology 2019, 146, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Millard, S.J.; Weston-Green, K.; Newell, K.A. The Wistar-Kyoto rat model of endogenous depression: A tool for exploring treatment resistance with an urgent need to focus on sex differences. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 101, 109908. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Oishi, N.; Sotozono, Y.; Watanabe, A.; Sakai, Y.; Yamada, S.; Matsuda, K.I.; Kido, M.; Ikoma, K.; Tanaka, M.; et al. Validation of Wistar-Kyoto rats kept in solitary housing as an animal model for depression using voxel-based morphometry. Sci. Rep. 2024, 14, 3601. [Google Scholar] [CrossRef]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Page, M.E.; Lucki, I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur. J. Pharmacol. 2002, 436, 197–205. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Keeney, A.; Hogg, S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur. J. Pharmacol. 2004, 492, 195–201. [Google Scholar] [CrossRef]
- Briley, M. New hope in the treatment of painful symptoms in depression. Curr. Opin. Investig. Drugs 2003, 4, 42–45. [Google Scholar] [PubMed]
- Bandapati, S.; Podila, K.S.; Yadala, V.R. Comparative study of anti-nociceptive effect of venlafaxine with tramadol by tail-flick test in animal model of mice. Natl. J. Physiol. Pharm. Pharmacol. 2021, 11, 633–637. [Google Scholar] [CrossRef]
- Jha, P.K.; Mazumdar, B.; Bhatt, J.D. Analgesic activity of venlafaxine and its interactions with tramadol, celecoxib and amlodipine in mice. Indian J. Pharmacol. 2006, 38, 181–184. [Google Scholar] [CrossRef]
- Mitsi, V.; Terzi, D.; Purushothaman, I.; Manouras, L.; Gaspari, S.; Neve, R.L.; Stratinaki, M.; Feng, J.; Shen, L.; Zachariou, V. RGS9-2–controlled adaptations in the striatum determine the onset of action and efficacy of antidepressants in neuropathic pain states. Proc. Natl. Acad. Sci. USA 2015, 112, 36. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2008, 18, 2483. [Google Scholar] [CrossRef] [PubMed]
- Hache, G.; Coudore, F.; Gardier, A.M.; Guiard, B.P. Monoaminergic Antidepressants in the Relief of Pain: Potential Therapeutic Utility of Triple Reuptake Inhibitors (TRIs). J. Pharm. 2011, 4, 285–342. [Google Scholar] [CrossRef]
- Pan, H.L.; Wu, Z.Z.; Zhou, H.Y.; Chen, S.R.; Zhang, H.M.; Li, D.P. Modulation of pain transmission by, G-protein-coupled receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Gruca, P.; Lason-Tyburkiewicz, M.; Willner, P. Antidepressant, anxiolytic and procognitive effects of rivastigmine and donepezil in the chronic mild stress model in rats. Psychopharmacology 2016, 233, 1235–1243. [Google Scholar] [CrossRef]
- Elizalde, N.; Gil-Bea, F.J.; Ramírez, M.J.; Aisa, B.; Lasheras, B.; Del Rio, J.; Tordera, R.M. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: Effect of antidepressant treatment. Psychopharmacology 2008, 199, 1–14. [Google Scholar] [CrossRef]
- Orsetti, M.; Colella, L.; Dellarole, A.; Canonico, P.L.; Ghi, P. Modification of spatial recognition memory and object discrimination after chronic administration of haloperidol, amitriptyline, sodium valproate or olanzapine in normal and anhedonic rats. Neuropsychopharmacology 2006, 10, 345–357. [Google Scholar]
- Gentsch, C.; Lichtsteiner, M.; Feer, H. Open field and elevated plus-maze: A behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto rats and the effects of chlordiazepoxide. Behav. Brain Res. 1987, 25, 101–107. [Google Scholar] [CrossRef]
- Paré, W.P. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol. Behav. 1994, 55, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Berton, O.; Mormede, P.; Chaouloff, F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav. Brain Res. 1997, 85, 57–69. [Google Scholar] [CrossRef]
- Nosek, K.; Dennis, K.; Andrus, B.M.; Ahmadiyeh, N.; Baum, A.E.; Solberg Woods, L.C.; Redei, E.E. Context and strain-dependent behavioral response to stress. Behav. Brain Funct. 2008, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Gruca, P.; Litwa, E.; Lason, M.; Willner, P. Optogenetic stimulation of transmission from prelimbic cortex to nucleus accumbens core overcomes resistance to venlafaxine in an animal model of treatment-resistant depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 123, 110715. [Google Scholar] [CrossRef]
- Wright, R.L.; Gilmour, G.; Dwyer, D.M. Wistar Kyoto Rats Display Anhedonia In Consumption but Retain Some Sensitivity to the Anticipation of Palatable Solutions. Front. Behav. Neurosci. 2020, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Harris, R. Failure to Change Exploration or Saccharin Preference in Rats Exposed to Chronic Mild Stress. Physiol. Behav. 1997, 63, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Strouthes, A.; Volo, A.M.; Unger, T. Hunger, Thirst and Their Interactive Effects on the Rat’s Drinking in a Saccharin-Water Choice. Physiol. Behav. 1974, 13, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.; Self, J.L.; Carter, D.J. Sucrose Preference Thresholds for Satiated and Water-Deprived Rats. Psychol. Rep. 1965, 16, 901–905. [Google Scholar] [CrossRef]
- Cohen, J.S.; Hachey, G.J. Development of Sucrose Preferences under Two Levels of Water Deprivation. Psychol. Rep. 1980, 46, 820–822. [Google Scholar] [CrossRef]
- Wang, C.H.; Gu, J.Y.; Zhang, X.L.; Dong, J.; Yang, J.; Zhang, Y.L.; Ning, Q.F.; Shan, X.W.; Li, Y. Venlafaxine ameliorates the depression-like behaviors and hippocampal S100B expression in a rat depression model. Behav. Brain Funct. 2016, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Lapmanee, S.; Charoenphandhu, N.; Krishnamra, N.; Charoenphandhu, J. Anxiolytic-like actions of reboxetine, venlafaxine and endurance swimming in stressed male rats. Behav. Brain Res. 2012, 16, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, R.; Barnaby, E.; Améndola, L.; Hea, S.Y.; Smith, B.; Webster, J.; Zobel, G. Voluntary Oral Ingestion of a Sedative Prior to Euthanasia with CO2: Behavioural Responses of Mice. Animals 2021, 11, 2879. [Google Scholar] [CrossRef] [PubMed]
- Viñas-Noguera, M.; Csatlósová, K.; Šimončičová, E.; Bögi, E.; Ujházy, E.; Dubovický, M.; Belovičová, K. Sex- and age- dependent effect of pre-gestational chronic stress and mirtazapine treatment on neurobehavioral development of Wistar rat offspring. PLoS ONE 2022, 17, e0255546. [Google Scholar] [CrossRef]
- Tjølsen, A.; Lund, A.; Berge, O.G.; Hole, K. An improved method for tail-flick testing with adjustment for tail-skin temperature. J. Neurosci. Methods 1989, 26, 259–265. [Google Scholar] [CrossRef]

| Stressor | Days of CMS |
|---|---|
| overcrowding | 6, 10, 16, 27 |
| air puff | 4, 8, 19, 24 |
| wet bedding | 2, 9, 20, 23 |
| tilted cage | 1, 11, 18, 25 |
| predator stress | 5, 13, 17, 26 |
| food deprivation | 3, 12, 15, 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvozilova, A.; Bukatova, S.; Koprdova, R.; Mach, M. Evaluation of New Approaches to Depression Treatment Using an Animal Model of Pharmacoresistant Depression. Int. J. Mol. Sci. 2024, 25, 5265. https://doi.org/10.3390/ijms25105265
Zvozilova A, Bukatova S, Koprdova R, Mach M. Evaluation of New Approaches to Depression Treatment Using an Animal Model of Pharmacoresistant Depression. International Journal of Molecular Sciences. 2024; 25(10):5265. https://doi.org/10.3390/ijms25105265
Chicago/Turabian StyleZvozilova, Alexandra, Stanislava Bukatova, Romana Koprdova, and Mojmir Mach. 2024. "Evaluation of New Approaches to Depression Treatment Using an Animal Model of Pharmacoresistant Depression" International Journal of Molecular Sciences 25, no. 10: 5265. https://doi.org/10.3390/ijms25105265
APA StyleZvozilova, A., Bukatova, S., Koprdova, R., & Mach, M. (2024). Evaluation of New Approaches to Depression Treatment Using an Animal Model of Pharmacoresistant Depression. International Journal of Molecular Sciences, 25(10), 5265. https://doi.org/10.3390/ijms25105265







