Puerarin—A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects
Abstract
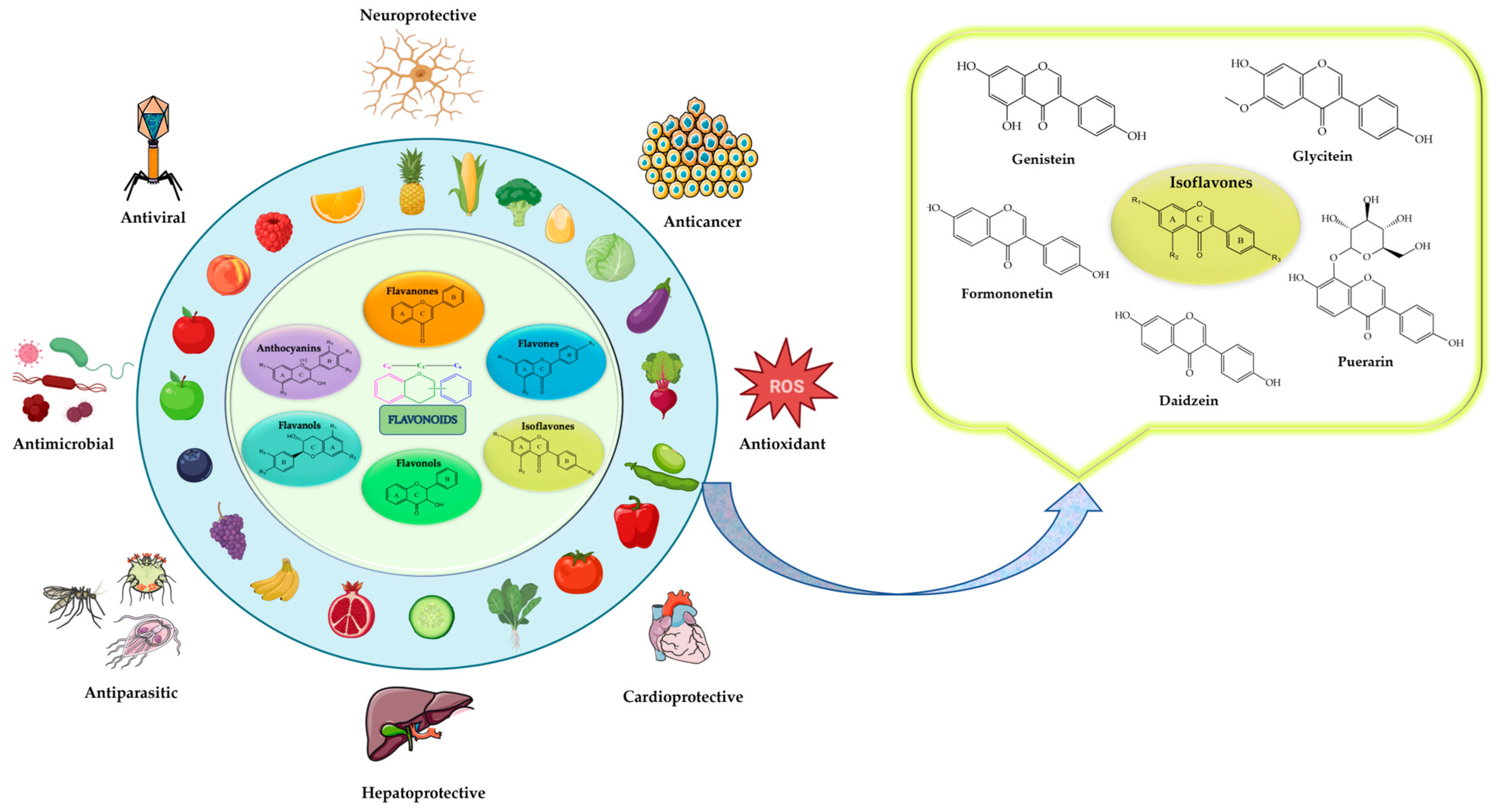
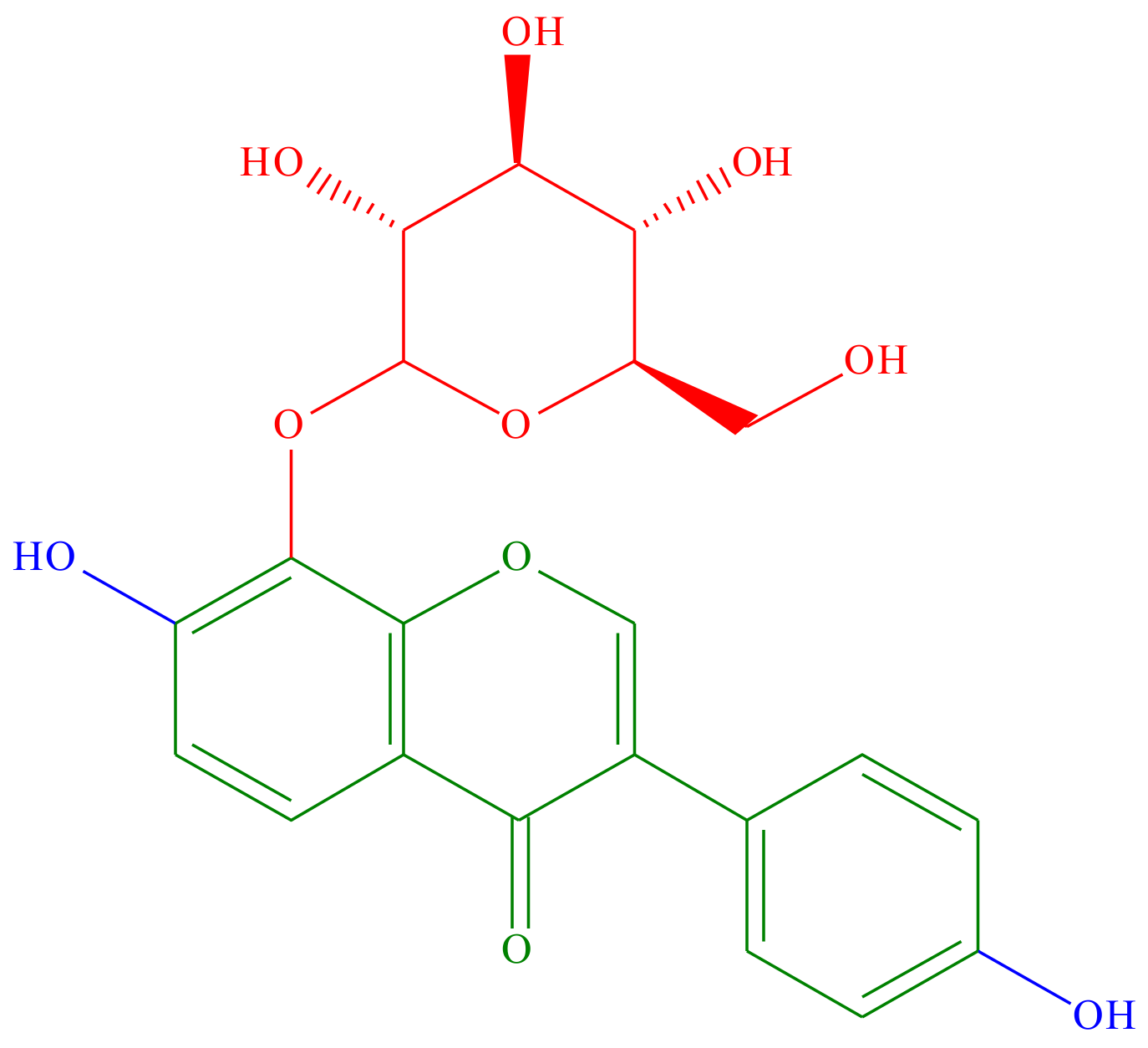
1. Introduction
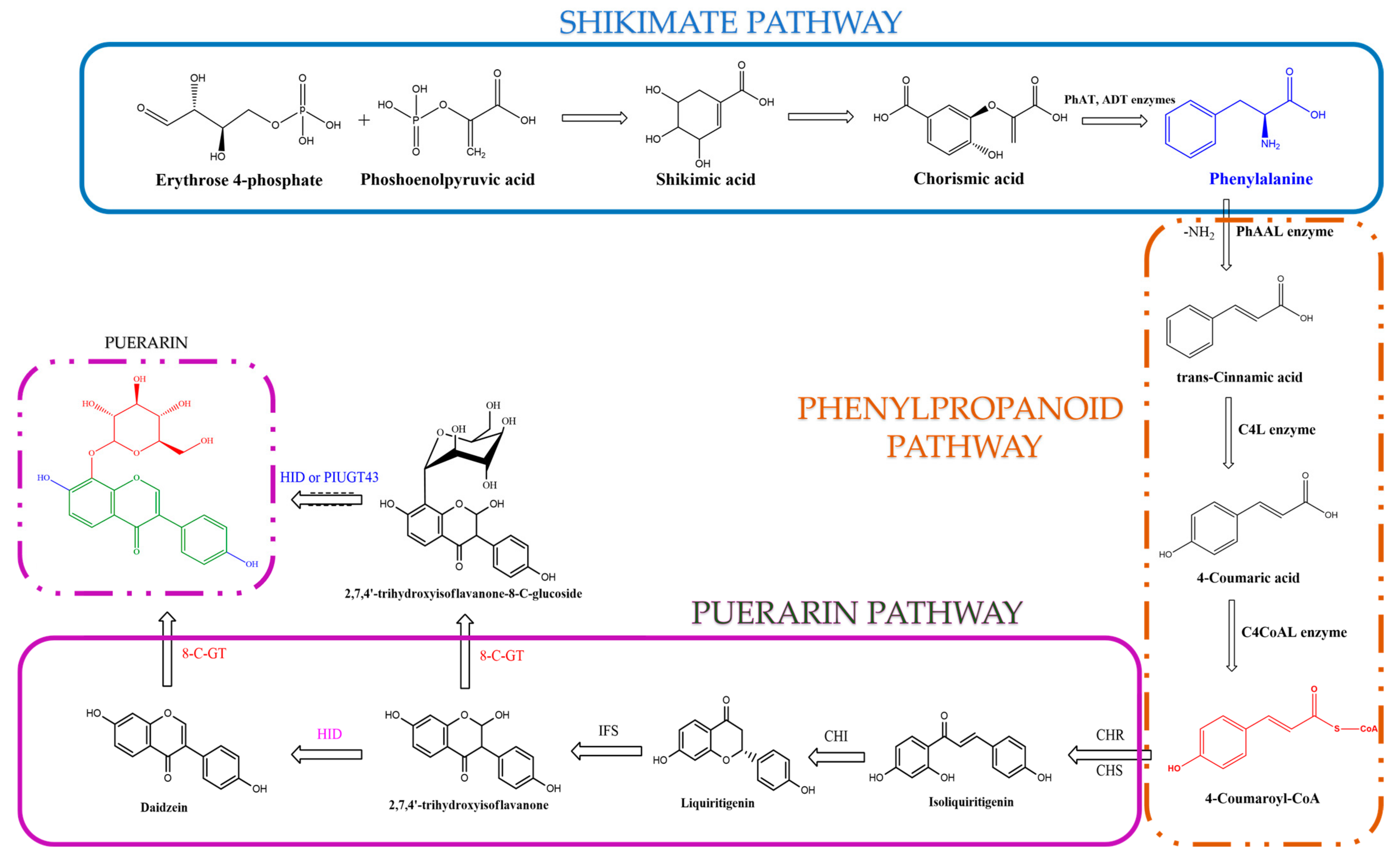
2. Biosynthesis of Puerarin
3. Extraction Methods and Analytical Techniques
4. Biological Effects of Puerarin
5. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moses, T.; Goossens, A. Plants for Human Health: Greening Biotechnology and Synthetic Biology. J. Exp. Bot. 2017, 68, 4009–4011. [Google Scholar] [CrossRef] [PubMed]
- Schaal, B. Plants and People: Our Shared History and Future. Plants People Planet 2019, 1, 14–19. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Pergola, M.; De Falco, E.; Belliggiano, A.; Ievoli, C. The Most Relevant Socio-Economic Aspects of Medicinal and Aromatic Plants through a Literature Review. Agriculture 2024, 14, 405. [Google Scholar] [CrossRef]
- Carrubba, A.; Marceddu, R.; Sarno, M. Bringing Spontaneous Plants to Cultivation: Issues and Constraints for Medicinal and Aromatic Plants. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Medicinal and Aromatic Plants: Domestication, Breeding, Cultivation and New Perspectives, Angers, France, 14–20 August 2022; Volume 1358, pp. 43–48. [Google Scholar]
- Ansari, M.K.A.; Iqbal, M.; Chaachouay, N.; Ansari, A.A.; Owens, G. The Concept and Status of Medicinal and Aromatic Plants: History, Pharmacognosy, Ecology, and Conservation. In Plants as Medicine and Aromatics; CRC Press: Boca Raton, FL, USA, 2023; pp. 129–144. [Google Scholar]
- Azaizeh, H.; Saad, B.; Cooper, E.; Said, O. Traditional Arabic and Islamic Medicine, a Re-Emerging Health Aid. Evid.-Based Complement. Altern. Med. 2010, 7, 340679. [Google Scholar] [CrossRef]
- Hamilton, A.C. Medicinal Plants, Conservation and Livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Woo, S.; Marquez, L.; Crandall, W.J.; Risener, C.J.; Quave, C.L. Recent Advances in the Discovery of Plant-Derived Antimicrobial Natural Products to Combat Antimicrobial Resistant Pathogens: Insights from 2018–2022. Nat. Prod. Rep. 2023, 40, 1271–1290. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, Y.; Song, C.; Chen, S. New Insights into the Roles of Fungi and Bacteria in the Development of Medicinal Plant. J. Adv. Res. 2024, in press. [CrossRef] [PubMed]
- Hui, Z.; Wen, H.; Zhu, J.; Deng, H.; Jiang, X.; Ye, X.Y.; Wang, L.; Xie, T.; Bai, R. Discovery of Plant-Derived Anti-Tumor Natural Products: Potential Leads for Anti-Tumor Drug Discovery. Bioorg Chem. 2024, 142, 106957. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. [Google Scholar] [CrossRef]
- Phillipson, J.D. Phytochemistry and Pharmacognosy. Phytochemistry 2007, 68, 2960–2972. [Google Scholar] [CrossRef] [PubMed]
- Teoh, E.S. Secondary Metabolites of Plants. In Medicinal Orchids of Asia; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 59–73. [Google Scholar]
- Zhao, J.H.; Wang, Y.W.; Yang, J.; Tong, Z.J.; Wu, J.Z.; Wang, Y.B.; Wang, Q.X.; Li, Q.Q.; Yu, Y.C.; Leng, X.J.; et al. Natural Products as Potential Lead Compounds to Develop New Antiviral Drugs over the Past Decade. Eur. J. Med. Chem. 2023, 260, 115726. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, W.; Xie, J. Natural Phenolic Compounds: Antimicrobial Properties, Antimicrobial Mechanisms, and Potential Utilization in the Preservation of Aquatic Products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, S.; Zhou, W.; Liu, Z.; Tan, J.; Wei, M. Microbial Regulation of Plant Secondary Metabolites: Impact, Mechanisms and Prospects. Microbiol. Res. 2024, 283, 127688. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Gao, W.; Wang, R.; Yang, W.; Zhang, H.; Huang, L.; Guo, L. Dynamic Metabolites: A Bridge between Plants and Microbes. Sci. Total Environ. 2023, 899, 165612. [Google Scholar] [CrossRef]
- Wrońska, N.; Szlaur, M.; Zawadzka, K.; Lisowska, K. The Synergistic Effect of Triterpenoids and Flavonoids—New Approaches for Treating Bacterial Infections? Molecules 2022, 27, 847. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 257–312. ISBN 1572-5995. [Google Scholar]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Alexander, V.S. Phytoestrogens and Their Effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Tan, S.T.; Tan, S.S.; Tan, C.X. Soy Protein, Bioactive Peptides, and Isoflavones: A Review of Their Safety and Health Benefits. PharmaNutrition 2023, 25, 100352. [Google Scholar] [CrossRef]
- Ren, Y.; Qu, S. Constituent Isoflavones of Puerariae radix as a Potential Neuroprotector in Cognitive Impairment: Evidence from Preclinical Studies. Ageing Res. Rev. 2023, 90, 102040. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific Evidence Supporting the Beneficial Effects of Isoflavones on Human Health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Puerarin: A Review of Pharmacological Effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Esch, H.L.; Kleider, C.; Scheffler, A.; Lehmann, L. Chapter 34—Isoflavones: Toxicological Aspects and Efficacy. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 465–487. ISBN 978-0-12-802147-7. [Google Scholar]
- Bacanlı, M.; Aydın, S.; Başaran, A.A.; Başaran, N. Chapter 33—A Phytoestrogen Puerarin and Its Health Effects. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 425–431. ISBN 978-0-12-813008-7. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid Production: Current Trends in Plant Metabolic Engineering and De Novo Microbial Production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Takahashi, H.; Nakamura, M.; Yoshimoto, N.; Suzuki, H.; Shibata, D.; Yamazaki, M.; Saito, K. Transcriptomic Landscape of Pueraria Lobata Demonstrates Potential for Phytochemical Study. Front. Plant Sci. 2015, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Zhou, C.; Li, J.; Zhang, Y. Molecular Characterization of the C-Glucosylation for Puerarin Biosynthesis in Pueraria Lobata. Plant J. 2017, 90, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, X.; Wu, S.; Wang, S.; Rao, S.; Li, L.; Cheng, S.; Li, L. Chromosome-Level Genome Assembly and Multi-Omics Dataset Provide Insights into Isoflavone and Puerarin Biosynthesis in Pueraria Lobata (Wild.) Ohwi. Biomolecules 2022, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Zhu, Y.; Sun, W.; Tang, N.; Xu, Z.; Shang, X.; Zhang, Y.; Yan, H.; Li, C. Comparative Transcriptome Analysis of Pueraria Lobata Provides Candidate Genes Involved in Puerarin Biosynthesis and Its Regulation. Biomolecules 2023, 13, 170. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, T.; Min, X.; He, J.; Hou, C.; Liu, X. Integrated Metabolomic and Transcriptomic Analysis of Puerarin Biosynthesis in Pueraria Montana Var. Thomsonii at Different Growth Stages. Genes 2023, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska-Turska, M.; Sieniawska, E. Puerarin: Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–30. ISBN 978-3-030-94753-8. [Google Scholar]
- Li, C.; Zhang, Y. Glycosylation and Methylation in the Biosynthesis of Isoflavonoids in Pueraria Lobata. Front. Plant Sci. 2023, 14, 1330586. [Google Scholar] [CrossRef] [PubMed]
- Adolfo, L.M.; Burks, D.; Rao, X.; Alvarez-Hernandez, A.; Dixon, R.A. Evaluation of Pathways to the C-Glycosyl Isoflavone Puerarin in Roots of Kudzu (Pueraria Montana Lobata). Plant Direct 2022, 6, e442. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Intharuksa, A.; Sasaki, Y. A Promising View of Kudzu Plant, Pueraria Montana Var. Lobata (Willd.) Sanjappa & Pradeep: Flavonoid Phytochemical Compounds, Taxonomic Data, Traditional Uses and Potential Biological Activities for Future Cosmetic Application. Cosmetics 2020, 7, 12. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The Impact and Invasive Mechanisms of Pueraria Montana Var. Lobata, One of the World’s Worst Alien Species. Plants 2023, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wei, P.; Jia, M.; Wang, L.; Li, Z.; Zhang, Z.; Liu, Y.; Shi, L. Research Progress in Modifications, Bioactivities, and Applications of Medicine and Food Homologous Plant Starch. Foods 2024, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Chopra, B.S.; Raut, S.; Khatri, N. Pueraria tuberosa: A Review on Traditional Uses, Pharmacology, and Phytochemistry. Front. Pharmacol. 2021, 11, 582506. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Jahan, M.S.; Tang, K.; Jiang, S.; Guo, J.; Luo, S.; Luo, W.; Li, G. Comparative Analysis of the Medicinal and Nutritional Components of Different Varieties of Pueraria Thomsonii and Pueraria Lobata. Front. Plant Sci. 2023, 14, 1115782. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.; Liu, Y.; Liu, R.; Liu, S.; Han, J.; Bai, X.; Wu, J.; Fan, R. Advances in Extraction, Purification, and Analysis Techniques of the Main Components of Kudzu Root: A Comprehensive Review. Molecules 2023, 28, 6577. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and Emerging Extraction Processes of Flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Abhari, K.; Mousavi Khaneghah, A. Alternative Extraction Techniques to Obtain, Isolate and Purify Proteins and Bioactive from Aquaculture and by-Products. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 92, pp. 35–52. [Google Scholar]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Zhu, H.; Xing, Y.; Akan, O.D.; Yang, T. Ultrafine Comminution-Assisted Ultrasonic-Microwave Synergistic Extraction of Pueraria Mirifica (Kudzu Flower and Root) Flavonoids. Heliyon 2023, 9, e21137. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Slesarev, G.P.; Aboushanab, S.A.; Kovalev, I.S.; Zeidler, D.M.; Kovaleva, E.G.; Bhat, R. An Eco-Friendly Approach to Enhance the Extraction and Recovery Efficiency of Isoflavones from Kudzu Roots and Soy Molasses Wastes Using Ultrasound-Assisted Extraction with Natural Deep Eutectic Solvents (NADES). Ind. Crops Prod. 2022, 182, 114886. [Google Scholar] [CrossRef]
- Blicharski, T.; Oniszczuk, A. Extraction Methods for the Isolation of Isoflavonoids from Plant Material. Open Chem. 2017, 15, 34–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Zou, L.; Zhang, M.; Chi, R. Development of an SVR Model for Microwave-Assisted Aqueous Two-Phase Extraction of Isoflavonoids from Radix Puerariae. Chem. Eng. Commun. 2021, 208, 1005–1016. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Yan, E.; Zhan, H.-Y.; Zhang, Z.-Q. Response Surface Optimization of Microwave-Assisted Extraction for HPLC-Fluorescence Determination of Puerarin and Daidzein in Radix Puerariae thomsonii. J. Pharm. Anal. 2011, 1, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nour, A.H.; Oluwaseun, A.R.; Nour, A.H.; Omer, M.S.; Ahmed, N. Microwave-Assisted Extraction of Bioactive Compounds (Review). In Microwave Heating; Churyumov, G.I., Ed.; IntechOpen: Rijeka, Croatia, 2021; pp. 1–31. ISBN 978-1-83968-227-8. [Google Scholar]
- Zou, Y.; Tian, M.; Liu, C. Optimization of Ultrasound-Assisted Extraction of Puerarin from Pueraria Lobata Dried Root. J. Food Process Preserv. 2016, 40, 431–436. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, J.; Jiang, X.; Guo, Q. Ultrasonic-Assisted Extraction of Puerarin Optimized by Response Surface Methodology. In Proceedings of the 2015 Chinese Intelligent Automation Conference, Fuzhou, China, 30 March 2015; Deng, Z., Li, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 499–508. [Google Scholar]
- Aihua, S.; Xiaoyan, C.; Xiaoguang, Y.; Jiang, F.; Yanmin, L.; Zhou, J. Applications and Prospects of Ultrasound-Assisted Extraction in Chinese Herbal Medicine. Open Access J. Biomed. Sci. 2019, 1. [Google Scholar] [CrossRef]
- Zeng, X.; Tan, H.; Liu, B.; Wen, Y. Optimization of Ultrasonic-Assisted Extraction and Purification of Total Flavonoids with Biological Activities from Radix Puerariae. Biomass Convers. Biorefin 2023. [Google Scholar] [CrossRef]
- Vinitha, U.G.; Sathasivam, R.; Muthuraman, M.S.; Park, S.U. Intensification of Supercritical Fluid in the Extraction of Flavonoids: A Comprehensive Review. Physiol. Mol. Plant Pathol. 2022, 118, 101815. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Majik, M.S.; Gawas, U.B. Chapter 2—Recent Advances in Extraction of Natural Compounds. In New Horizons in Natural Compound Research; Meena, S.N., Nandre, V., Kodam, K., Meena, R.S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 17–33. ISBN 978-0-443-15232-0. [Google Scholar]
- Huang, Y.; Yang, J.; Zhao, Y.; Yu, L.; He, Y.; Wan, H.; Li, C. Screening, Optimization, and Bioavailability Research of Natural Deep Eutectic Solvent Extracts from Radix Pueraria. Molecules 2021, 26, 729. [Google Scholar] [CrossRef]
- Makkliang, F.; Siriwarin, B.; Yusakul, G.; Phaisan, S.; Sakdamas, A.; Chuphol, N.; Putalun, W.; Sakamoto, S. Biocompatible Natural Deep Eutectic Solvent-Based Extraction and Cellulolytic Enzyme-Mediated Transformation of Pueraria Mirifica Isoflavones: A Sustainable Approach for Increasing Health-Bioactive Constituents. Bioresour. Bioprocess. 2021, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, Q.; Yang, H.; Gao, W.; Li, P. PH-Controlled Reversible Deep-Eutectic Solvent Based Enzyme System for Simultaneous Extraction and in-Situ Separation of Isoflavones from Pueraria Lobata. Sep. Purif. Technol. 2022, 292, 120992. [Google Scholar] [CrossRef]
- Kaoui, S.; Chebli, B.; Zaidouni, S.; Basaid, K.; Mir, Y. Deep Eutectic Solvents as Sustainable Extraction Media for Plants and Food Samples: A Review. Sustain. Chem. Pharm. 2023, 31, 100937. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Jurinjak Tušek, A.; Šamec, D.; Šalić, A. Modern Techniques for Flavonoid Extraction—To Optimize or Not to Optimize? Appl. Sci. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Ameta, S.C.; Ameta, R. Green Chemistry: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2023; ISBN 1000932648. [Google Scholar]
- Medina Valderrama, C.J.; Morales Huamán, H.I.; Valencia-Arias, A.; Vasquez Coronado, M.H.; Cardona-Acevedo, S.; Delgado-Caramutti, J. Trends in Green Chemistry Research between 2012 and 2022: Current Trends and Research Agenda. Sustainability 2023, 15, 13946. [Google Scholar] [CrossRef]
- Singh, D.; Isharani, R. A Detailed Review on Analytical Methods to Manage the Impurities in Drug Substances. OAlib 2023, 10, 1–18. [Google Scholar] [CrossRef]
- Mattrey, F.T.; Makarov, A.A.; Regalado, E.L.; Bernardoni, F.; Figus, M.; Hicks, M.B.; Zheng, J.; Wang, L.; Schafer, W.; Antonucci, V.; et al. Current Challenges and Future Prospects in Chromatographic Method Development for Pharmaceutical Research. TrAC-Trends Anal. Chem. 2017, 95, 36–46. [Google Scholar] [CrossRef]
- Dispas, A.; Sacré, P.Y.; Ziemons, E.; Hubert, P. Emerging Analytical Techniques for Pharmaceutical Quality Control: Where Are We in 2022? J. Pharm. Biomed. Anal. 2022, 221, 115071. [Google Scholar] [CrossRef] [PubMed]
- Maji, A.K.; Banerjee, D.; Maity, N.; Banerji, P. A Validated RP-HPLC-UV Method for Quantitative Determination of Puerarin in Pueraria tuberosa DC Tuber Extract. Pharm. Methods 2012, 3, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Y.; Yang, L.-L.; Liu-Qing, Y.; Zou, Y.-M.; Lu, J.-M. Simultaneous RP-HPLC Determination of Puerarin, Daidzin and Daidzein in Roots, Stems and Leaves of Pueraria Lobata (Wild) Ohwi. Food Sci. 2009, 30, 248–252. [Google Scholar]
- Chauhan, S.K.; Singh, B.; Agrawal, S. Determination of Puerarin from Pueraria tuberosa DC by Hplc. Anc. Sci. Life 2004, 23, 22–25. [Google Scholar] [PubMed]
- Chew, Y.L.; Khor, M.A.; Lim, Y.Y. Choices of Chromatographic Methods as Stability Indicating Assays for Pharmaceutical Products: A Review. Heliyon 2021, 7, e06553. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Adrjan, B.; Li, J.; Hu, B.; Roszak, S. NMR Studies of Daidzein and Puerarin: Active Anti-Oxidants in Traditional Chinese Medicine. J. Mol. Model. 2019, 25, 202. [Google Scholar] [CrossRef] [PubMed]
- Shockcor, J.P. HPLC–NMR, Pharmaceutical Applications☆. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 141–151. ISBN 978-0-12-803224-4. [Google Scholar]
- Gebretsadik, T.; Linert, W.; Thomas, M.; Berhanu, T.; Frew, R. LC–NMR for Natural Product Analysis: A Journey from an Academic Curiosity to a Robust Analytical Tool. Science 2021, 3, 6. [Google Scholar] [CrossRef]
- Seger, C.; Sturm, S. NMR-Based Chromatography Readouts: Indispensable Tools to “Translate” Analytical Features into Molecular Structures. Cells 2022, 11, 3526. [Google Scholar] [CrossRef]
- Gao, D.; Cho, C.W.; Kim, J.H.; Lee, E.J.; Kim, C.T.; Kang, J.S. A New HPLC Method for the Analysis of Puerarin for Quality Control of the Extract of Pueraria Lobate Stem and Puerarin Cream. J. Pharm. Sci. 2020, 35, 88–93. [Google Scholar]
- Gao, D.; Kim, J.H.; Kim, C.T.; Jeong, W.S.; Kim, H.M.; Sim, J.; Kang, J.S.; Attanzio, A. Molecular Sciences Evaluation of Anti-Melanogenesis Activity of Enriched Pueraria Lobata Stem Extracts and Characterization of Its Phytochemical Components Using HPLC-PDA-ESI-MS/MS. Int. J. Mol. Sci. 2021, 22, 8105. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Song, K.; Zhang, Q.; Guo, J.; Huang, J. Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity. Molecules 2020, 25, 344. [Google Scholar] [CrossRef]
- Shang, X.; Huang, D.; Wang, Y.; Xiao, L.; Ming, R.; Zeng, W.; Cao, S.; Lu, L.; Wu, Z.; Yan, H. Identification of Nutritional Ingredients and Medicinal Components of Pueraria Lobata and Its Varieties Using Uplc-Ms/Ms-Based Metabolomics. Molecules 2021, 26, 6587. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, X.; Chen, X.; Chen, X.; Liu, Y.; Xu, H.; Wang, Q.; Tang, Z. A Study on Puerarin in Situ Gel Eye Drops: Formulation Optimization and Pharmacokinetics on Rabbits by Microdialysis. Int. J. Pharm. 2023, 642, 123176. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Liu, D.; Wen, S.; Wu, Y.; Li, T.; Tang, T.; Wang, Y.; Zou, T.; Zhao, C.; et al. Water Extracts of Pueraria Thomsonii Radix Ameliorates Alcoholic Liver Disease via PI3K/AKT and NOX4/ROS Pathways. J. Funct. Foods 2023, 110, 105830. [Google Scholar] [CrossRef]
- Baranyika, J.B.; Bakire, S.; Shoucheng, P.; Meihao, S.; Hirwa, H. Application of the Selected Macroporous Resin for the Separation and Identification of Flavonoids from Chinese Radix Pueraria Lobata by HPLC-Q-TOF-MS. Microchem. J. 2024, 196, 109662. [Google Scholar] [CrossRef]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xu, Q.; Ma, J.; Li, X.; Tian, Y.; Wen, Y.; Chen, T. Ginseng and Health Outcomes: An Umbrella Review. Front. Pharmacol. 2023, 14, 1069268. [Google Scholar] [CrossRef]
- Akaberi, M.; Baharara, H.; Amiri, M.S.; Moghadam, A.T.; Sahebkar, A.; Emami, S.A. Ginkgo Biloba: An Updated Review on Pharmacological, Ethnobotanical, and Phytochemical Studies. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100331. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Q.; Lin, A.; Chen, X.; Wu, Y.; Zhang, B.; Zhang, Y.; Min, H.; Wen, Y.; Song, S.; et al. Puerarin Increases Survival and Protects Against Organ Injury by Suppressing NF-ΚB/JNK Signaling in Experimental Sepsis. Front. Pharmacol. 2020, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.P.; Zeng, J.H.; Lin, X.; Ni, Y.H.; Jiang, C.S.; Li, D.Z.; He, X.J.; Wang, R.; Wang, W. Puerarin Ameliorates Caerulein-Induced Chronic Pancreatitis via Inhibition of MAPK Signaling Pathway. Front. Pharmacol. 2021, 12, 686992. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.L.; Han, L.L.; Qian, J.H.; Wang, H.Z. Molecular Mechanism of Puerarin Against Diabetes and Its Complications. Front. Pharmacol. 2022, 12, 780419. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Ye, C.; Bayliss, G.; Zhuang, S. New Insights Into the Effects of Individual Chinese Herbal Medicines on Chronic Kidney Disease. Front. Pharmacol. 2021, 12, 774414. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Yan, H.L.; Wang, L.X.; Xu, J.F.; Peng, C.; Ao, H.; Tan, Y.Z. Review of Natural Resources with Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front. Pharmacol. 2021, 12, 627458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Zhang, H.; Peng, C. Effects of Puerarin on the Prevention and Treatment of Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 771793. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Y.; Huang, C.; Liu, Q.; Huang, M.; Chen, B.; Peng, Z.; Zhu, W.; Ding, B. Efficacy and Safety of Puerarin Injection on Acute Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 934598. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, S.; Lin, C.; Dong, D.; Xiao, J.; Ye, Y.; Wang, M. Roles of Flavonoids in Ischemic Heart Disease: Cardioprotective Effects and Mechanisms against Myocardial Ischemia and Reperfusion Injury. Phytomedicine 2024, 126, 155409. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, T.; He, Q.; Guo, C.; Xu, L.; Zhang, K.; Duan, X. Puerarin, Isolated from Kudzu Root (Willd.), Attenuates Hepatocellular Cytotoxicity and Regulates the GSK-3β/NF-ΚB Pathway for Exerting the Hepatoprotection against Chronic Alcohol-Induced Liver Injury in Rats. Int. Immunopharmacol. 2013, 17, 71–78. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yuan, M.H.; Zhang, C.Y.; Liu, H.M.; Liu, J.R.; Wei, A.L.; Ye, Q.; Zeng, B.; Li, M.F.; Guo, Y.P.; et al. Puerariae Lobatae Radix Flavonoids and Puerarin Alleviate Alcoholic Liver Injury in Zebrafish by Regulating Alcohol and Lipid Metabolism. Biomed. Pharmacother. 2021, 134, 111121. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xia, L.; Song, J.; Hu, H.; Zang, N.; Yang, J.; Zou, Y.; Wang, L.; Zheng, X.; He, Q.; et al. Puerarin Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease by Inhibiting Ferroptosis and Inflammation. Lipids Health Dis. 2023, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, Z.; Cao, F.; Wang, L.; Liao, Y.; Liu, X.; Pan, R.; Chang, Q. Brain Pharmacokinetics and the Pharmacological Effects on Striatal Neurotransmitter Levels of Pueraria Lobata Isoflavonoids in Rat. Front. Pharmacol. 2017, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, Z.; Lai, K.; Deng, Y.; Zhao, C.; Lu, Z.; Geng, Q. Puerarin: A Protective Drug against Ischemia-Reperfusion Injury. Front. Pharmacol. 2022, 13, 927611. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, X.; Wang, H.; Nie, K.; Meng, Q.; He, J.; Zheng, C. Puerarin: A Potential Therapeutic for SARS-CoV-2 and Hantavirus Co-Infection. Front. Immunol. 2022, 13, 892350. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Shi, S.; Zhang, B.; Xu, X.; Zheng, H.; Li, Y.; Cui, X.; Wu, H.; Song, Q. Role of Puerarin in Pathological Cardiac Remodeling: A Review. Pharmacol. Res. 2022, 178, 106152. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Cui, X.; Qu, P.; Shang, C.; Xiang, M.; Wang, J. Roles and Mechanisms of Puerarin on Cardiovascular Disease: A Review. Biomed. Pharmacother. 2022, 147, 112655. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Honglei, Y.; Yun, W.; Sheng, D.; Yun, H.; Anhua, Z.; Na, F.; Min, L.; Dandan, S.; Jing, W.; et al. Puerarin Ameliorates Myocardial Remodeling of Spontaneously Hypertensive Rats through Inhibiting TRPC6-CaN-NFATc3 Pathway. Eur. J. Pharmacol. 2022, 933, 175254. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Guo, J.; Gou, W.; Wu, S.; Guo, N.; Zhao, Y.; Hou, W. Molecular Mechanisms of Isoflavone Puerarin against Cardiovascular Diseases: What We Know and Where We Go. Chin. Herb. Med. 2022, 14, 234–243. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Zhao, X.; Lai, S.; He, X.; Fan, Q.; He, H.; He, M. Puerarin Attenuates Lipopolysaccharide-Induced Myocardial Injury via the 14-3-3γ/PKCε Pathway Activating Adaptive Autophagy. Int. Immunopharmacol. 2022, 108, 108905. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Zhang, Z.; He, X.; Fan, Q.; Cheng, X.; Qiao, Y.; Huang, H.; Lai, S.; Wan, Q.; et al. Puerarin Activates Adaptive Autophagy and Protects the Myocardium against Doxorubicin-Induced Cardiotoxicity via the 14–3-3γ/PKCε Pathway. Biomed. Pharmacother. 2022, 153, 113403. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Z.; Li, Z.; Du, X.; Cui, Y.; Wang, J.; Gao, M.; Hou, Y. Puerarin-Tanshinone IIA Suppresses Atherosclerosis Inflammatory Plaque via Targeting Succinate/HIF-1α/IL-1β Axis. J. Ethnopharmacol. 2023, 317, 116675. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Ge, J.; Li, F.; Jiang, Y.; Sun-Waterhouse, D.; Li, D. MiR-34a-5p/Sirt1 Axis: A Novel Pathway for Puerarin-Mediated Hepatoprotection against Benzo(a)Pyrene. Free Radic. Biol. Med. 2022, 186, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Keskin Alkaç, Z.; Ahmet Korkak, F.; Dağoğlu, G.; Akdeniz İncili, C.; Dağoğlu Hark, B.; Tanyıldızı, S. Puerarin Mitigates Oxidative Injuries, Opening of Mitochondrial Permeability Transition Pores and Pathological Damage Associated with Liver and Kidney in Xanthium Strumarium-Intoxicated Rats. Toxicon 2022, 213, 13–22. [Google Scholar] [CrossRef] [PubMed]
- HU, Y.; WANG, S.; WU, L.; YANG, K.; YANG, F.; YANG, J.; HU, S.; YAO, Y.; XIA, X.; LIU, Y.; et al. Puerarin Inhibits Inflammation and Lipid Accumulation in Alcoholic Liver Disease through Regulating MMP8. Chin. J. Nat. Med. 2023, 21, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, H.; Chen, Z. Puerarin Inhibits Ferroptosis and Inflammation of Lung Injury Caused by Sepsis in LPS Induced Lung Epithelial Cells. Front. Pediatr. 2021, 9, 706327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Y.; Wang, L.; Wang, X.; Xu, S.; Zhai, Z.; Wang, C.; Cai, H. Reversal of NADPH Oxidase-Dependent Early Oxidative and Inflammatory Responses in Chronic Obstructive Pulmonary Disease by Puerarin. Oxid. Med. Cell Longev. 2022, 2022, 5595781. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.T.; Liu, H. Puerarin Attenuates LPS-Induced Inflammatory Injury in Gastric Epithelial Cells by Repressing NLRP3 Inflammasome-Mediated Apoptosis. Toxicol. In Vitro 2022, 81, 105350. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Yan, J.; He, J.C.; Li, Y.; Zhong, Y. Additive Renal Protective Effects between Arctigenin and Puerarin in Diabetic Kidney Disease. Biomed. Pharmacother. 2024, 171, 116107. [Google Scholar] [CrossRef]
- Hou, B.; Ma, P.; Yang, X.; Zhao, X.; Zhang, L.; Zhao, Y.; He, P.; Du, G.; Qiang, G. In Silico Prediction and Experimental Validation to Reveal the Protective Mechanism of Puerarin against Excessive Extracellular Matrix Accumulation through Inhibiting Ferroptosis in Diabetic Nephropathy. J. Ethnopharmacol. 2024, 319, 117281. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, B.; Wang, L.; Sun, Y.; Jin, Z.; Liu, X.; Ouyang, L.; Liao, Y. Chitosan@Puerarin Hydrogel for Accelerated Wound Healing in Diabetic Subjects by MiR-29ab1 Mediated Inflammatory Axis Suppression. Bioact. Mater. 2023, 19, 653–665. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Ding, S.; Wang, Y.; Ye, L.; Chen, X.; Ma, H. Exploring the Potential Antidepressant Mechanisms of Puerarin: Anti-Inflammatory Response via the Gut-Brain Axis. J. Affect. Disord. 2022, 310, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sui, X.; Zhang, Y.; Yue, R.; Yin, S. Efficacy of Puerarin in Rats with Focal Cerebral Ischemia through Modulation of the SIRT1/HIF-1α/VEGF Signaling Pathway and Its Effect on Synaptic Plasticity. Heliyon 2023, 9, e15872. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, R.; Wan, J. Puerarin: A Potential Natural Neuroprotective Agent for Neurological Disorders. Biomed. Pharmacother. 2023, 162, 114581. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Zhu, L.; Shi, H.; Ye, S.; Li, Q.; Yin, X.; Xie, Q.; Xu, Q.; Wei, J.X.; Mei, F.; et al. Puerarin Prevents Sepsis-Associated Encephalopathy by Regulating the AKT1 Pathway in Microglia. Phytomedicine 2023, 121, 155119. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Hong, Y.; Zhong, Z.; Zhao, M. Puerarin Protects against Sepsis-Associated Encephalopathy by Inhibiting NLRP3/Caspase-1/GSDMD Pyroptosis Pathway and Reducing Blood-Brain Barrier Damage. Eur. J. Pharmacol. 2023, 945, 175616. [Google Scholar] [CrossRef]
- Liu, T.; Su, K.; Cai, W.; Ao, H.; Li, M. Therapeutic Potential of Puerarin against Cerebral Diseases: From Bench to Bedside. Eur. J. Pharmacol. 2023, 953, 175695. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Lan, Y.; Chen, S.; Xiang, X.; Tan, Y.; Zeng, G.; Guo, Z.; Li, K.; Zhang, J. Puerarin Promotes Apoptosis and Senescence of Bladder Cancer Cells. J. Funct. Foods 2022, 91, 105032. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, L.; Zhao, Y.; Li, Y. Puerarin Action on Stem Cell Proliferation, Differentiation and Apoptosis: Therapeutic Implications for Geriatric Diseases. Phytomedicine 2022, 96, 153915. [Google Scholar] [CrossRef]
- Li, T.; Shi, C.; Mi, Z.; Xu, H.; Xu, J.; Wang, L.; Zhang, X. Biocompatible Puerarin Injectable-Hydrogel Using Self-Assembly Tetrapeptide for Local Treatment of Osteoarthritis in Rats. J. Drug Deliv. Sci. Technol. 2022, 78, 103909. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, L.; Huang, Y.; Shi, K.; Zhang, L.; Huang, C.; Liang, J.; Zeng, Q.; Wang, J.; He, X.; et al. Puerarin Specifically Disrupts Osteoclast Activation via Blocking Integrin-Β3 Pyk2/Src/Cbl Signaling Pathway. J. Orthop. Translat 2022, 33, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Gong, S.; Yao, W.; Gao, H.; Liu, M.; Wei, M. Puerarin Improves OVX-Induced Osteoporosis by Regulating Phospholipid Metabolism and Biosynthesis of Unsaturated Fatty Acids Based on Serum Metabolomics. Phytomedicine 2022, 102, 154198. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Guo, Y.; Han, X.; Xie, X. Effect and Mechanisms of Puerarin on the Treatment of Postmenopausal Osteoporosis: A Preliminary Pre-Clinical Study. Asian J. Surg. 2023, 46, 1332–1333. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Guo, B.; Ma, Y.-q.; Li, K.-w.; Niu, F.-j. Puerarin: A Review of Its Mechanisms of Action and Clinical Studies in Ophthalmology. Phytomedicine 2022, 107, 154465. [Google Scholar]
- Dong, Y.; Ding, Y.Y.; Gao, W.P. Puerarin Alleviates Hyperosmotic Stress-Induced Oxidative Stress, Inflammation, Apoptosis and Barrier Damage of Human Corneal Epithelial Cells by Targeting SIRT1/NLRP3 Signaling. Toxicol. In Vitro 2024, 94, 105722. [Google Scholar] [CrossRef]
- Xu, B.; Li, J.; Chen, X.; Kou, M. Puerarin Attenuates Cisplatin-Induced Apoptosis of Hair Cells through the Mitochondrial Apoptotic Pathway. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119208. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, X.; Zhang, X.; Li, X.; Lin, X.; Huang, Y.; Wu, J. Puerarin Protects against H2O2-Induced Apoptosis of HTR-8/SVneo Cells by Regulating the MiR-20a-5p/VEGFA/Akt Axis. Placenta 2022, 126, 202–208. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Scherer, M.; Fleishman, S.J.; Jones, P.R.; Dandekar, T.; Bencurova, E. Computational Enzyme Engineering Pipelines for Optimized Production of Renewable Chemicals. Front. Bioeng. Biotechnol. 2021, 9, 673005. [Google Scholar] [CrossRef]
- Nam, K.; Shao, Y.; Major, D.T.; Wolf-Watz, M. Perspectives on Computational Enzyme Modeling: From Mechanisms to Design and Drug Development. ACS Omega 2024, 9, 7393–7412. [Google Scholar] [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Moacă, E.A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, W.; Xiong, S.; Li, D.; Fang, S.; Wu, Z.; Wang, Q.; Chen, X. Nanoparticles Mediating the Sustained Puerarin Release Facilitate Improved Brain Delivery to Treat Parkinson’s Disease. ACS Appl. Mater. Interfaces 2019, 11, 45276–45289. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guan, Z.Y.; Zhu, W.F.; Zhong, L.Y.; Qiu, Z.Q.; Yue, P.F.; Wu, W.T.; Liu, J.; Huang, X. Preparation of Puerarin Chitosan Oral Nanoparticles by Ionic Gelation Method and Its Related Kinetics. Pharmaceutics 2020, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chen, K.; Su, C.; Liu, X.; Luo, X. Puerarin Loaded PLGA Nanoparticles: Optimization Processes of Preparation and Anti-Alcohol Intoxication Effects in Mice. AAPS PharmSciTech 2021, 22, 217. [Google Scholar] [CrossRef]
- Qiang, S.; Gu, L.; Kuang, Y.; Zhao, M.; You, Y.; Han, Q. Changes in the Content of Puerarin-PLGA Nanoparticles in Mice under the Influence of Alcohol and Analysis of Their Antialcoholism. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000221148100. [Google Scholar] [CrossRef]
| Extraction Techniques | Advantages | Disadvantages | References |
|---|---|---|---|
| Traditional (e.g., Maceration, Percolation, Decoction, Soxhlet) |
|
| [47,48,53] |
| Microwave-Assisted Extraction |
|
| [46,47,48,53,54,55,56] |
| Ultrasound-Assisted Extraction |
|
| [47,48,53,57,58,59,60] |
| Supercritical Fluid Extraction |
|
| [46,47,48,61,62] |
| Enzyme-Assisted Extraction |
|
| [46,47,48,63] |
| Deep Eutectic Solvents Extraction |
|
| [64,65,66,67] |
| Analyte | Column; Mobile Phase | Flow Rate; Temperature; Detection Wavelength | Combined Technique Parameters | Results | References |
|---|---|---|---|---|---|
| Puerarin (Pueraria lobata stem extract, puerarin cream) | Optimapark C18 column (250 × 4.6 mm, 5 μm); A: 0.5% aqueous acetic acid; B: methanol (77:23, v/v) | 1 mL/min; 30 °C; 250 nm | - |
| [84] |
| Puerarin (Pueraria lobata) | Optimapark C18 column (4.6 mm × 250 mm, 5 μm); A: 0.1% formic acid/aqueous solution; B: acetonitrile | 1 mL/min | PDA–ESI–MS/MS:
|
| [85] |
| Puerarin (Pueraria lobata radix) | ZORBAX SB C18 reversed-phase column (4.6 mm × 250 mm, 5 μm); A: 0.2% phosphoric acid/ water; B: methanol | 1 mL/min; 35 °C; 475 nm | - |
| [86] |
| Puerarin (Pueraria lobata) | Agilent SB-C18 (2.1 mm × 100 mm, 1.8 μm); A: water/0.1% formic acid; B: acetonitrile/0.1% formic acid | 40 °C | ESI–(QTRAP)–MS:
|
| [87] |
| Puerarin (gel eye drops) | Agilent Zorbax SB-C18 column (3.0 × 150 mm, 3.5 μm); A: acetonitrile gradient; B: 0.1% formic acid (15:85, v:v) | 0.6 mL/min; 35 °C; 250 nm | MS:
|
| [88] |
| Puerarin (Pueraria thomsonii radix) | Waters BEH C18 column (2.1 mm × 100 mm, 1.7 μm); A: 0.1% formic acid/water; B: 0.1% formic acid/acetonitrile | 0.3 mL/min; 30 °C | Q-TOF-MS:
|
| [89] |
| Puerarin (Pueraria tuberosa) | C18 (250 mm × 4.6 mm); A: methanol; B: water (25:27 ratio) | 1 mL/min; 25 °C; 250 nm | Q-TOF-MS |
| [90] |
| Type of Disease/Disorder | Biological Effects of Puerarin | References |
|---|---|---|
| Cardiovascular disease |
| [109] |
| [110] | |
| [111] | |
| [112] | |
| [113] | |
| [114] | |
| [115] | |
| Liver disease |
| [116] |
| [117] | |
| [118] | |
| Respiratory disease |
| [119] |
| [120] | |
| Gastric disease |
| [121] |
| Kidney disease |
| [122] |
| [123] | |
| Metabolic disease |
| [124] |
| Neurological disorders |
| [125] |
| [126] | |
| [127] | |
| [128] | |
| [129] | |
| [130] | |
| Urologic disease |
| [131] |
| Geriatric disease |
| [132] |
| [133] | |
| Bone disease |
| [134] |
| [135] | |
| [136] | |
| Ophthalmology disease |
| [137] |
| [138] | |
| Sensorial disorders |
| [139] |
| Pregnancy-specific disorder |
| [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liga, S.; Paul, C. Puerarin—A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects. Int. J. Mol. Sci. 2024, 25, 5222. https://doi.org/10.3390/ijms25105222
Liga S, Paul C. Puerarin—A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects. International Journal of Molecular Sciences. 2024; 25(10):5222. https://doi.org/10.3390/ijms25105222
Chicago/Turabian StyleLiga, Sergio, and Cristina Paul. 2024. "Puerarin—A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects" International Journal of Molecular Sciences 25, no. 10: 5222. https://doi.org/10.3390/ijms25105222
APA StyleLiga, S., & Paul, C. (2024). Puerarin—A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects. International Journal of Molecular Sciences, 25(10), 5222. https://doi.org/10.3390/ijms25105222








