Diagnosis of Fabry Disease Using Alpha-Galactosidase A Activity or LysoGb3 in Blood Fails to Identify Up to Two Thirds of Female Patients
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Patients
3.2. Genetic Analysis
3.3. α-Galactosidase A Activity Assay
3.4. LysoGb3 Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vardarli, I.; Rischpler, C.; Herrmann, K.; Weidemann, F. Diagnosis and screening of patients with Fabry disease. Ther. Clin. Risk Manag. 2020, 16, 551–558. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Gahl, W.A. Lysosomal storage diseases. Transl. Sci. Rare Dis. 2017, 2, 1–71. [Google Scholar] [CrossRef]
- Brady, R.O.; Gal, A.E.; Bradley, R.M.; Martensson, E.; Warshaw, A.L.; Laster, L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967, 276, 1163–1167. [Google Scholar] [CrossRef]
- Bertoldi, G.; Caputo, I.; Driussi, G.; Stefanelli, L.F.; Di Vico, V.; Carraro, G.; Nalesso, F.; Calò, L.A. Biochemical Mechanisms beyond Glycosphingolipid Accumulation in Fabry Disease: Might They Provide Additional Therapeutic Treatments? J. Clin. Med. 2023, 12, 2063. [Google Scholar] [CrossRef]
- Desnick, R.J.; Ioannou, Y.A.; Eng, C.M. Alpha-galactosidase A deficiency: Fabry disease. In Metabolic and Molecular Basis of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3733–3774. [Google Scholar] [CrossRef]
- Rombach, S.M.; Dekker, N.; Bouwman, M.G.; Linthorst, G.E.; Zwinderman, A.H.; Wijburg, F.A.; Kuiper, S.; Vd Bergh Weerman, M.A.; Groener, J.E.; Poorthuis, B.J.; et al. Plasma globotriaosylsphingosine: Diagnostic value and relation to clinical manifestations of Fabry disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 741–748. [Google Scholar] [CrossRef]
- Nowak, A.; Mechtler, T.P.; Hornemann, T.; Gawinecka, J.; Theswet, E.; Hilz, M.J.; Kasper, D.C. Genotype, phenotype and disease severity reflected by serum LysoGb3 levels in patients with Fabry disease. Mol. Genet. Metab. 2018, 123, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Weidemann, F. Biomarkers for diagnosing and staging of Fabry disease. Curr. Med. Chem. 2018, 25, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, R.; Desnick, R.J.; Bishop, D.F. Nucleotide sequence of the human alpha-galactosidase A gene. Nucleic Acids Res. 1989, 17, 3301–3302. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P. Fabry disease. Orphanet. J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef]
- Verrecchia, E.; Zampetti, A.; Antuzzi, D.; Ricci, R.; Ferri, L.; Morrone, A.; Feliciani, C.; Dagna, L.; Manna, R. The impact of fever/hyperthermia in the diagnosis of Fabry: A retrospective analysis. Eur. J. Intern. Med. 2016, 32, 26–30. [Google Scholar] [CrossRef]
- Duro, G.; Zizzo, C.; Cammarata, G.; Burlina, A.; Burlina, A.; Polo, G.; Scalia, S.; Oliveri, R.; Sciarrino, S.; Francofonte, D.; et al. Mutations in the GLA Gene and LysoGb3: Is It Really Anderson-Fabry Disease? Int. J. Mol. Sci. 2018, 19, 3726. [Google Scholar] [CrossRef]
- Arends, M.; Wanner, C.; Hughes, D.; Mehta, A.; Oder, D.; Watkinson, O.T.; Elliott, P.M.; Linthorst, G.E.; Wijburg, F.A.; Biegstraaten, M.; et al. Characterization of Classical and Nonclassical Fabry Disease: A Multicenter Study. J. Am. Soc. Nephrol. 2017, 28, 1631–1641. [Google Scholar] [CrossRef]
- Nakao, S.; Takenaka, T.; Maeda, M.; Kodama, C.; Tanaka, A.; Tahara, M.; Yoshida, A.; Kuriyama, M.; Hayashibe, H.; Sakuraba, H.; et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N. Engl. J. Med. 1995, 333, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Kodama, C.; Takenaka, T.; Tanaka, A.; Yasumoto, Y.; Yoshida, A.; Kanzaki, T.; Enriquez, A.L.D.; Eng, C.M.; Tanaka, H.; et al. Fabry disease: Detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 2003, 64, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, E.; Politano, L. X Chromosome Inactivation in Carriers of Fabry Disease: Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 7663. [Google Scholar] [CrossRef] [PubMed]
- Elstein, D.; Schachamorov, E.; Beeri, R.; Altarescu, G. X-inactivation in Fabry disease. Gene 2012, 505, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M. Gene action in the X-chromosome of the mouse (Mus musculus L). Nature 1961, 190, 372–373. [Google Scholar] [CrossRef]
- Morey, C.; Avner, P. Genetics and epigenetics of the X chromosome. Ann. N. Y. Acad. Sci. 2010, 1214, E18–E33. [Google Scholar] [CrossRef] [PubMed]
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; Jabbour, F.; Beldjord, C.; De Mazancourt, P.; Germain, D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016, 89, 44–54. [Google Scholar] [CrossRef]
- Juchniewicz, P.; Kloska, A.; Tilky-Szymanska, A.; Jakòbkiewicz-Banecka, J.; Wegrzyn, G.; Moskot, M.; Gabig-Ciminska, M.; Piotrowska, E. Female Fabry disease patients and X-chromosome inactivation. Gene 2018, 641, 259–264. [Google Scholar] [CrossRef]
- Dobrovolny, R.; Dvorakova, L.; Ledvinova, J.; Magage, S.; Bultas, J.; Lubanda, J.C.; Elleder, M.; Karetova, D.; Pavlikova, M.; Hrebicek, M. Relationship between X-inactivation and clinical involvement in Fabry heterozygotes. Eleven novel mutations in the alpha-galactosidase A gene in the Czech and Slovak population. J. Mol. Med. 2005, 83, 647–654. [Google Scholar] [CrossRef]
- Deegan, P.B.; Baehner, A.F.; Barba Romero, M.A.; Hughes, D.A.; Kampmann, C.; Beck, M.; European FOS Investigators. Natural History of Fabry disease in females in the Fabry Outcome Survey. J. Med. Genet. 2006, 43, 347–352. [Google Scholar] [CrossRef]
- Wang, R.Y.; Lelis, A.; Mirocha, J.; Wilcox, W.R. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet. Med. 2007, 9, 34–45. [Google Scholar] [CrossRef]
- Chamoles, N.A.; Blanco, M.; Gaggioli, D. Fabry disease: Enzymatic diagnosis in dried blood spots on filter paper. Clin. Chim. Acta 2001, 308, 195–196. [Google Scholar] [CrossRef]
- Polo, G.; Burlina, A.P.; Kolamunnage, T.B.; Zampieri, M.; Dionisi-Vici, C.; Strisciuglio, P.; Zaninotto, M.; Plebani, M.; Burlina, A.B. Diagnosis of sphingolipidoses: A new simultaneous measurement of lysosphingolipids by LC-MS/MS. Clin. Chem. Lab. Med. 2017, 55, 403–414. [Google Scholar] [CrossRef]
- Marchesoni, C.L.; Roa, N.; Pardal, A.M.; Neumann, P.; Cáceres, G.; Martínez, P.; Kisinovsky, I.; Bianchi, S.; Tarabuso, A.L.; Reisin, R.C. Misdiagnosis in Fabry disease. J. Pediatr. 2010, 156, 828–831. [Google Scholar] [CrossRef]
- Linthorst, G.E.; Vedder, A.C.; Aerts, J.M.; Hollak, C.E. Screening for Fabry disease using whole blood spots fails to identify one-third of female carriers. Clin. Chim. Acta. 2005, 353, 201–203. [Google Scholar] [CrossRef]
- Burton, B.K.; Charrow, J.; Hoganson, G.E.; Waggoner, D.; Tinkle, B.; Braddock, S.R.; Schneider, M.; Grange, D.K.; Nash, C.; Shryock, H.; et al. Newborn Screening for Lysosomal Storage Disorders in Illinois: The Initial 15-Month Experience. J. Pediatr. 2017, 190, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Gragnaniello, V.; Burlina, A.P.; Polo, G.; Giuliani, A.; Salviati, L.; Duro, G.; Cazzorla, C.; Rubert, L.; Maines, E.; Germain, D.P.; et al. Newborn Screening for Fabry Disease in Northeastern Italy: Results of Five Years of Experience. Biomolecules 2021, 11, 951. [Google Scholar] [CrossRef]
| Overall | Patients | Male Patients | Female Patients |
|---|---|---|---|
| Patients (%) | 827 | 374 (45.2%) | 453 (54.8%) |
| Average age (min-max) | 41.3 (0–87) | 40.5 (0–75) | 41.9 (0–87) |
| Average α-Gal A activity | 3.8 | 0.8 | 6.1 |
| Average LysoGb3 | 14.2 | 26.2 | 4.5 |
| Classic Phenotype | Patients | Male Patients | Female Patients |
| Patients (%) | 456 | 188 (41.2%) | 268 (58.8%) |
| Average age (min-max) | 40.2 (0–87) | 38.1 (0–63) | 41.7 (0–87) |
| Average α-Gal A activity | 3.7 | 0.2 | 6.0 |
| Average LysoGb3 | 21.9 | 44.4 | 6.3 |
| Late-Onset | Patients | Male Patients | Female Patients |
| Patients (%) | 371 | 186 (50.1%) | 185 (49.9%) |
| Average age (min-max) | 42.6 (0–86) | 42.9 (0–75) | 42.2 (6–78) |
| Average α-Gal A activity | 4.1 | 1.3 | 6.9 |
| Average LysoGb3 | 4.0 | 6.3 | 1.8 |
| Overall | Patients with Mutations | Patients with Mutations and Activity < 3 nmol/mL/h | % of Patients with Mutations and Activity < 3 nmol/mL/h | Patients with Mutations and Activity > 3 nmol/mL/h | % of Patients with Mutations and Activity > 3 nmol/mL/h |
|---|---|---|---|---|---|
| Male Patients | 374 | 374 | 100% | - | - |
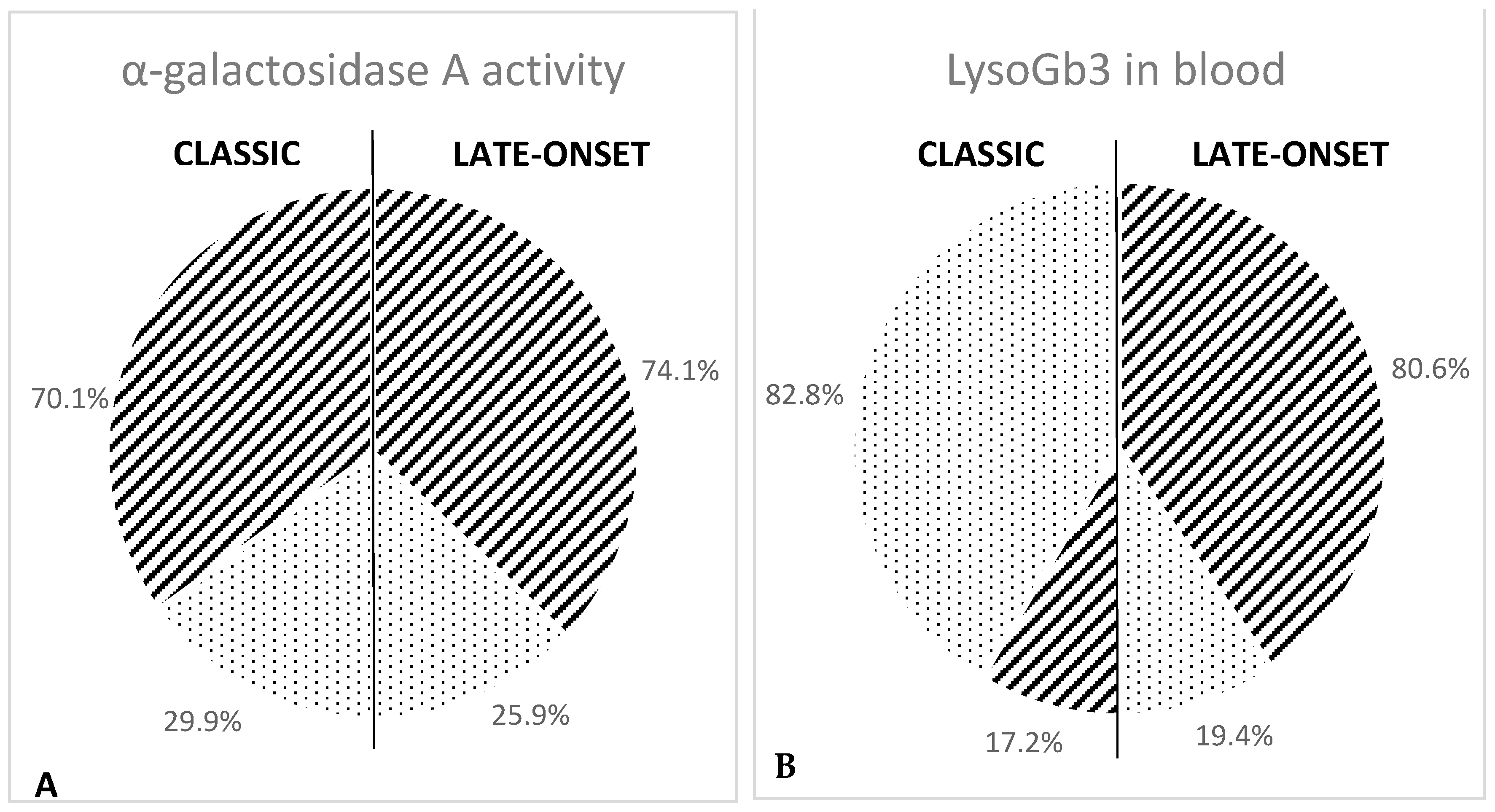
| Female Patients | 453 | 128 | 28.2% | 325 | 71.8% |
| Classic | |||||
| Male Patients | 188 | 188 | 100% | - | - |
| Female Patients | 268 | 80 | 29.9% | 188 | 70.1% |
| Late-Onset | |||||
| Male Patients | 186 | 186 | 100% | - | - |
| Female Patients | 185 | 48 | 25.9% | 137 | 74.1% |
| Overall | Patients with Mutations | Patients with Mutations and LysoGb3 > 2.3 nmol/L | % of Patients with Mutations and LysoGb3 > 2.3 nmol/L | Patients with Mutations and LysoGb3 < 2.3 nmol/L | % of Patients with Mutations and LysoGb3 < 2.3 nmol/L |
|---|---|---|---|---|---|
| Male Patients | 374 | 374 | 100% | - | - |
| Female Patients | 453 | 261 | 57.6% | 192 | 42.4% |
| Classic | |||||
| Male Patients | 188 | 188 | 100% | - | - |
| Female Patients | 268 | 222 | 82.8% | 46 | 17.2% |
| Late-Onset | |||||
| Male Patients | 186 | 186 | 100% | - | - |
| Female Patients | 185 | 36 | 19.4% | 149 | 80.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duro, G.; Anania, M.; Zizzo, C.; Francofonte, D.; Giacalone, I.; D’Errico, A.; Marsana, E.M.; Colomba, P. Diagnosis of Fabry Disease Using Alpha-Galactosidase A Activity or LysoGb3 in Blood Fails to Identify Up to Two Thirds of Female Patients. Int. J. Mol. Sci. 2024, 25, 5158. https://doi.org/10.3390/ijms25105158
Duro G, Anania M, Zizzo C, Francofonte D, Giacalone I, D’Errico A, Marsana EM, Colomba P. Diagnosis of Fabry Disease Using Alpha-Galactosidase A Activity or LysoGb3 in Blood Fails to Identify Up to Two Thirds of Female Patients. International Journal of Molecular Sciences. 2024; 25(10):5158. https://doi.org/10.3390/ijms25105158
Chicago/Turabian StyleDuro, Giovanni, Monia Anania, Carmela Zizzo, Daniele Francofonte, Irene Giacalone, Annalisa D’Errico, Emanuela Maria Marsana, and Paolo Colomba. 2024. "Diagnosis of Fabry Disease Using Alpha-Galactosidase A Activity or LysoGb3 in Blood Fails to Identify Up to Two Thirds of Female Patients" International Journal of Molecular Sciences 25, no. 10: 5158. https://doi.org/10.3390/ijms25105158
APA StyleDuro, G., Anania, M., Zizzo, C., Francofonte, D., Giacalone, I., D’Errico, A., Marsana, E. M., & Colomba, P. (2024). Diagnosis of Fabry Disease Using Alpha-Galactosidase A Activity or LysoGb3 in Blood Fails to Identify Up to Two Thirds of Female Patients. International Journal of Molecular Sciences, 25(10), 5158. https://doi.org/10.3390/ijms25105158






