Vascular Endothelial Growth Factor A VEGFA Inhibition: An Effective Treatment Strategy for Psoriasis
Abstract
1. Introduction
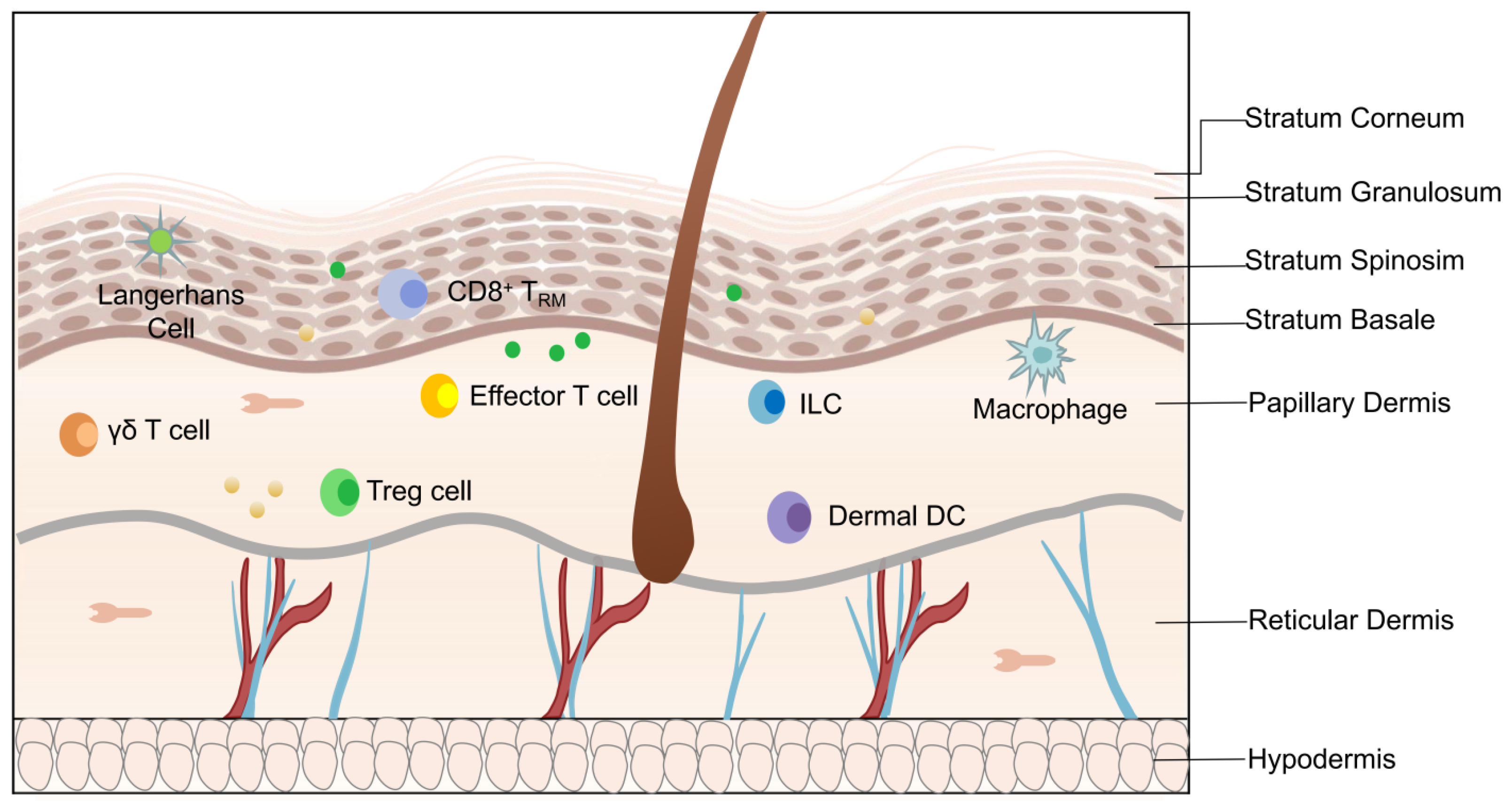
2. Angiogenesis in the Pathogenesis of Psoriasis
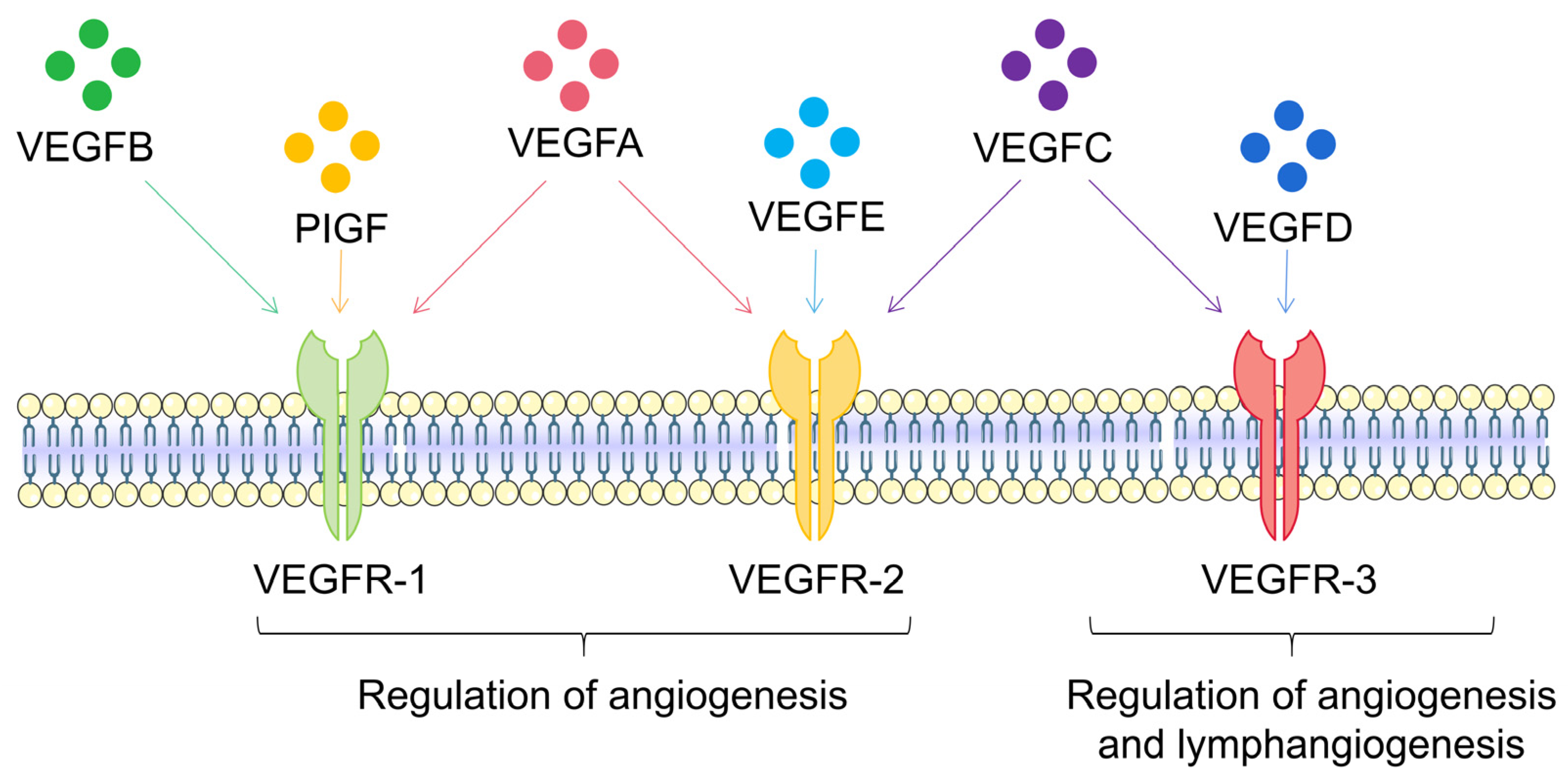
3. VEGFA Is the Most Important Vascular Endothelial Growth Factor
4. VEGFA Regulates the EIME in Psoriasis
4.1. VEGFA Promotes Keratinocyte Proliferation
4.2. VEGFA Promotes Keratin Expression in Psoriatic Epithelium
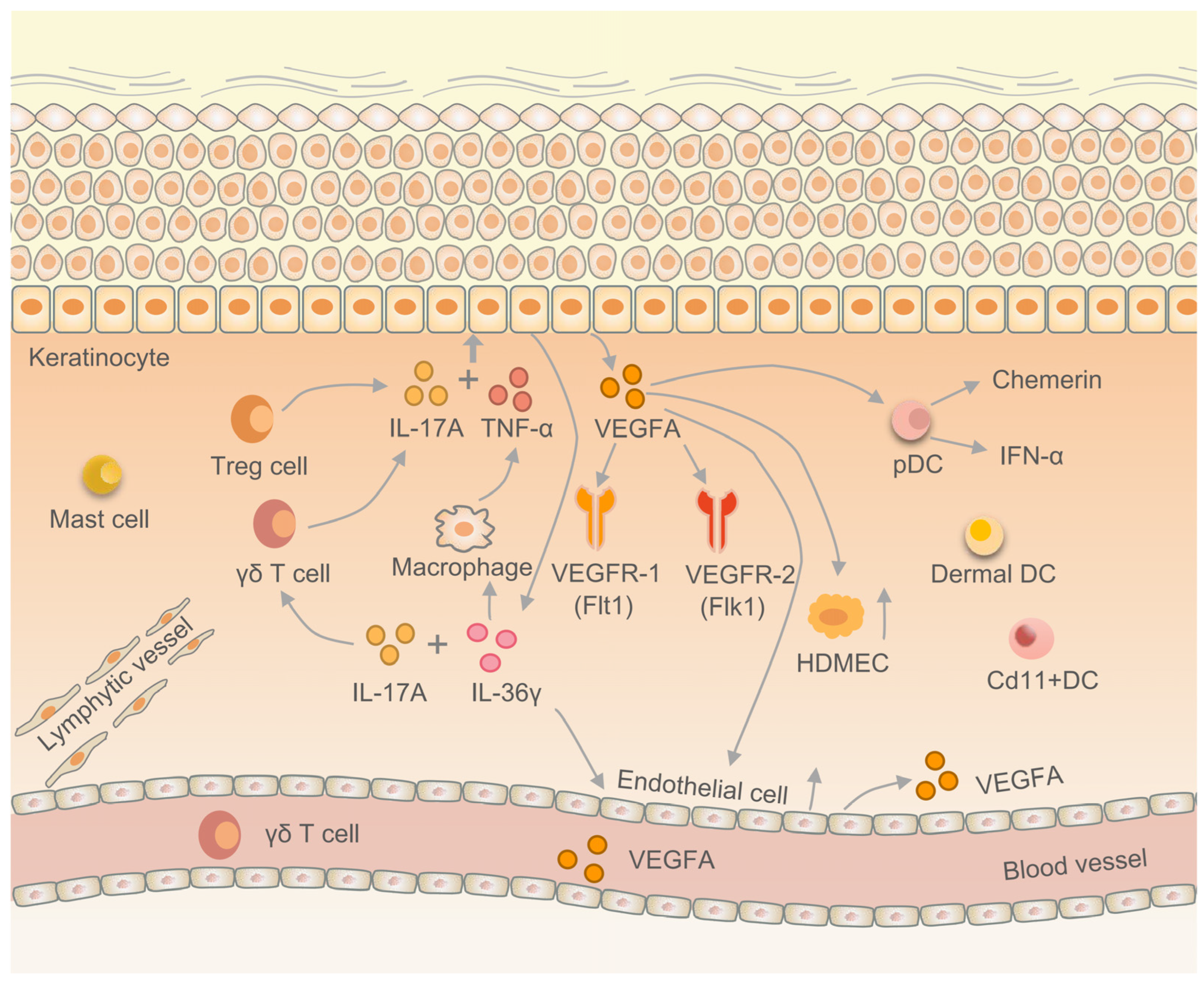
4.3. VEGFA Regulates the Immune Response in EIME
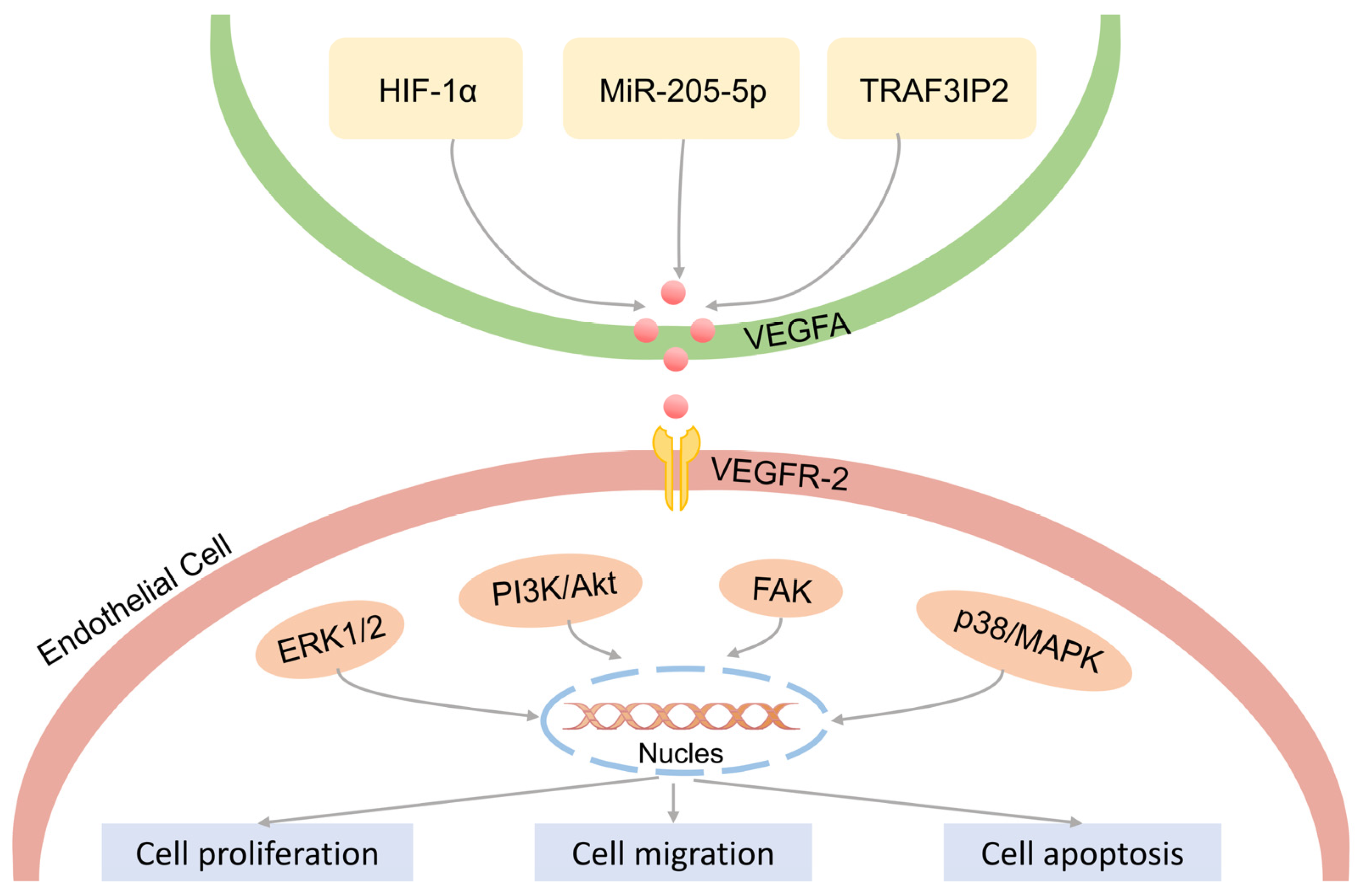
4.4. VEGFA Regulates the Proliferation of Vascular Endothelial Cells
4.5. VEGFA Inhibitors in Psoriasis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perera, G.K.; Di Meglio, P.; Nestle, F.O. Psoriasis. Annu. Rev. Pathol. 2012, 7, 385–422. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, F.; Chen, X.Y.; Yan, B.X.; Wang, Z.Y.; Chen, S.Q.; Zheng, M.; Man, X.Y. The epidermal immune microenvironment plays a dominant role in psoriasis development, as revealed by mass cytometry. Cell Mol. Immunol. 2022, 19, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Hanakawa, S.; Kabashima, K. Classification of inflammatory skin diseases: A proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J. Dermatol. Sci. 2014, 76, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int. J. Mol. Sci. 2021, 22, 12035. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, X.; Pei, H.; Xie, C.; Qiu, N.; Li, S.; Wang, W.; Cheng, X.; Chen, L. Anti-psoriatic effects of Honokiol through the inhibition of NF-κB and VEGFR-2/Flk1 in animal model of K14-VEGF transgenic mouse. J. Pharmacol. Sci. 2015, 128, 116–124. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Sun, X.; Yu, H.; Hu, L.; Wang, D. Biological distinctions between juvenile nasopharyngeal angiofibroma and vascular malformation: An immunohistochemical study. Acta Histochem. 2011, 113, 626–630. [Google Scholar] [CrossRef]
- Výbohová, D.; Adamicová, K.; Mellová, Y.; Hešková, G. Microvascular changes in relation to inflammation and epidermal hyperplasia in chronic cutaneous lesions of psoriasis vulgaris. Histol. Histopathol. 2017, 32, 461–470. [Google Scholar]
- Rajan, P.T.; Suresh, T.N.; Rajashekar, T.S. Expression of Vascular Endothelial Growth Factor and Microvessel Density in Psoriatic Skin Lesions. Indian Dermatol. Online J. 2018, 9, 418–421. [Google Scholar]
- Bellafiore, M.; Battaglia, G.; Bianco, A.; Palma, A. Expression Pattern of Angiogenic Factors in Healthy Heart in Response to Physical Exercise Intensity. Front. Physiol. 2019, 10, 238. [Google Scholar] [CrossRef]
- Reich, K.; Gooderham, M.; Thaçi, D.; Crowley, J.J.; Ryan, C.; Krueger, J.G.; Tsai, T.F.; Flack, M.; Gu, Y.; Williams, D.A.; et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): A randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019, 394, 576–586. [Google Scholar] [CrossRef]
- Andrade, M.M.D.L.; Tejada, G.L.; Peruzzo, J.; Bonamigo, R.R. Pustular psoriasis triggered by therapy with atezolizumab and bevacizumab. An. Bras. Dermatol. 2023, 30. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Deng, J.; Xu, K.; Zhu, T.; Han, L.; Yan, Y.; Yan, D.; Deng, H.; Wang, D.; Sun, Y.; et al. In-depth serum proteomics reveals biomarkers of psoriasis severity and response to traditional Chinese medicine. Theranostics 2019, 9, 2475–2488. [Google Scholar] [CrossRef] [PubMed]
- De Luisi, A.; Mangialardi, G.; Ria, R.; Acuto, G.; Ribatti, D.; Vacca, A. Anti-angiogenic activity of carebastine: A plausible mechanism affecting airway remodelling. Eur. Respir. J. 2009, 34, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gerkowicz, A.; Socha, M.; Pietrzak, A.; Zubilewicz, T.; Krasowska, D. The role of VEGF in psoriasis: An update. Acta Angiol. 2018, 24, 134–140. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Dudley, A.C. Angiogenesis: A year in review. Angiogenesis 2021, 24, 195–196. [Google Scholar] [CrossRef]
- Kaliyadan, F. The Dermoscopic Auspitz Sign. Indian Dermatol. Online J. 2018, 9, 290–291. [Google Scholar] [CrossRef]
- Varricchi, G.; Granata, F.; Loffredo, S.; Genovese, A.; Marone, G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J. Am. Acad. Dermatol. 2015, 73, 144–153. [Google Scholar] [CrossRef]
- Luengas-Martinez, A.; Paus, R.; Young, H.S. Antivascular endothelial growth factor-A therapy: A novel personalized treatment approach for psoriasis. Br. J. Dermatol. 2022, 186, 782–791. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in psoriasis: Therapeutic implications. J. Investig. Dermatol. 1972, 59, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Perruzzi, C.A.; Feder, J.; Dvorak, H.F. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986, 46, 5629–5632. [Google Scholar] [PubMed]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989, 161, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.T.; Heuvelman, D.M.; Nelson, R.; Olander, J.V.; Eppley, B.L.; Delfino, J.J.; Siegel, N.R.; Leimgruber, R.M.; Feder, J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Investig. 1989, 84, 1470–1478. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Haneda, Y.; Hasegawa, S.; Hirano, R.; Hashimoto, K.; Ohsaki, A.; Ichiyama, T. Leukotriene D4 enhances tumor necrosis factor-α-induced vascular endothelial growth factor production in human monocytes/macrophages. Cytokine 2011, 55, 24–28. [Google Scholar] [CrossRef] [PubMed]
- White, A.L.; Bix, G.J. VEGFA Isoforms as Pro-Angiogenic Therapeutics for Cerebrovascular Diseases. Biomolecules 2023, 13, 702. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Z.; Mo, X.; Song, Y.; Li, X.; Li, X.; Zhang, M. The circular RNA 001971/miR-29c-3p axis modulates colorectal cancer growth, metastasis, and angiogenesis through VEGFA. J. Exp. Clin. Cancer. Res. 2020, 39, 91. [Google Scholar] [CrossRef]
- Tanabe, K.; Wada, J.; Sato, Y. Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat. Rev. Nephrol. 2020, 16, 289–303. [Google Scholar] [CrossRef]
- Cho, E.J.; Yang, J.; Mohamedali, K.A.; Lim, E.K.; Kim, E.J.; Farhangfar, C.J.; Suh, J.S.; Haam, S.; Rosenblum, M.G.; Huh, Y.M. Sensitive angiogenesis imaging of orthotopic bladder tumors in mice using a selective magnetic resonance imaging contrast agent containing VEGF121/rGel. Investig. Radiol. 2011, 46, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Li, X.; Harper, S.J.; Bates, D.O.; Claesson-Welsh, L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008, 68, 4683–4692. [Google Scholar] [CrossRef]
- Kinney, C.M.; Chandrasekharan, U.M.; Mavrakis, L.; DiCorleto, P.E. VEGF and thrombin induce MKP-1 through distinct signaling pathways: Role for MKP-1 in endothelial cell migration. Am. J. Physiol. Cell Physiol. 2008, 294, C241–C250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. 64Cu-1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid-quantum dot-vascular endothelial growth factor. In Molecular Imaging and Contrast Agent Database (MICAD); Created 1 July 2008; Updated 12 August 2008; 2004–2013; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2008. [Google Scholar]
- Cheng, S.; Zhang, X.; Feng, Q.; Chen, J.; Shen, L.; Yu, P.; Yang, L.; Chen, D.; Zhang, H.; Sun, W.; et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 2019, 227, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef]
- Lechertier, T.; Reynolds, L.E.; Kim, H.; Pedrosa, A.R.; Gómez-Escudero, J.; Muñoz-Félix, J.M.; Batista, S.; Dukinfield, M.; Demircioglu, F.; Wong, P.P.; et al. Pericyte FAK negatively regulates Gas6/Axl signalling to suppress tumour angiogenesis and tumour growth. Nat. Commun. 2020, 11, 2810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, J.; Lin, D.; Xu, R.; Chen, Y.; Hu, X. Exosomes derived from dental pulp stem cells accelerate cutaneous wound healing by enhancing angiogenesis via the Cdc42/p38 MAPK pathway. Int. J. Mol. Med. 2022, 50, 143. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Ai, X.; Kilic, E.; Hermann, D.M.; Venkataramani, V.; Bähr, M.; Doeppner, T.R. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway. Cell Death Dis. 2021, 12, 1068. [Google Scholar] [CrossRef]
- Pang, J.; Hoefen, R.; Pryhuber, G.S.; Wang, J.; Yin, G.; White, R.J.; Xu, X.; O’Dell, M.R.; Mohan, A.; Michaloski, H.; et al. G-protein-coupled receptor kinase interacting protein-1 is required for pulmonary vascular development. Circulation 2009, 119, 1524–1532. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, W.; Zhang, B.; Lv, M.; Wang, J.; Zhang, L. Single nucleotide polymorphisms of VEGF gene and Psoriasis risk. J. Dermatol. Sci. 2008, 49, 263–265. [Google Scholar] [CrossRef]
- Young, H.S.; Kamaly-Asl, I.D.; Laws, P.M.; Pemberton, P.; Griffiths, C.E.M. Genetic interaction between placental growth factor and vascular endothelial growth factor A in psoriasis. Clin. Exp. Dermatol. 2020, 45, 302–308. [Google Scholar] [CrossRef]
- Benhadou, F.; Glitzner, E.; Brisebarre, A.; Swedlund, B.; Song, Y.; Dubois, C.; Rozzi, M.; Paulissen, C.; Del Marmol, V.; Sibilia, M.; et al. Epidermal autonomous VEGFA/Flt1/Nrp1 functions mediate psoriasis-like disease. Sci. Adv. 2020, 6, eaax5849. [Google Scholar] [CrossRef]
- Yan, B.X.; Zheng, Y.X.; Li, W.; Chen, J.Q.; Zhou, J.; Cai, S.Q.; Zheng, M.; Man, X.Y. Comparative expression of PEDF and VEGF in human epidermal keratinocytes and dermal fibroblasts: From normal skin to psoriasis. Discov. Med. 2018, 25, 47–56. [Google Scholar]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Bhushan, M.; McLaughlin, B.; Weiss, J.B.; Griffiths, C.E. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br. J. Dermatol. 1999, 141, 1054–1060. [Google Scholar] [CrossRef]
- Canavese, M.; Altruda, F.; Ruzicka, T.; Schauber, J. Vascular endothelial growth factor (VEGF) in the pathogenesis of psoriasis—A possible target for novel therapies? J. Dermatol. Sci. 2010, 8, 171–176. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Z.; Zhang, J.; Ma, T.; Wu, W.; Wei, X.; Wang, Z.; Zhen, H.; Zhou, H.; Huang, N.; et al. IL-30 ameliorates imiquimod and K14-VEGF induced psoriasis-like disease by inhibiting both innate and adaptive immunity disorders. Biochem. Biophys. Res. Commun. 2021, 579, 97–104. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.Y. Nerve growth factor regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes via PI3K/mTOR pathway. Genet. Mol. Res. 2014, 13, 9324–9335. [Google Scholar] [CrossRef]
- Wu, H.H.; Xie, W.L.; Zhao, Y.K.; Liu, J.H.; Luo, D.Q. Imiquimod Increases Cutaneous VEGF Expression in Imiquimod-induced Psoriatic Mouse Model. Curr. Vasc. Pharmacol. 2016, 14, 275–279. [Google Scholar] [CrossRef]
- Barile, S.; Medda, E.; Nisticò, L.; Bordignon, V.; Cordiali-Fei, P.; Carducci, M.; Rainaldi, A.; Marinelli, R.; Bonifati, C. Vascular endothelial growth factor gene polymorphisms increase the risk to develop psoriasis. Exp. Dermatol. 2006, 15, 368–376. [Google Scholar] [CrossRef]
- Qi, M.; Huang, X.; Zhou, L.; Zhang, J. Four polymorphisms of VEGF (+405C>G, -460T>C, -2578C>A, and -1154G>A) in susceptibility to psoriasis: A meta-analysis. DNA Cell Biol. 2014, 33, 234–244. [Google Scholar] [CrossRef]
- Socha, M.; Kicinski, P.; Feldo, M.; Zubilewicz, T.; Pietrzak, A. Assessment of selected angiogenesis markers in the serum of middle-aged male patients with plaque psoriasis. Dermatol. Ther. 2021, 34, e14727. [Google Scholar] [CrossRef]
- Luengas-Martinez, A.; Kamaly-Asl, A.; Chaudhry, I.H.; Brenchley, P.E.C.; Young, H.S. Cutaneous vascular structure and perfusion in patients with chronic plaque psoriasis. Clin. Exp. Dermatol. 2023, 48, 181–187. [Google Scholar] [CrossRef]
- Datta-Mitra, A.; Riar, N.K.; Raychaudhuri, S.P. Remission of psoriasis and psoriatic arthritis during bevacizumab therapy for renal cell cancer. Indian J. Dermatol. 2014, 59, 632. [Google Scholar]
- Wu, R.; Luo, X.; Zhang, P.; Li, S.; Zhao, M.; Su, Z.; Deng, M.; Zhu, Y.; Tang, G.; Kuang, Q. Application of Auranofin in the Preparation of Drugs for the Prevention, Treatment and/or Alleviation of Psoriasis. CN202111564985, 1 February 2022. [Google Scholar]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786. [Google Scholar] [CrossRef]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Hoegler, K.M.; John, A.M.; Handler, M.Z.; Schwartz, R.A. Generalized pustular psoriasis: A review and update on treatment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1645–1651. [Google Scholar] [CrossRef]
- Ferrari, D.; Casciano, F.; Secchiero, P.; Reali, E. Purinergic Signaling and Inflammasome Activation in Psoriasis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9449. [Google Scholar] [CrossRef]
- de Rie, M.A.; Goedkoop, A.Y.; Bos, J.D. Overview of psoriasis. Dermatol. Ther. 2004, 17, 341–349. [Google Scholar]
- Yang, L.; Zhang, S.; Wang, G. Keratin 17 in disease pathogenesis: From cancer to dermatoses. J. Pathol. 2019, 247, 158–165. [Google Scholar] [CrossRef]
- Wallace, L.; Roberts-Thompson, L.; Reichelt, J. Deletion of K1/K10 does not impair epidermal stratification but affects desmosomal structure and nuclear integrity. J. Cell Sci. 2012, 125, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Szeverenyi, I.; Cassidy, A.J.; Chung, C.W.; Lee, B.T.; Common, J.E.; Ogg, S.C.; Chen, H.; Sim, S.Y.; Goh, W.L.; Ng, K.W.; et al. The Human Intermediate Filament Database: Comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 2008, 29, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.C.; Piña-Paz, S.; Rotty, J.D.; Hickerson, R.P.; Kaspar, R.L.; Balmain, A.; Coulombe, P.A. Keratin 16 regulates innate immunity in response to epidermal barrier breach. Proc. Natl. Acad. Sci. USA 2013, 110, 19537–19542. [Google Scholar] [CrossRef]
- Kerns, M.L.; Hakim, J.M.; Lu, R.G.; Guo, Y.; Berroth, A.; Kaspar, R.L.; Coulombe, P.A. Oxidative stress and dysfunctional NRF2 underlie pachyonychia congenita phenotypes. J. Clin. Investig. 2016, 126, 2356–2366. [Google Scholar] [CrossRef]
- Paramio, J.M.; Casanova, M.L.; Segrelles, C.; Mittnacht, S.; Lane, E.B.; Jorcano, J.L. Modulation of cell proliferation by cytokeratins K10 and K16. Mol. Cell Biol. 1999, 19, 3086–3094. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, L.; Liu, Y.F.; Gao, T.W.; Wang, G.; Fan, X.L.; Fan, J.Y.; Fan, P.S.; Li, C.Y.; Liu, B.; et al. Altered keratin 17 peptide ligands inhibit in vitro proliferation of keratinocytes and T cells isolated from patients with psoriasis. J. Am. Acad. Dermatol. 2006, 54, 992–1002. [Google Scholar] [CrossRef]
- Jiang, M.; Li, B.; Zhang, J.; Hu, L.; Dang, E.; Wang, G. Vascular endothelial growth factor driving aberrant keratin expression pattern contributes to the pathogenesis of psoriasis. Exp. Cell Res. 2017, 360, 310–319. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirakawa, S.; Shimauchi, T.; Ito, T.; Sakabe, J.; Detmar, M.; Tokura, Y. VEGFA promotes IL-17A-producing γδ T cell accumulation in mouse skin and serves as a chemotactic factor for plasmacytoid dendritic cells. J. Dermatol. Sci. 2014, 74, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Matsuo, H.; Morita, E. Vascular endothelial growth factor 121 is the predominant isoform in psoriatic scales. Exp. Dermatol. 2005, 14, 758–764. [Google Scholar] [CrossRef]
- Henno, A.; Blacher, S.; Lambert, C.; Colige, A.; Seidel, L.; Noël, A.; Lapière, C.; de la Brassinne, M.; Nusgens, B.V. Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque-type psoriasis. Br. J. Dermatol. 2009, 160, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, H.; Chen, L.; Xu, J.; Wang, M.; Li, D.; Lu, P. Effects of interleukin-17 on human retinal vascular endothelial cell capillary tube formation in vitro. Mol. Med. Rep. 2017, 16, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Tsilioni, I.; Weng, Z.; Theoharides, T.C. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp. Dermatol. 2018, 27, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, L.; Failla, C.M.; Capriotti, L.; Scarponi, C.; Facchiano, F.; Morelli, M.; Rossi, S.; Pagnanelli, G.; Albanesi, C.; Cavani, A.; et al. Interleukin (IL)-17/IL-36 axis participates to the crosstalk between endothelial cells and keratinocytes during inflammatory skin responses. PLoS ONE 2020, 15, e0222969. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, D.; Ronca, R.; Salvi, V.; Presta, M.; Sozzani, S. Dendritic cells in inflammatory angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Bluth, M.J.; Zaba, L.C.; Moussai, D.; Suárez-Fariñas, M.; Kaporis, H.; Fan, L.; Pierson, K.C.; White, T.R.; Pitts-Kiefer, A.; Fuentes-Duculan, J.; et al. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J. Investig. Dermatol. 2009, 129, 2451–2462. [Google Scholar] [CrossRef]
- Ding, X.C.; Wang, L.L.; Zhang, X.D.; Xu, J.L.; Li, P.F.; Liang, H.; Zhang, X.B.; Xie, L.; Zhou, Z.H.; Yang, J.; et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J. Hematol. Oncol. 2021, 14, 92. [Google Scholar] [CrossRef]
- Luo, D.; Wang, Z.; Wu, J.; Jiang, C.; Wu, J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed. Res. Int. 2014, 2014, 409272. [Google Scholar] [CrossRef]
- Song, Y.; Chen, L.; Li, Y.; Lin, Q.; Liu, W.; Zhang, L. Knockdown of TRAF3IP2 suppresses the expression of VEGFA and the proliferation of keratinocytes and vascular endothelial cells. Heliyon 2019, 5, e01642. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Y.; Bian, X.; Zhang, Y.; Li, Y.; Zhang, Q.; Yin, M. miR-205-5p inhibits psoriasis-associated proliferation and angiogenesis: Wnt/β-catenin and mitogen-activated protein kinase signaling pathway are involved. J. Dermatol. 2020, 47, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Gille, H.; Kowalski, J.; Li, B.; LeCouter, J.; Moffat, B.; Zioncheck, T.F.; Pelletier, N.; Ferrara, N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J. Biol. Chem. 2001, 276, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, N.S.; Reynolds, A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Bovone, C.; Spena, R.; Senni, C.; Scorcia, V.; Busin, M. Anti-VEGF Treatment in Corneal Diseases. Curr. Drug Targets 2020, 21, 1159–1180. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.A.; Yan, M.N.; Hanna, N. The Story of Angiogenesis Inhibitors in Non-small-cell Lung Cancer: The Past, Present, and Future. Clin. Lung Cancer 2020, 21, 308–313. [Google Scholar] [CrossRef]
- Shaker, O.G.; Khairallah, M.; Rasheed, H.M.; Abdel-Halim, M.R.; Abuzeid, O.M.; El Tawdi, A.M.; El Hadidi, H.H.; Ashmaui, A. Antiangiogenic effect of methotrexate and PUVA on psoriasis. Cell Biochem. Biophys. 2013, 67, 735–742. [Google Scholar] [CrossRef]
- Chen, H.Q.; Li, X.; Tang, R. Effects of Narrow Band Ultraviolet B on Serum Levels of Vascular Endothelial Growth Factor and Interleukin-8 in Patients with Psoriasis. Am. J. Ther. 2016, 23, e655–e662. [Google Scholar] [CrossRef]
- Chen, W.; Wu, L.; Zhu, W.; Chen, X. The polymorphisms of growth factor genes (VEGFA & EGF) were associated with response to acitretin in psoriasis. Pers. Med. 2018, 15, 181–188. [Google Scholar]
- Akman, A.; Yilmaz, E.; Mutlu, H.; Ozdogan, M. Complete remission of psoriasis following bevacizumab therapy for colon cancer. Clin. Exp. Dermatol. 2009, 34, e202–e204. [Google Scholar] [CrossRef]
- Hanssen, S.C.A.; van der Vleuten, C.J.M.; van Erp, P.E.J.; Seyger, M.M.B.; van de Kerkhof, P.C.M. The effect of adalimumab on the vasculature in psoriatic skin lesions. J. Dermatol. Treat. 2019, 30, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shucheng, H.; Fu, L.; Pei, B.; Xu, W.; Jiang, X. Overexpression and potential roles of midkine via regulation of vascular endothelial growth factor A in psoriasis. Exp. Dermatol. 2023, 23, 1383–1393. [Google Scholar] [CrossRef]
- Tang, J.; Liu, C.; Liu, S.; Zhou, X.; Lu, J.; Li, M.; Zhu, L. Inhibition of JAK1/STAT3 pathway by 2-methoxyestradiol ameliorates psoriatic features in vitro and in an imiquimod-induced psoriasis-like mouse model. Eur. J. Pharmacol. 2022, 933, 175276. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Wu, H.H.; Zhao, Y.K.; Wang, F.; Gao, Q.; Luo, D.Q. Thalidomide Improves Psoriasis-like Lesions and Inhibits Cutaneous VEGF Expression without Alteration of Microvessel Density in Imiquimod-induced Psoriatic Mouse Model. Curr. Vasc. Pharmacol. 2018, 16, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.; Lin, Z.C.; Chen, Y.L.; Tzeng, C.C.; Fang, J.Y.; Tseng, C.H. Synthesis and Biological Evaluation of Thalidomide Derivatives as Potential Anti-Psoriasis Agents. Int. J. Mol. Sci. 2018, 19, 3061. [Google Scholar] [CrossRef]
- Amagai, R.; Takahashi, T.; Terui, H.; Fujimura, T.; Yamasaki, K.; Aiba, S.; Asano, Y. The Antimicrobial Peptide Cathelicidin Exerts Immunomodulatory Effects via Scavenger Receptors. Int. J. Mol. Sci. 2023, 24, 875. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Chasuna; Bagenna. Astilbin reduces ROS accumulation and VEGF expression through Nrf2 in psoriasis-like skin disease. Biol. Res. 2019, 52, 49. [Google Scholar] [CrossRef]
- Capriotti, L.; Didona, B.; Madonna, S.; Scarponi, C.; Pilla, M.A.; Facchiano, F.; Cordella, M.; Cavani, A.; Failla, C.M. Eosin treatment for psoriasis reduces skin leukocyte infiltration and secretion of inflammatory chemokines and angiogenic factors. Eur. J. Dermatol. 2018, 28, 457–466. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Y.; Yi, X.; Ding, Y. Ginsenoside Rh2 Suppresses Neovascularization in Xenograft Psoriasis Model. Cell Physiol. Biochem. 2015, 36, 980–987. [Google Scholar] [CrossRef]
- Wen, J.; Pei, H.; Wang, X.; Xie, C.; Li, S.; Huang, L.; Qiu, N.; Wang, W.; Cheng, X.; Chen, L. Gambogic acid exhibits anti-psoriatic efficacy through inhibition of angiogenesis and inflammation. J. Dermatol. Sci. 2014, 74, 242–250. [Google Scholar] [CrossRef]
- Lin, P.T.; Wang, S.H.; Chi, C.C. Drug survival of biologics in treating psoriasis: A meta-analysis of real-world evidence. Sci. Rep. 2018, 8, 16068. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.M.; Ahn, H.Y. Biological products for the treatment of psoriasis: Therapeutic targets, pharmacodynamics and disease-drug-drug interaction implications. AAPS J. 2014, 16, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lebwohl, M.G. Biologics and Psoriasis: The Beat Goes On. Dermatol. Clin. 2019, 37, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Li, M.; Kroetz, D.L. Bevacizumab-induced hypertension: Clinical presentation and molecular understanding. Pharmacol. Ther. 2018, 182, 152–160. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
| Research Stage | Discovery | Ref. |
|---|---|---|
| Preclinical research | Transgenic mouse models overexpressing VEGFA in keratinocytes lead to the main hallmarks of human psoriasis. | [48] |
| VEGFA expression is enhanced in IL-17A-induced keratinocytes. | [49] | |
| In an imiquimod-induced psoriasis mouse model, the expression of VEGFA in skin lesions and serum is increased. | [50] | |
| SNPs of the VEGF gene, such as C at −460 to T in the promoter region and C at +405 to G in the 5′-untranslated region, confer a genetic predisposition associated with psoriasis risk. | [51,52] | |
| Clinical research | The blood vessels in the papillary dermis of patients with psoriasis elongate, extend, and are tortuous. The expression of VEGFA and other pro-angiogenic growth factors in the skin lesions is increased. | [53,54] |
| Anti-angiogenic therapy, e.g., bevacizumab and auranofin, may improve psoriatic lesions. | [55,56] |
| Type of Treatment | Drug | Findings | Ref. |
|---|---|---|---|
| Phototherapy | PUVA | Suppresses keratinocytes proliferation; Induces keratinocytes apoptosis; Regulates cytokine production. | [90] |
| NB-UVB | Decrease the serum levels of VEGF and IL-8 in patients with psoriasis. | [91] | |
| Standard Systemic Therapy | Retinoids | Normalization of keratinocyte proliferation/differentiation; Anti-proliferative effects. | [92] |
| Methotrexate (MTX) | Reduces keratinocytes and lymphocyte proliferation; Induces lymphocyte apoptosis; Inhibits T-cell inflammatory action. | [90] | |
| Biologic Therapy | Bevacizumab | Binds to VEGFA and its isoforms and inhibits interaction of VEGF with its receptors; Inhibition of new vessel formation and regression of existing vasculature. | [51,93] |
| Adalimumab | Inhibition of endothelial cell proliferation; Narrowing of a vascular network; Reduction of vascular diameter. | [94] | |
| Midkine Monoclonal Antibody | Regulating VEGFA expression through the Notch Receptor 2 (Notch 2)/Hes Family bHLH Transcription Factor 1 (HES1)/Janus Kinase 2 (JAK2)-STAT5A pathway; Inhibition of keratinocyte proliferation; Reduces blood vessel density and inhibits angiogenesis. | [95] | |
| Molecular Compounds | 2-methoxyestradiol (2-ME) | 2-ME induces apoptosis in keratinocytes; 2-ME blocked the G2/M phase and inhibited the proliferation of keratinocytes; 2-ME inhibits VEGFA induced by IL-17A; 2-ME alleviates psoriasis by inhibiting the JAK1/STAT3 pathway in vitro and in vivo. | [96] |
| Thalidomide | Inhibition of keratinocyte activity; Decreased secretion of VEGF and TNF-a. | [97,98] | |
| Auranofin | Reduce mRNA expression levels of the VEGFA gene; Increased mRNA expression of IL-4 and IL-10 that was relevant to Th2/Treg cells; Reduce the mRNA expression levels of IFN-γ and IL-17A that were related to Th1/Th17 cells. | [56] | |
| Calcipotriol | LL-37 is implicated in the pathogenesis of psoriasis, induced angiogenesis; Inhibitors of scavenger receptors decrease the expression of VEGFA induced by LL-37 in keratinocytes. | [99] | |
| Astilbin | Astilbin could induce Nrf2 nucleus translocation; Reduce the ROS accumulation and VEGFA expression; Inhibit the proliferation of keratinocytes. | [100] | |
| Eosin | Dampens the release of pro-inflammatory chemokines (CCL2 and CCL5) and VEGFA. | [101] | |
| Ginsenoside Rh2 | Inhibited VEGFA levels in the PN skin grafts. | [102] | |
| Gambogic acid | Inhibited proliferation of keratinocytes; Suppressed hyperplastic and inflamed vessels of K14-VEGF transgenic mice. | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tai, Z.; Zhu, C.; Yu, Q.; Zhu, Q.; Chen, Z. Vascular Endothelial Growth Factor A VEGFA Inhibition: An Effective Treatment Strategy for Psoriasis. Int. J. Mol. Sci. 2024, 25, 59. https://doi.org/10.3390/ijms25010059
Chen Y, Tai Z, Zhu C, Yu Q, Zhu Q, Chen Z. Vascular Endothelial Growth Factor A VEGFA Inhibition: An Effective Treatment Strategy for Psoriasis. International Journal of Molecular Sciences. 2024; 25(1):59. https://doi.org/10.3390/ijms25010059
Chicago/Turabian StyleChen, Ya, Zongguang Tai, Congcong Zhu, Qin Yu, Quangang Zhu, and Zhongjian Chen. 2024. "Vascular Endothelial Growth Factor A VEGFA Inhibition: An Effective Treatment Strategy for Psoriasis" International Journal of Molecular Sciences 25, no. 1: 59. https://doi.org/10.3390/ijms25010059
APA StyleChen, Y., Tai, Z., Zhu, C., Yu, Q., Zhu, Q., & Chen, Z. (2024). Vascular Endothelial Growth Factor A VEGFA Inhibition: An Effective Treatment Strategy for Psoriasis. International Journal of Molecular Sciences, 25(1), 59. https://doi.org/10.3390/ijms25010059






