Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression
Abstract
1. Introduction
2. Results
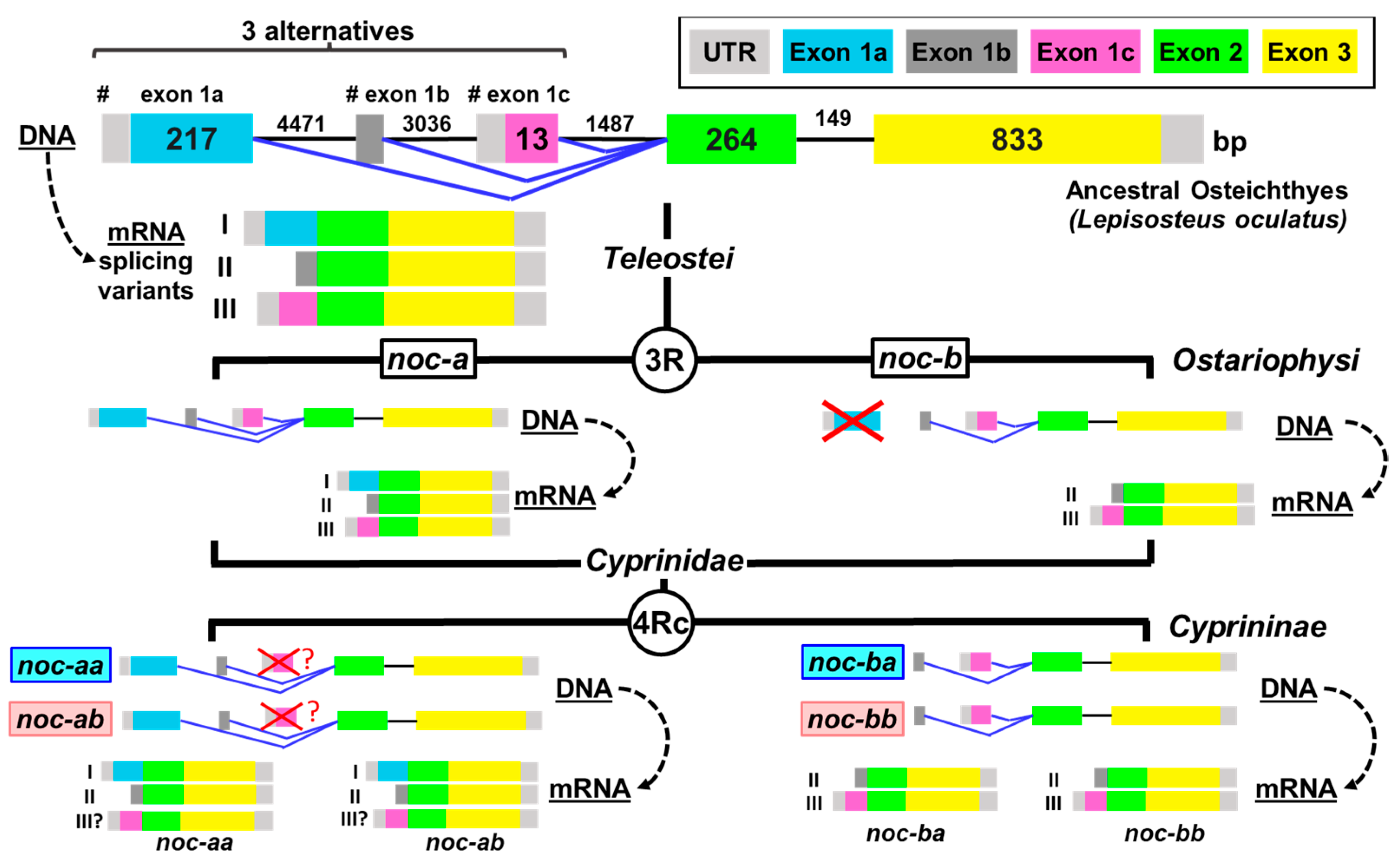
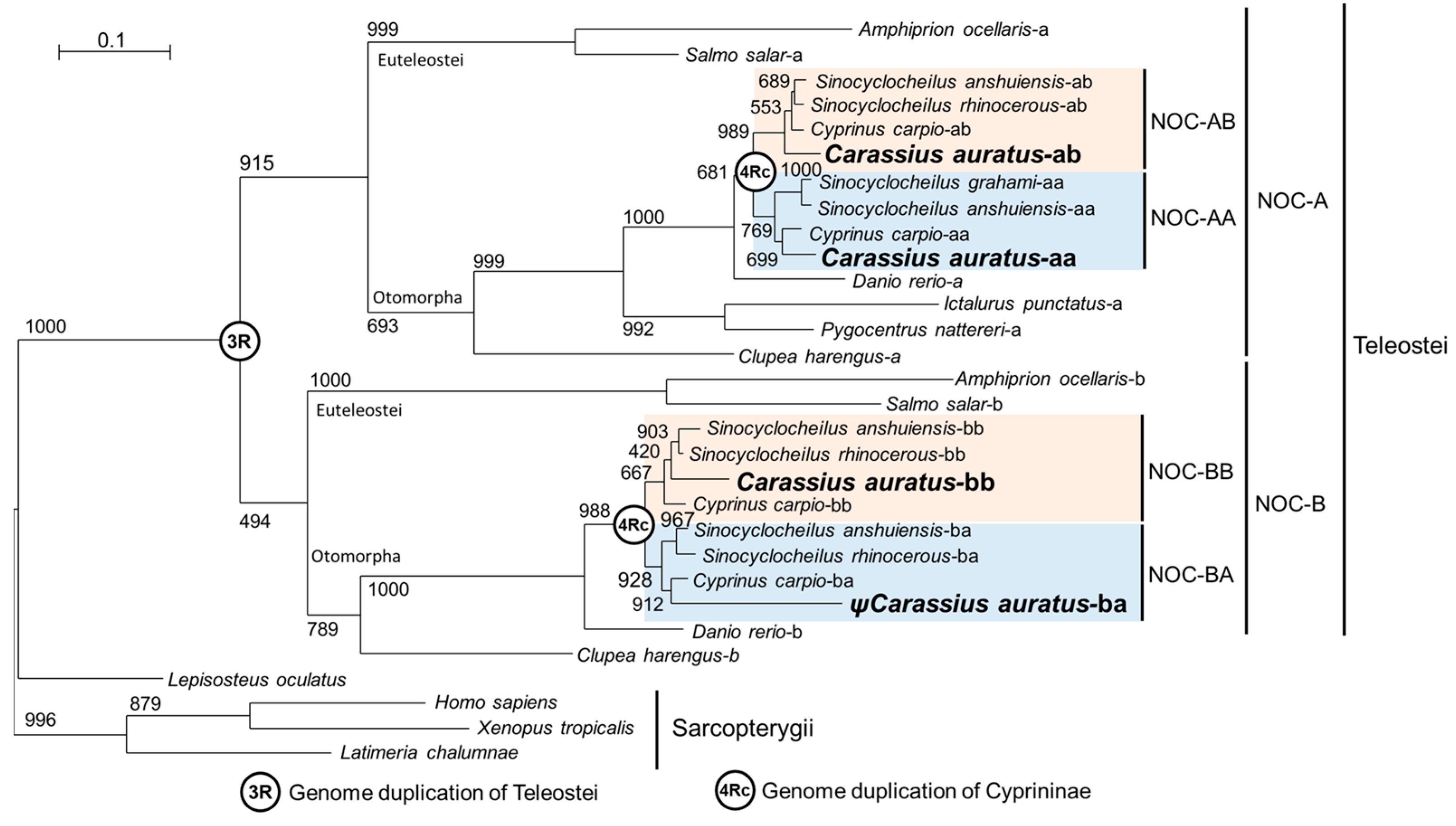
2.1. Teleostean Nocturnins: Evolutive Origin
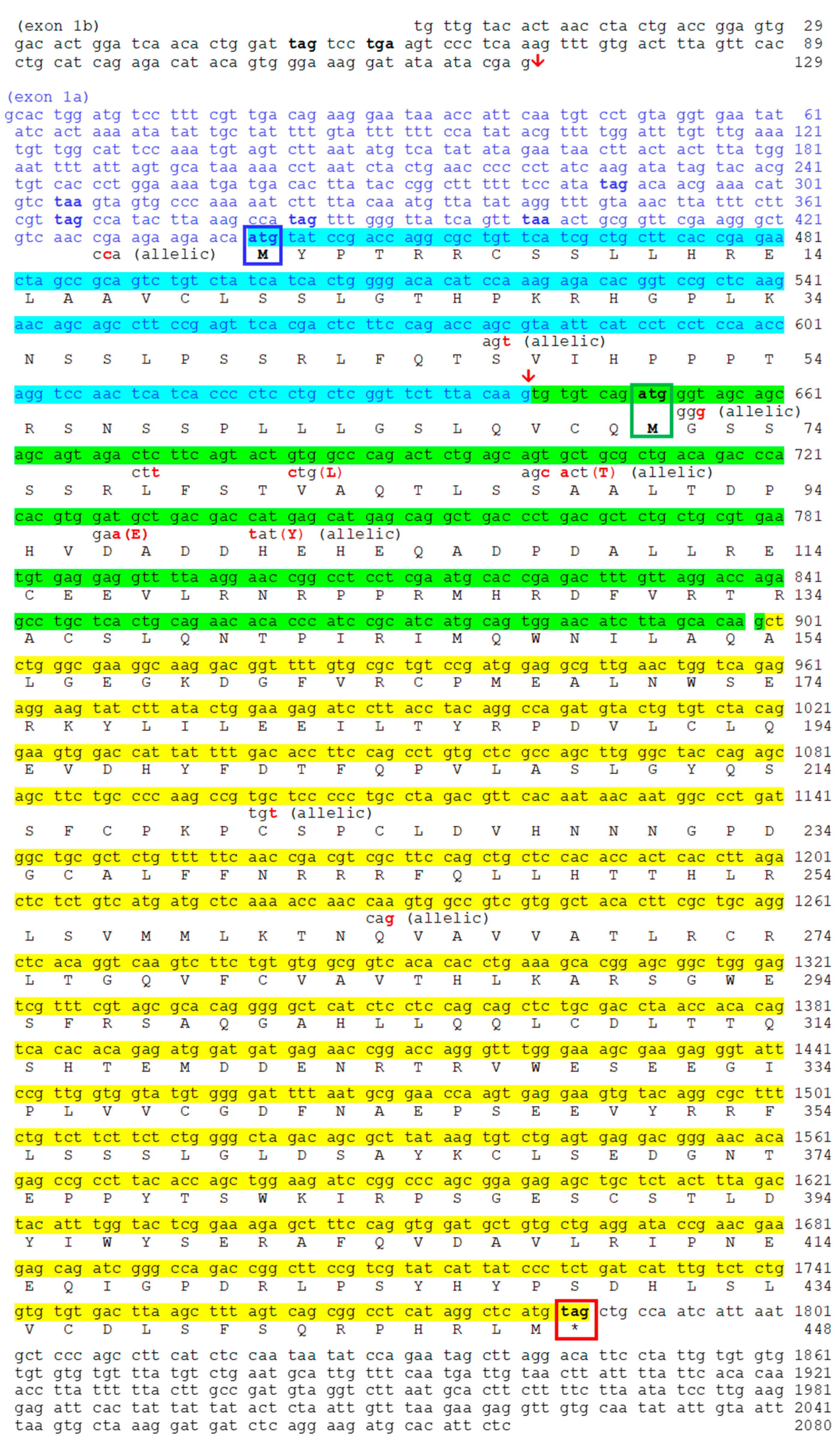
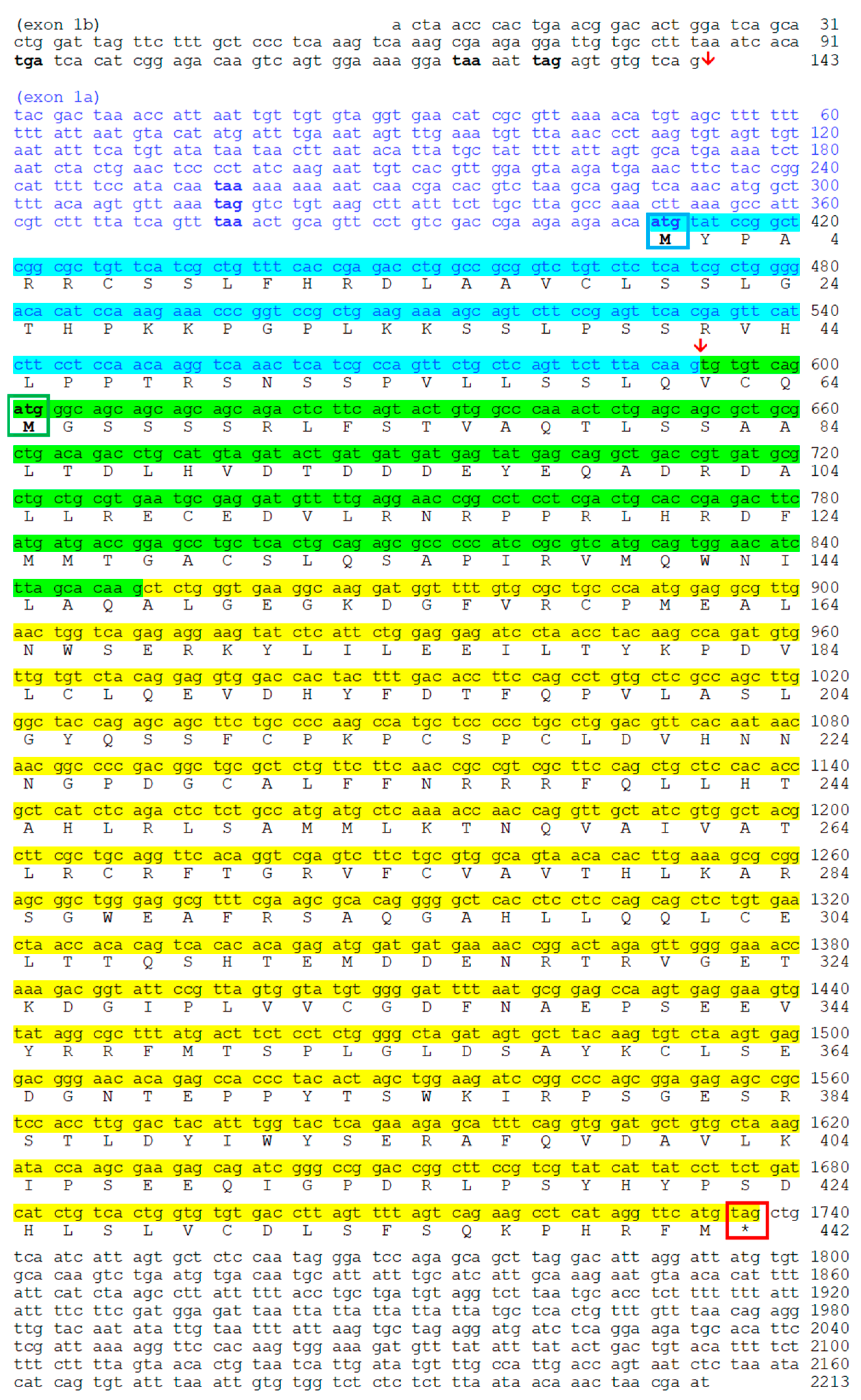
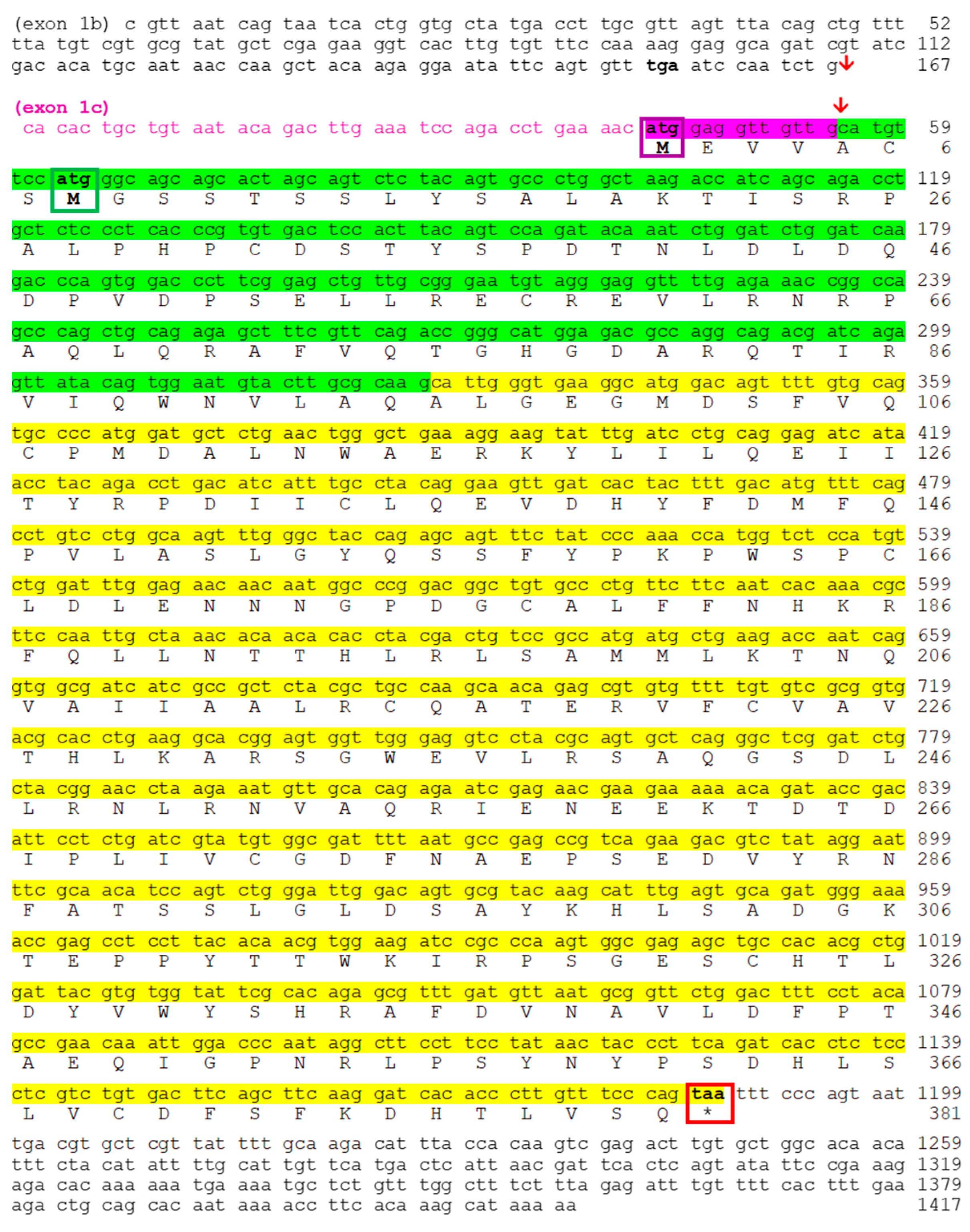
2.2. Nocturnin Sequences in Goldfish
2.2.1. Transcription of Nocturnin aa (noc-aa) and Nocturnin ab (noc-ab) Genes
2.2.2. Transcription of Nocturnin bb (noc-bb) Gene
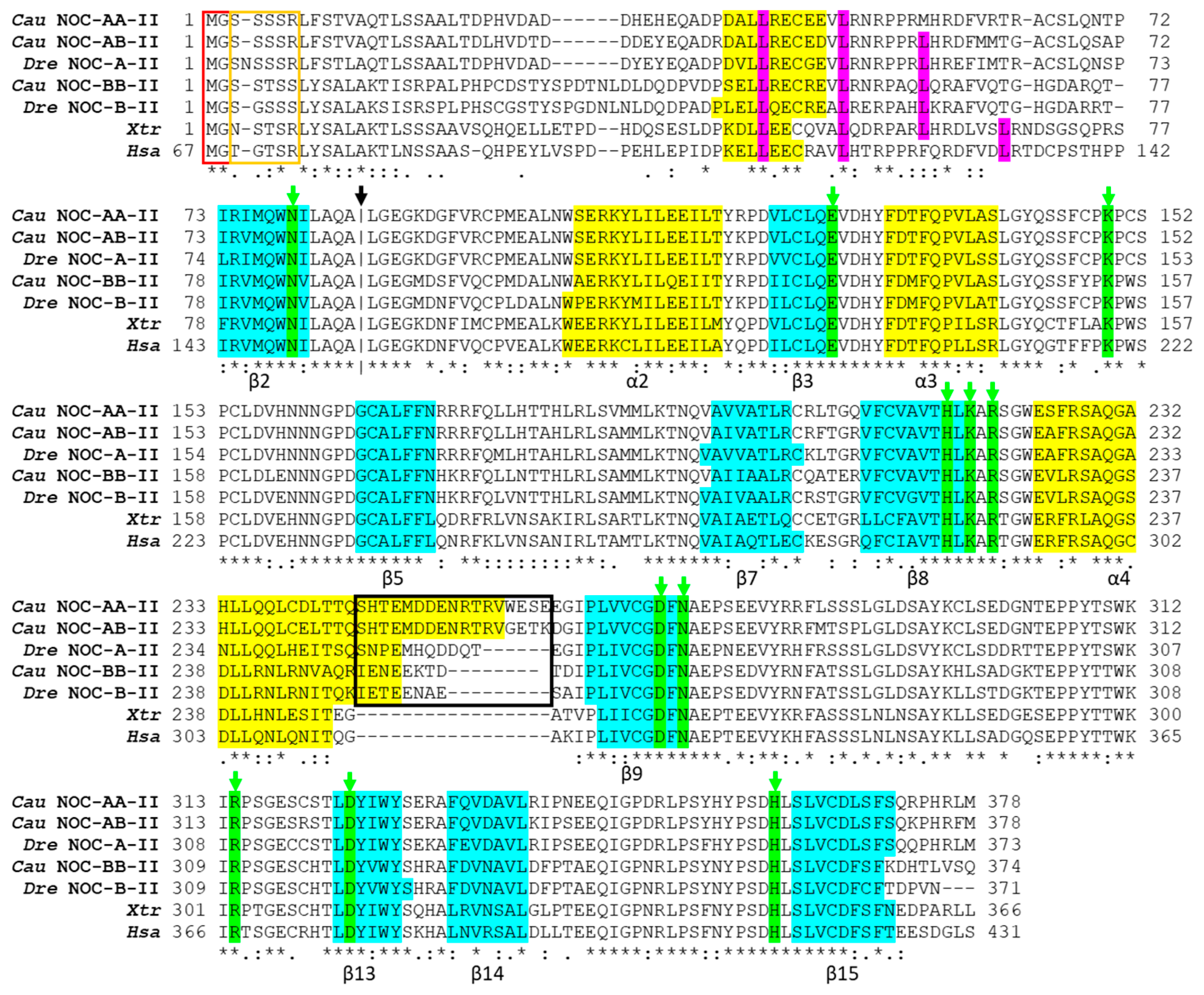
2.2.3. Protein Alignment and Deduced Secondary Structure
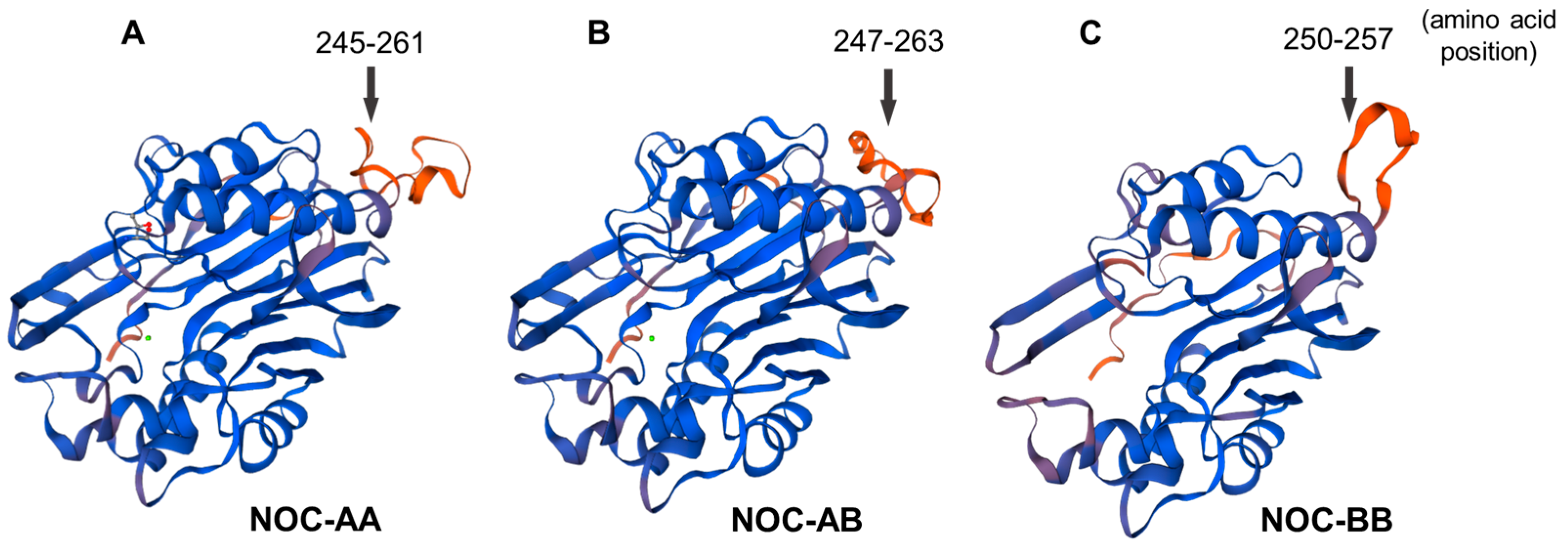
2.2.4. Tertiary 3D Structure
2.3. Proposed Classification for Cyprinine Nocturnins
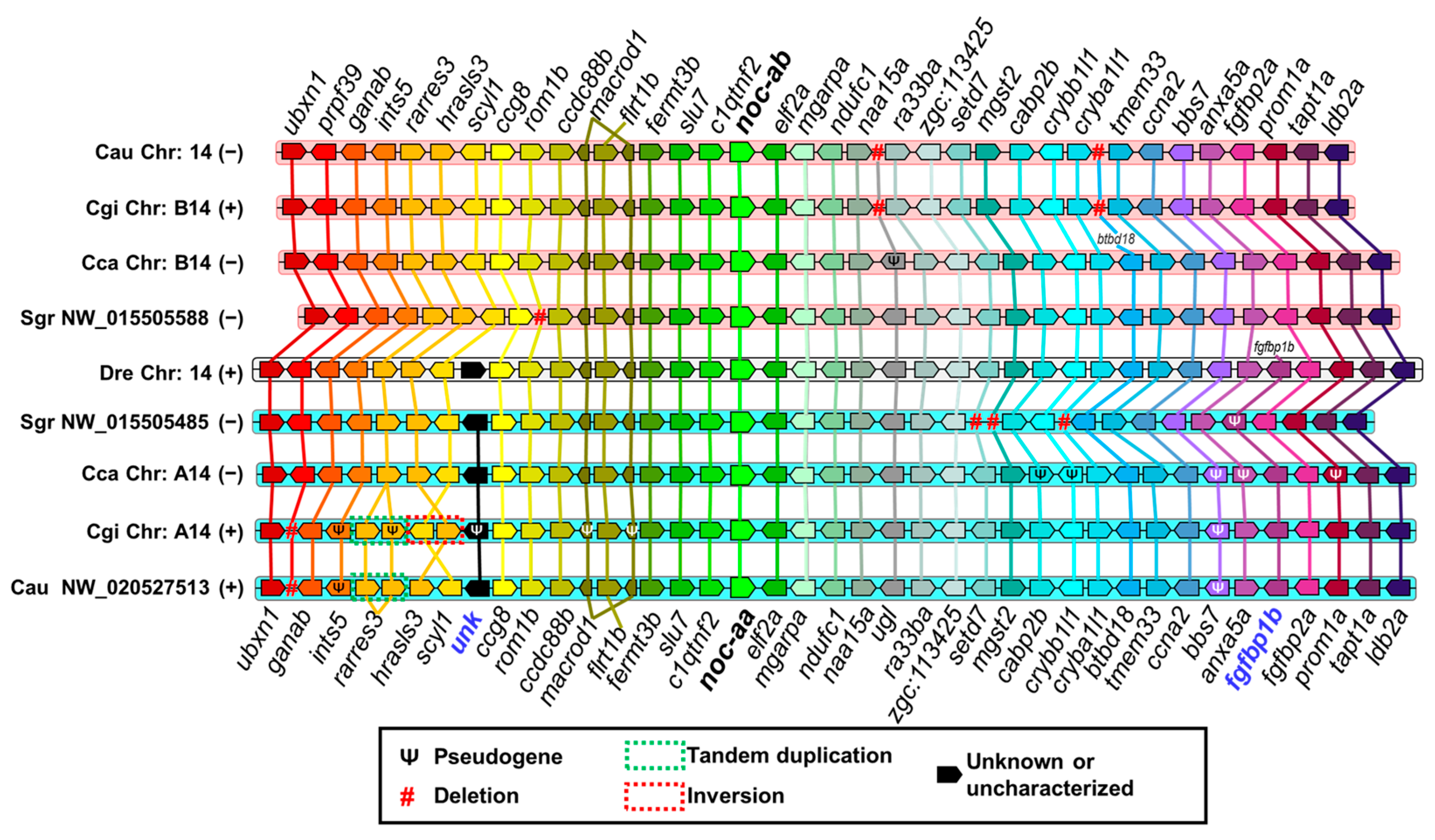
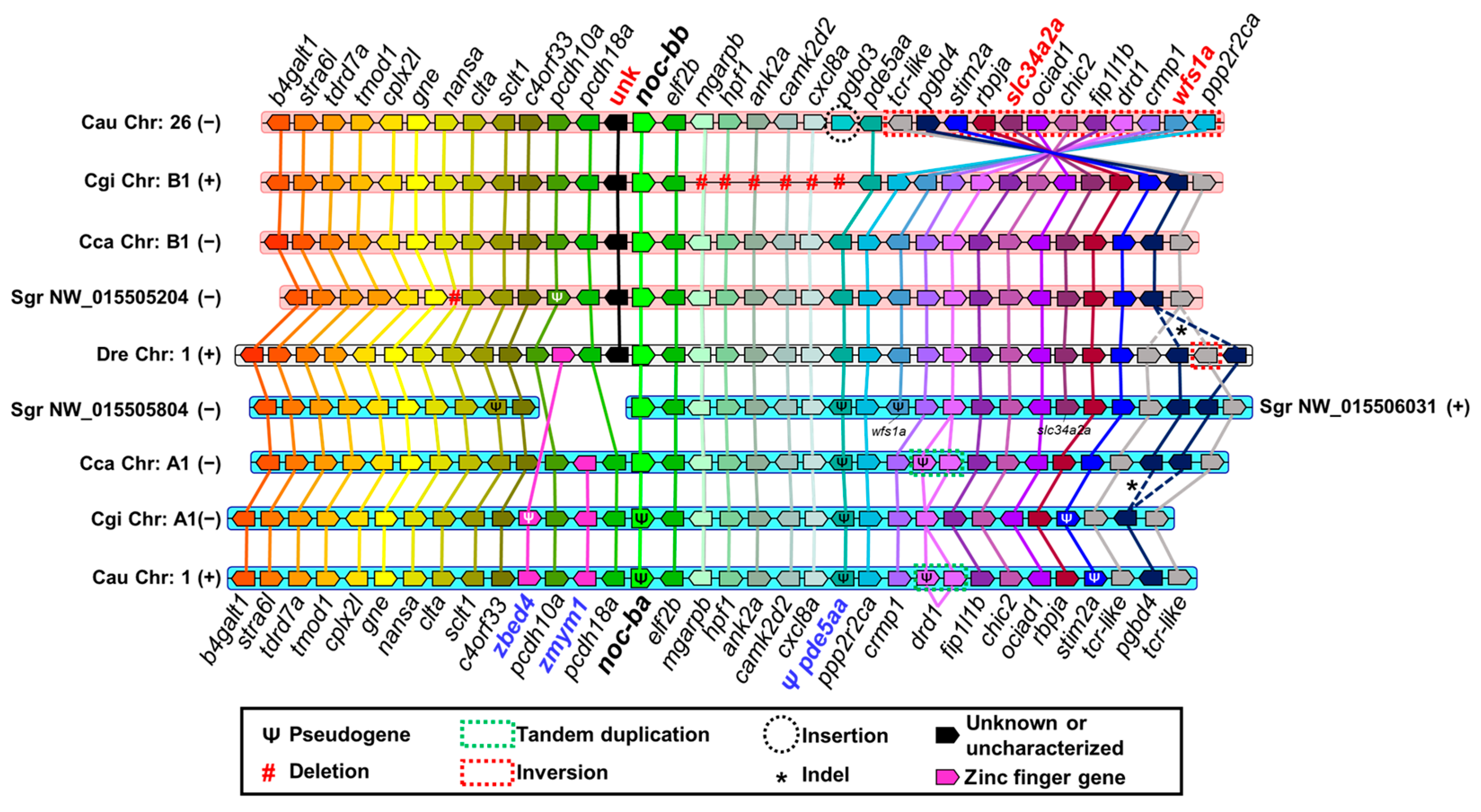
2.3.1. Synteny Analysis
2.3.2. Phylogenetic Tree
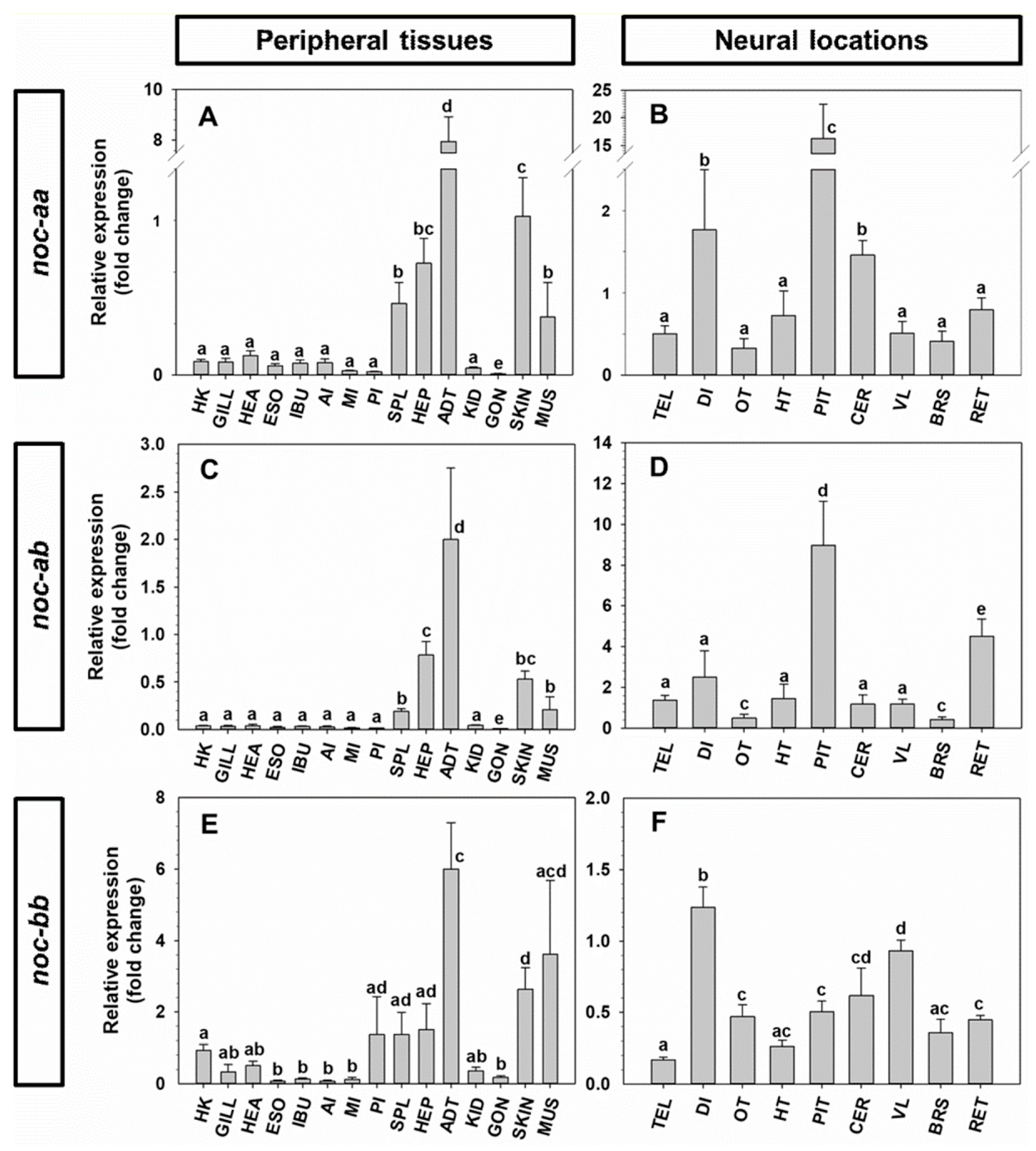
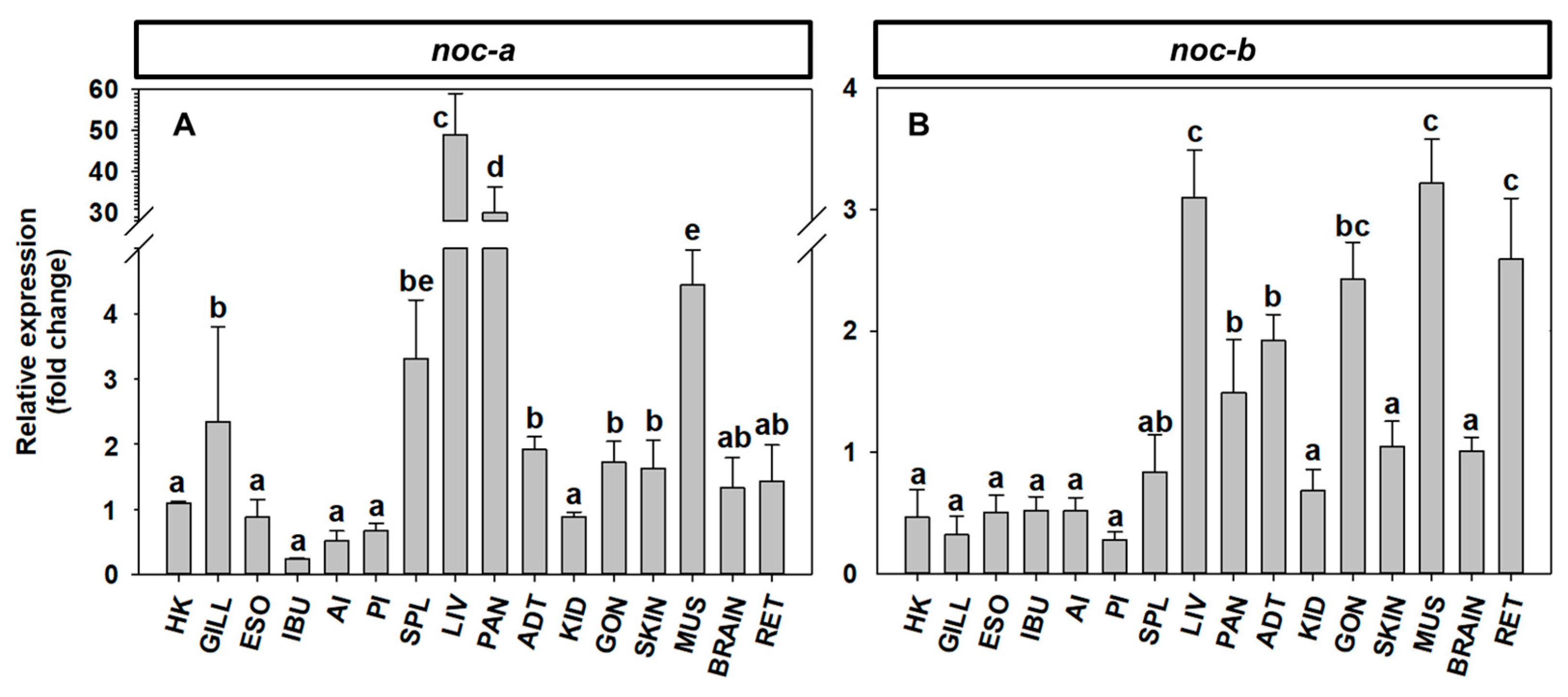
2.4. Tissue Expression of noc mRNA in Goldfish and Zebrafish
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance
4.2. mRNA Sequencing of Goldfish Nocturnins
4.3. Blast Analysis of mRNA SRA Libraries
4.4. In Silico Structural Analysis and Phylogenetic Tree Construction
4.5. Synteny Analysis
4.6. Tissue Expression of noc mRNA Using RT-qPCR
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- -
- Gene in common (#). This is a gene present in two chromosomes, regardless of its position.
- -
- Conserved Relative Position (*). The position of a gene is conserved when the upstream and downstream genes are the same on both chromosomes, and the sense of transcription is also conserved.
- The Synteny Index (SI) determines the ratio between conserved and non-conserved relative positions in segments of two orthologous chromosomes. A synteny index higher than 1 indicates more conserved relative positions than non-conserved ones. In the case of high conservation (in which the non-conserved relative positions tend to be 0), the SI tends to be infinite.
- 2.
- The Synteny Conservation Rate (SCR) follows the Laplace law for probability. This index expresses the percentage of conservation of relative positions with respect to the total number of genes in common in two orthologous chromosomes.
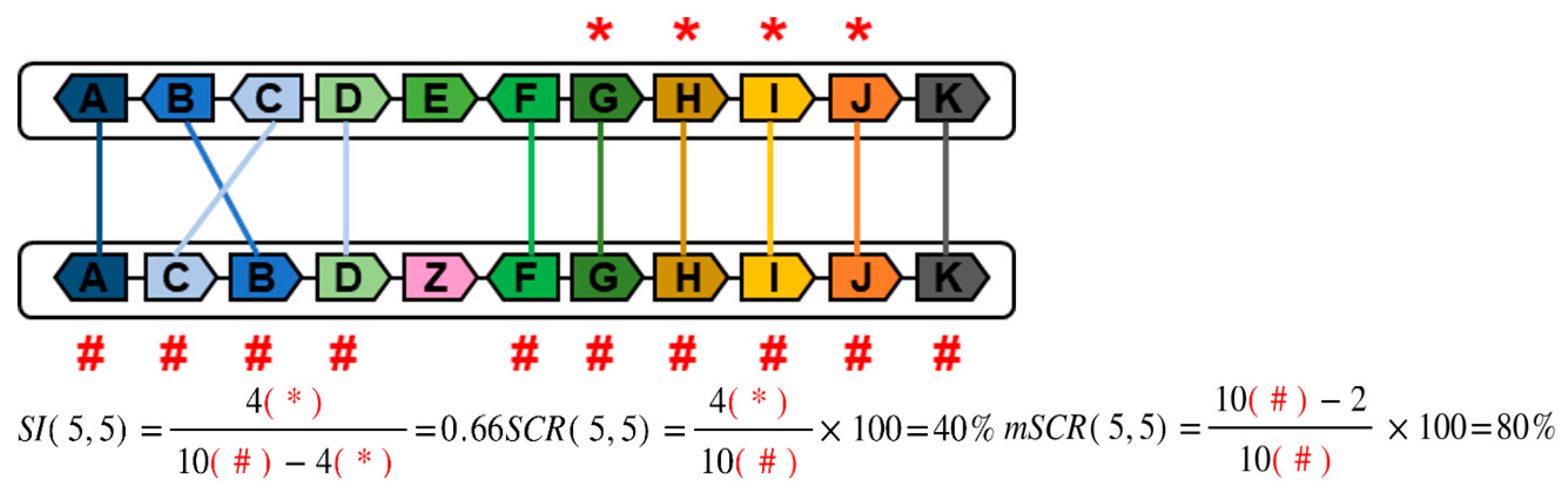
- -
- Tandem Duplications
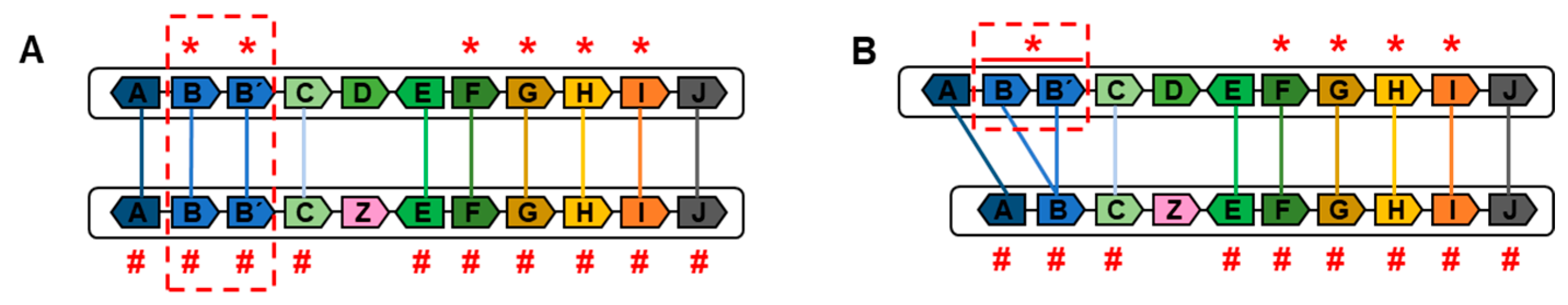
- -
- Inversions

- -
- Translocations

References
- Green, C.B.; Besharse, J.C. Identification of a novel vertebrate circadian clock-regulated gene encoding the protein nocturnin. Proc. Natl. Acad. Sci. USA 1996, 93, 14884–14888. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, X.; Grebenjuk, V.A.; Korzhev, M.; Wiens, M.; Schlossmacher, U.; Schröder, H.C. Nocturnin in the demosponge Suberites domuncula: A potential circadian clock protein controlling glycogenin synthesis in sponges. Biochem. J. 2012, 448, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.; Meuti, M.E. Circadian transcription factors differentially regulate features of the adult overwintering diapause in the Northern house mosquito, Culex pipiens. Insect Biochem. Mol. Biol. 2020, 121, 103365. [Google Scholar] [CrossRef] [PubMed]
- Grönke, S.; Bickmeyer, I.; Wunderlich, R.; Jäckle, H.; Kühnlein, R.P. Curled encodes the Drosophila homolog of the vertebrate circadian deadenylase nocturnin. Genetics 2009, 183, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fu, J.; Wei, X. Expression patterns of zebrafish nocturnin genes and the transcriptional activity of the frog nocturnin promoter in zebrafish rod photoreceptors. Mol. Vis. 2017, 23, 1039–1047. [Google Scholar]
- Blanco, A.M.; Gómez-Boronat, M.; Madera, D.; Valenciano, A.I.; Alonso-Gómez, A.L.; Delgado, M.J. First evidence of nocturnin in fish: Two isoforms in goldfish differentially regulated by feeding. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 314, R304–R312. [Google Scholar] [CrossRef]
- Wang, Y.; Osterbur, D.L.; Megaw, P.L.; Tosini, G.; Fukuhara, C.; Green, C.B.; Besharse, J.C. Rhythmic expression of Nocturnin mRNA in multiple tissues of the mouse. BMC Dev. Biol. 2001, 1, 9–15. [Google Scholar] [CrossRef]
- Li, R.; Yue, J.; Zhang, Y.; Zhou, L.; Hao, W.; Yuan, J.; Qiang, B.; Ding, J.M.; Peng, X.; Cao, J.M. CLOCK/BMAL1 regulates human nocturnin transcription through binding to the E-box of nocturnin promoter. Mol. Cell. Biochem. 2008, 317, 169–177. [Google Scholar] [CrossRef]
- Curran, K.L.; LaRue, S.; Bronson, B.; Solis, J.; Trow, A.; Sarver, N.; Zhu, H. Circadian genes are expressed during early development in Xenopus laevis. PLoS ONE 2008, 3, e2749. [Google Scholar] [CrossRef]
- Bracci, M.; Copertaro, A.; Ciarapica, V.; Barbaresi, M.; Esposito, S.; Albanesi, A.; Valentino, M.; Ledda, C.; Rapisarda, V.; Santarelli, L. Nocturnin gene diurnal variation in healthy volunteers and expression levels in shift workers. Biomed. Res. Int. 2019, 31, 7582734. [Google Scholar] [CrossRef]
- Liu, X.; Green, C.B. Circadian regulation of nocturnin transcription by phosphorylated CREB in Xenopus retinal photoreceptor cells. Mol. Cell. Biol. 2002, 22, 7501–7511. [Google Scholar] [CrossRef] [PubMed]
- Baggs, J.E.; Green, C.B. Nocturnin, a deadenylase in Xenopus laevis retina: A mechanism for postranscriptional control of circadian-related mRNA. Curr. Biol. 2003, 13, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Gendreau, K.L.; Sher-Chen, E.L.; Gao, P.; Green, C.B. Changes in poly(A) tail length dynamics from the loss of the circadian deadenylase Nocturnin. Sci. Rep. 2015, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Stubblefield, J.J.; Terrien, J.; Green, C.B. Nocturnin: At the crossroads of clocks and metabolism. Trends Endocrinol. Metab. 2012, 23, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Estrella, M.A.; Du, J.; Chen, L.; Rath, S.; Prangley, E.; Chitrakar, A.; Aoki, T.; Schedl, P.; Rabinowitz, J.; Korennykh, A. The metabolites NADP+ and NADPH are the targets of the circadian protein Nocturnin (Curled). Nat. Comm. 2019, 10, 2367. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B.; Douris, N.; Kojima, S.; Strayer, C.A.; Fogerty, J.; Lourim, D.; Keller, S.R.; Besharse, J.C. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet induced obesity. Proc. Natl. Acad. Sci. USA 2007, 104, 9888–9893. [Google Scholar] [CrossRef] [PubMed]
- Douris, N.; Kojima, S.; Pan, X.; Lerch-Gaggl, A.F.; Duong, S.Q.; Hussain, M.; Green, C.B. Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes. Curr. Biol. 2011, 21, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Douris, N.; Green, C.B. NOC out the fat: A short review of the circadian deadenylase Nocturnin. Ann. Med. 2008, 40, 622–626. [Google Scholar] [CrossRef]
- Stubblefield, J.J.; Gao, P.; Kilaru, G.; Mukadam, B.; Terrien, J.; Green, C.B. Temporal control of metabolic amplitude by nocturnin. Cell Rep. 2018, 22, 1225–1235. [Google Scholar] [CrossRef]
- Kawai, M.; Green, C.B.; Lecka-Czernik, B.; Douris, N.; Gilbert, M.R.; Kojima, S.; Ackert-Bicknell, C.; Garg, N.; Horowitz, M.C.; Adamo, M.L.; et al. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc. Natl. Acad. Sci. USA 2010, 107, 10508–10513. [Google Scholar] [CrossRef]
- Kawai, M.; Delany, A.M.; Green, C.B.; Adamo, M.L.; Rosen, C.J. Nocturnin suppresses igf1 expression in bone by targeting the 3′ untranslated region of igf1 mRNA. Endocrinology 2010, 151, 4861–4870. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Hatanaka, Y.; Tokoro, M.; Shin, S.-W.; Shimizu, N.; Nishihara, T.; Kato, R.; Takemoto, A.; Amano, T.; Anzai, M.; et al. Functional analysis of nocturnin, a circadian deadenylase, at maternal-to-zygotic transition in mice. J. Reprod. Dev. 2013, 59, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.L.; Allen, L.; Porter, B.B.; Dodge, J.; Lope, C.; Willadsen, G.; Fisher, R.; Johnson, N.; Campbell, E.; VonBergen, B.; et al. Circadian genes, xBmal1 and xNocturnin, modulate the timing and differentiation of somites in Xenopus laevis. PLoS ONE 2014, 9, e108266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, A.; Van de Peer, Y. From 2R to 3R: Evidence for a fish-specific genome duplication (FSGD). BioEssays 2005, 27, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Macqueen, D.J.; Johnston, I.A. A well-constrained estimate for the timing of the salmonid whole genome duplication reveals major decoupling from species diversification. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132881. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sado, T.; Vincent Hirt, M.; Pasco-Viel, E.; Arunachalam, M.; Li, J.; Wang, X.; Freyhof, J.; Saitoh, K.; Simons, A.M.; et al. Phylogeny and polyploidy: Resolving the classification of cyprinine fishes (Teleostei: Cypriniformes). Mol. Phylogenet. Evol. 2015, 85, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Abshire, E.T.; Chasseur, J.; Bohn, J.A.; Del Rizzo, P.A.; Freddolino, P.L.; Goldstrohm, A.C.; Trievel, R.C. The structure of human Nocturnin reveals a conserved ribonuclease domain that represses target transcript translation and abundance in cells. Nucleic Acids Res. 2018, 46, 6257–6270. [Google Scholar] [CrossRef]
- Glasauer, S.M.; Neuhauss, S.C. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genomics. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Luo, J.; Chai, J.; Wen, Y.; Tao, M.; Lin, G.; Liu, X.; Ren, L.; Chen, Z.; Wu, S.; Li, S.; et al. From asymmetrical to balanced genomic diversification during rediploidization: Subgenomic evolution in allotetraploid fish. Sci. Adv. 2020, 6, eaaz7677. [Google Scholar] [CrossRef]
- Laothamatas, I.; Gao, P.; Wickramaratne, A.; Quintanilla, C.G.; Dino, A.; Khan, C.A.; Liou, J.; Green, C.B. Spatiotemporal regulation of NADP(H) phosphatase Nocturnin and its role in oxidative stress response. Proc. Natl. Acad. Sci. USA 2020, 117, 993–999. [Google Scholar] [CrossRef]
- Moe, S.E.; Sorbo, J.G.; Holen, T. Huntingtin triplet-repeat locus is stable under long-term Fen1 knockdown in human cells. J. Neurosci. Methods 2008, 171, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, E.; Nobile, V.; Pucci, C.; Chiurazzi, P. Mechanisms of the FMR1 repeat instability: How does the CGG sequence expand? Int. J. Mol. Sci. 2022, 23, 5425. [Google Scholar] [CrossRef] [PubMed]
- Abshire, E.T.; Hughes, K.L.; Diao, R.; Pearce, S.; Gopalakrishna, S.; Trievel, R.C.; Rorbach, J.; Freddolino, P.L.; Goldstrohm, A.C. Differential processing and localization of human Nocturnin controls metabolism of mRNA and nicotinamide adenine dinucleotide cofactors. J. Biol. Chem. 2020, 295, 15112–15133. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Huertes, D.; Mulica, P.; Vögtle, F.N. The versatility of the mitochondrial presequence processing machinery: Cleavage, quality control and turnover. Cell Tissue Res. 2017, 367, 73–81. [Google Scholar] [CrossRef]
- Sriram, S.M.; Kim, B.Y.; Kwon, Y.T. The N-end rule pathway: Emerging functions and molecular principles of substrate recognition. Nat. Rev. Mol. Cell. Biol. 2011, 12, 735–747. [Google Scholar] [CrossRef]
- Alonso-Gómez, A.L.; Iuvone, P.M. Melatonin biosynthesis in cultured chick retinal photoreceptor cells: Calcium and cyclic AMP protect serotonin N-acetyltransferase from inactivation in cycloheximide-treated cells. J. Neurochem. 1995, 65, 1054–1060. [Google Scholar] [CrossRef]
- Clark, L.B.; Viswanathan, P.; Quigley, G.; Chiang, Y.C.; McMahon, J.S.; Yao, G.; Chen, J.; Nelsbach, A.; Denis, C.L. Systematic mutagenesis of the leucine-rich repeat (LRR) domain of CCR4 reveals specific sites for binding to CAF1 and a separate critical role for the LRR in CCR4 deadenylase activity. J. Biol. Chem. 2004, 279, 13616–13623. [Google Scholar] [CrossRef]
- Dupressoir, A.; Morel, A.P.; Barbot, W.; Loireau, M.P.; Corbo, L.; Heidmann, T. Identification of four families of yCCR4- and Mg2+-dependent endonuclease-related proteins in higher eukaryotes, and characterization of orthologs of yCCR4 with a conserved leucine-rich repeat essential for hCAF1/hPOP2 binding. BMC Genom. 2001, 2, 9. [Google Scholar] [CrossRef]
- Godwin, A.R.; Kojima, S.; Green, C.B.; Wilusz, J. Kiss your tail goodbye: The role of PARN, Nocturnin, and Angel deadenylases in mRNA biology. Biochim. Biophys. Acta 2013, 1829, 571–579. [Google Scholar] [CrossRef]
- Landschulz, W.H.; Johnson, P.F.; McKnight, S.L. The leucine zipper: A hypothetical structure common to a new class of DNA binding proteins. Science 1988, 240, 1759–1764. [Google Scholar] [CrossRef]
- Sinha, A.U.; Meller, J. Cinteny: Flexible analysis and visualization of synteny and genome rearrangements in multiple organisms. BMC Bioinform. 2007, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.H.; Muhammad, S.A.; Arvestad, L. GenFamClust: An accurate, synteny-aware and reliable homology inference algorithm. BMC Evol. Biol. 2016, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Ghiurcuta, C.G.; Moret, B.M. Evaluating synteny for improved comparative studies. Bioinformatics 2014, 30, i9–i18. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Naylor, G.J.P.; Mayden, R.L. Deciphering reticulate evolution of the largest group of polyploid vertebrates, the subfamily cyprininae (Teleostei: Cypriniformes). Mol. Phylogenet. Evol. 2022, 166, 107323. [Google Scholar] [CrossRef] [PubMed]
- Session, A.M.; Uno, Y.; Kwon, T.; Chapman, J.A.; Toyoda, A.; Takahashi, S.; Fukui, A.; Hikosaka, A.; Suzuki, A.; Kondo, M.; et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 2016, 538, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Barbot, W.; Wasowicz, M.; Dupressoir, A.; Versaux-Botteri, C.; Heidmann, T. A murine gene with circadian expression revealed by transposon insertion: Self-sustained rhythmicity in the liver and the photoreceptors. Biochim. Biophys. Acta 2002, 1576, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- Drozdetskiy, A.; Cole, C.; Procter, J.; Barton, G.J. JPred4: A protein secondary structure prediction server. Nucleic Acids Res. 2015, 43, W389–W394. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Tsuji, J.; Fu, S.C.; Tomii, K.; Horton, P.; Imai, K. MitoFates: Improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol. Cell. Proteom. 2015, 14, 1113–1126. [Google Scholar] [CrossRef]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Casadio, R. TPpred3 detects and discriminates mitochondrial and chloroplastic targeting peptides in eukaryotic proteins. Bioinformatics 2015, 31, 3269–3275. [Google Scholar] [CrossRef] [PubMed]
- Bologna, G.; Yvon, C.; Duvaud, S.; Veuthey, A.L. N-Terminal myristoylation predictions by ensembles of neural networks. Proteomics 2004, 4, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Michael, S.; Alvarado-Valverde, J.; Mészáros, B.; Sámano-Sánchez, H.; Zeke, A.; Dobson, L.; Lazar, T.; Örd, M.; Nagpal, A.; et al. The eukaryotic linear motif resource: 2022 release. Nucleic Acids Res. 2022, 50, D497–D508. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Perrière, G.; Gouy, M. WWW-Query: An on-line retrieval system for biological sequence banks. Biochimie 1996, 78, 364–369. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Vincens, P.; Dufayard, J.F.; Roest Crollius, H.; Louis, A. Genomicus in 2022: Comparative tools for thousands of genomes and reconstructed ancestors. Nucleic Acids Res 2022, 50, D1025–D1031. [Google Scholar] [CrossRef]
- Bradford, Y.M.; Van Slyke, C.E.; Ruzicka, L.; Singer, A.; Eagle, A.; Fashena, D.; Howe, D.G.; Frazer, K.; Martin, R.; Paddock, H.; et al. Zebrafish information network, the knowledgebase for Danio rerio research. Genetics 2022, 220, iyac016. [Google Scholar] [CrossRef]
- Sánchez-Bretaño, A.; Blanco, A.M.; Unniappan, S.; Kah, O.; Gueguen, M.M.; Bertucci, J.I.; Alonso-Gómez, A.L.; Valenciano, A.I.; Isorna, E.; Delgado, M.J. In Situ localization and rhythmic expression of ghrelin and ghs-r1 ghrelin receptor in the brain and gastrointestinal tract of goldfish (Carassius auratus). PLoS ONE 2015, 10, e0141043. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Huang, J.; Tang, L.; Zhang, Y.; Wu, J.; Lin, S.; Wang, H. Heme acts through the Bach1b/Nrf2a-MafK pathway to regulate exocrine peptidase precursor genes in porphyric zebrafish. Dis. Model. Mech. 2014, 7, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Omori, Y.; Koren, S.; Shirokiya, T.; Kuroda, T.; Miyamoto, A.; Wada, H.; Fujiyama, A.; Toyoda, A.; Zhang, S.; et al. De novo assembly of the goldfish (Carassius auratus) genome and the evolution of genes after whole-genome duplication. Sci. Adv. 2019, 5, eaav0547. [Google Scholar] [CrossRef]
- Kuhl, H.; Du, K.; Schartl, M.; Kalous, L.; Stöck, M.; Lamatsch, D.K. Equilibrated evolution of the mixed auto-/allopolyploid haplotype-resolved genome of the invasive hexaploid Prussian carp. Nat. Commun. 2022, 13, 4092. [Google Scholar] [CrossRef]
- Xu, P.; Xu, J.; Liu, G.; Chen, L.; Zhou, Z.; Peng, W.; Jiang, Y.; Zhao, Z.; Jia, Z.; Sun, Y.; et al. The allotetraploid origin and asymmetrical genome evolution of the common carp Cyprinus carpio. Nat. Commun. 2019, 10, 4625. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Bai, J.; Fang, D.; Qiu, Y.; Jiang, W.; Yuan, H.; Bian, C.; Lu, J.; He, S.; et al. The Sinocyclocheilus cavefish genome provides insights into cave adaptation. BMC Biol. 2016, 14, 1. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madera, D.; Alonso-Gómez, A.; Delgado, M.J.; Valenciano, A.I.; Alonso-Gómez, Á.L. Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression. Int. J. Mol. Sci. 2024, 25, 54. https://doi.org/10.3390/ijms25010054
Madera D, Alonso-Gómez A, Delgado MJ, Valenciano AI, Alonso-Gómez ÁL. Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression. International Journal of Molecular Sciences. 2024; 25(1):54. https://doi.org/10.3390/ijms25010054
Chicago/Turabian StyleMadera, Diego, Aitana Alonso-Gómez, María Jesús Delgado, Ana Isabel Valenciano, and Ángel Luis Alonso-Gómez. 2024. "Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression" International Journal of Molecular Sciences 25, no. 1: 54. https://doi.org/10.3390/ijms25010054
APA StyleMadera, D., Alonso-Gómez, A., Delgado, M. J., Valenciano, A. I., & Alonso-Gómez, Á. L. (2024). Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression. International Journal of Molecular Sciences, 25(1), 54. https://doi.org/10.3390/ijms25010054





