Probing the Cellular Fate of the Protein Corona around Nanoparticles with Nanofocused X-ray Fluorescence Imaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gold Nanoparticles Used for Treatment
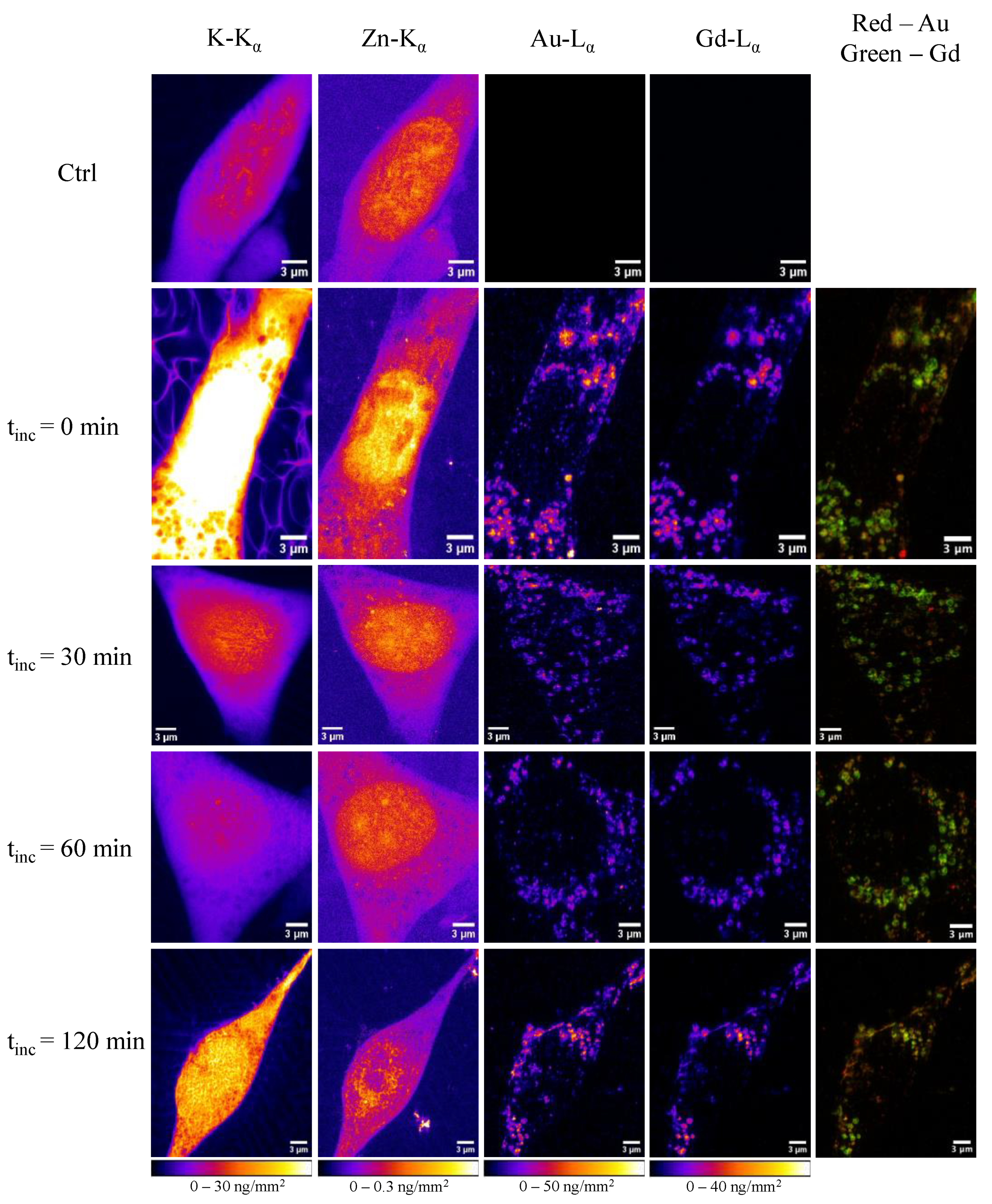
2.2. X-ray Fluorescence Imaging
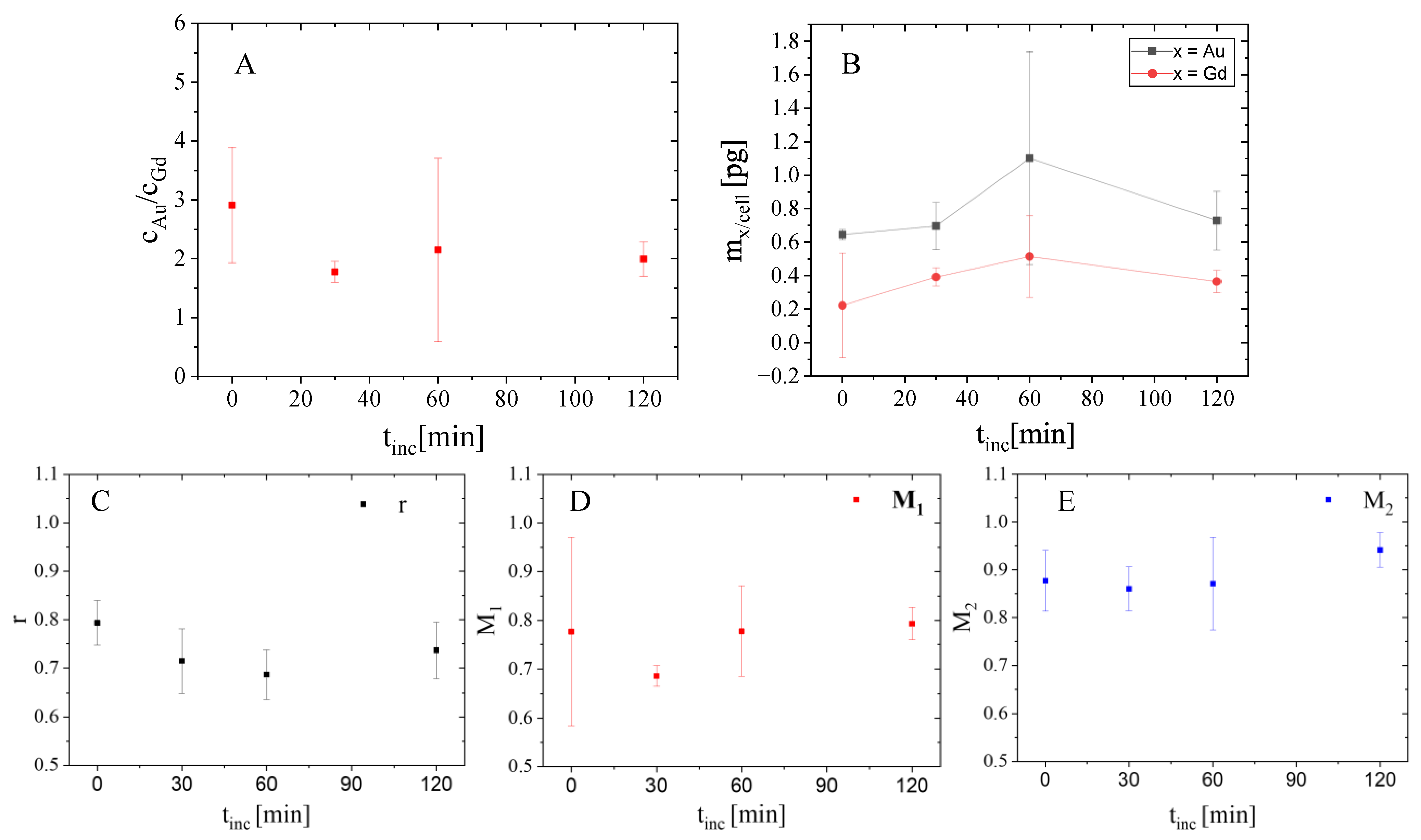
2.3. Analysis in Whole Cells
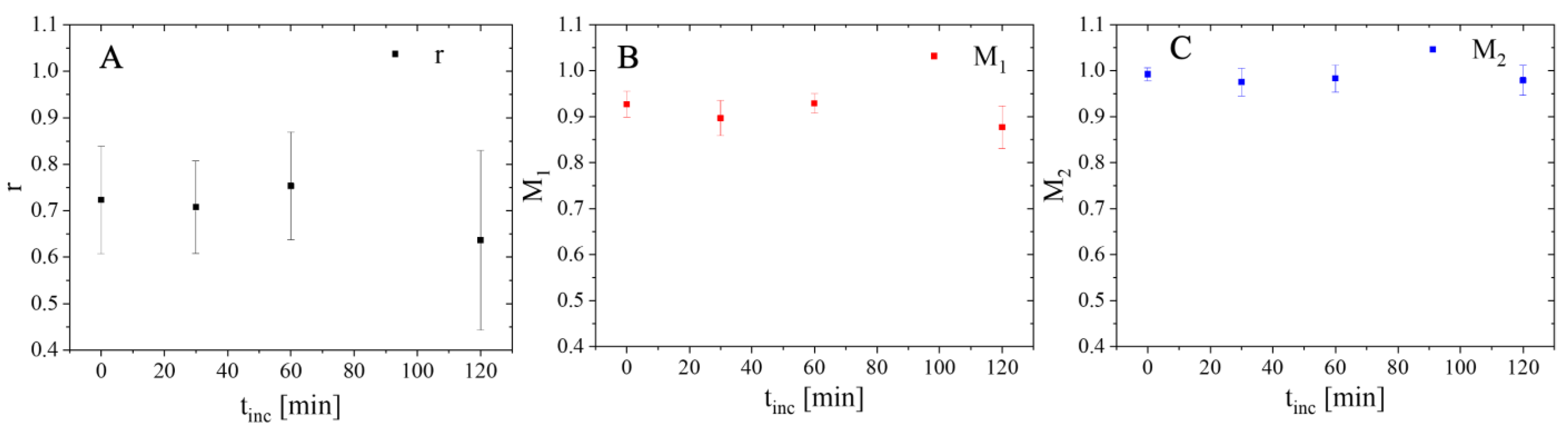
2.4. Analysis Inside Cellular Vesicles
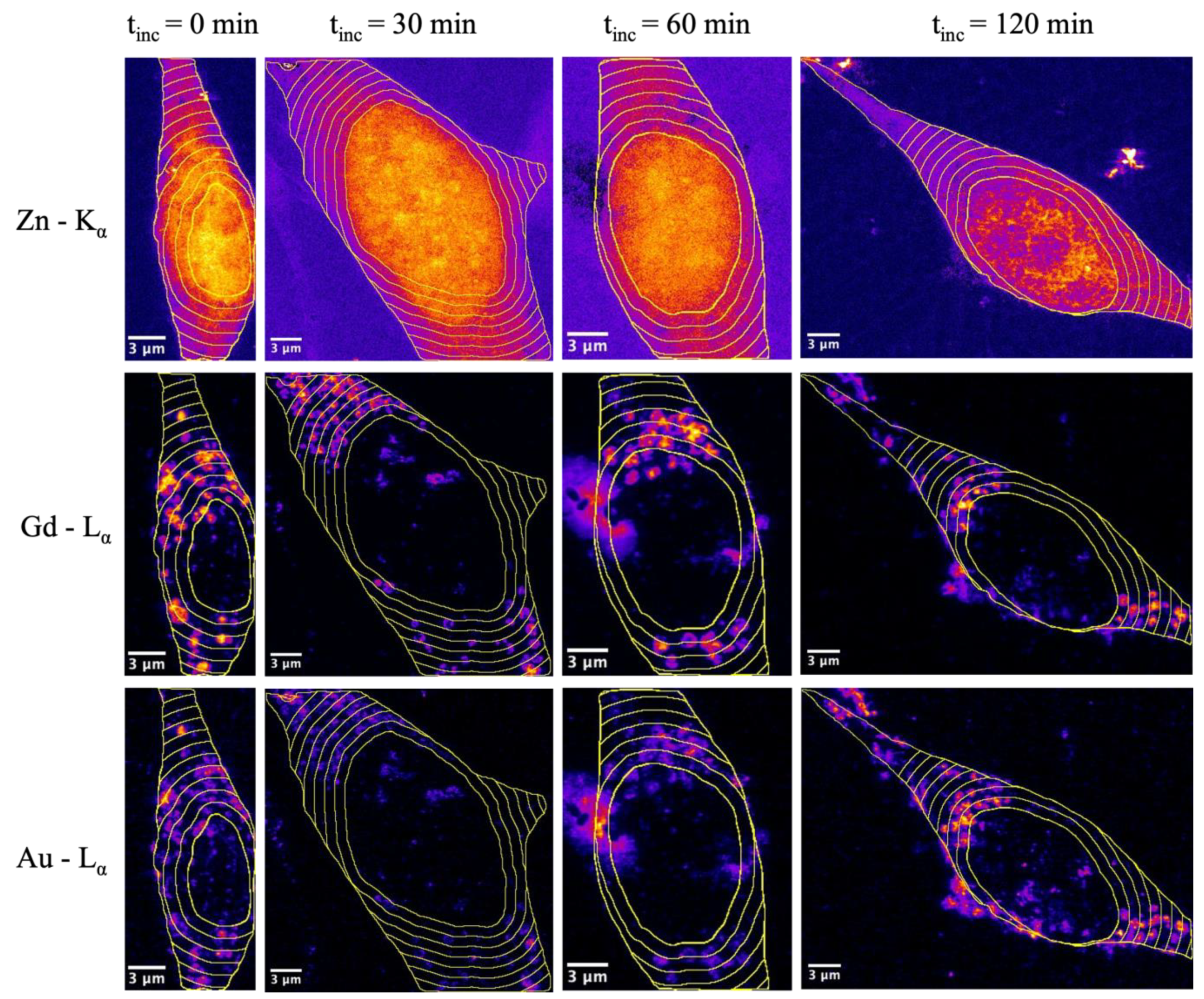
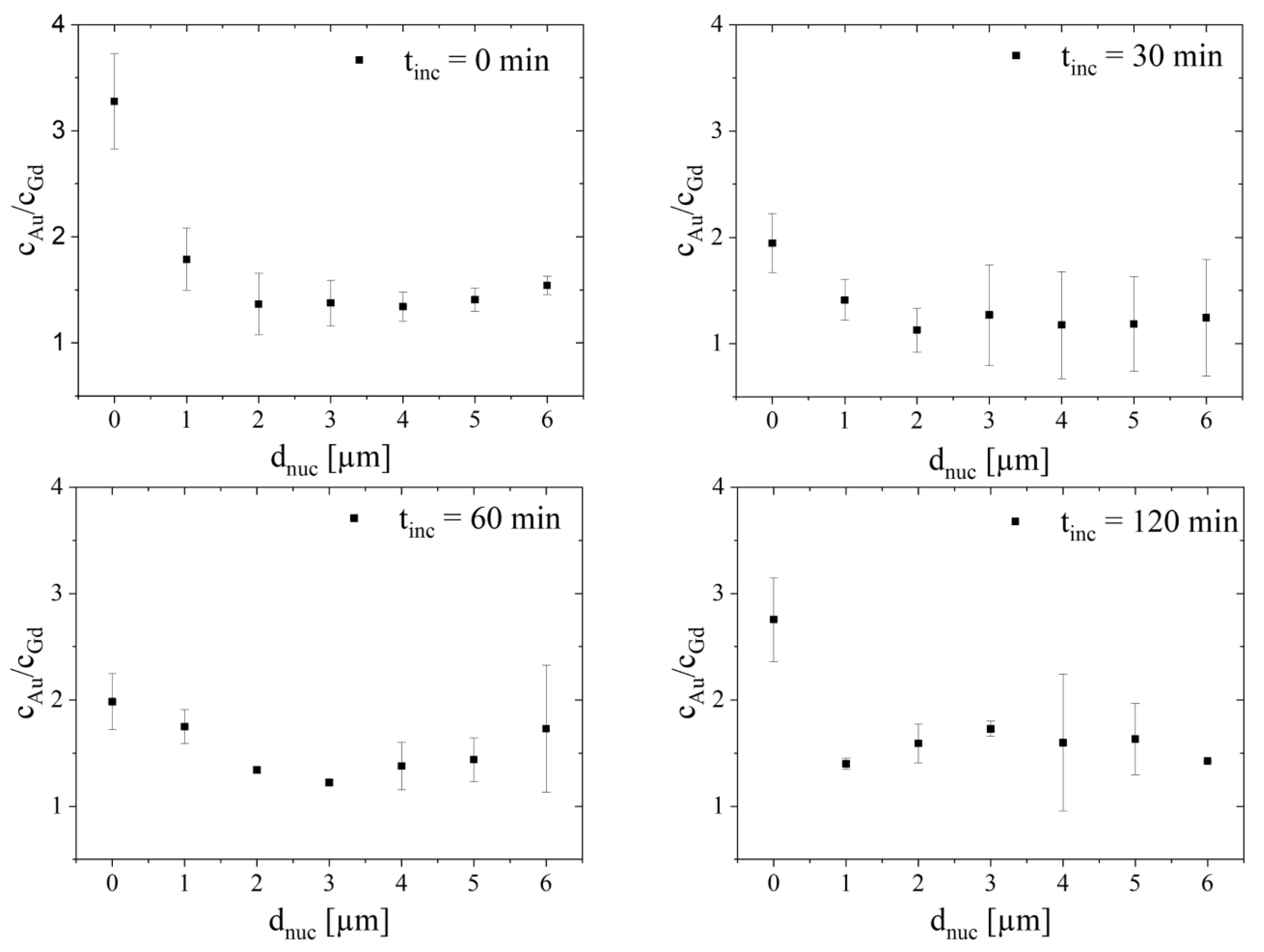
2.5. Analysis Dependent on Distance to Nucleus
2.6. Post Hoc Sample Size Estimation
3. Materials and Methods
3.1. Nanoparticle Synthesis
3.2. Surface Modification of Nanoparticles
3.3. X-ray Fluorescence Imaging Sample Preparation
3.4. Synchrotron X-ray Fluorescence Imaging
3.5. Colocalization Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegner, K.D.; Hildebrandt, N. Quantum Dots: Bright and Versatile in Vitro and in Vivo Fluorescence Imaging Biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Nieves, L.M.; Betzer, O.; Sadan, T.; Noël, P.B.; Popovtzer, R.; Cormode, D.P. Nanoparticle Contrast Agents for X-Ray Imaging Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1642. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kutsevol, N.; Naumenko, A.; Harahuts, Y.; Chumachenko, V.; Shton, I.; Shishko, E.; Lukianova, N.; Chekhun, V. New Hybrid Composites for Photodynamic Therapy: Synthesis, Characterization and Biological Study. Appl. Nanosci. 2019, 9, 881–888. [Google Scholar] [CrossRef]
- Zhu, G.H.; Gray, A.B.C.; Patra, H.K. Nanomedicine: Controlling Nanoparticle Clearance for Translational Success. Trends Pharmacol. Sci. 2022, 43, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular Coronas Provide the Biological Identity of Nanosized Materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-Functionalized Nanoparticles Lose Their Targeting Capabilities When a Biomolecule Corona Adsorbs on the Surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef]
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano 2019, 13, 11107–11121. [Google Scholar] [CrossRef]
- Yang, H.; Wang, M.; Zhang, Y.; Li, F.; Yu, S.; Zhu, L.; Guo, Y.; Yang, L.; Yang, S. Conformational-Transited Protein Corona Regulated Cell-Membrane Penetration and Induced Cytotoxicity of Ultrasmall Au Nanoparticles. RSC Adv. 2019, 9, 4435–4444. [Google Scholar] [CrossRef] [PubMed]
- Böhmert, L.; Voß, L.; Stock, V.; Braeuning, A.; Lampen, A.; Sieg, H. Isolation Methods for Particle Protein Corona Complexes from Protein-Rich Matrices. Nanoscale Adv. 2020, 2, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Balmori, A.; Sandu, R.; Gheorghe, D.; Botea-Petcu, A.; Precupas, A.; Tanasescu, S.; Sánchez-García, D.; Borrós, S. Revising Protein Corona Characterization and Combining ITC and Nano-DSC to Understand the Interaction of Proteins With Porous Nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 650281. [Google Scholar] [CrossRef]
- Tan, X.; Welsher, K. Particle-by-Particle In Situ Characterization of the Protein Coronavia Real-Time 3D Single-Particle-Tracking Spectroscopy. Angew. Chem. 2021, 133, 22533–22541. [Google Scholar] [CrossRef]
- Dolci, M.; Wang, Y.; Nooteboom, S.W.; Soto Rodriguez, P.E.D.; Sánchez, S.; Albertazzi, L.; Zijlstra, P. Real-Time Optical Tracking of Protein Corona Formation on Single Nanoparticles in Serum. ACS Nano 2023, 17, 20167–20178. [Google Scholar] [CrossRef] [PubMed]
- Feiner-Gracia, N.; Beck, M.; Pujals, S.; Tosi, S.; Mandal, T.; Buske, C.; Linden, M.; Albertazzi, L. Super-Resolution Microscopy Unveils Dynamic Heterogeneities in Nanoparticle Protein Corona. Small 2017, 13, 1701631. [Google Scholar] [CrossRef]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Kostarelos, K. Time-Evolution of in Vivo Protein Corona onto Blood-Circulating PEGylated Liposomal Doxorubicin (DOXIL) Nanoparticles. Nanoscale 2016, 8, 6948–6957. [Google Scholar] [CrossRef]
- Otto, F.; Sun, X.; Schulz, F.; Sanchez-cano, C.; Feliu, N.; Westermeier, F.; Parak, W.J. X-ray Photon Correlation Spectroscopy Towards Measuring Nanoparticle Diameters in Biological Environments Allowing for the In Situ Analysis of Their Bio-Nano Interface. Small 2022, 2201324, 2201324. [Google Scholar] [CrossRef]
- Padro, D.; Cienskowski, P.; Lopez-Fernandez, S.; Chakraborty, I.; Carrillo-Carrion, C.; Feliu, N.; Parak, W.J.; Carril, M. Toward Diffusion Measurements of Colloidal Nanoparticles in Biological Environments by Nuclear Magnetic Resonance. Small 2020, 16, 2001160. [Google Scholar] [CrossRef]
- Iranpour Anaraki, N.; Liebi, M.; Ong, Q.; Blanchet, C.; Maurya, A.K.; Stellacci, F.; Salentinig, S.; Wick, P.; Neels, A. In-Situ Investigations on Gold Nanoparticles Stabilization Mechanisms in Biological Environments Containing HSA. Adv. Funct. Mater. 2022, 32, 2110253. [Google Scholar] [CrossRef]
- Carrillo-Carrion, C.; Bocanegra, A.I.; Arnaiz, B.; Feliu, N.; Zhu, D.; Parak, W.J. Triple-Labeling of Polymer-Coated Quantum Dots and Adsorbed Proteins for Tracing Their Fate in Cell Cultures. ACS Nano 2019, 13, 4631–4639. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; da Costa Marques, R.; Simon, J.; Kaltbeitzel, A.; Koynov, K.; Landfester, K.; Mailänder, V.; Lieberwirth, I. Endosomal Sorting Results in a Selective Separation of the Protein Corona from Nanoparticles. Nat. Commun. 2023, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Grüner, F.; Blumendorf, F.; Schmutzler, O.; Staufer, T.; Bradbury, M.; Wiesner, U.; Rosentreter, T.; Loers, G.; Lutz, D.; Richter, B.; et al. Localising Functionalised Gold-Nanoparticles in Murine Spinal Cords by X-ray Fluorescence Imaging and Background-Reduction through Spatial Filtering for Human-Sized Objects. Sci. Rep. 2018, 8, 16561. [Google Scholar] [CrossRef] [PubMed]
- Beckhoff, B.; Kanngießer, B.; Langhoff, N.; Wedell, R.; Wolff, H. Handbook of Practical X-ray Fluorescence Analysis; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2006; ISBN 9783540286035. [Google Scholar]
- Villar, F.; Andre, L.; Baker, R.; Bohic, S.; da Silva, J.C.; Guilloud, C.; Hignette, O.; Meyer, J.; Pacureanu, A.; Perez, M.; et al. Nanopositioning for the ESRF ID16A Nano-Imaging Beamline. Synchrotron Radiat. News 2018, 31, 9–14. [Google Scholar] [CrossRef]
- Kikongi, P.; Salvas, J.; Gosselin, R. Curve-Fitting Regression: Improving Light Element Quantification with XRF. X-ray Spectrom. 2017, 46, 347–355. [Google Scholar] [CrossRef]
- Uribe, K.B.; Guisasola, E.; Aires, A.; López-Martínez, E.; Guedes, G.; Sasselli, I.R.; Cortajarena, A.L. Engineered Repeat Protein Hybrids: The New Horizon for Biologic Medicines and Diagnostic Tools. Acc. Chem. Res. 2021, 54, 4166–4177. [Google Scholar] [CrossRef] [PubMed]
- Cortajarena, A.L.; Aires, A.; Sousaraei, A.; Möller, M.; Cabanillas-Gonzalez, J. Boosting the Photoluminescent Properties of Protein-Stabilized Gold Nanoclusters through Protein Engineering. Nano Lett. 2021, 21, 9347–9353. [Google Scholar] [CrossRef]
- Schulz, F.; Homolka, T.; Bastús, N.G.; Puntes, V.; Weller, H.; Vossmeyer, T. Little Adjustments Significantly Improve the Turkevich Synthesis of Gold Nanoparticles. Langmuir 2014, 30, 10779–10784. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT Assay: Direct and off-Target Effects of Inhibitors Can Result in over/Underestimation of Cell Viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Sousa De Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Li, Y.; Kröger, M.; Liu, W.K. Endocytosis of PEGylated Nanoparticles Accompanied by Structural and Free Energy Changes of the Grafted Polyethylene Glycol. Biomaterials 2014, 35, 8467–8478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Monteiro-Riviere, N.A. Mechanisms of Cell Uptake, Inflammatory Potential and Protein Corona Effects with Gold Nanoparticles. Nanomedicine 2016, 11, 3185–3203. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hartmann, R.; Jimenez De Aberasturi, D.; Yang, F.; Soenen, S.J.H.; Manshian, B.B.; Franz, J.; Valdeperez, D.; Pelaz, B.; Feliu, N.; et al. Colloidal Gold Nanoparticles Induce Changes in Cellular and Subcellular Morphology. ACS Nano 2017, 11, 7807–7820. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome Biogenesis and Lysosomal Membrane Proteins: Trafficking Meets Function. Nat. Rev. Mol. Cell. Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Chanana, M.; Rivera-gil, P.; Correa-Duarte, M.A.; Liz-Marzán, L.M.; Parak, W.J. Physicochemical Properties of Protein-Coated Gold Nanoparticles in Biological Fluids and Cells before and after Proteolytic Digestion. Angew. Chem.—Int. Ed. 2013, 52, 4179–4183. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Yue, J.; Feliciano, T.J.; Li, W.; Lee, A.; Odom, T.W. Gold Nanoparticle Size and Shape Effects on Cellular Uptake and Intracellular Distribution of SiRNA Nanoconstructs. Bioconjug. Chem. 2017, 28, 1791–1800. [Google Scholar] [CrossRef]
- Lu, H.; Su, J.; Mamdooh, R.; Li, Y.; Stenzel, M.H. Cellular Uptake of Gold Nanoparticles and Their Movement in 3D Multicellular Tumor Spheroids: Effect of Molecular Weight and Grafting Density of Poly(2-Hydroxyl Ethyl Acrylate). Macromol. Biosci. 2020, 20, 1900221. [Google Scholar] [CrossRef]
- Sun, X.; Gamal, M.; Nold, P.; Said, A.; Chakraborty, I.; Pelaz, B.; Schmied, F.; von Pückler, K.; Figiel, J.; Zhao, Y.; et al. Tracking Stem Cells and Macrophages with Gold and Iron Oxide Nanoparticles—The Choice of the Best Suited Particles. Appl. Mater. Today 2019, 15, 267–279. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareekd, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Morteza, M. Cellular Uptake of Nanoparticles: Journey Inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Gunduz, N.; Ceylan, H.; Guler, M.O.; Tekinay, A.B. Intracellular Accumulation of Gold Nanoparticles Leads to Inhibition of Macropinocytosis to Reduce the Endoplasmic Reticulum Stress. Sci. Rep. 2017, 7, 40493. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.R.; Trowbridge, I.S. Internalization and Processing of Transferrin and the Transferrin Receptor in Human Carcinoma A431 Cells. J. Cell Biol. 1983, 97, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988; ISBN 0805802835. [Google Scholar]
- Martinez-Criado, G.; Villanova, J.; Tucoulou, R.; Salomon, D.; Suuronen, J.P.; Laboure, S.; Guilloud, C.; Valls, V.; Barrett, R.; Gagliardini, E.; et al. ID16B: A Hard X-Ray Nanoprobe Beamline at the ESRF for Nano-Analysis. J. Synchrotron Radiat. 2016, 23, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Cesar da Silva, J.; Pacureanu, A.; Yang, Y.; Bohic, S.; Morawe, C.; Barrett, R.; Cloetens, P. Efficient Concentration of High-Energy x-Rays for Diffraction-Limited Imaging Resolution. Optica 2017, 4, 492. [Google Scholar] [CrossRef]
- Schroer, C.G.; Boye, P.; Feldkamp, J.M.; Patommel, J.; Samberg, D.; Schropp, A.; Schwab, A.; Stephan, S.; Falkenberg, G.; Wellenreuther, G.; et al. Hard X-Ray Nanoprobe at Beamline P06 at PETRA III. Nucl. Instrum. Methods Phys. Res. A 2010, 616, 93–97. [Google Scholar] [CrossRef]
- Quinn, P.D.; Alianelli, L.; Gomez-Gonzalez, M.; Mahoney, D.; Cacho-Nerin, F.; Peach, A.; Parker, J.E. The Hard X-ray Nanoprobe Beamline at Diamond Light Source. J. Synchrotron Radiat. 2021, 28, 1006–1013. [Google Scholar] [CrossRef]
- Bissardon, C.; Reymond, S.; Salomé, M.; André, L.; Bayat, S.; Cloetens, P.; Bohic, S. Cell Culture on Silicon Nitride Membranes and Cryopreparation for Synchrotron X-Ray Fluorescence Nano-Analysis. J. Vis. Exp. 2019, 2019, e60461. [Google Scholar] [CrossRef]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A Multiplatform Code for the Analysis of Energy-Dispersive X-Ray Fluorescence Spectra. Spectrochim. Acta Part. B Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Carril, M.; Padro, D.; del Pino, P.; Carrillo-Carrion, C.; Gallego, M.; Parak, W.J. In situ detection of the protein corona in complex Environments. Nat. Commun. 2017, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium Salts and Formazan Products in Cell Biology: Viability Assessment, Fluorescence Imaging, and Labeling Perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction Coefficient of Gold Nanoparticles with Different Sizes and Different Capping Ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Milani, S.; Baldelli Bombelli, F.; Pitek, A.S.; Dawson, K.A.; Rädler, J. Reversible versus Irreversible Binding of Transferrin to Polystyrene Nanoparticles: Soft and Hard Corona. ACS Nano 2012, 6, 2532–2541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, H.; Li, Y.; Chen, B.; Liu, S.; Shi, D. Bioinspired Synthesis of Gadolinium-Based Hybrid Nanoparticles as MRI Blood Pool Contrast Agents with High Relaxivity. J. Mater. Chem. 2012, 22, 14494–14501. [Google Scholar] [CrossRef]
- Pearson, K. Mathematical Contributions to the Theory of Evolution—III. Regression, Heredity, and Panmixia. Philos. Trans. R. Soc. 1895, 191, 229–311. [Google Scholar]
- Pike, J.A.; Styles, I.B.; Rappoport, J.Z.; Heath, J.K. Quantifying Receptor Trafficking and Colocalization with Confocal Microscopy. Methods 2017, 115, 42–54. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelières, F.P. A Guided Tour into Subcellular Colocalization Analysis in Light Microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of Co-localization of Objects in Dual-colour Confocal Images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Aaron, J.S.; Taylor, A.B.; Chew, T.L. Image Co-Localization—Co-Occurrence versus Correlation. J. Cell Sci. 2018, 131, jcs211847. [Google Scholar] [CrossRef] [PubMed]
- Costes, S.V.; Daelemans, D.; Cho, E.H.; Dobbin, Z.; Pavlakis, G.; Lockett, S. Automatic and Quantitative Measurement of Protein-Protein Colocalization in Live Cells. Biophys. J. 2004, 86, 3993–4003. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiba, M.; Guedes, G.; Karpov, D.; Feliu, N.; L. Cortajarena, A.; Parak, W.J.; Sanchez-Cano, C. Probing the Cellular Fate of the Protein Corona around Nanoparticles with Nanofocused X-ray Fluorescence Imaging. Int. J. Mol. Sci. 2024, 25, 528. https://doi.org/10.3390/ijms25010528
Skiba M, Guedes G, Karpov D, Feliu N, L. Cortajarena A, Parak WJ, Sanchez-Cano C. Probing the Cellular Fate of the Protein Corona around Nanoparticles with Nanofocused X-ray Fluorescence Imaging. International Journal of Molecular Sciences. 2024; 25(1):528. https://doi.org/10.3390/ijms25010528
Chicago/Turabian StyleSkiba, Marvin, Gabriela Guedes, Dmitry Karpov, Neus Feliu, Aitziber L. Cortajarena, Wolfgang J. Parak, and Carlos Sanchez-Cano. 2024. "Probing the Cellular Fate of the Protein Corona around Nanoparticles with Nanofocused X-ray Fluorescence Imaging" International Journal of Molecular Sciences 25, no. 1: 528. https://doi.org/10.3390/ijms25010528
APA StyleSkiba, M., Guedes, G., Karpov, D., Feliu, N., L. Cortajarena, A., Parak, W. J., & Sanchez-Cano, C. (2024). Probing the Cellular Fate of the Protein Corona around Nanoparticles with Nanofocused X-ray Fluorescence Imaging. International Journal of Molecular Sciences, 25(1), 528. https://doi.org/10.3390/ijms25010528





