Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Criteria
2.3. Article Selection
2.4. Data Extraction
2.5. Additional Analysis
2.6. Risk of Bias
2.7. Synthesis of Data
2.8. Assessment of Certainty of the Evidence
3. Results
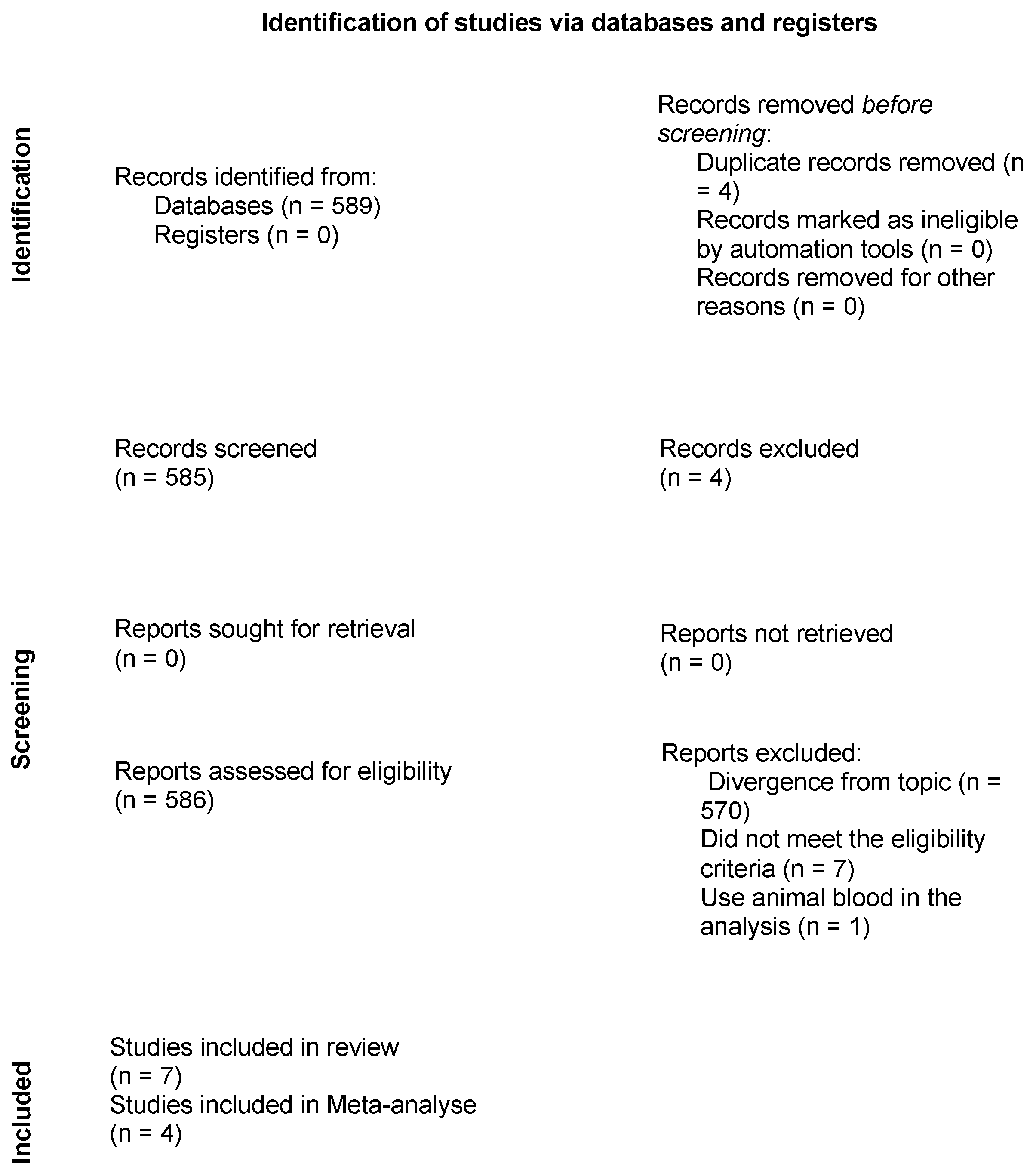
3.1. Article Selection
3.2. Characteristics of Studies
3.3. Release of Growth Factors
3.4. Platelet Recovery
3.5. Cell Viability
3.6. Cell Distribution
3.7. Fibrin Network
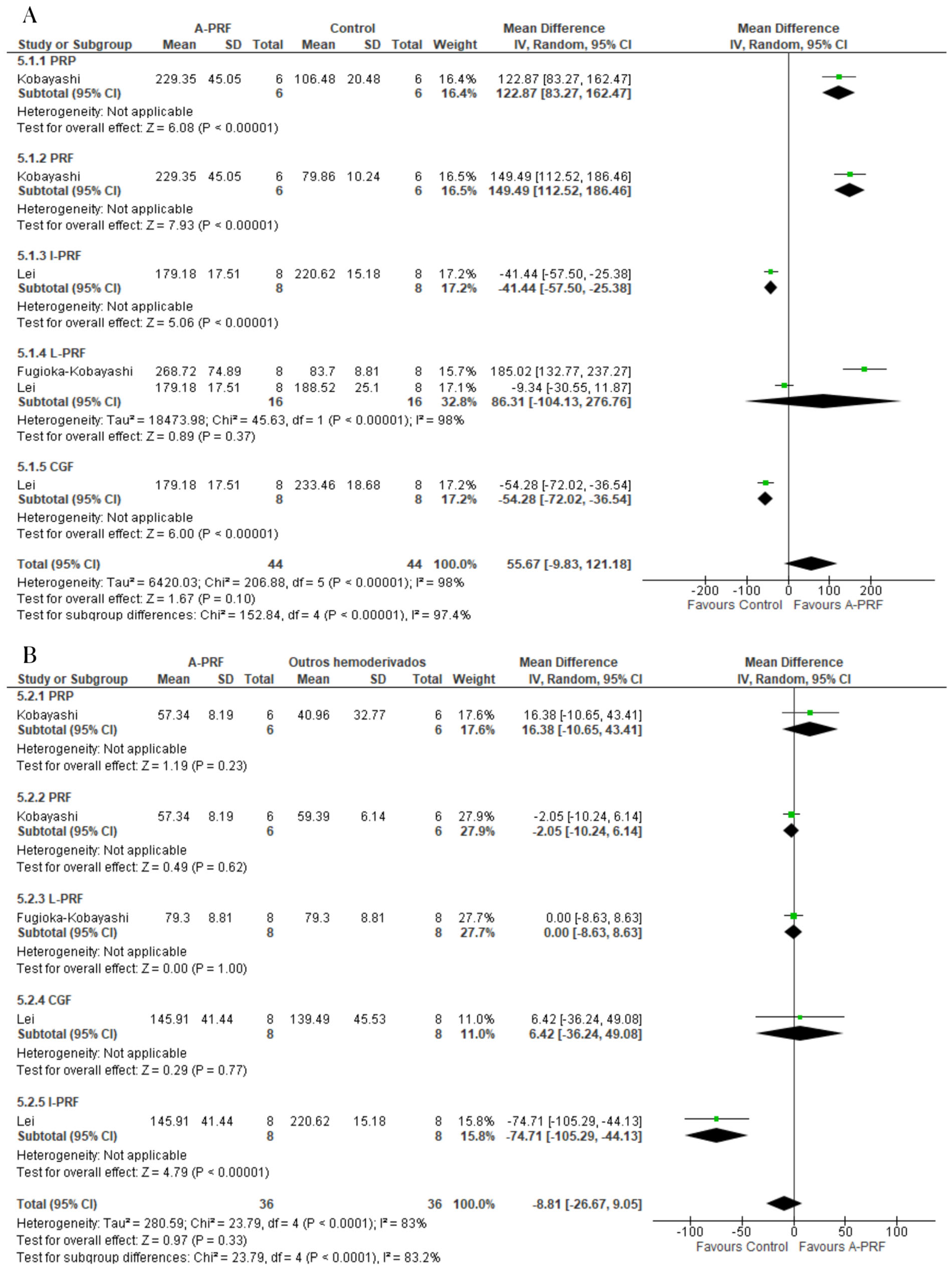
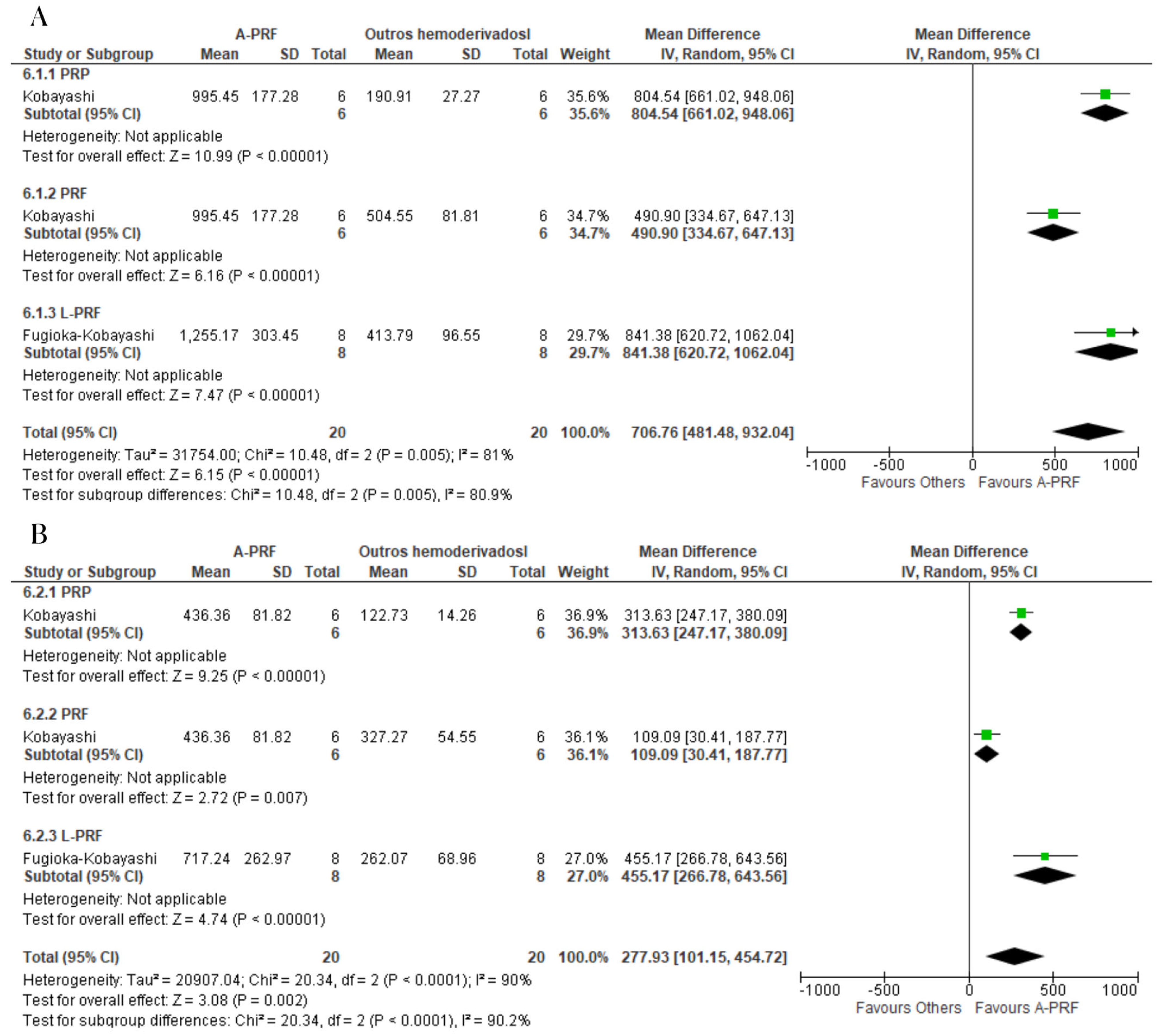
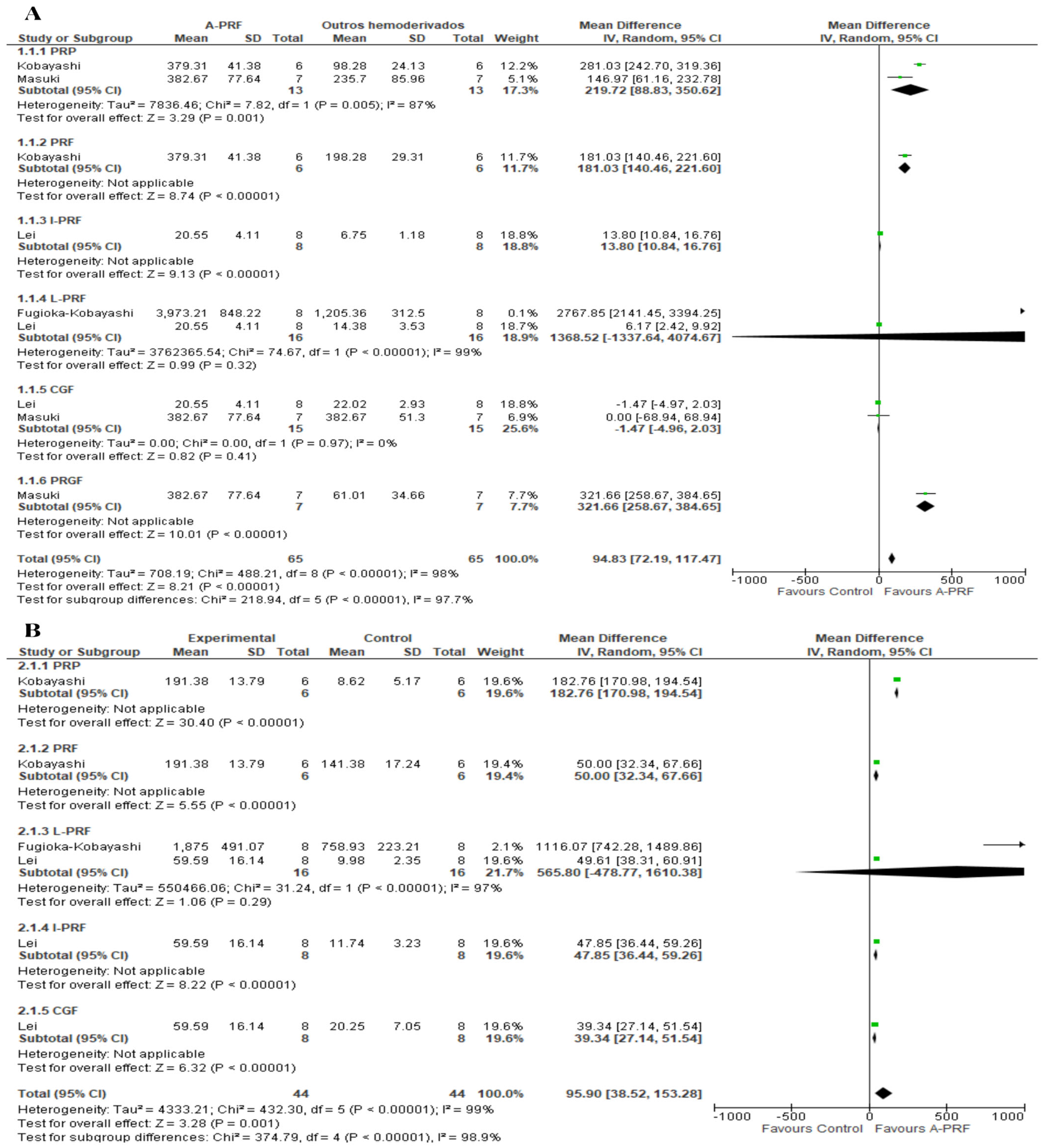
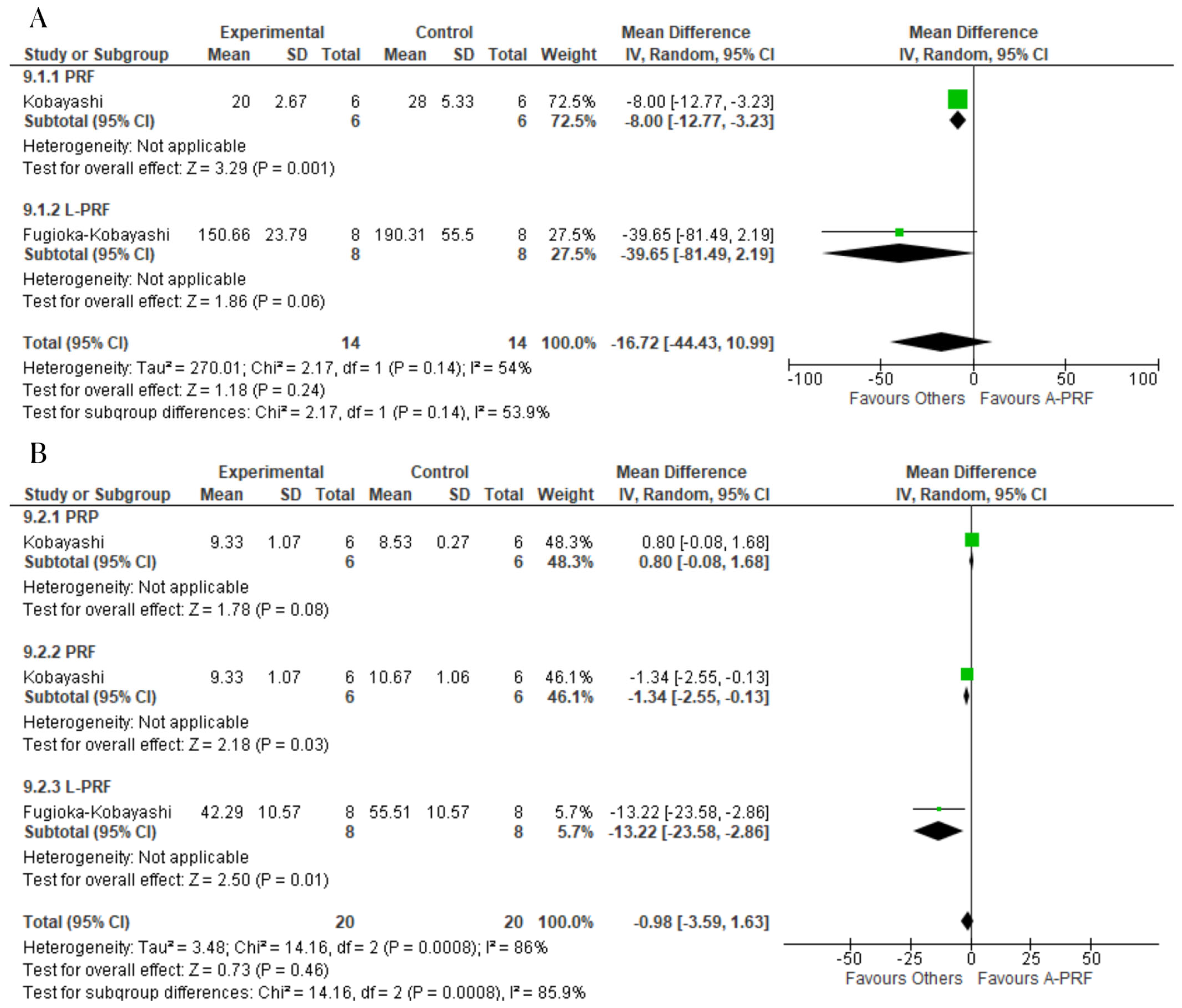
3.8. Meta-Analysis
3.9. Grading of Recommendations, Assessment, Development and Evaluation (GRADE)
4. Discussion
5. Conclusions
Protocol and Registration
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck, T.M.; Mealey, B.L. Histologic analysis of healing after tooth extraction with ridge preservation using mineralized human bone allograft. J. Periodontol. 2010, 81, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 82–91. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [PubMed]
- Melville, J.C.; Mañón, V.A.; Blackburn, C.; Young, S. Current methods of maxillofacial tissue engineering. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Dohan, E.D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Hoboken, NJ, USA; Hartlebury, UK, 2023; Available online: https://training.cochrane.org/handbook (accessed on 20 October 2023).
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2022; in press. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin with the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Yu, Y.; Han, J.; Shi, D.; Sun, W.; Zhang, D.; Chen, L. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J. Periodontol. 2020, 91, 462–472. [Google Scholar] [CrossRef]
- Pitzurra, L.; Jansen, I.D.C.; de Vries, T.J.; Hoogenkamp, M.A.; Loos, B.G. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J. Periodontal Res. 2020, 55, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Esfahrood, Z.R.; Ardakani, M.T.; Shokri, M.; Shokri, M. Effects of leukocyte-platelet-rich fibrin and advanced platelet-rich fibrin on the viability and migration of human gingival fibroblasts. J. Indian Soc. Periodontol. 2020, 24, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; The GRADE Working Group: London, UK; Denver, CO, USA, 2013; Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 20 October 2023).
- GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. 2023. Available online: https://www.gradepro.org/ (accessed on 20 October 2023).
- Santos Pereira, V.B.; Barbirato, D.D.S.; Lago, C.A.P.D.; Vasconcelos, B.C.D.E. The Effect of Advanced Platelet-Rich Fibrin in Tissue Regeneration in Reconstructive and Graft Surgery: Systematic Review. J. Craniofacial Surg. 2023, 34, 1217–1221. [Google Scholar] [CrossRef]
- Tabrizi, R.; Arabion, H.; Karagah, T. Does platelet-rich fibrin increase the stability of implants in the posterior of the maxilla? A split-mouth randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2018, 47, 672–675. [Google Scholar] [CrossRef]
- Shah, R.M.G.T.; Thomas, R.; Mehta, D.S. An update on the protocols and biologic actions of platelet rich fibrin in dentistry. Eur. J. Prosthodont. Restor. Dent. 2017, 25, 64–72. [Google Scholar]
- Gaur, S.; Chugh, A.; Chaudhry, K.; Bajpayee, A.; Jain, G.; Chugh, V.K.; Kumar, P.; Singh, S. Efficacy and Safety of Concentrated Growth Factors and Platelet- Rich Fibrin on Stability and Bone Regeneration in Patients with Immediate Dental Implants: A Randomized Controlled Trial. Int. J. Oral Maxillofac. Implant. 2022, 37, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Benalcázar Jalkh, E.B.; Tovar, N.; Arbex, L.; Kurgansky, G.; Torroni, A.; Gil, L.F.; Wall, B.; Kohanbash, K.; Bonfante, E.A.; Coelho, P.G.; et al. Effect of leukocyte-platelet-rich fibrin in bone healing around dental implants placed in conventional and wide osteotomy sites: A pre-clinical study. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Lyris, V.; Millen, C.; Besi, E.; Pace-Balzan, A. Effect of leukocyte and platelet rich fibrin (L-PRF) on stability of dental implants. A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, 1130–1139. [Google Scholar] [CrossRef] [PubMed]




| Terms | Database |
|---|---|
| (guided tissue regeneration OR tissue regeneration, guided OR regeneration, guided tissue OR guided regeneration, periodontal OR regeneration, periodontal guided tissue OR guided periodontal tissue regeneration OR periodontal guided tissue regeneration OR bone regeneration OR bone regenerations OR regeneration, bone OR regenerations, bone OR osteoconduction OR bone transplantation OR grafting, bone OR bone grafting OR transplantation, bone OR alveolar bone grafting OR alveolar cleft grafting OR alveolar ridge augmentation OR alveolar ridge augmentations OR augmentation, alveolar ridge OR augmentations, alveolar ridge OR ridge augmentation, alveolar OR ridge augmentations, alveolar OR mandibular ridge augmentation OR augmentation, mandibular ridge OR augmentations, mandibular ridge OR mandibular ridge augmentations OR ridge augmentation, mandibular OR ridge augmentations, mandibular OR maxillary ridge augmentation OR augmentation, maxillary ridge OR augmentations, maxillary ridge OR maxillary ridge augmentations OR ridge augmentation, maxillary OR ridge augmentations, maxillary OR sinus floor augmentation OR augmentation, sinus floor OR augmentations, sinus floor OR floor augmentation, sinus OR floor augmentations, sinus OR sinus floor augmentations OR maxillary sinus floor augmentation OR sinus augmentation therapy OR augmentation therapies, sinus OR augmentation therapy, sinus OR sinus augmentation therapies OR therapies, sinus augmentation OR therapy, sinus augmentation OR regeneration OR guided tissue regeneration, periodontal) AND (A-PRF OR advanced platelet-rich fibrin) AND (L-PRF OR platelet-rich fibrin OR fibrin, platelet-rich OR platelet rich fibrin OR L-PRF OR leukocyte- and platelet-rich fibrin OR leucocyte and platelet rich fibrin) | PubMed|Medline, Web of Science |
| (ALL (tissue AND regeneration) OR ALL (bone AND conduction) OR ALL (bone AND graft) OR ALL (alveolar AND ridge AND augmentation) OR ALL (sinus AND floor AND augmentation) AND ALL (platelet-rich AND fibrin) OR ALL (platelet-rich AND fibrin AND matrix)) AND (LIMIT-TO (DOCTYPE, “ar”)) | Scopus |
| ((“tissue regeneration” OR “bone conduction” OR “bone graft” OR “alveolar ridge augmentation” OR “sinus floor augmentation”) AND (“platelet-rich fibrin” OR “platelet rich fibrin matrix”)) AND “article”/it | Embase |
| (tissue regeneration OR bone conduction OR bone graft OR alveolar ridge augmentation OR sinus floor augmentation) AND (platelet-rich fibrin OR platelet rich plasma) | LILACS|bvs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, V.B.S.; Lago, C.A.P.; Almeida, R.d.A.C.; Barbirato, D.d.S.; Vasconcelos, B.C.d.E. Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 482. https://doi.org/10.3390/ijms25010482
Pereira VBS, Lago CAP, Almeida RdAC, Barbirato DdS, Vasconcelos BCdE. Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(1):482. https://doi.org/10.3390/ijms25010482
Chicago/Turabian StylePereira, Vinicius Balan Santos, Carlos Augusto Pereira Lago, Renata de Albuquerque Cavalcanti Almeida, Davi da Silva Barbirato, and Belmiro Cavalcanti do Egito Vasconcelos. 2024. "Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 25, no. 1: 482. https://doi.org/10.3390/ijms25010482
APA StylePereira, V. B. S., Lago, C. A. P., Almeida, R. d. A. C., Barbirato, D. d. S., & Vasconcelos, B. C. d. E. (2024). Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 25(1), 482. https://doi.org/10.3390/ijms25010482





