Melatonin as a Promising Anti-Inflammatory Agent in an In Vivo Animal Model of Sepsis-Induced Rat Liver Damage
Abstract
:1. Introduction
2. Results
2.1. Blood Analysis
2.2. Hematological Indices
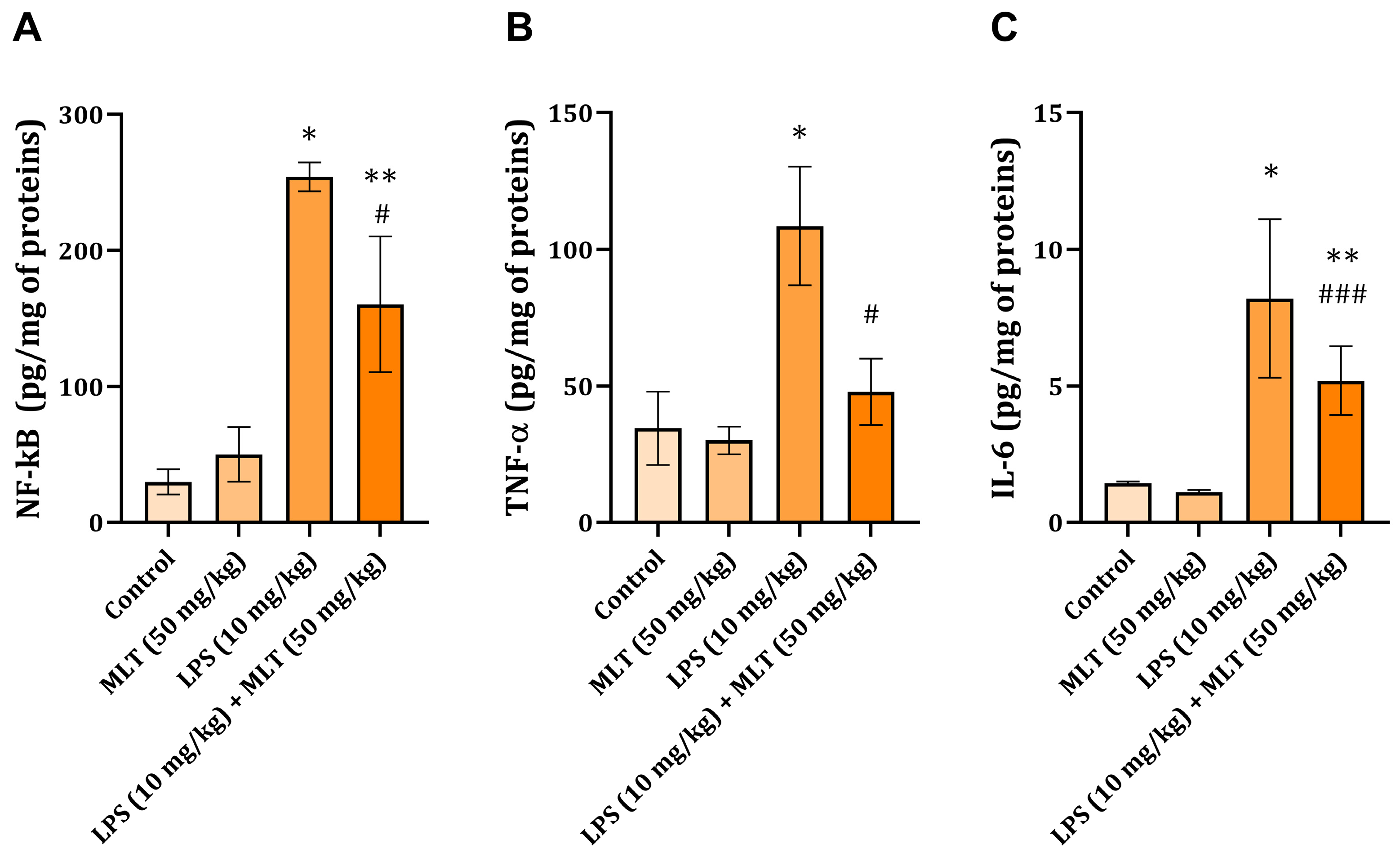
2.3. Liver NF-kB, TNF-α, and IL-6 Levels
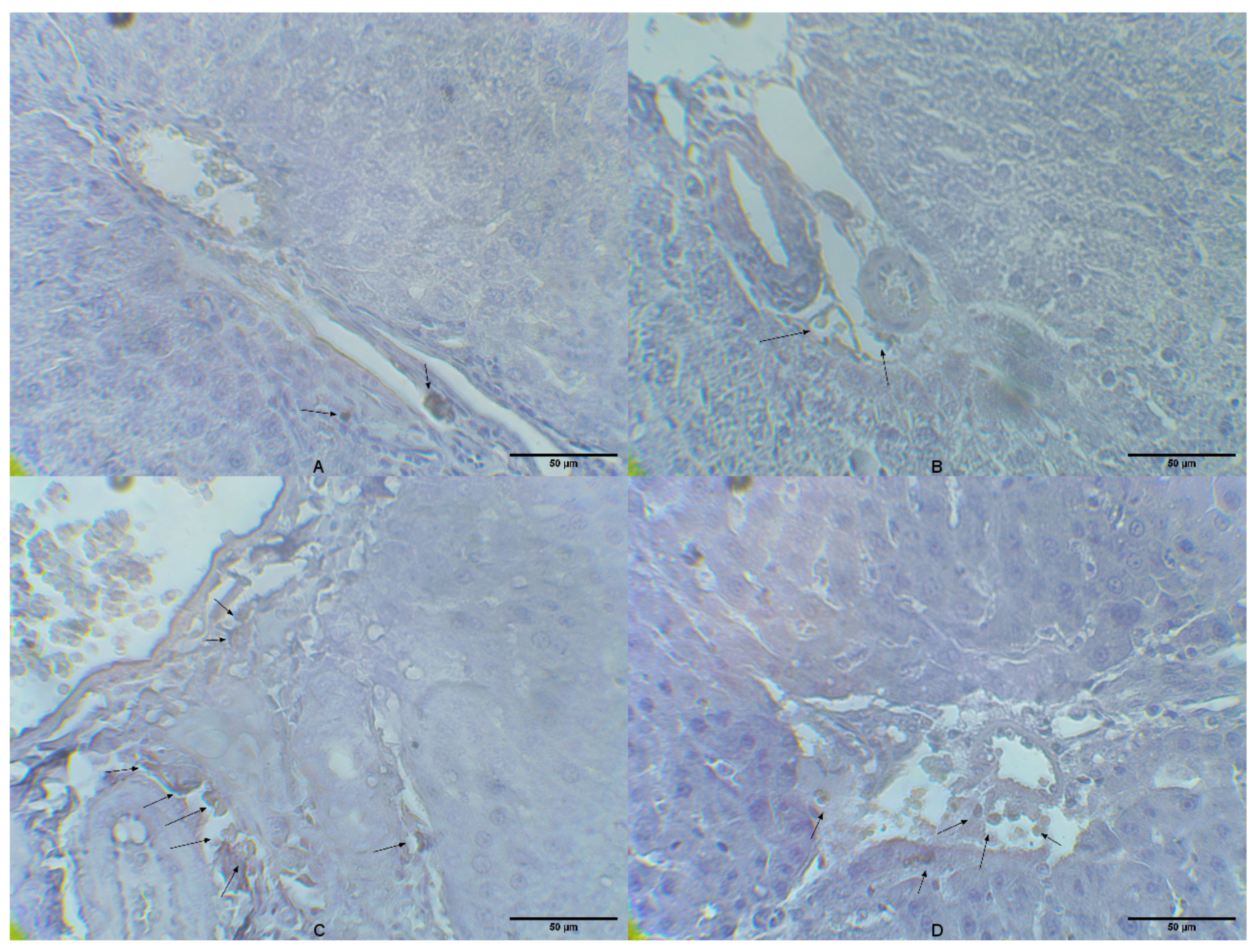
2.4. Liver Tissue CD14 Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Housing
4.3. Experimental Design
4.4. Serum and Blood Hematological Analysis
4.5. Liver Tissue Homogenate Preparation
4.6. ELISA Assays
4.7. Liver Tissue Immunohistochemical Analyses
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annane, D.; Bellissant, E.; Cavaillon, J.M. Septic shock. Lancet 2005, 365, 63–78. [Google Scholar] [CrossRef]
- Gardlund, B.; Sjolin, J.; Nilsson, A.; Roll, M.; Wickerts, C.-J.; Wretlind, B. Plasma levels of cytokines in primary septic shock in humans: Correlation with disease severity. J. Infect. Dis. 1995, 172, 296–301. [Google Scholar] [CrossRef]
- Skirecki, T.; Borkowska-Zielińska, U.; Złotorowicz, M.; Hoser, G. Sepsis immunopathology: Perspectives of monitoring and modulation of the immune disturbances. Arch. Immunol. Ther. Exp. 2012, 60, 123–135. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Annane, D.; Sanquer, S.; Sébille, V.; Faye, A.; Djuranovic, D.; Raphaël, J.C.; Gajdos, P.; Bellisasant, E. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet 2000, 355, 1143–1148. [Google Scholar] [CrossRef]
- Sokolović, D.; Lazarević, M.; Milić, D.; Stanojković, Z.; Mitić, K.; Sokolović, D.T. Melatonin arrests excessive inflammatory response and apoptosis in lipopolysaccharide-damaged rat liver: A deeper insight into its mechanism of action. Tissue Cell 2022, 79, 101904. [Google Scholar] [CrossRef]
- Sokolović, D.; Lazarević, M.; Milić, D.; Stanojković, Z.; Petković, M.N.; Stojanović, N.M.; Sokolović, D.T. Melatonin reduces lipopolysaccharide-induced kidney damage in rats Acta Med. Medianae 2023, 62, 15–20. [Google Scholar]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef]
- Deng, M.; Scott, M.J.; Loughran, P.; Gibson, G.; Sodhi, C.; Watkins, S.; Hackam, D.; Billiar, T.R. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J. Immunol. 2013, 190, 5152–5160. [Google Scholar] [CrossRef] [PubMed]
- Gaitantzi, H.; Karch, J.; Germann, L.; Cai, C.; Rausch, V.; Birgin, E.; Rahbari, N.; Seitz, T.; Hellerbrand, C.; König, C.; et al. BMP-9 modulates the hepatic responses to LPS. Cells 2020, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Akashi, S.; Ogata, H.; Kirikae, F.; Kawasaki, K.; Nishijima, M.; Shimazu, R.; Nagai, Y.; Fukudome, K.; Kimoto, M.; Miyake, K. Regulatory roles for CD14 and phosphatidylinositol in the signaling via Toll-like receptor 4- MD-2. Biochem. Biophys. Res. Commun. 2000, 268, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.A.; Reiter, R.J.; Lupi, L.A. Melatonin as a promising agent to treat ovarian cancer: Molecular mechanisms. Carcinogenesis 2017, 38, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chang, Q.; Cai, J.; Fan, J.; Zhang, X.; Xu, G. Protective effects of melatonin on retinal inflammation and oxidative stress in experimental diabetic retinopathy. Oxid. Med. Cell Longev. 2016, 2016, 3528274. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, H.; Zhang, L.; Zhang, L.; Zhang, H.; Wang, D.; Tao, X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed. Pharmacother. 2019, 117, 109150. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Mohammed, Y.H.; Marik, P.E. Melatonin for the treatment of sepsis: The scientific rationale. J. Thorac. Dis. 2020, 12, S54–S65. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Lapshina, E.A.; Zavodnik, L.B.; Labieniec, M.; Bryszewska, M.; Reiter, R.J. Hypochlorous acid-induced oxidative stress in Chinese hamster B14 cells: Viability, DNA and protein damage and the protective action of melatonin. Mutat. Res. 2004, 559, 39–48. [Google Scholar] [CrossRef]
- Sener, G.; Toklu, H.; Kapucu, C.; Ercan, F.; Erkanli, G.; KaÇmaz, A.; Tilki, M.; Yegen, B.Ç. Melatonin protects against organ injury in rat model of sepsis. Surg. Today 2005, 35, 52–59. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Naji, L.; Fernández-Santos, J.M.; Martín-Lacave, I.; Guerrero, J.M.; Calvo, J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: Regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 2005, 39, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Rigato, O.; Silva, E.; Kallas, E.G.; Brunialti, M.K.C.; Martins, P.S.; Salomao, R. Pathogenetic aspects of sepsis and possible targets for adjunctive therapy. Curr. Drug Targets Immune. Endocrin. Metabol. Dis. 2001, 1, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Karzai, W.; Reinhart, K. Modulation of neutrophil function in sepsis. In Yearbook of Intensive Care and Emergency Medicine; Vincent, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 41–50. [Google Scholar]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.M.; Conti, A. Melatonin in relation to the immune system. In Melatonin. Biosynthesis, Physiological Effect, and Clinical Applications; Yu, H.S., Reiter, R., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 289–311. [Google Scholar]
- Ben-Nathan, D.; Maestroni, G.J.M.; Lustig, S.; Conti, A. Protective effects of melatonin in mice infected with encephalitis viruses. Arch. Virol. 1996, 140, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity: III. Melatonin antagonizes the immunosuppressive effect of acute stress via an opiatergic mechanism. Immunology 1988, 63, 465–469. [Google Scholar] [PubMed]
- Pioli, C.; Caroleo, M.C.; Nistico, G.; Doria, G. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int. J. Immunopharmacol. 1993, 15, 463–468. [Google Scholar] [CrossRef]
- Mavrommatis, A.C.; Theodoridis, T.; Orfanidou, A.; Roussos, C.; Christopoulou-Kokkinou, V.; Zakynthinos, S. Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit. Care Med. 2000, 28, 451–457. [Google Scholar] [CrossRef]
- Zielinski, T.; Wachowicz, B.; Saluk-Juszczak, J.; Kaca, W. Poly-saccharide part of Proteus mirabilis lipopolysaccharide may beresponsible for the stimulation of platelet adhesion to collagen. Platelets 2002, 13, 419–424. [Google Scholar] [CrossRef]
- Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M.; Stafforini, D.M. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit. Care Med. 2002, 30, S294–S301. [Google Scholar] [CrossRef]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12, S16–S21. [Google Scholar] [CrossRef]
- Ljungström, L.; Pernestig, A.-K.; Jacobsson, G.; Andersson, R.; Usener, B.; Tilevik, D. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS ONE 2017, 12, e0181704. [Google Scholar] [CrossRef]
- Vincent, J.L.; Lefrant, J.-Y.; Kotfis, K.; Nanchal, R.; Martin-Loeches, I.; Wittebole, X.; Sakka, S.G.; Pickkers, P.; Moreno, R.; Sakr, Y. Comparison of European ICU patients in 2012 (ICON) versus 2002 (SOAP). Intensive Care Med. 2018, 44, 337–344. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Gall, J.-R.L.; Payen, D. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, X.; Zhang, W. Platelet-to lymphocyte ratio as a prognostic predictor of mortality for sepsis: Interaction effect with disease severity-a retrospective study. BMJ Open 2019, 9, e022896. [Google Scholar] [CrossRef]
- Bharadwaj, N.; Singh, H.B.; Anjum, S.; Tadury, A. Does the platelet to neutrophil ratio and platelet to lymphocyte ratio predict newborn sepsis? A case control study. Paripex Indian J. Res. 2018, 7, 192–193. [Google Scholar]
- May, M.J.; Ghosh, S. Signal transduction through NF-kappa B. Immunol. Today 1998, 19, 80–88. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Reiter, R.J. Pharmacological actions of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2002, 2, 153–165. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Reiter, R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef]
- Mohan, N.; Sadeghi, K.; Reiter, R.J.; Meltz, M.L. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem. Mol. Biol. Int. 1995, 37, 1063–1070. [Google Scholar]
- Gilad, E.; Wong, H.R.; Zingarelli, B.; Virag, L.; O’Connor, M.; Salzman, A.L.; Szabo, C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: Role of inhibition of NF kappa B activation. FASEB J. 1998, 12, 685–693. [Google Scholar] [CrossRef]
- Bruck, R.; Aeed, H.; Avni, Y.; Shirin, H.; Matas, Z.; Shahmurov, M.; Avinoach, I.; Zozulya, G.; Weizman, N.; Hochman, A. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J. Hepatol. 2004, 40, 86–93. [Google Scholar] [CrossRef]
- Srinivasan, V.; Mohamed, M.; Kato, H. Melatonin in bacterial and viral infections with focus on sepsis: A review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 30–39. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Ničković, V.P.; Novaković, T.; Lazarević, S.; Šulović, L.; Živković, Z.; Živković, J.; Mladenović, B.; Stojanović, N.M.; Petrović, V.; Sokolović, D.T. Pre- vs. posttreatment with melatonin in CCl4-induced liver damage: Oxidative stress inferred from biochemical and pathohistological studies. Life Sci. 2018, 202, 28–34. [Google Scholar] [CrossRef]
- Maestroni, G.J. Melatonin as a therapeutic agent in experimental endotoxic shock. J. Pineal Res. 1996, 20, 84–89. [Google Scholar] [CrossRef]
- Sener, G.; Tosun, O.; Sehirli, A.O.; Kacmaz, A.; Arbak, S.; Ersoy, Y.; Ayanoglu-Dulger, G. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003, 72, 2707–2718. [Google Scholar] [CrossRef]
- Dong, X.; Liu, J.; Xu, Y.; Cao, H. Role of macrophages in experimental liver injury and repair in mice. Exp. Ther. Med. 2019, 17, 3835–3847. [Google Scholar] [CrossRef]
- Selzner, M.; Clavien, P.-A. Failure of regeneration of the steatotic rat liver-disruption at two different levels of the regeneration pathway. Hepatology 2000, 31, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Debonera, F.; Aldeguer, X.; Shen, X.; Gelman, A.E.; Gao, F.; Que, X.; Greenbaum, L.E.; Furth, E.E.; Taub, R.; Olthoff, K.M. Activation of interleukin-6/STAT3 and liver regeneration following transplantation. J. Surg. Res. 2001, 96, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Z.; Liang, Y.L.; Wang, H.; Chen, X.; Huang, Y.Y.; Zhang, Z.H.; Chen, Y.H.; Zhang, C.; Zhao, M.; Xu, D.X.; et al. Melatonin modulates TLR4-mediated inflammatory genes through MYD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW 264.7 cells. J. Pineal Res. 2012, 53, 325–334. [Google Scholar] [CrossRef]
- Choi, E.Y.; Jin, J.Y.; Lee, J.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. Melatonin inhibits Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-6 in murine macrophages by suppressing NF-kappa B and STAT1 activity. J. Pineal Res. 2011, 50, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Cao, X.J.; Wei, W. Melatonin decreases tlr3-mediated inflammatory factor expression via inhibition of NF-kappa B activation in respiratory syncytial virus-infected raw 264.7 macrophages. J. Pineal Res. 2008, 45, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tocharus, J.; Khonthun, C.; Chongthammakun, S.; Govitrapong, P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res. 2010, 48, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, J.B.; Frozza, R.L.; Horn, A.P.; Comiran, R.A.; Bernardi, A.; Campos, M.M.; Battastini, A.M.; Salbego, C. Amyloid-beta neurotoxicity in organotypic culture is attenuated by melatonin: Involvement of gsk-3beta, tau and neuroinflammation. J. Pineal Res. 2010, 48, 230–238. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Randjelović, P.J.; Mladenović, M.Z.; Ilić, I.R.; Petrović, V.; Stojiljković, N.; Ilić, S.; Radulović, N.S. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 2019, 133, 110794. [Google Scholar] [CrossRef]
| Parameter | Control | Melatonin | LPS | LPS+MLT |
|---|---|---|---|---|
| Red blood cell number (×1012/L) | 7.53 ± 0.44 | 7.20 ± 0.2 | 7.31 ± 0.61 | 6.97 ± 0.73 |
| Hematocrit | 0.42 ± 0.02 | 0.41 ± 0.01 | 0.40 ± 0.03 | 0.39 ± 0.04 |
| Hemoglobin (g/L) | 141.9 ± 6.2 | 136.8 ± 1.5 | 142.1 ± 7.4 | 137.9 ± 5.1 |
| MCV (fl) | 55.7 ± 1.52 | 57.6 ± 2.35 | 55.5 ± 1.98 | 57.3 ± 1.95 |
| MCH (pg) | 18.85 ± 0.41 | 19.03 ± 0.59 | 19.23 ± 0.72 | 19.13 ± 0.48 |
| MCHC (g/L) | 338 ± 7.3 | 330 ± 7.9 | 347 ± 12.8 | 334 ± 5.3 |
| RDW (%) | 20.9 ± 0.94 | 20.03 ± 1.15 | 20.31 ± 0.93 | 19.66 ± 0.75 |
| White blood cell number (×109/L) | 6.55 ± 3.22 | 7.94 ± 1.4 | 3.95 ± 1.73 | 5.68 ± 1.59 |
| Neutrophiles number (×109/L) | 0.77 ± 0.53 | 0.67 ± 0.24 | 1.4 ± 0.84 *** | 1.31 ± 0.45 |
| Lymphocytes number (×109/L) | 4.84 ± 1.83 | 6.39 ± 0.85 * | 2.37 ± 0.58 * | 3.61 ± 0.83 # |
| Monocytes number (×109/L) | 0.11 ± 0.07 | 0.17 ± 0.06 | 0.19 ± 0.06 | 0.25 ± 0.02 # |
| Eosinophiles number (×109/L) | 0.025 ± 0.018 | 0.026 ± 0.005 | 0.041 ± 0.014 | 0.07 ± 0.064 |
| Basophile number (×109/L) | 0.041 ± 0.026 | 0.043 ± 0.005 | 0.073 ± 0.052 | 0.075 ± 0.046 |
| Platelets number (×109/L) | 313 ± 89 | 437 ± 159 | 168 ± 74 *** | 126 ± 21 |
| MPV (fl) | 7.98 ± 1.00 | 8.13 ± 0.61 | 9.09 ± 1.49 | 10.43 ± 1.37 |
| ALT (U/L) | 50.1 ± 7.2 | 54.3 ± 0.9 | 281.4 ± 76.2 * | 133 ± 27.3 # |
| AST (U/L) | 162 ± 18.9 | 158 ± 24.3 | 663 ± 119.7 * | 571 ± 211 * |
| LDH (U/L) | 2008 ± 114 | 2004 ± 298 | 2477 ± 446 *** | 2171 ± 445 ## |
| Parameter | Control | Melatonin | LPS | LPS+MLT |
|---|---|---|---|---|
| Platelet to lymphocyte ratio (PLR) | 72.2 ± 49.8 | 73.2 ± 49.9 | 77.4 ± 45 | 39.4 ± 11.7 # |
| Neutrophil to lymphocyte ratio (NLR) | 0.15 ± 0.07 | 0.11 ± 0.04 | 0.59 ± 0.32 * | 0.35 ± 0.07 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedeto-Stojanov, D.; Ničković, V.P.; Petrović, G.; Rancić, A.; Grgov, I.; Nikolić, G.R.; Marčetić, Z.P.; Popović, M.R.; Lazarević, M.; Mitić, K.V.; et al. Melatonin as a Promising Anti-Inflammatory Agent in an In Vivo Animal Model of Sepsis-Induced Rat Liver Damage. Int. J. Mol. Sci. 2024, 25, 455. https://doi.org/10.3390/ijms25010455
Benedeto-Stojanov D, Ničković VP, Petrović G, Rancić A, Grgov I, Nikolić GR, Marčetić ZP, Popović MR, Lazarević M, Mitić KV, et al. Melatonin as a Promising Anti-Inflammatory Agent in an In Vivo Animal Model of Sepsis-Induced Rat Liver Damage. International Journal of Molecular Sciences. 2024; 25(1):455. https://doi.org/10.3390/ijms25010455
Chicago/Turabian StyleBenedeto-Stojanov, Daniela, Vanja P. Ničković, Gordana Petrović, Andrija Rancić, Ivan Grgov, Gordana R. Nikolić, Zoran P. Marčetić, Milica R. Popović, Milan Lazarević, Katarina V. Mitić, and et al. 2024. "Melatonin as a Promising Anti-Inflammatory Agent in an In Vivo Animal Model of Sepsis-Induced Rat Liver Damage" International Journal of Molecular Sciences 25, no. 1: 455. https://doi.org/10.3390/ijms25010455
APA StyleBenedeto-Stojanov, D., Ničković, V. P., Petrović, G., Rancić, A., Grgov, I., Nikolić, G. R., Marčetić, Z. P., Popović, M. R., Lazarević, M., Mitić, K. V., & Sokolović, D. (2024). Melatonin as a Promising Anti-Inflammatory Agent in an In Vivo Animal Model of Sepsis-Induced Rat Liver Damage. International Journal of Molecular Sciences, 25(1), 455. https://doi.org/10.3390/ijms25010455






