Effect of Mycolic Acids on Host Immunity and Lipid Metabolism
Abstract
:1. Introduction
2. Results
2.1. The Synthesis of Mycolic Acid
2.2. Immunomodulatory Effects of Glycosylated Mycolic Acids of Mycobacteria
2.2.1. Arabinose Monomycolates (AraMM)
2.2.2. Trehalose Mycolates
2.2.3. Glucose Monomycolate (GMM)
2.3. Immunomodulatory Effects of Non-Glycosylated Mycolic Acids of Mycobacteria
2.3.1. Glycerol Monomycolate (GroMM)
2.3.2. Free Mycolic Acids (fMAs)
2.4. Regulation of Lipid Metabolism in Macrophages by Mycolic Acids
2.4.1. MAs Attract Cholesterol to Release into the Biofilm
2.4.2. MAs Direct Host Macrophages Phenotype
3. Conclusions
- (1)
- Development of novel anti-tuberculosis drugs: MAs represent a reservoir of new targets urgently needed to combat Mtb strains. Targeting MAs biosynthesis and other pathways is important for novel and promising drug candidates being developed. For example, through molecular docking techniques, targeted drugs can be designed to inhibit the transcription of the cmaA2 or mmaA4 genes, thereby greatly diminishing the pathogenicity of Mycobacterium and achieving therapeutic efficacy against tuberculosis;
- (2)
- Vaccine and adjuvant development: The effects of MAs on immunity are wide-ranging, and can mediate the development of innate immunity such as secretion of inflammatory factors by macrophages, neutrophils, or dendritic cells. It can also mediate the generation of adaptive immunity, e.g., effector cells and memory T cells respond rapidly upon re-encountering MAs. The modification of MAs is important for the development of vaccines as well as adjuvants. For example, TDB is a short-acyl-chain structural analogue of TDM, which is currently used as the active ingredient in CAF01, a TB vaccine undergoing phase I clinical trials (Table 1).
- (3)
- MAs may serve as a novel diagnostic marker: MAs appear to be a marker of Mtb adaptation to the external environment. For example, Mtb is converted from TDM to GMM upon cell invasion due to the presence of large amounts of glucose inside the cell. In addition, during the active phase, MAs in Mtb are present in the form of TDM, whereas during the dormant phase, MAs in Mtb exist in a free state. Thus, MAs may be included in studies of markers for detection of latent infection.
- (1)
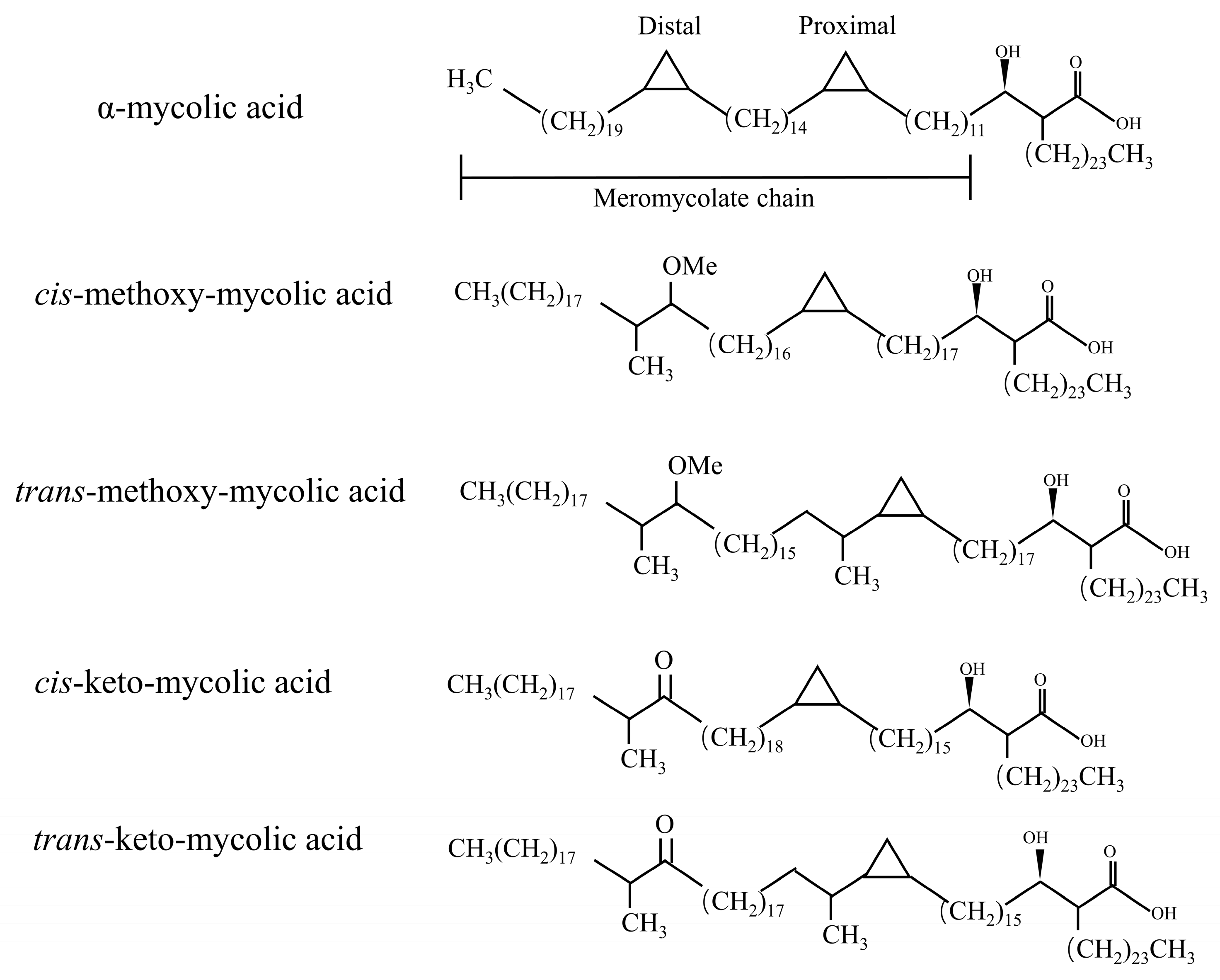
- The functions of many genes involved in the synthesis of MAs are still unknown, e.g., in Mycobacterium, all mycobacteria have two positions, i.e., distal and proximal, which initially contain a double bond that is subsequently modified to cis-cyclopropane, trans-double bond, cyclopropane, epoxide, or hydroxyl groups with adjacent methyl branches. The proteins that catalyze these desaturation/isomerization steps, as well as the underlying mechanisms, remain to be discovered and investigated;
- (2)
- Designing targeted drugs for a single gene may not result in good bacterial inhibition. For example, there are many genes that introduce methoxy or keto groups to MAs, and the absence of a single gene does not inhibit bacterial viability at all;
- (3)
- The inflammatory response induced by MAs has long been recognized as a double-edged sword, and how to rationally control the inflammation induced by MAs is a major problem to be solved in the future. Certainly, we cannot overlook the issue of MA-induced lipid accumulation in macrophages. The substantial lipid content within macrophages provides ample nutrition for Mtb. Balancing inflammation and lipid metabolism is also a challenge that needs to be addressed in the future;
- (4)
- Although Mtb changes the presence of MAs in different environments, it is too difficult to detect the pathogens in clinical practice. If the structure of MAs can be found to be associated with certain proteins in the host body, it seems that it can be an important diagnostic marker to distinguish between latent and active infections.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.Y.; Yates, T.A.; Osman, M.; Warren, R.M.; van der Heijden, Y.; Padayatchi, N.; Nardell, E.A.; Moore, D.; Mathema, B.; Gandhi, N.; et al. Transmission of drug-resistant tuberculosis in HIV-endemic settings. Lancet Infect. Dis. 2019, 19, e77–e88. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, D.W.; Behr, M.A. Are we underestimating the annual risk of infection with Mycobacterium tuberculosis in high-burden settings? Lancet Infect. Dis. 2022, 22, e271–e278. [Google Scholar] [CrossRef] [PubMed]
- Asselineau, J.; Lederer, E. Recent studies on lipid chemistry of the tubercle bacillus. Experientia 1951, 7, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Jankute, M.; Cox, J.A.; Harrison, J.; Besra, G.S. Assembly of the Mycobacterial Cell Wall. Annu. Rev. Microbiol. 2015, 69, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.E.; Gawronski, J.D.; Dejesus, M.A.; Ioerger, T.R.; Akerley, B.J.; Sassetti, C.M. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011, 7, e1002251. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, N.; Fodran, P.; Jayaraman, D.; Buter, J.; Witte, M.D.; Ocampo, T.A.; Moody, D.B.; Van Rhijn, I.; Minnaard, A.J. Total Synthesis of a Mycolic Acid from Mycobacterium tuberculosis. Angew. Chem. Int. Ed. Engl. 2020, 59, 7555–7560. [Google Scholar] [CrossRef]
- Lea-Smith, D.J.; Pyke, J.S.; Tull, D.; McConville, M.J.; Coppel, R.L.; Crellin, P.K. The reductase that catalyzes mycolic motif synthesis is required for efficient attachment of mycolic acids to arabinogalactan. J. Biol. Chem. 2007, 282, 11000–11008. [Google Scholar] [CrossRef] [PubMed]
- Lanéelle, M.A.; Launay, A.; Spina, L.; Marrakchi, H.; Laval, F.; Eynard, N.; LeMAssu, A.; Tropis, M.; Daffé, M.; Etienne, G. A novel mycolic acid species defines two novel genera of the Actinobacteria, Hoyosella and Amycolicicoccus. Microbiology 2012, 158, 843–855. [Google Scholar] [CrossRef]
- Dautin, N.; de Sousa-d’Auria, C.; Constantinesco-Becker, F.; Labarre, C.; Oberto, J.; Li de la Sierra-Gallay, I.; Dietrich, C.; Issa, H.; Houssin, C.; Bayan, N. Mycoloyltransferases: A large and major family of enzymes shaping the cell envelope of Corynebacteriales. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3581–3592. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.Y.; Laval, F.; Lawson, E.H.; Groger, R.K.; Woodruff, A.; Morisaki, J.H.; Cox, J.S.; Daffe, M.; Brown, E.J. Requirement for kasB in Mycobacterium mycolic acid biosynthesis, cell wall impermeability and intracellular survival: Implications for therapy. Mol. Microbiol. 2003, 49, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Lanéelle, M.A.; Eynard, N.; Spina, L.; LeMAssu, A.; Laval, F.; Huc, E.; Etienne, G.; Marrakchi, H.; Daffé, M. Structural elucidation and genomic scrutiny of the C60-C100 mycolic acids of Segniliparus rotundus. Microbiology 2013, 159, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Daffé, M.; Lanéelle, M.A.; Asselineau, C.; Lévy-Frébault, V.; David, H. Taxonomic value of mycobacterial fatty acids: Proposal for a method of analysis. Ann. Microbiol. 1983, 134, 241–256. [Google Scholar]
- Laval, F.; Lanéelle, M.A.; Déon, C.; Monsarrat, B.; Daffé, M. Accurate molecular MAss determination of mycolic acids by MALDI-TOF MAss spectrometry. Anal. Chem. 2001, 73, 4537–4544. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Aoyagi, Y.; Ridell, M.; Minnikin, D.E. Separation and characterization of individual mycolic acids in representative mycobacteria. Microbiology 2001, 147, 1825–1837. [Google Scholar] [CrossRef]
- Li, M.; Huang, Q.; Zhang, W.; Cao, Y.; Wang, Z.; Zhao, Z.; Zhang, X.; Zhang, J. A Novel Acyl-AcpM-Binding Protein Confers Intrinsic Sensitivity to Fatty Acid Synthase Type II Inhibitors in Mycobacterium smegmatis. Front. Microbiol. 2022, 13, 846722. [Google Scholar] [CrossRef]
- Miyauchi, M.; Murata, M.; Shibuya, K.; Koga-Yamakawa, E.; Uenishi, Y.; Kusunose, N.; Sunagawa, M.; Yano, I.; Kashiwazaki, Y. Arabino-mycolates derived from cell-wall skeleton of Mycobacterium bovis BCG as a prominent structure for recognition by host immunity. Drug Discov. Ther. 2011, 5, 130–135. [Google Scholar] [CrossRef]
- Ishikawa, E.; Ishikawa, T.; Morita, Y.S.; Toyonaga, K.; Yamada, H.; Takeuchi, O.; Kinoshita, T.; Akira, S.; Yoshikai, Y.; Yamasaki, S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009, 206, 2879–2888. [Google Scholar] [CrossRef]
- Hoving, J.C.; Wilson, G.J.; Brown, G.D. Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol. 2014, 16, 185–194. [Google Scholar] [CrossRef]
- Hou, H.; Guo, Y.; Chang, Q.; Luo, T.; Wu, X.; Zhao, X. C-type Lectin Receptor: Old Friend and New Player. Med. Chem. 2017, 13, 536–543. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tanaka, T.; Kaisho, T.; Sanjo, H.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Akira, S. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 1999, 163, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Ishikawa, E.; Sakuma, M.; Hara, H.; Ogata, K.; Saito, T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008, 9, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Hollwedel, F.D.; Maus, R.; Stolper, J.; Khan, A.; Stocker, B.L.; Timmer, M.S.M.; Lu, X.; Pich, A.; Welte, T.; Yamasaki, S.; et al. Overexpression of Macrophage-Inducible C-Type Lectin Mincle Aggravates Proinflammatory Responses to Streptococcus pneumoniae with Fatal Outcome in Mice. J. Immunol. 2020, 205, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflamMAsomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, H.; Bodendorfer, B.; Hitchens, K.; Manzanero, S.; Werninghaus, K.; Nimmerjahn, F.; Agger, E.M.; Stenger, S.; Andersen, P.; Ruland, J.; et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 2010, 184, 2756–2760. [Google Scholar] [CrossRef]
- Del Fresno, C.; Iborra, S.; Saz-Leal, P.; Martínez-López, M.; Sancho, D. Flexible Signaling of Myeloid C-Type Lectin Receptors in Immunity and Inflammation. Front. Immunol. 2018, 9, 804. [Google Scholar] [CrossRef]
- Miyake, Y.; Toyonaga, K.; Mori, D.; Kakuta, S.; Hoshino, Y.; Oyamada, A.; Yamada, H.; Ono, K.; Suyama, M.; Iwakura, Y.; et al. C-type lectin MCL is an FcRγ-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 2013, 38, 1050–1062. [Google Scholar] [CrossRef]
- Miyake, Y.; MAsatsugu, O.H.; Yamasaki, S. C-Type Lectin Receptor MCL Facilitates Mincle Expression and Signaling through Complex Formation. J. Immunol. 2015, 194, 5366–5374. [Google Scholar] [CrossRef]
- Kerscher, B.; Dambuza, I.M.; Christofi, M.; Reid, D.M.; Yamasaki, S.; Willment, J.A.; Brown, G.D. Signalling through MyD88 drives surface expression of the mycobacterial receptors MCL (Clecsf8, Clec4d) and Mincle (Clec4e) following microbial stimulation. Microbes Infect. 2016, 18, 505–509. [Google Scholar] [CrossRef]
- Gavin, A.L.; Hoebe, K.; Duong, B.; Ota, T.; Martin, C.; Beutler, B.; Nemazee, D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 2006, 314, 1936–1938. [Google Scholar] [CrossRef]
- Axelrod, S.; Oschkinat, H.; Enders, J.; Schlegel, B.; Brinkmann, V.; Kaufmann, S.H.; Haas, A.; Schaible, U.E. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol. 2008, 10, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, M.B.; Aliprantis, A.; Hu, B.; Glimcher, L.H. Calcineurin regulates innate antifungal immunity in neutrophils. J. Exp. Med. 2010, 207, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.B.; Kang, J.S.; Yan, J.J.; Lee, M.S.; Jeon, B.Y.; Cho, S.N.; Kim, Y.J. Neutrophils Promote Mycobacterial Trehalose Dimycolate-Induced Lung Inflammation via the Mincle Pathway. PLoS Pathog. 2012, 8, e1002614. [Google Scholar] [CrossRef] [PubMed]
- Middlebrook, G.; Dubos, R.J.; Pierce, C. Virulence and morphological characteristics of mammalian Tubercle bacilli. J. Exp. Med. 1947, 86, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.S.; Cox, J.S.; Jacobs, W.R., Jr. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 2000, 5, 717–727. [Google Scholar] [CrossRef]
- Rao, V.; Fujiwara, N.; Porcelli, S.A.; Glickman, M.S. Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J. Exp. Med. 2005, 201, 535–543. [Google Scholar] [CrossRef]
- Greinert, U.; Ernst, M.; Schlaak, M.; Entzian, P. Interleukin-12 as successful adjuvant in tuberculosis treatment. Eur. Respir. J. 2001, 17, 1049–1051. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Triebold, K.J.; Sypek, J.; Wolf, S.; Bloom, B.R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 1995, 155, 2515–2524. [Google Scholar] [CrossRef]
- Dao, D.N.; Sweeney, K.; Hsu, T.; Gurcha, S.S.; Nascimento, I.P.; Roshevsky, D.; Besra, G.S.; Chan, J.; Porcelli, S.A.; Jacobs, W.R. Mycolic acid modification by the mmaA4 gene of M. tuberculosis modulates IL-12 production. PLoS Pathog. 2008, 4, e1000081. [Google Scholar] [CrossRef]
- Rao, V.; Gao, F.; Chen, B.; Jacobs, W.R., Jr.; Glickman, M.S. Trans-cyclopropanation of mycolic acids on trehalose dimycolate suppresses Mycobacterium tuberculosis-induced inflammation and virulence. J. Clin. Investig. 2006, 116, 1660–1667. [Google Scholar] [CrossRef]
- Su, C.C.; Klenotic, P.A.; Bolla, J.R.; Purdy, G.E.; Robinson, C.V.; Yu, E.W. MmpL3 is a lipid transporter that binds trehalose monomycolate and phosphatidylethanolamine. Proc. Natl. Acad. Sci. USA 2019, 116, 11241–11246. [Google Scholar] [CrossRef] [PubMed]
- Pohane, A.A.; Carr, C.R.; Garhyan, J.; Swarts, B.M.; Siegrist, M.S. Trehalose Recycling Promotes Energy-Efficient Biosynthesis of the Mycobacterial Cell Envelope. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Naka, T.; Doi, T.; Yano, I. Direct molecular MAss determination of trehalose monomycolate from 11 species of mycobacteria by MALDI-TOF MAss spectrometry. Microbiology 2005, 151, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef] [PubMed]
- Agger, E.M.; Rosenkrands, I.; Hansen, J.; Brahimi, K.; Vandahl, B.S.; Aagaard, C.; Werninghaus, K.; Kirschning, C.; Lang, R.; Christensen, D.; et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): A versatile adjuvant for vaccines with different immunological requirements. PLoS ONE 2008, 3, e3116. [Google Scholar] [CrossRef]
- Pahari, S.; Negi, S.; Aqdas, M.; Arnett, E.; Schlesinger, L.S.; Agrewala, J.N. Induction of autophagy through CLEC4E in combination with TLR4: An innovative strategy to restrict the survival of Mycobacterium tuberculosis. Autophagy 2020, 16, 1021–1043. [Google Scholar] [CrossRef] [PubMed]
- Schweneker, K.; Gorka, O.; Schweneker, M.; Poeck, H.; Tschopp, J.; Peschel, C.; Ruland, J.; Gross, O. The mycobacterial cord factor adjuvant analogue trehalose-6,6’-dibehenate (TDB) activates the Nlrp3 inflamMAsome. Immunobiology 2013, 218, 664–673. [Google Scholar] [CrossRef]
- Desel, C.; Werninghaus, K.; Ritter, M.; Jozefowski, K.; Wenzel, J.; Russkamp, N.; Schleicher, U.; Christensen, D.; Wirtz, S.; Kirschning, C.; et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS ONE 2013, 8, e53531. [Google Scholar] [CrossRef]
- Matsunaga, I.; Naka, T.; Talekar, R.S.; McConnell, M.J.; Katoh, K.; Nakao, H.; Otsuka, A.; Behar, S.M.; Yano, I.; Moody, D.B.; et al. Mycolyltransferase-mediated glycolipid exchange in Mycobacteria. J. Biol. Chem. 2008, 283, 28835–28841. [Google Scholar] [CrossRef]
- Tima, H.G.; Al Dulayymi, J.R.; Denis, O.; Lehebel, P.; Baols, K.S.; Mohammed, M.O.; L’Homme, L.; Sahb, M.M.; Potemberg, G.; Legrand, S.; et al. Inflammatory Properties and Adjuvant Potential of Synthetic Glycolipids Homologous to Mycolate Esters of the Cell Wall of Mycobacterium tuberculosis. J. Innate Immun. 2017, 9, 162–180. [Google Scholar] [CrossRef]
- James, C.A.; Xu, Y.; Aguilar, M.S.; Jing, L.; Layton, E.D.; Gilleron, M.; Minnaard, A.J.; Scriba, T.J.; Day, C.L.; Warren, E.H.; et al. CD4 and CD8 co-receptors modulate functional avidity of CD1b-restricted T cells. Nat. Commun. 2022, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Moody, D.B.; Guy, M.R.; Grant, E.; Cheng, T.Y.; Brenner, M.B.; Besra, G.S.; Porcelli, S.A. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J. Exp. Med. 2000, 192, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Sugita, M.; Matsunaga, I.; Naka, T.; Sato, A.; Kawashima, T.; Shimizu, K.; Takahashi, H.; Norose, Y.; Yano, I. Temperature-dependent biosynthesis of glucose monomycolate and its recognition by CD1-restricted T cells. Biochem. Biophys. Res. Commun. 2005, 337, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.; Alderwick, L.J.; Allnutt, J.A.; Gabasova, E.; Watson, R.; Hatch, K.A.; Clark, S.O.; Jeeves, R.E.; Marriott, A.; Rayner, E.; et al. Non-replicating Mycobacterium tuberculosis elicits a reduced infectivity profile with corresponding modifications to the cell wall and extracellular matrix. PLoS ONE 2014, 9, e87329. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Morita, D.; Fujiwara, N.; Mori, D.; Nakamura, T.; Harashima, H.; Yamasaki, S.; Sugita, M. Glycerol monomycolate is a novel ligand for the human, but not mouse macrophage inducible C-type lectin, Mincle. J. Biol. Chem. 2014, 289, 15405–15412. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Mori, D.; Yamasaki, S. Recognition of Mycobacterial Lipids by Immune Receptors. Trends Immunol. 2017, 38, 66–76. [Google Scholar] [CrossRef]
- Korf, J.; Stoltz, A.; Verschoor, J.; De Baetselier, P.; Grooten, J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur. J. Immunol. 2005, 35, 890–900. [Google Scholar] [CrossRef]
- Finelli, D.; Rollinson, S.; Harris, J.; Jones, M.; Richardson, A.; Gerhard, A.; Snowden, J.; Mann, D.; Pickering-Brown, S. TREM2 analysis and increased risk of Alzheimer’s disease. Neurobiol. Aging 2015, 36, 546.e9–546.e13. [Google Scholar] [CrossRef]
- Kiialainen, A.; Hovanes, K.; Paloneva, J.; Kopra, O.; Peltonen, L. Dap12 and Trem2, molecules involved in innate immunity and neurodegeneration, are co-expressed in the CNS. Neurobiol. Dis. 2005, 18, 314–322. [Google Scholar] [CrossRef]
- Ulland, T.K.; Song, W.M.; Huang, S.C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663.e613. [Google Scholar] [CrossRef]
- Lee, C.Y.D.; Daggett, A.; Gu, X.; Jiang, L.L.; Langfelder, P.; Li, X.; Wang, N.; Zhao, Y.; Park, C.S.; Cooper, Y.; et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron 2018, 97, 1032–1048.e1035. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Prinz, M.; Stagi, M.; Chechneva, O.; Neumann, H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007, 4, e124. [Google Scholar] [CrossRef] [PubMed]
- Dabla, A.; Liang, Y.C.; Rajabalee, N.; Irwin, C.; Moonen, C.G.J.; Willis, J.V.; Berton, S.; Sun, J. TREM2 Promotes Immune Evasion by Mycobacterium tuberculosis in Human Macrophages. mBio 2022, 13, e0145622. [Google Scholar] [CrossRef] [PubMed]
- Iizasa, E.; Chuma, Y.; Uematsu, T.; Kubota, M.; Kawaguchi, H.; Umemura, M.; Toyonaga, K.; Kiyohara, H.; Yano, I.; Colonna, M.; et al. TREM2 is a receptor for non-glycosylated mycolic acids of mycobacteria that limits anti-mycobacterial macrophage activation. Nat. Commun. 2021, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Vander Beken, S.; Al Dulayymi, J.R.; Naessens, T.; Koza, G.; Maza-Iglesias, M.; Rowles, R.; Theunissen, C.; De Medts, J.; Lanckacker, E.; Baird, M.S.; et al. Molecular structure of the Mycobacterium tuberculosis virulence factor, mycolic acid, determines the elicited inflammatory pattern. Eur. J. Immunol. 2011, 41, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Sambandan, D.; Dao, D.N.; Weinrick, B.C.; Vilchèze, C.; Gurcha, S.S.; Ojha, A.; Kremer, L.; Besra, G.S.; Hatfull, G.F.; Jacobs, W.R., Jr. Keto-mycolic acid-dependent pellicle formation confers tolerance to drug-sensitive Mycobacterium tuberculosis. mBio 2013, 4, e00222-00213. [Google Scholar] [CrossRef]
- Ojha, A.K.; Trivelli, X.; Guerardel, Y.; Kremer, L.; Hatfull, G.F. Enzymatic hydrolysis of trehalose dimycolate releases free mycolic acids during mycobacterial growth in biofilms. J. Biol. Chem. 2010, 285, 17380–17389. [Google Scholar] [CrossRef]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef]
- Venkateswaran, A.; Laffitte, B.A.; Joseph, S.B.; Mak, P.A.; Wilpitz, D.C.; Edwards, P.A.; Tontonoz, P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. USA 2000, 97, 12097–12102. [Google Scholar] [CrossRef] [PubMed]
- Gatfield, J.; Pieters, J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 2000, 288, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- de Chastellier, C.; Thilo, L. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell Microbiol. 2006, 8, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sassetti, C.M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. USA 2008, 105, 4376–4380. [Google Scholar] [CrossRef] [PubMed]
- Brzostek, A.; Pawelczyk, J.; Rumijowska-Galewicz, A.; Dziadek, B.; Dziadek, J. Mycobacterium tuberculosis is able to accumulate and utilize cholesterol. J. Bacteriol. 2009, 191, 6584–6591. [Google Scholar] [CrossRef]
- Puissegur, M.P.; Botanch, C.; Duteyrat, J.L.; Delsol, G.; Caratero, C.; Altare, F. An in vitro dual model of mycobacterial granuloMAs to investigate the molecular interactions between mycobacteria and human host cells. Cell Microbiol. 2004, 6, 423–433. [Google Scholar] [CrossRef]
- Pieters, J. Mycobacterium tuberculosis and the macrophage: Maintaining a balance. Cell Host Microbe 2008, 3, 399–407. [Google Scholar] [CrossRef]
- Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Dkhar, H.K.; Nanduri, R.; Mahajan, S.; Dave, S.; Saini, A.; Somavarapu, A.K.; Arora, A.; Parkesh, R.; Thakur, K.G.; Mayilraj, S.; et al. Mycobacterium tuberculosis keto-mycolic acid and macrophage nuclear receptor TR4 modulate foamy biogenesis in granuloMAs: A case of a heterologous and noncanonical ligand-receptor pair. J. Immunol. 2014, 193, 295–305. [Google Scholar] [CrossRef]
- Vermeulen, I.; Baird, M.; Al-Dulayymi, J.; Smet, M.; Verschoor, J.; Grooten, J. Mycolates of Mycobacterium tuberculosis modulate the flow of cholesterol for bacillary proliferation in murine macrophages. J. Lipid Res. 2017, 58, 709–718. [Google Scholar] [CrossRef] [PubMed]
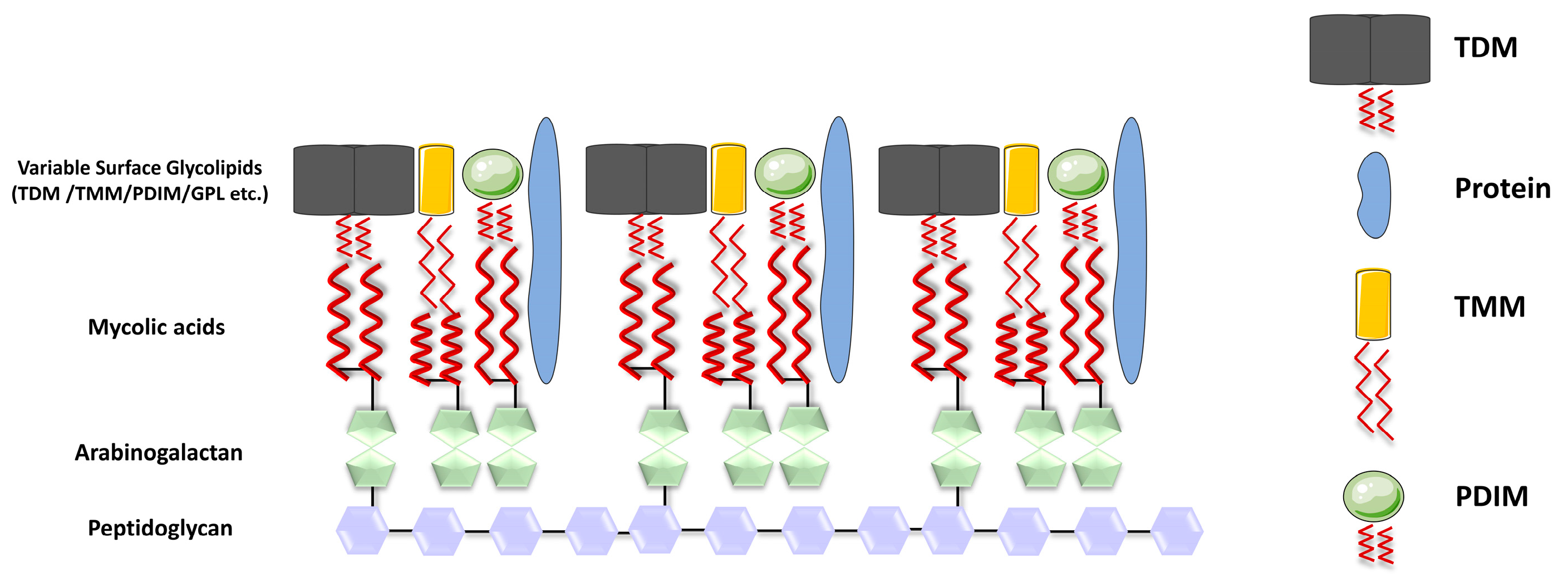

| Type of Mycolic Acids | Receptor | Immunomodulatory Action | Ref. |
|---|---|---|---|
| Trehalose dimycolate (TDM) | Mincle, MCL | 1. Production of inflammatory cytokines; 2. Promotes neutrophil adhesion. | [23,24,31] |
| Trehalose dibehenate (TDB) | Mincle | 1. Production of inflammatory cytokines; 2. Promoted macrophage induced autophagy | [41,43] |
| Glucose monomycolate (GMM) | CD-1 | 1. Activation of adaptive immune; 2. As a good indicator of local invasion of mycobacteria | [48,49,50] |
| Glycerol monomycolate (GroMM) | Mincle, CD-1 | 1. Production of inflammatory cytokines; 2. Distinguishing latent and active tuberculosis | [52,53] |
| Free mycolic acids (fMAs) | TREM2 | 1. Inhibition of inflammatory cytokines; 2. Induction of secretion INF-β | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, D.; Zhou, X. Effect of Mycolic Acids on Host Immunity and Lipid Metabolism. Int. J. Mol. Sci. 2024, 25, 396. https://doi.org/10.3390/ijms25010396
Wang H, Liu D, Zhou X. Effect of Mycolic Acids on Host Immunity and Lipid Metabolism. International Journal of Molecular Sciences. 2024; 25(1):396. https://doi.org/10.3390/ijms25010396
Chicago/Turabian StyleWang, Haoran, Dingpu Liu, and Xiangmei Zhou. 2024. "Effect of Mycolic Acids on Host Immunity and Lipid Metabolism" International Journal of Molecular Sciences 25, no. 1: 396. https://doi.org/10.3390/ijms25010396
APA StyleWang, H., Liu, D., & Zhou, X. (2024). Effect of Mycolic Acids on Host Immunity and Lipid Metabolism. International Journal of Molecular Sciences, 25(1), 396. https://doi.org/10.3390/ijms25010396







