Metabolomic Analysis of the Effect of Freezing on Leaves of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings
Abstract
:1. Introduction
2. Results
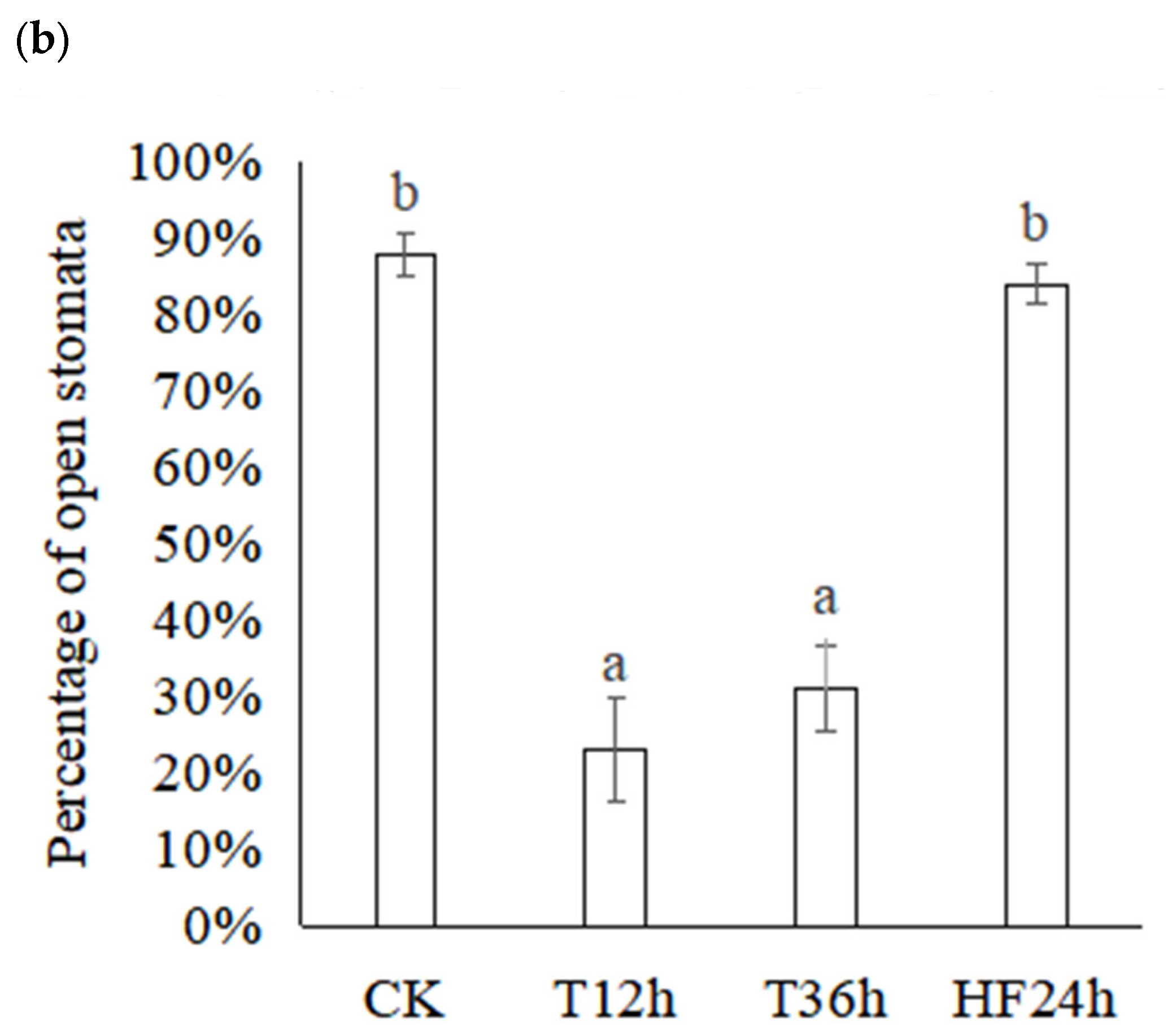
2.1. Effect of Freezing on Morphology and Leaf Stomata of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings
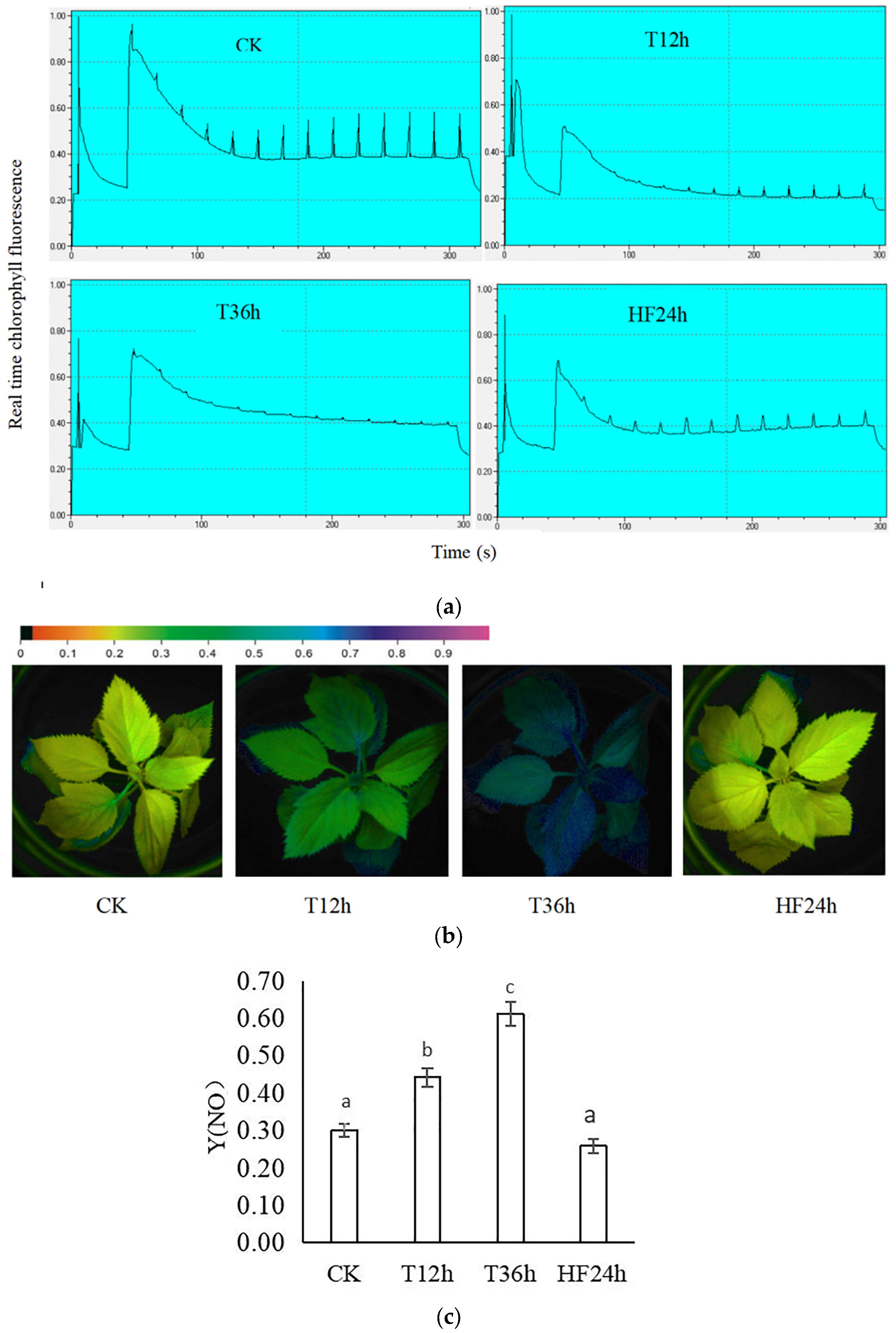
2.2. Effect of Freezing on Chlorophyll Fluorescence of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings
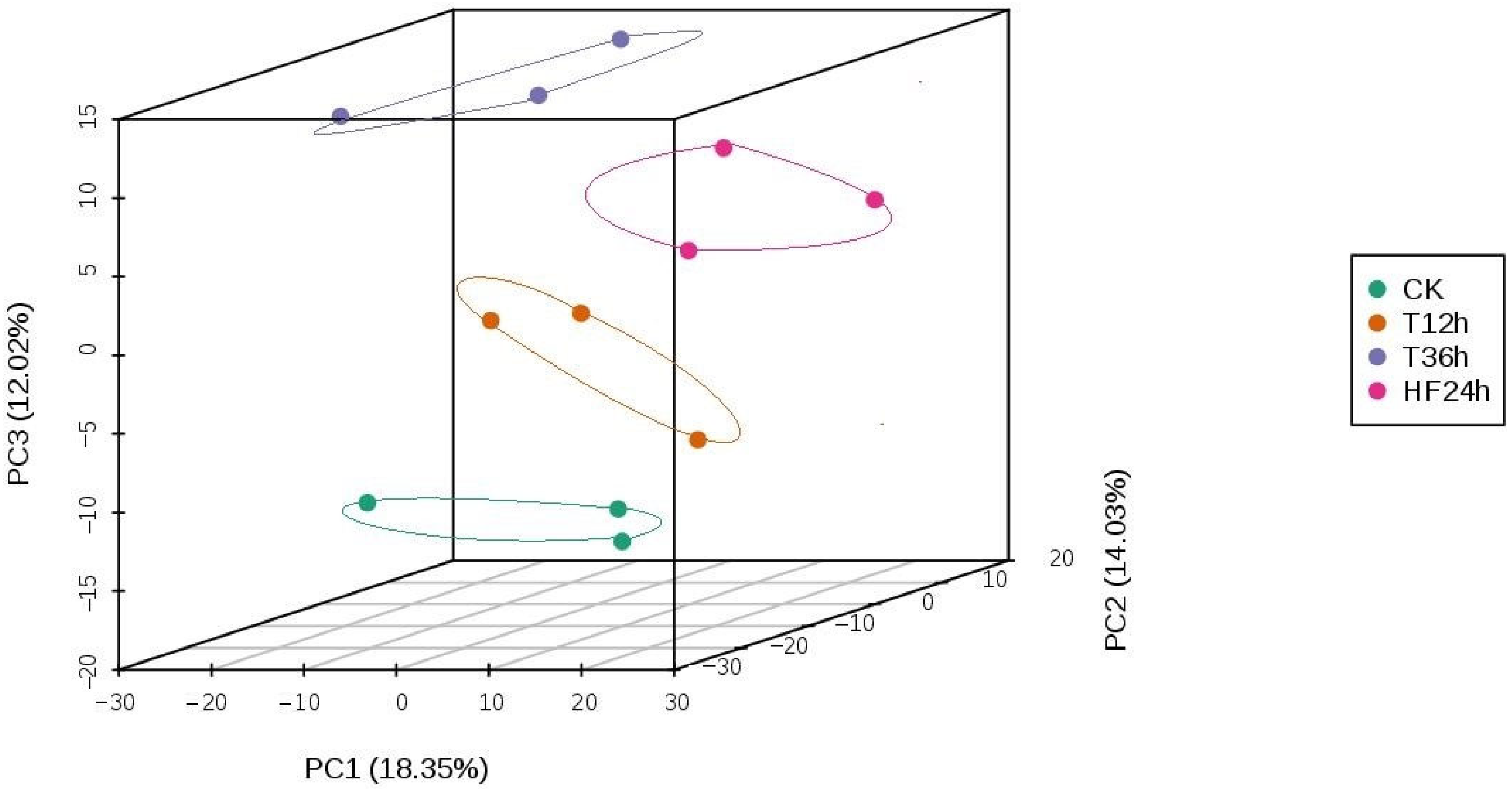
2.3. Metabolomics Data Analysis
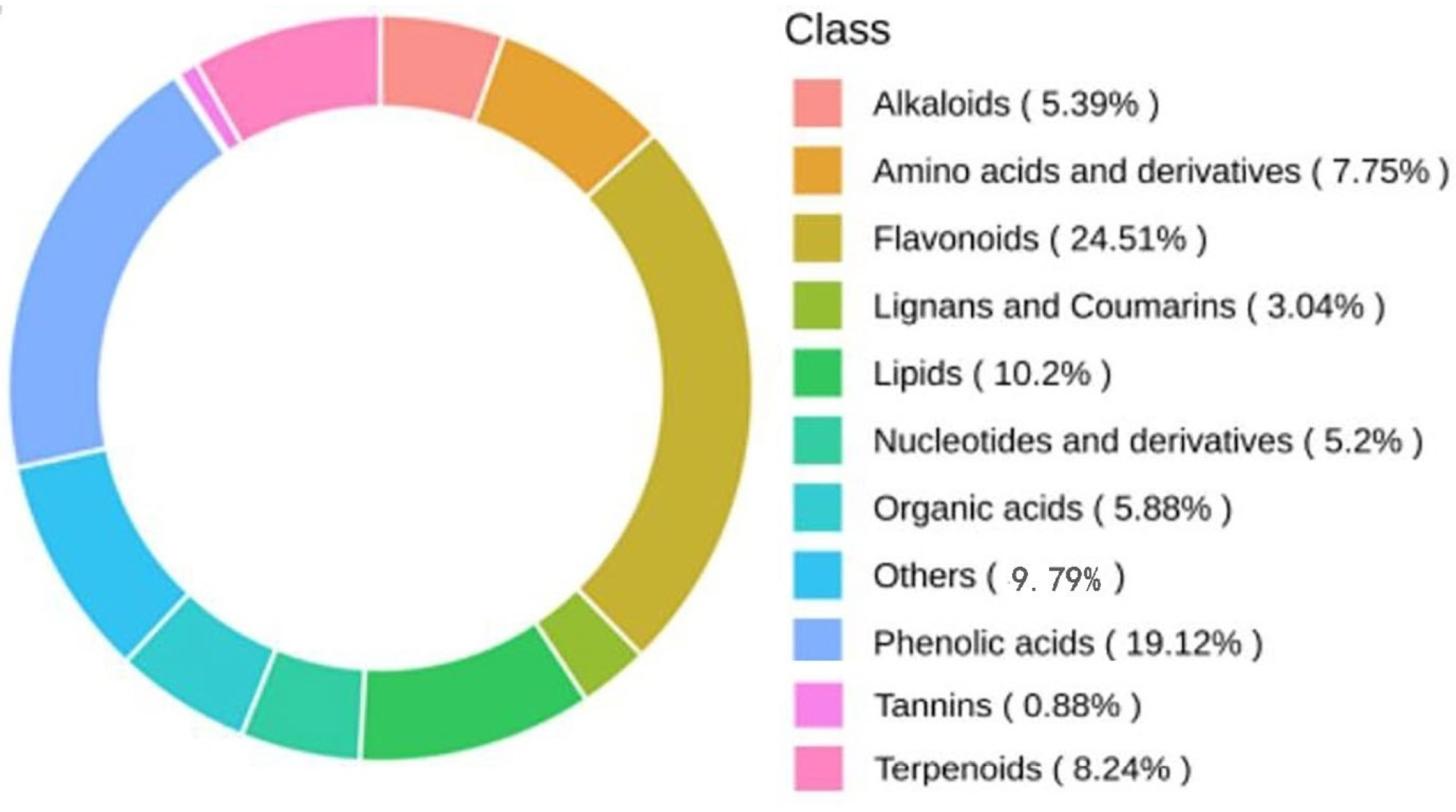
2.3.1. Metabolite Classification of Leaf Metabolites in Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings under Freezing Stress
2.3.2. Qualitative Analysis of Leaf Metabolites in Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings under Freezing Stress
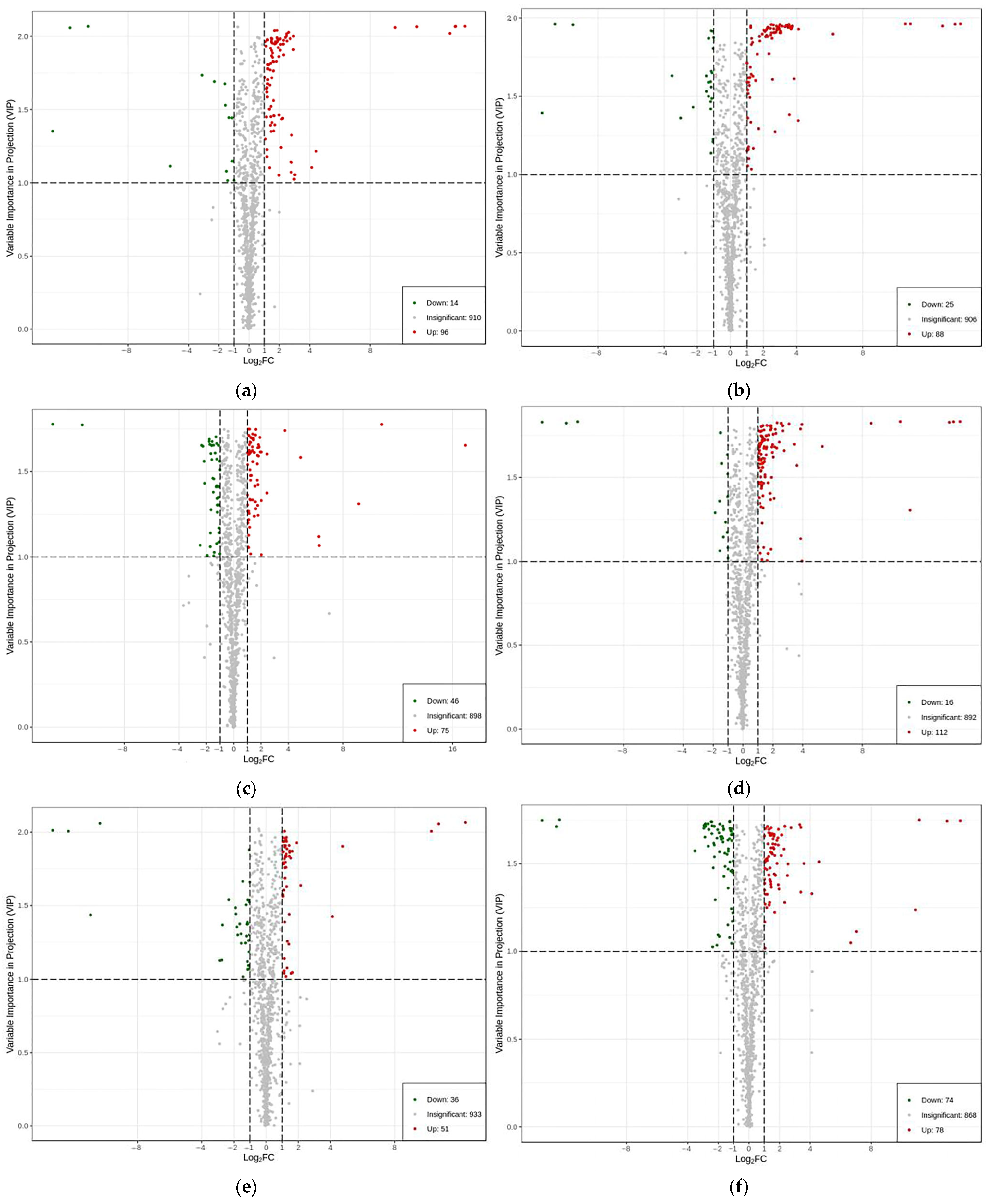
2.3.3. Metabolite Differential Analysis
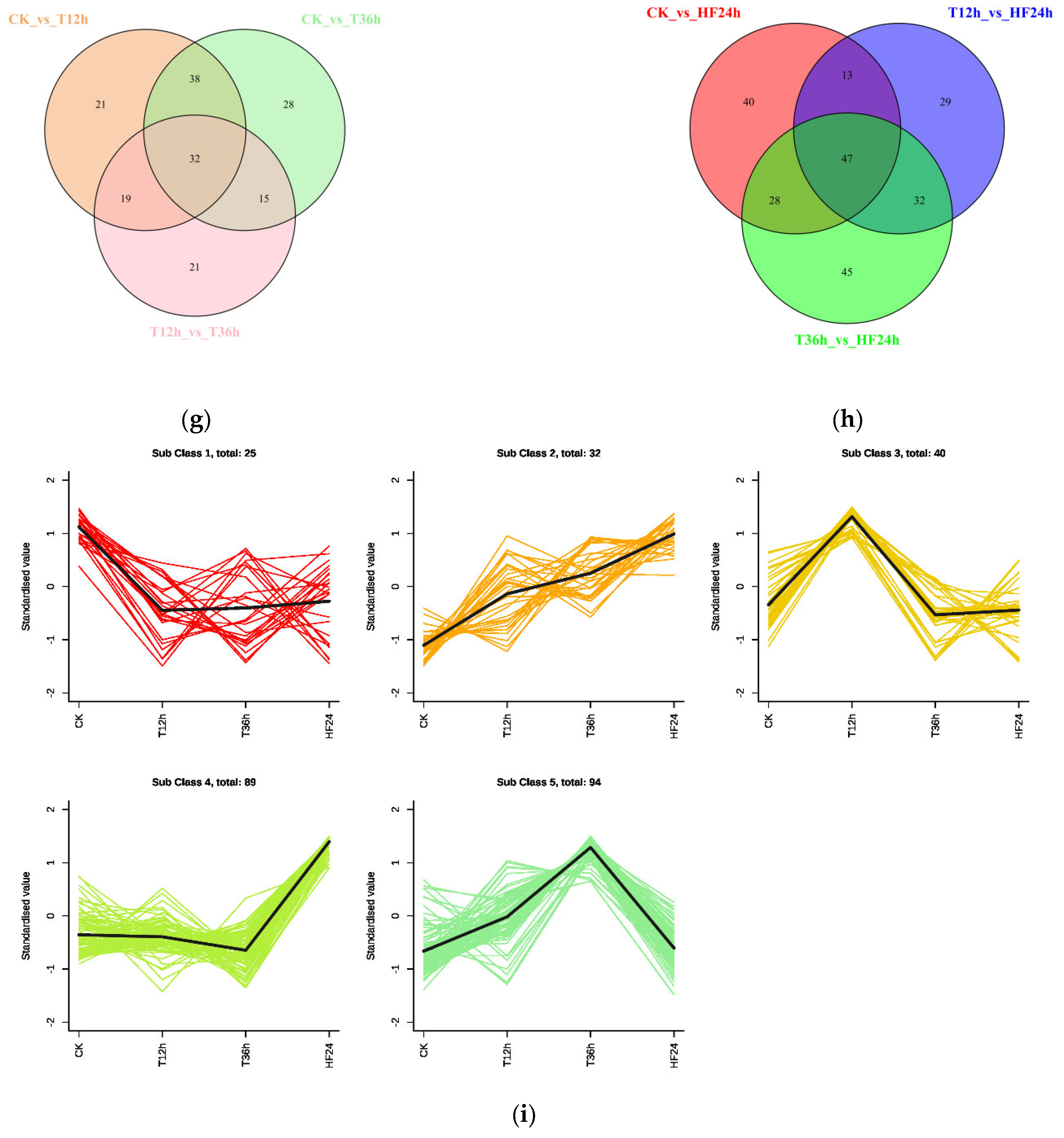
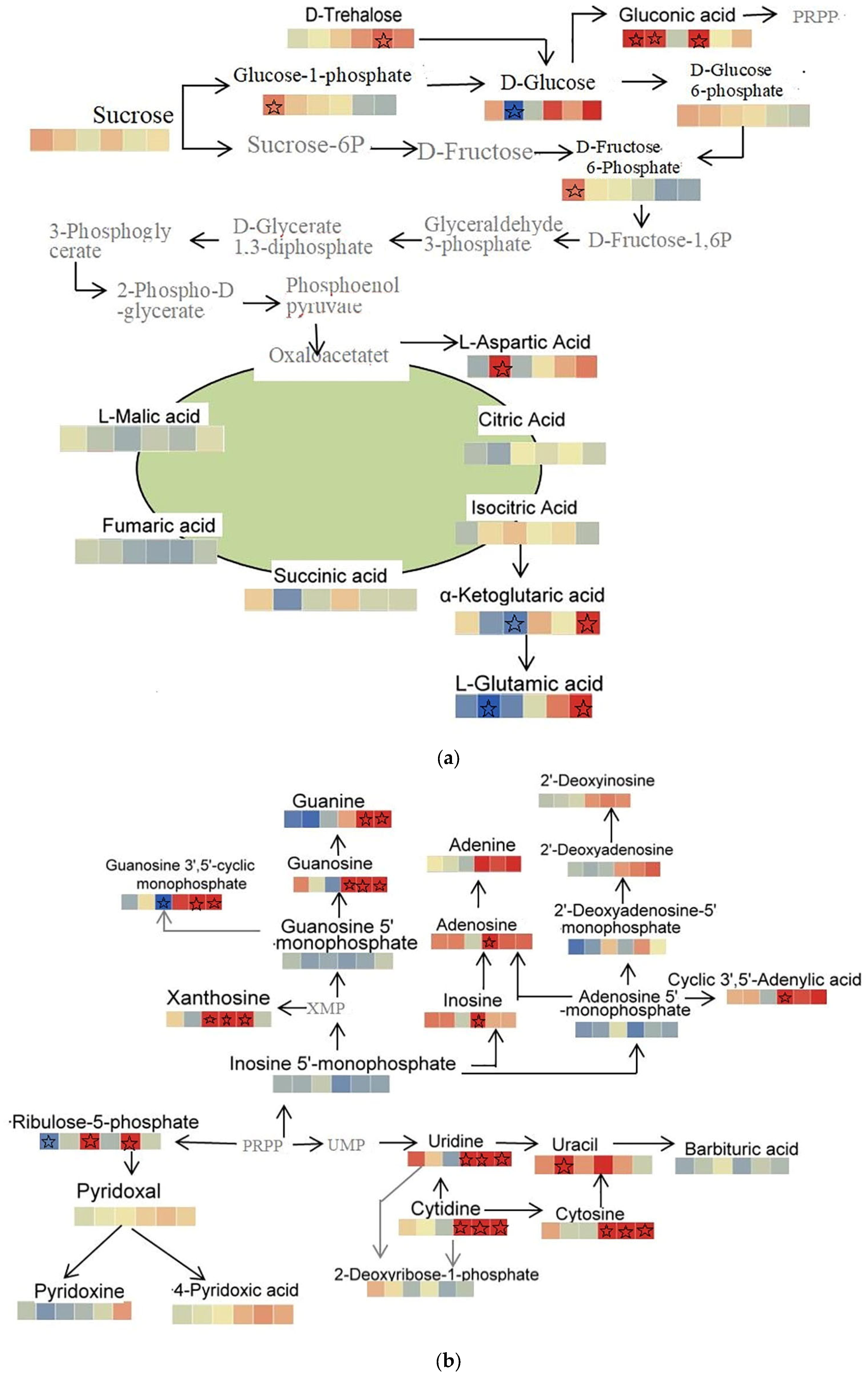
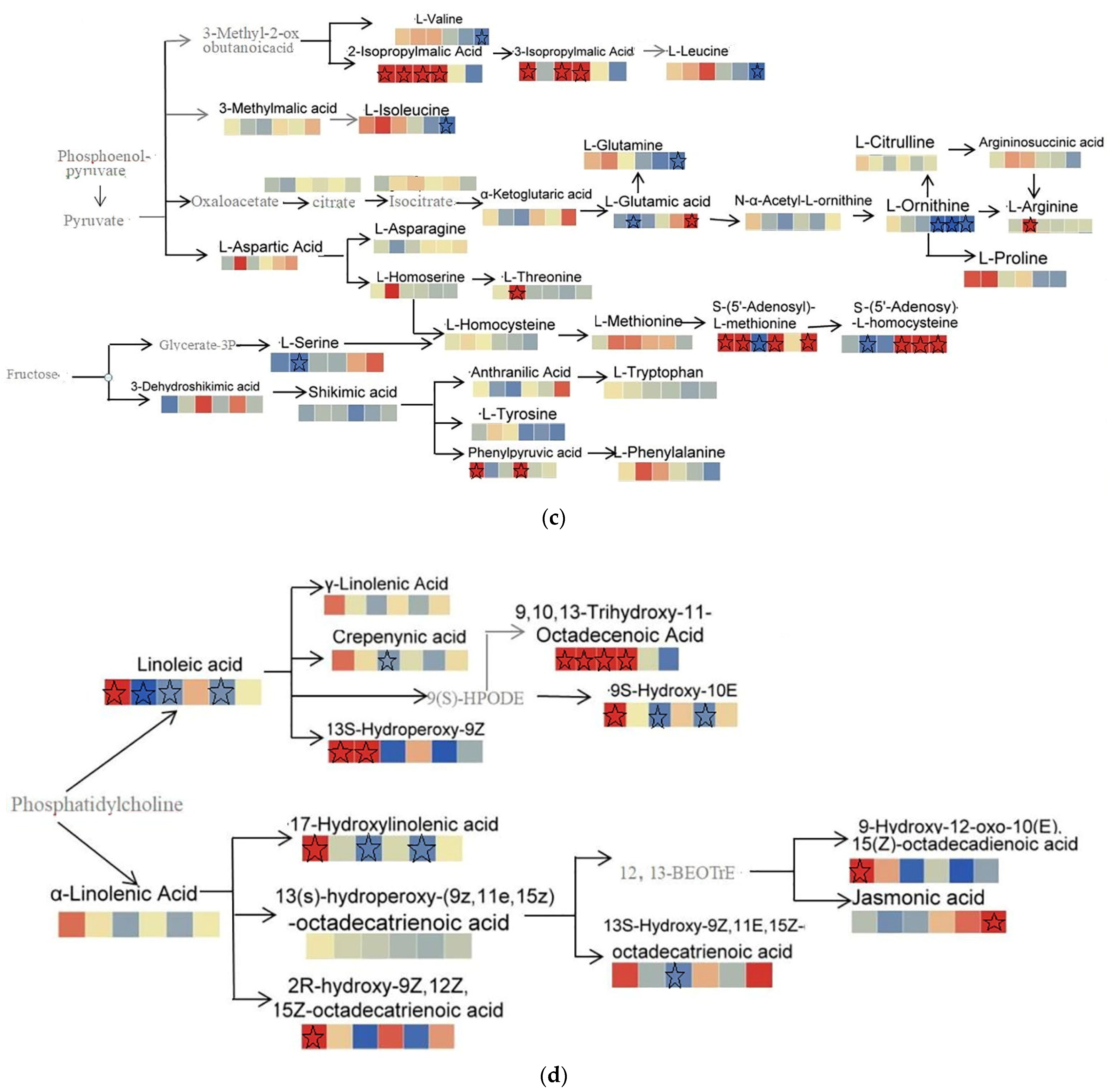
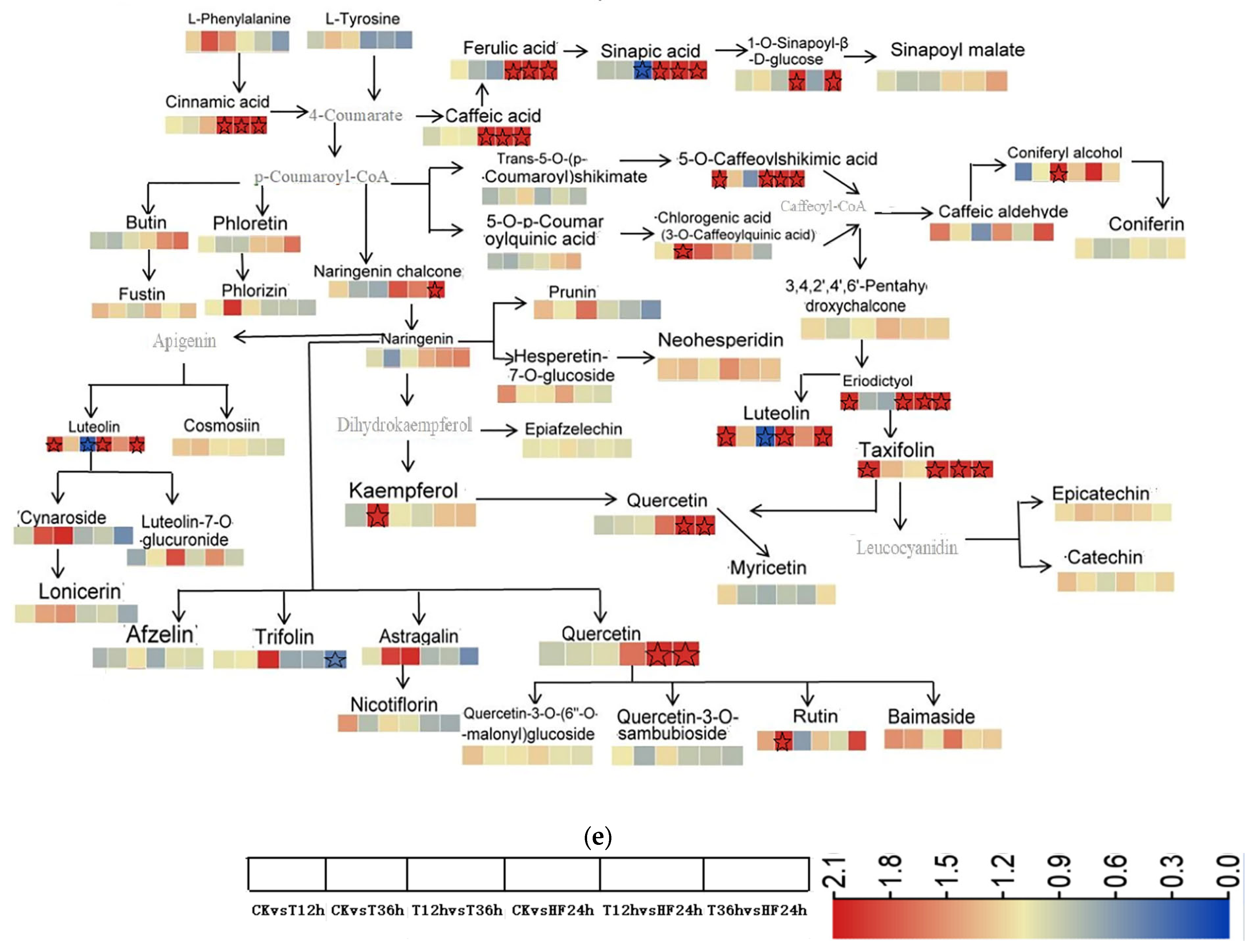
2.3.4. Integrated Metabolic Network Analysis of Malus sieversii (Ledeb.) M.Roem. Histocultures under Freezing Stress
3. Discussion
4. Materials and Methods
4.1. Succession and Culture of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings
4.2. Observations on Morphology and Stomatal Structure of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings under Freezing Stress
4.3. Chlorophyll Fluorescence Induction Curve and Chlorophyll Fluorescence Imaging Analysis of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings under Freezing Stress
4.4. Determination of Metabolites in Leaf of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings under Low-Temperature Stress
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volk, G.M.; Henk, A.D.; Richards, C.M.; Forsline, P.L.; Chao, C.T. Malus sieversii: A Diverse Central Asian Apple Species in the USDA-ARS National Plant Germplasm System. HortScience 2013, 48, 1440–1444. [Google Scholar] [CrossRef]
- Li, Y.N. Researches of Germplasm Resources of MalusMill; China Agriculture Press: Beijing, China, 2001; pp. 20–134. [Google Scholar] [CrossRef]
- Yan, G.R.; Long, H.; Song, W.Q.; Chen, R.Y. Genetic polymorphism of Malus sieversii populations in Xinjiang, China. Genet. Resour. Crop Evol. 2008, 55, 171–181. [Google Scholar] [CrossRef]
- Xu, J.I.; Liu, G.; Ma, P.; Huang, Y.P.; Yang, Y.L. Research Progress of Adversity Response Mechanism of Malus sieversii. Shandong Agric. Sci. 2014, 46, 4. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, N.; Deswal, R. The molecular biology of the low-temperature response in plants. Bioessays 2005, 27, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Chen, S.; Jua, Z.; Yao, Y. Joint transcriptomic and metabolomic analysis reveals the mechanism of low temperature tolerance in Hosta ventricosa. PLoS ONE 2021, 16, e0259455. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Zhao, L.; Ren, Y.; Cui, D.; Chen, J.; Wang, Y.; Yu, P.; Chen, F. iTRAQ and virus-induced gene silencing revealed three proteins involved in cold response in bread wheat. Sci. Rep. 2017, 7, 7524. [Google Scholar] [CrossRef]
- Clemente-Moreno, N.; Omranian, P.L.; Sáez, C.M.; Figueroa, N.; Del-Saz, M.; Elso, L.; Poblete, I.; Orf, A.; Cuadros-Inostroza, L.A.; Cavieres, L.; et al. Low-temperature tolerance of the Antarctic species Deschampsia antarctica: A complex metabolic response associated with nutrient remobilization. Plant Cell Environ. 2020, 43, 1376–1393. [Google Scholar] [CrossRef]
- Fiehn, O.; Kloska, S.; Altmann, T. Integrated studies on plant biology using multiparallel techniques. Curr. Opin. Biotechnol. 2001, 12, 82–86. [Google Scholar] [CrossRef]
- Nielsen, J.; Jewett, M.C. The Role of Metabolomics in Systems Biology; Springer: Berlin/Heidelberg, Germany, 2007; Chapter 228; pp. 1–10. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, N.; Kleessen, S.; Nikoloski, Z. Integration of metabolomics data into metabolic networks. Front. Plant Sci. 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M.F. A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 15243–15248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Di, Y.G.; Qiu, Q.L.; Cai, J.L.; Liu, Y.X.; Xu, H.X.; Li, K.Z. Effects of Low Temperature Stress on Growth and Development of G. elata were Analyzed by Transcriptome and Metabolome. Mol. Plant Breed. 2023, 21, 110–122. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, Y.L.; Jiang, L.; Shao, H.B. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8. [Google Scholar] [CrossRef]
- Zhu, A.D.; Li, W.Y.; Ye, J.L.; Sun, X.H.; Ding, Y.D.; Cheng, Y.J.; Deng, X.X. Microarray expression profiling of postharvest ponkan mandarin (Citrus reticulata) fruit under cold storage reveals regulatory gene candidates and implications on soluble sugars metabolism. J. Int. Plant Biol. 2011, 53, 358–374. [Google Scholar] [CrossRef] [PubMed]
- Halman, J.M.; Schaberg, P.G.; Hawley, G.J.; Eagar, C. Calcium addition at the Hubbard Brook Experimental Forest increases sugar storage, antioxidant activity and cold tolerance in native red spruce (Picea rubens). Tree Physiol. 2008, 28, 855–862. [Google Scholar] [CrossRef]
- Taïbi, K.; Del Campo, A.D.; Vilagrosa, A.; Bellés, J.M.; López-Gresa, M.; López-Nicolás, J.M.; Mulet, J.M. Distinctive physiological and molecular responses to cold stress among cold-tolerant and cold-sensitive Pinus halepensis seed sources. BMC Plant Biol. 2018, 18, 236. [Google Scholar] [CrossRef]
- Zuther, E.; Buchel, K.; Hundertmark, M.; Stitt, M.; Hincha, D.K.; Heyer, A.G. The role of raffinose in the cold acclimation response of Arabidopsis thaliana. FEBS Lett. 2004, 576, 169–173. [Google Scholar] [CrossRef]
- Yazdanpanah, P.; Jonoubi, P.; Zeinalabedini, M.; Rajael, H.; Ghaffar, M.R.; Vazifeshenas, M.R.; Abdirad, S. Seasonal metabolicinvestigation in pomegranate (Punica granatum L.) highlights the role of amino acids in genotype- and organ-specifc adaptive responses to freezing Stress. Front. Plant Sci. 2021, 12, 699139. [Google Scholar] [CrossRef]
- Saito, K.; Matsuda, F. Metabolomics for Functional Genomics, Systems Biology and Biotechnology. Annu. Rev. Plant Biol. 2010, 61, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Adamski, J.; Suhre, K. Metabolomics platforms for genome wide association studies—Linking the genome to the metabolome. Curr. Opin. Biotechnol. 2013, 24, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T. Proteomic and Metabolomic Studies on Seedlings of Two Desert Quinoa Species under Low-Temperature Stress; Shihezi University: Shihezi, China, 2018. [Google Scholar]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold Acclimation of Arabidopsis thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Browse, J.; Xin, Z. Temperature sensing and cold acclimation. Curr. Opin. Plant Biol. 2001, 4, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2023, 63, 1593. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Wang, L.H.; Zhu, L.; Lang, Z.H.; Lun, X.G. Research on the Mechanism of Gamma-aminobutyric Acid Shunt in Plant Response to Adversity Stresses. Curr. Biotechnol. 2014, 4, 77–84. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, A.S. Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef]
- Miquel, M.; Browse, J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J. Biol. Chem. 1992, 267, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Kunst, L.; Browse, J.; Somerville, C.A. Mutant of Arabidopsis Deficient in Desaturation of Palmitic Acid in Leaf Lipids. Plant Physiol. 1989, 90, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Durrett, T.P.; Benning, C. Functional diversity of glycerolipid acylhydrolases in plant metabolism and physiology. Prog. Lipid Res. 2019, 75, 100987. [Google Scholar] [CrossRef] [PubMed]
- Degenkolbe, T.; Giavalisco, P.; Zuther, E.; Seiwert, B.; Hincha, D.K.; Willmitzer, L. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana. Plant J. 2012, 72, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Ahmad, F.; Shamsul, H. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Tuteja, N.; Gill, S.S. Crop Improvement under Adverse Conditions; Springer: New York, NY, USA, 2012; pp. 285–307. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van, D.E.; Wim, C.A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Darin, P.; Rudy, V.; Andrea, M.; Va, H.; Wim, V.D.E. Towards understanding vacuolar antioxidant mechanisms: A role for fructans? J. Exp. Bot. 2013, 4, 1025–1038. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Anchordoguy, T.J. Interactions of sugars with membranes. Biochim. Biophys. Acta 1988, 947, 367–384. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E.; Heyer, A.G. The preservation of liposomes by raffinose family oligosaccharides during drying is mediated by effects on fusion and lipid phase transitions. Biochim. Et Biophys. Acta (BBA)—Rev. Biomembr. 2003, 1612, 172–177. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Valluru, R.; Ende, W.V.D. Plant fructans in stress environments: Emerging concepts and future prospects. J. Exp. Bot. 2008, 59, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Zhang, T.; Wang, W.S.; Zhang, F.; Zhu, L.H.; Fu, B.Y.; Li, Z.K. Time-Course Metabolic Profiling in Rice under Low Temperature Treatment. Acta Agron. Sin. 2013, 39, 720–726. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2020, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ou-Lee, T.M.; Raba, R.; Amundson, R.G.; Last, R.L. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Zeng, L.; Wanamaker, S.; Mandal, J.; Xu, J.; Cui, X. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol. 2005, 139, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.; Peterek, S.; Mock, H.P.; Heyer, A.G.; Hincha, D.K. Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant Cell Environ. 2008, 31, 813–827. [Google Scholar] [CrossRef]
- Dixon, R.; Paiva, N. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Su, Y.F.; Liu, L.J.; Ma, H.X.; Yuan, Y.Y.; Zhang, D.E.; Lu, X.Y. Screening of genes related to glutathione metabolism responding to freezing stress in Malus sieversii seedlings in vitro based on transcriptome sequencing. J. Fruit Sci. 2023, 40, 829–840. [Google Scholar] [CrossRef]
- Hu, H.; Fei, X.; He, B.; Luo, Y.; Qi, Y.; Wei, A. Integrated Analysis of Metabolome and Transcriptome Data for Uncovering Flavonoid Components of Zanthoxylum bungeanum Maxim. Leaves Under Drought Stress. Front. Nutr. 2022, 8, 801244. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Fang, J.; Lin, M.; Hu, C.; Qi, X.; Chen, J.; Zhong, Y.; Muhammad, A.; Li, Z.; Li, Y. Comparative Metabolomic and Transcriptomic Studies Reveal Key Metabolism Pathways Contributing to Freezing Tolerance Under Cold Stress in Kiwifruit. Front. Plant Sci. 2021, 12, 628969. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, X.; Zhang, H. Comprehensive transcriptome and metabolome profiling reveal metabolic mechanisms of Nitraria sibirica Pall. to salt stress. Sci. Rep. 2021, 11, 12878. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, G.; Ma, L.; Zhang, C.; Yan, H.; Bao, J.; Nai, G.; Wang, W.; Chen, B.; Ma, S.; et al. Integrated transcriptome and metabolome analysis reveals antioxidant machinery in grapevine exposed to salt and alkali stress. Physiol. Plant 2023, 175, e13950. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Simon-Sarkadi, L.; Szűcs, A.; Kocsy, G. Differential effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 2010, 38, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Odlum, K.; Blake, T.; Kim, Y.; Glerum, C. Influence of photoperiod and temperature on frost hardiness and free amino acid concentrations in black spruce seedlings. Tree Physiol. 1993, 13, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Glerum, C. Seasonal free amino acid fluctuations in red pine and white spruce needles. Can. J. For. Res. 1995, 25, 697–703. [Google Scholar] [CrossRef]
- Angelcheva, L.; Mishra, Y.; Antti, H. Metabolomic analysis of extreme freezing tolerance in Siberian spruce (Picea obovata). New Phytol. 2015, 204, 545–555. [Google Scholar] [CrossRef]
- He, C.C.; Liu, L.J.; Lu, X.Y. Analysis of genes related to glycolysis in the leaves and roots of Malus sieversii under NaCl stress based on transcriptome sequencing. J. Fruit Sci. 2020, 37, 951–961. [Google Scholar] [CrossRef]
- Fan, Z.M.; Sun, J.L.; Zhao, B.L.; Liu, H.F.; Yu, K.; Zhang, Z.J.; Liu, J.J. Evaluation of cold resistance of one-year shoots from Cabernet Sauvignon. J. Fruit Sci. 2020, 37, 215–225. [Google Scholar]

| CKvsT12h | CKvsT36h | CKvsHF24h | T12hvsT36h | T12hvsHF24h | T36hvsHF24h | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | Up | Down | Up | Down | Up | Down | |
| Amino acids and their derivatives | 5 | 0 | 8 | 3 | 12 | 2 | 4 | 2 | 6 | 2 | 8 | 11 |
| Phenolic acids | 5 | 6 | 5 | 4 | 14 | 2 | 7 | 11 | 14 | 3 | 17 | 3 |
| Nucleotides and their derivatives | 1 | 0 | 4 | 1 | 19 | 0 | 1 | 2 | 16 | 1 | 16 | 1 |
| Flavonoids | 10 | 3 | 13 | 5 | 22 | 6 | 5 | 6 | 6 | 27 | 20 | 14 |
| Lignans and coumarins | 5 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Sugars | 5 | 1 | 2 | 0 | 4 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Alkaloids | 3 | 1 | 0 | 3 | 2 | 1 | 0 | 2 | 1 | 1 | 2 | 4 |
| Terpenoids | 0 | 3 | 0 | 4 | 3 | 0 | 1 | 2 | 2 | 1 | 1 | 5 |
| Organic acids | 7 | 0 | 6 | 2 | 10 | 0 | 4 | 1 | 4 | 2 | 4 | 3 |
| Lipids | 61 | 0 | 48 | 0 | 28 | 0 | 9 | 25 | 1 | 27 | 1 | 36 |
| Vitamins | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Liu, L.; Ma, H.; Yuan, Y.; Zhang, D.; Lu, X. Metabolomic Analysis of the Effect of Freezing on Leaves of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings. Int. J. Mol. Sci. 2024, 25, 310. https://doi.org/10.3390/ijms25010310
Su Y, Liu L, Ma H, Yuan Y, Zhang D, Lu X. Metabolomic Analysis of the Effect of Freezing on Leaves of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings. International Journal of Molecular Sciences. 2024; 25(1):310. https://doi.org/10.3390/ijms25010310
Chicago/Turabian StyleSu, Yongfeng, Lijun Liu, Hongxi Ma, Yinyan Yuan, Deen Zhang, and Xiaoyan Lu. 2024. "Metabolomic Analysis of the Effect of Freezing on Leaves of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings" International Journal of Molecular Sciences 25, no. 1: 310. https://doi.org/10.3390/ijms25010310
APA StyleSu, Y., Liu, L., Ma, H., Yuan, Y., Zhang, D., & Lu, X. (2024). Metabolomic Analysis of the Effect of Freezing on Leaves of Malus sieversii (Ledeb.) M.Roem. Histoculture Seedlings. International Journal of Molecular Sciences, 25(1), 310. https://doi.org/10.3390/ijms25010310





