Multiple Genomic Alterations, Including a Novel AFF4::IRF1 Fusion Gene, in a Treatment-Refractory Blastic Plasmacytoid Dendritic-Cell Neoplasm: A Case Report and Literature Review
Abstract
:1. Introduction
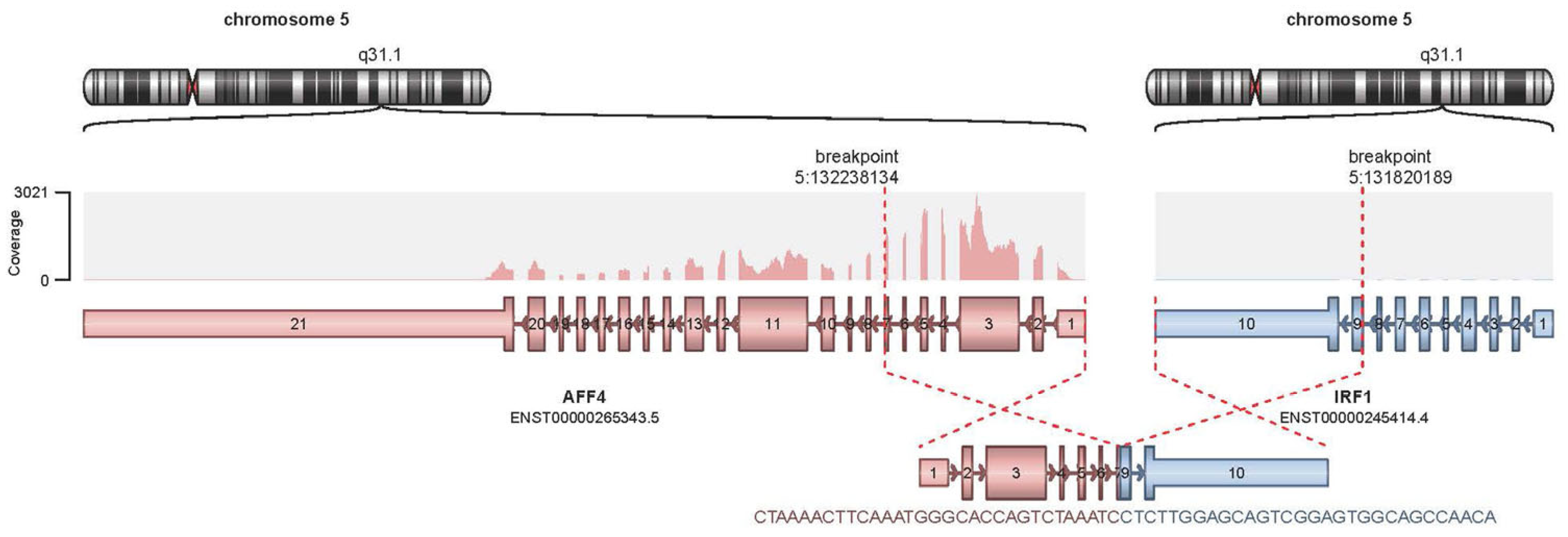
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guru Murthy, G.S.; Pemmaraju, N.; Atallah, E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk. Res. 2018, 73, 21–23. [Google Scholar] [CrossRef]
- Jain, A.; Sweet, K. Blastic Plasmacytoid Dendritic Cell Neoplasm. J. Natl. Compr. Cancer Netw. JNCCN 2023, 21, 515–521. [Google Scholar] [CrossRef]
- Pagano, L.; Valentini, C.G.; Pulsoni, A.; Fisogni, S.; Carluccio, P.; Mannelli, F.; Lunghi, M.; Pica, G.; Onida, F.; Cattaneo, C.; et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: An Italian multicenter study. Haematologica 2013, 98, 239–246. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.; Park, S.H.; Jo, J.C. Plasmacytoid dendritic cell neoplasms. Blood Res. 2023, 58, 90–95. [Google Scholar] [CrossRef]
- Bueno, C.; Almeida, J.; Lucio, P.; Marco, J.; Garcia, R.; de Pablos, J.M.; Parreira, A.; Ramos, F.; Ruiz-Cabello, F.; Suarez-Vilela, D.; et al. Incidence and characteristics of CD4(+)/HLA DRhi dendritic cell malignancies. Haematologica 2004, 89, 58–69. [Google Scholar]
- Tang, Z.; Tang, G.; Wang, S.A.; Lu, X.; Young, K.H.; Bueso-Ramos, C.E.; Alvarado, Y.; Medeiros, L.J.; Khoury, J.D. Simultaneous deletion of 3′ETV6 and 5′EWSR1 genes in blastic plasmacytoid dendritic cell neoplasm: Case report and literature review. Mol. Cytogenet. 2016, 9, 23. [Google Scholar] [CrossRef]
- Leroux, D.; Mugneret, F.; Callanan, M.; Radford-Weiss, I.; Dastugue, N.; Feuillard, J.; Le Mée, F.; Plessis, G.; Talmant, P.; Gachard, N.; et al. CD4(+), CD56(+) DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: A study of 21 cases by the Groupe Français de Cytogénétique Hématologique. Blood 2002, 99, 4154–4159. [Google Scholar] [CrossRef]
- Petrella, T.; Bagot, M.; Willemze, R.; Beylot-Barry, M.; Vergier, B.; Delaunay, M.; Meijer, C.J.; Courville, P.; Joly, P.; Grange, F.; et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): A review. Am. J. Clin. Pathol. 2005, 123, 662–675. [Google Scholar] [CrossRef]
- Alayed, K.; Patel, K.P.; Konoplev, S.; Singh, R.R.; Routbort, M.J.; Reddy, N.; Pemmaraju, N.; Zhang, L.; Shaikh, A.A.; Aladily, T.N.; et al. TET2 mutations, myelodysplastic features, and a distinct immunoprofile characterize blastic plasmacytoid dendritic cell neoplasm in the bone marrow. Am. J. Hematol. 2013, 88, 1055–1061. [Google Scholar] [CrossRef]
- Bohlander, S.K. ETV6: A versatile player in leukemogenesis. Semin. Cancer Biol. 2005, 15, 162–174. [Google Scholar] [CrossRef]
- Ceribelli, M.; Hou, Z.E.; Kelly, P.N.; Huang, D.W.; Wright, G.; Ganapathi, K.; Evbuomwan, M.O.; Pittaluga, S.; Shaffer, A.L.; Marcucci, G.; et al. A Druggable TCF4- and BRD4-Dependent Transcriptional Network Sustains Malignancy in Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancer Cell 2016, 30, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Beziat, G.; Ysebaert, L. Tagraxofusp for the Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN): A Brief Report on Emerging Data. OncoTargets Ther. 2020, 13, 5199–5205. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Stephansky, J.; Cai, T.; Griffin, G.K.; Cabal-Hierro, L.; Togami, K.; Hogdal, L.J.; Galinsky, I.; Morgan, E.A.; Aster, J.C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discov. 2017, 7, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, L.; Yin, L.; Zhou, L.; Deng, Y.; Deng, H. Diagnosis, treatment, and genetic characteristics of blastic plasmacytoid dendritic cell neoplasm: A review. Medicine 2023, 102, e32904. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N. Novel Pathways and Potential Therapeutic Strategies for Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN): CD123 and Beyond. Curr. Hematol. Malig. Rep. 2017, 12, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fowle, H.; Valentine, H.; Liu, Z.; Tan, Y.; Pei, J.; Badal, S.; Testa, J.R.; Graña, X. Immortalization of human primary prostate epithelial cells via CRISPR inactivation of the CDKN2A locus and expression of telomerase. Prostate Cancer Prostatic Dis. 2021, 24, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Xiong, Q.C.; Zhu, X.X.; Du, W.; Deng, P.; Li, X.B.; Jiang, Y.Z.; Zou, S.J.; Wang, C.Y.; Yuan, Q. AFF1 and AFF4 differentially regulate the osteogenic differentiation of human MSCs. Bone Res. 2017, 5, 17044. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, H.; Gong, X.; Chen, Y.; Zhuang, Y.; Zhou, S.; Chen, Y.; Jiang, Y.; Ji, X.; Peng, H.; et al. AFF4 Predicts the Prognosis of Colorectal Cancer Patients and Suppresses Colorectal Cancer Metastasis via Promoting CDH1 Expression. Front. Oncol. 2022, 12, 797392. [Google Scholar] [CrossRef]
- Deng, P.; Wang, J.; Zhang, X.; Wu, X.; Ji, N.; Li, J.; Zhou, M.; Jiang, L.; Zeng, X.; Chen, Q. AFF4 promotes tumorigenesis and tumor-initiation capacity of head and neck squamous cell carcinoma cells by regulating SOX2. Carcinogenesis 2018, 39, 937–947. [Google Scholar] [CrossRef]
- Cheng, M.; Sheng, L.; Gao, Q.; Xiong, Q.; Zhang, H.; Wu, M.; Liang, Y.; Zhu, F.; Zhang, Y.; Zhang, X.; et al. The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-κB/MYC signaling network. Oncogene 2019, 38, 3667–3680. [Google Scholar] [CrossRef]
- Romeo, G.; Fiorucci, G.; Chiantore, M.V.; Percario, Z.A.; Vannucchi, S.; Affabris, E. IRF-1 as a negative regulator of cell proliferation. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2002, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tzoanopoulos, D.; Speletas, M.; Arvanitidis, K.; Veiopoulou, C.; Kyriaki, S.; Thyphronitis, G.; Sideras, P.; Kartalis, G.; Ritis, K. Low expression of interferon regulatory factor-1 and identification of novel exons skipping in patients with chronic myeloid leukaemia. Br. J. Haematol. 2002, 119, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Boultwood, J.; Fidler, C.; Lewis, S.; MacCarthy, A.; Sheridan, H.; Kelly, S.; Oscier, D.; Buckle, V.J.; Wainscoat, J.S. Allelic loss of IRF1 in myelodysplasia and acute myeloid leukemia: Retention of IRF1 on the 5q- chromosome in some patients with the 5q-syndrome. Blood 1993, 82, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Alsamman, K.; El-Masry, O.S. Interferon regulatory factor 1 inactivation in human cancer. Biosci. Rep. 2018, 38, BSR20171672. [Google Scholar] [CrossRef]
- AbuSara, N.; Razavi, S.; Derwish, L.; Komatsu, Y.; Licursi, M.; Hirasawa, K. Restoration of IRF1-dependent anticancer effects by MEK inhibition in human cancer cells. Cancer Lett. 2015, 357, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Sweet, K. Blastic plasmacytoid dendritic cell neoplasm: Diagnosis, manifestations, and treatment. Curr. Opin. Hematol. 2020, 27, 103–107. [Google Scholar] [CrossRef]
- Khoury, J.D. Blastic Plasmacytoid Dendritic Cell Neoplasm. Curr. Hematol. Malig. Rep. 2018, 13, 477–483. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Kantarjian, H.M.; Cortes, J.E.; Duvic, M.; Khoury, J.D.; Patel, K.; Daver, N.; O’Brien, S.; Pierce, S.; Garcia-Manero, G. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): A large single-center experience: Analysis of clinical and molecular characteristics and patient outcomes. Blood 2015, 126, 3746. [Google Scholar] [CrossRef]
- Menezes, J.; Acquadro, F.; Wiseman, M.; Gómez-López, G.; Salgado, R.N.; Talavera-Casañas, J.G.; Buño, I.; Cervera, J.V.; Montes-Moreno, S.; Hernández-Rivas, J.M.; et al. Exome sequencing reveals novel and recurrent mutations with clinical impact in blastic plasmacytoid dendritic cell neoplasm. Leukemia 2014, 28, 823–829. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Yang, M.; Wang, L.; Jin, J. New perspectives in genetics and targeted therapy for blastic plasmacytoid dendritic cell neoplasm. Crit. Rev. Oncol./Hematol. 2020, 149, 102928. [Google Scholar] [CrossRef]
- Stenzinger, A.; Endris, V.; Pfarr, N.; Andrulis, M.; Jöhrens, K.; Klauschen, F.; Siebolts, U.; Wolf, T.; Koch, P.S.; Schulz, M.; et al. Targeted ultra-deep sequencing reveals recurrent and mutually exclusive mutations of cancer genes in blastic plasmacytoid dendritic cell neoplasm. Oncotarget 2014, 5, 6404–6413. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Yang, M.; Wang, L.; Jin, J.; Yu, W. Mutational analysis in different foci revealing the clonal evolution of blastic plasmacytoid dendritic cell neoplasm. Leuk. Lymphoma 2021, 62, 988–991. [Google Scholar] [CrossRef]
- Sakamoto, K.; Katayama, R.; Asaka, R.; Sakata, S.; Baba, S.; Nakasone, H.; Koike, S.; Tsuyama, N.; Dobashi, A.; Sasaki, M.; et al. Recurrent 8q24 rearrangement in blastic plasmacytoid dendritic cell neoplasm: Association with immunoblastoid cytomorphology, MYC expression, and drug response. Leukemia 2018, 32, 2590–2603. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, Y.; Hama, A.; Muramatsu, H.; Nakatochi, M.; Gunji, M.; Ichikawa, D.; Hamada, M.; Taniguchi, R.; Kataoka, S.; et al. Recurrent MYB rearrangement in blastic plasmacytoid dendritic cell neoplasm. Leukemia 2017, 31, 1629–1633. [Google Scholar] [CrossRef]
- Fiandrino, G.; Arra, M.; Riboni, R.; Lucioni, M.; Dallera, E.; Arcaini, L.; Berti, E.; Paulli, M. Absence of MYD88 L265P mutation in blastic plasmacytoid dendritic cell neoplasm. Br. J. Dermatol. 2013, 168, 883–884. [Google Scholar] [CrossRef]
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011, 470, 115–119. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, Y.; Watkins, A.J.; Kocialkowski, S.; Zeng, N.; Hamoudi, R.A.; Isaacson, P.G.; de Leval, L.; Wotherspoon, A.; Du, M.Q. BCR and TLR signaling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica 2012, 97, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lawrence, M.S.; Wan, Y.; Stojanov, P.; Sougnez, C.; Stevenson, K.; Werner, L.; Sivachenko, A.; DeLuca, D.S.; Zhang, L.; et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N. Engl. J. Med. 2011, 365, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Sheehy, P.; Manning, R.J.; Patterson, C.J.; Tripsas, C.; et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N. Engl. J. Med. 2012, 367, 826–833. [Google Scholar] [CrossRef]
- Muñoz, L.; Nomdedéu, J.F.; López, O.; Carnicer, M.J.; Bellido, M.; Aventín, A.; Brunet, S.; Sierra, J. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica 2001, 86, 1261–1269. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; WHO Press: Geneve, Switzerland, 2017; Volume 2. [Google Scholar]
- Sapienza, M.R.; Fuligni, F.; Agostinelli, C.; Tripodo, C.; Righi, S.; Laginestra, M.A.; Pileri, A., Jr.; Mancini, M.; Rossi, M.; Ricci, F.; et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014, 28, 1606–1616. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers 2019, 11, 1358. [Google Scholar] [CrossRef]
- Adimora, I.J.; Wilson, N.R.; Pemmaraju, N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): A promising future in the era of targeted therapeutics. Cancer 2022, 128, 3019–3026. [Google Scholar] [CrossRef]
- Taylor, J.; Haddadin, M.; Upadhyay, V.A.; Grussie, E.; Mehta-Shah, N.; Brunner, A.M.; Louissaint, A., Jr.; Lovitch, S.B.; Dogan, A.; Fathi, A.T.; et al. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood 2019, 134, 678–687. [Google Scholar] [CrossRef]
- Lin, X.; Wang, L.; Hu, Q.; Zhu, J.; Tao, Y.; Huang, L.; Niu, T. Incidence, prognostic factors, and survival outcomes in patients with blastic plasmacytoid dendritic cell neoplasm: A retrospective study in the Surveillance, Epidemiology, and End Results database. Eur. J. Haematol. 2023, 110, 743–753. [Google Scholar] [CrossRef]
- Yin, C.C.; Pemmaraju, N.; You, M.J.; Li, S.; Xu, J.; Wang, W.; Tang, Z.; Alswailmi, O.; Bhalla, K.N.; Qazilbash, M.H.; et al. Integrated Clinical Genotype-Phenotype Characteristics of Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancers 2021, 13, 5888. [Google Scholar] [CrossRef]
- Julia, F.; Dalle, S.; Duru, G.; Balme, B.; Vergier, B.; Ortonne, N.; Vignon-Pennamen, M.D.; Costes-Martineau, V.; Lamant, L.; Dalac, S.; et al. Blastic plasmacytoid dendritic cell neoplasms: Clinico-immunohistochemical correlations in a series of 91 patients. Am. J. Surg. Pathol. 2014, 38, 673–680. [Google Scholar] [CrossRef]
- Lucioni, M.; Novara, F.; Fiandrino, G.; Riboni, R.; Fanoni, D.; Arra, M.; Venegoni, L.; Nicola, M.; Dallera, E.; Arcaini, L.; et al. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: Focus on biallelic locus 9p21.3 deletion. Blood 2011, 118, 4591–4594. [Google Scholar] [CrossRef]

| Gene | Protein Change | cDNA Change | Allele Frequency | Reference |
|---|---|---|---|---|
| MYD88 | p.Ala6fs | c.10_28delGACCGCGCTGAGGCTCCAG | 27.5% | ENST00000396334 |
| TET2 | p.Ser825* | c.2474C>G | 22.5% | ENST00000380013 |
| TET2 | p.Pro851fs | c.2551delC | 22% | ENST00000380013 |
| ASXL1 | p.Arg693* | c.2077C>T | 19% | ENST00000375687 |
| Gene | Protein Change | cDNA Change | Allele Frequency | Reference |
|---|---|---|---|---|
| KMT2C | p.Tyr366Cys | c.1097A>G | 6.4% | ENST00000262189 |
| TET2 | p.Ser825* | c.2474C>G | 46.1% | ENST00000380013 |
| TET2 | p.Pro851fs | c.2551delC | 44.0% | ENST00000380013 |
| ASXL1 | p.Arg693* | c.2077C>T | 44.9% | ENST00000375687 |
| GNAS | p.Ala331Thr | c.991G>A | 47.0% | ENST00000371100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin, Y.; Wang, Y.L.; Pei, J.; Mansoor, N.; Styler, M.; Testa, J.R.; Nejati, R. Multiple Genomic Alterations, Including a Novel AFF4::IRF1 Fusion Gene, in a Treatment-Refractory Blastic Plasmacytoid Dendritic-Cell Neoplasm: A Case Report and Literature Review. Int. J. Mol. Sci. 2024, 25, 305. https://doi.org/10.3390/ijms25010305
Sahin Y, Wang YL, Pei J, Mansoor N, Styler M, Testa JR, Nejati R. Multiple Genomic Alterations, Including a Novel AFF4::IRF1 Fusion Gene, in a Treatment-Refractory Blastic Plasmacytoid Dendritic-Cell Neoplasm: A Case Report and Literature Review. International Journal of Molecular Sciences. 2024; 25(1):305. https://doi.org/10.3390/ijms25010305
Chicago/Turabian StyleSahin, Yavuz, Y. Lynn Wang, Jianming Pei, Nashwa Mansoor, Michael Styler, Joseph R. Testa, and Reza Nejati. 2024. "Multiple Genomic Alterations, Including a Novel AFF4::IRF1 Fusion Gene, in a Treatment-Refractory Blastic Plasmacytoid Dendritic-Cell Neoplasm: A Case Report and Literature Review" International Journal of Molecular Sciences 25, no. 1: 305. https://doi.org/10.3390/ijms25010305





