Preclinical and Clinical Observations Implying Combination Therapy to Enhance the Efficacy of the Her-2/neu B-Cell Peptide-Based Vaccine HER-Vaxx and to Prevent Immune Evasion
Abstract
:1. Introduction
2. Results
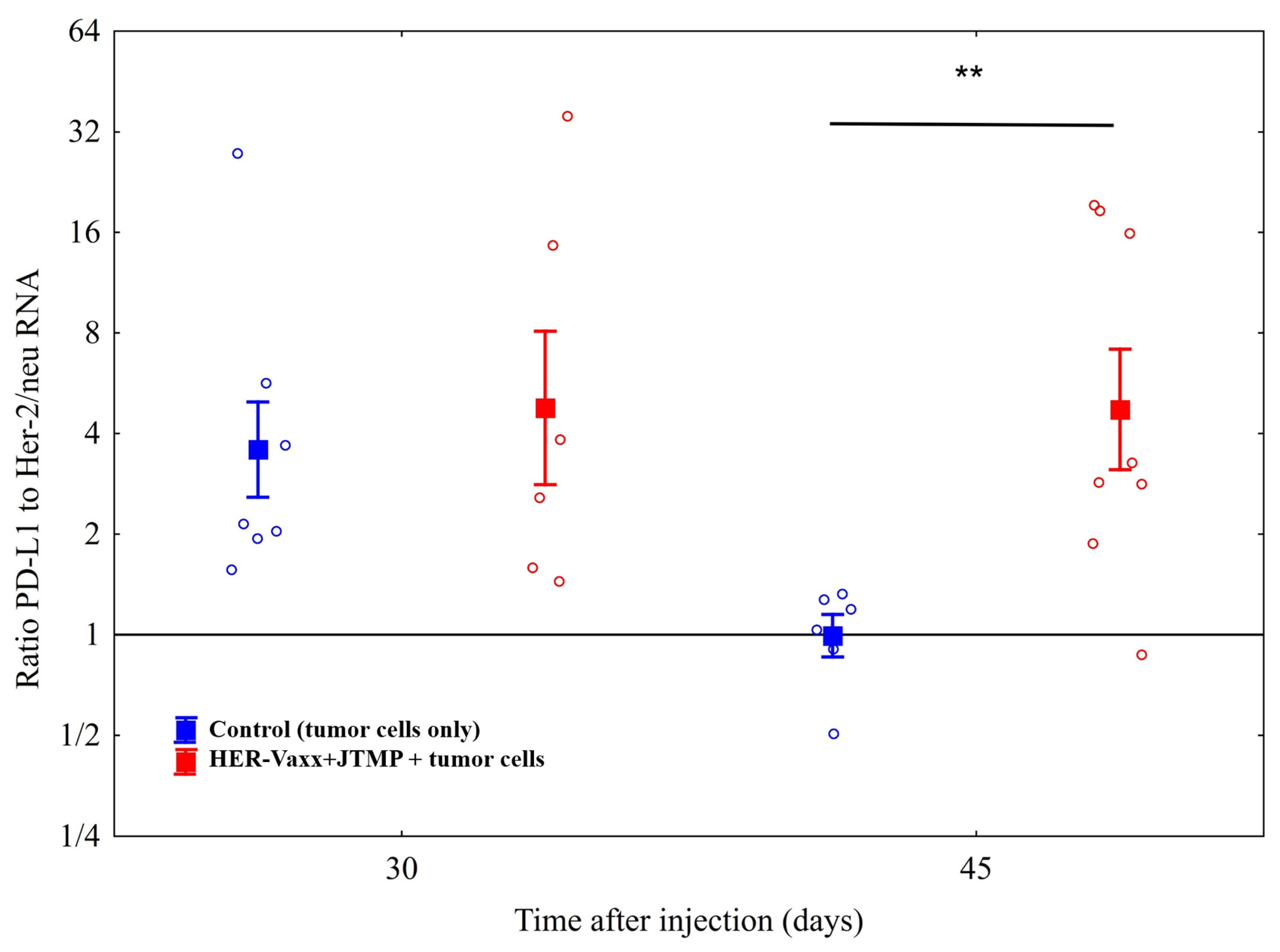
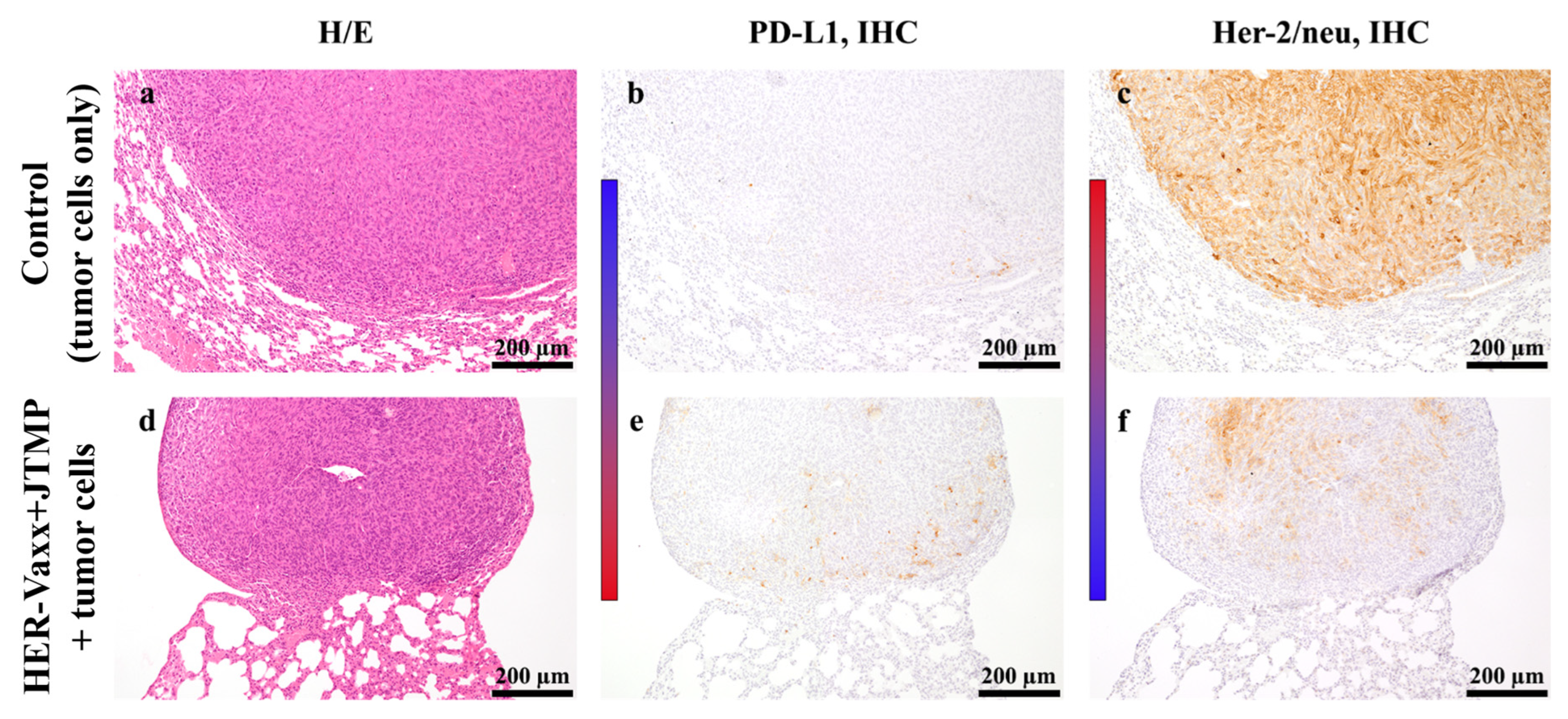
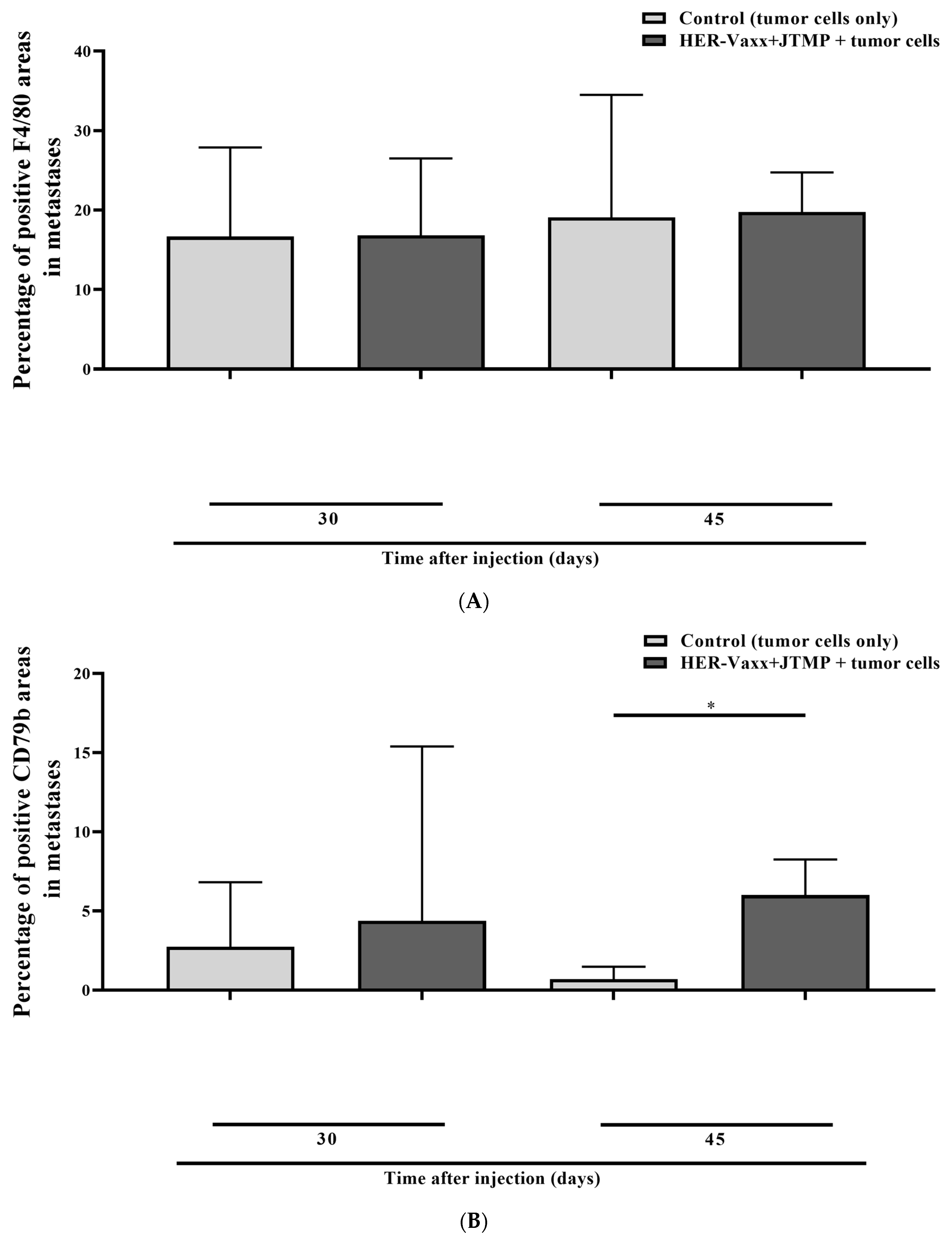
2.1. Her-2/neu-Targeted Therapy Upregulates PD-L1 Expression in Association with Loss of Her-2/meu Expression—Preclinical Observation
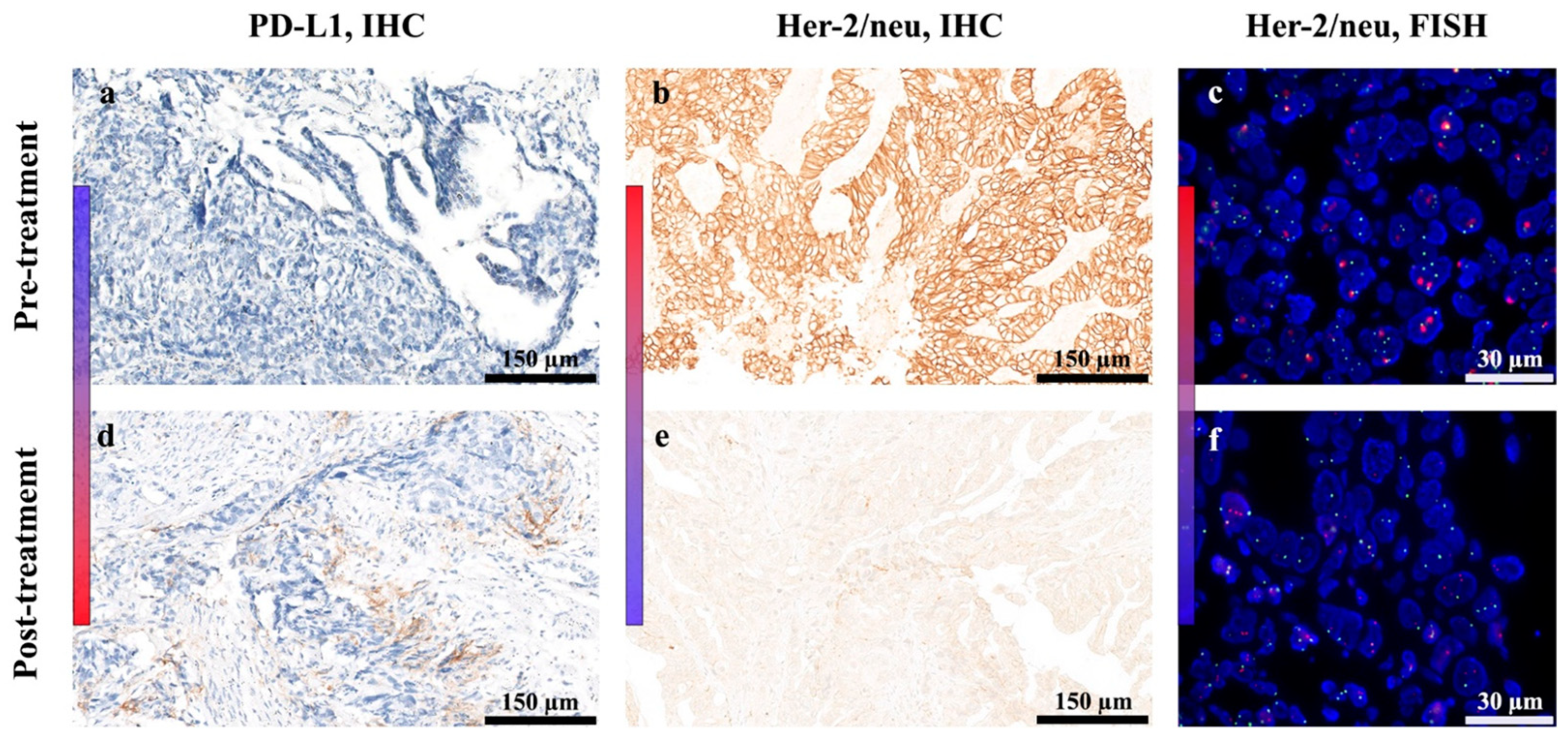
2.2. Treatment with HER-Vaxx Is Associated with Upregulation of PD-L1 Expression in Concomitance with Downregulation of Her-2/meu Expression—Clinical Observation
3. Discussion
4. Materials and Methods
4.1. Cell Line and Culture Conditions
4.2. Preclinical Setting
4.2.1. Animals and Immunization Settings
4.2.2. Pathohistological Assessment
4.2.3. Assessment of PD-L1 and Her-2/neu Expression using RT-PCR
4.3. Clinical Setting
4.3.1. The Phase Ib Dose-Escalation Trial (IMU.ACS.001, NCT02795988)
4.3.2. Biopsies from the Vaccinated Patient
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cell cytotoxicity |
| CD4 | Cluster of differentiation 4 |
| CD79b | Cluster of differentiation 79b |
| CPS | Combined üositive score |
| CR | Complete response |
| CTL | Cytotoxic T-lymphocytes |
| FISH | Fluorescence in situ hybridization |
| IC | Immune checkpoint |
| IHC | Immunohistochemistry |
| IFNγ | Interferon gamma |
| MHC-I | Major histocompatibility complex |
| mRNA | Messenger RNA |
| PBS | Phosphate-buffered saline |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
| RNA | Ribonucleic acid |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SOD | Sum of diameters |
References
- Arienti, C.; Pignatta, S.; Tesei, A. Epidermal Growth Factor Receptor Family and its Role in Gastric Cancer. Front. Oncol. 2019, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lv, M.; Jiang, H.; Wang, Y.; Yu, S.; Li, W.; Yu, Y.; Liu, T. A prospective observational study on the optimal maintenance strategy in HER2-positive advanced gastric cancer treated with trastuzumab-based therapy. J. Cancer Res. Clin. Oncol. 2020, 146, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, S.; Knutson, K.L. Resistance to Trastuzumab. Cancers 2022, 14, 5115. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Ladjemi, M.Z.; Jacot, W.; Chardes, T.; Pelegrin, A.; Navarro-Teulon, I. Anti-HER2 vaccines: New prospects for breast cancer therapy. Cancer Immunol. Immunother. 2010, 59, 1295–1312. [Google Scholar] [CrossRef]
- Tobias, J.; Garner-Spitzer, E.; Drinic, M.; Wiedermann, U. Vaccination against Her-2/neu, with focus on peptide-based vaccines. ESMO Open 2022, 7, 100361. [Google Scholar] [CrossRef]
- Tobias, J.; Drinic, M.; Hogler, S.; Ambroz, K.; Baier, K.; Kodajova, P.; Tomasich, E.; Berghoff, A.S.; Schmid, A.; Garner-Spitzer, E.; et al. Active immunization with a Her-2/neu-targeting Multi-peptide B cell vaccine prevents lung metastases formation from Her-2/neu breast cancer in a mouse model. Transl. Oncol. 2022, 19, 101378. [Google Scholar] [CrossRef]
- Wiedermann, U.; Garner-Spitzer, E.; Chao, Y.; Maglakelidze, M.; Bulat, I.; Dechaphunkul, A.; Arpornwirat, W.; Charoentum, C.; Yen, C.J.; Yau, T.C.; et al. Clinical and Immunologic Responses to a B-Cell Epitope Vaccine in Patients with HER2/neu-Overexpressing Advanced Gastric Cancer-Results from Phase Ib Trial IMU.ACS.001. Clin. Cancer Res. 2021, 27, 3649–3660. [Google Scholar] [CrossRef]
- Tobias, J.; Kundi, M.; Garner-Spitzer, E.; Zielinski, C.; Maglakelidze, M.; Andric, Z.; Petrovic, Z.; Nagarkar, R.; Chawla, T.; Chong, L.; et al. PD-8 HERIZON: A phase 2 study of HER-Vaxx (IMU-131), a HER2-targeting peptide vaccine plus SOC chemotherapy in patients with HER2+ advanced stomach cancer—Correlation of the antibody responses and clinical outcome. Ann. Oncol. 2023, 34, S4. [Google Scholar] [CrossRef]
- Maglakelidze, M.; Ryspayeva, D.; Bulat, I.; Andric, Z.; Nikolic, I.; Chawla, T.; Nagarkar, R.; Chourdhary, V.; Venkata, G.; Singh, R.K.; et al. A phase 1b/2 open-label study with randomization in phase 2 of Imu-131 Her2/neu peptide vaccine plus standard of care chemotherapy in patients with Her2/neu overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junction. Cancer Res. 2021, 81, CT107. [Google Scholar] [CrossRef]
- Tobias, J.; Kundi, M.; Garner-Spitzer, E.; Zielinski, C.; Maglakelidze, M.; Andric, Z.G.; Petrovic, Z.; Nagarkar, R.; Chawla, T.; Chong, L.O.; et al. 1536P HERIZON: A phase II study of HER-Vaxx (IMU-131), a HER2-targeting peptide vaccine plus standard of care chemotherapy in patients with HER2+ advanced stomach cancer—Dose-dependent anti-cancer antibodies correlating with improved clinical outcome. Ann. Oncol. 2023, 34, S864. [Google Scholar] [CrossRef]
- Tobias, J.; Drinic, M.; Högler, S.; Schmid, A.; Garner-Spitzer, E.; Kenner, L.; Kundi, M.; Zieleinski, C.; Wiedermann, U. 1676P Active immunization with a multi-peptide B cell vaccine, targeting trastuzumab and pertuzumab binding sites, prevents the formation of HER-2/neu expressing lung metastases. Ann. Oncol. 2022, 33, S1309. [Google Scholar] [CrossRef]
- Chaganty, B.K.R.; Qiu, S.; Gest, A.; Lu, Y.; Ivan, C.; Calin, G.A.; Weiner, L.M.; Fan, Z. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Iwatsuki, M.; Yasuda-Yoshihara, N.; Morinaga, T.; Nakao, Y.; Harada, K.; Eto, K.; Kurashige, J.; Hiyoshi, Y.; Ishimoto, T.; et al. Trastuzumab upregulates programmed death ligand-1 expression through interaction with NK cells in gastric cancer. Br. J. Cancer 2021, 124, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Gorbunow, F.; Eggemann, H.; Ortmann, O.; Ignatov, A. Loss of HER2 after HER2-targeted treatment. Breast Cancer Res. Treat. 2019, 175, 401–408. [Google Scholar] [CrossRef]
- Seo, S.; Ryu, M.H.; Park, Y.S.; Ahn, J.Y.; Park, Y.; Park, S.R.; Ryoo, B.Y.; Lee, G.H.; Jung, H.Y.; Kang, Y.K. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: Results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer 2019, 22, 527–535. [Google Scholar] [CrossRef]
- Maruyama, T.; Mimura, K.; Sato, E.; Watanabe, M.; Mizukami, Y.; Kawaguchi, Y.; Ando, T.; Kinouchi, H.; Fujii, H.; Kono, K. Inverse correlation of HER2 with MHC class I expression on oesophageal squamous cell carcinoma. Br. J. Cancer 2010, 103, 552–559. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2019, 29, 3766. [Google Scholar] [CrossRef]
- Wei, W.Z.; Shi, W.P.; Galy, A.; Lichlyter, D.; Hernandez, S.; Groner, B.; Heilbrun, L.; Jones, R.F. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int. J. Cancer 1999, 81, 748–754. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, Y.; Liu, S.; Zhang, Y.; Wang, J.; Qin, T.; Chen, P.; Li, K. Immune-independent acquired resistance to PD-L1 antibody initiated by PD-L1 upregulation via PI3K/AKT signaling can be reversed by anlotinib. Cancer Med. 2023, 12, 15337–15349. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Nami, B.; Maadi, H.; Wang, Z. The Effects of Pertuzumab and Its Combination with Trastuzumab on HER2 Homodimerization and Phosphorylation. Cancers 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Peranzoni, E.; Ingangi, V.; Masetto, E.; Pinton, L.; Marigo, I. Myeloid Cells as Clinical Biomarkers for Immune Checkpoint Blockade. Front. Immunol. 2020, 11, 1590. [Google Scholar] [CrossRef]
- Kurbel, S.; Dmitrovic, B.; Marjanovic, K.; Vrbanec, D.; Juretic, A. Distribution of Ki-67 values within HER2 & ER/PgR expression variants of ductal breast cancers as a potential link between IHC features and breast cancer biology. BMC Cancer 2017, 17, 231. [Google Scholar] [CrossRef]
- Qin, M.; Jin, Y.; Pan, L.Y. Tertiary lymphoid structure and B-cell-related pathways: A potential target in tumor immunotherapy. Oncol. Lett. 2021, 22, 836. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front. Immunol. 2022, 13, 1063711. [Google Scholar] [CrossRef]
- Schaafsma, E.; Jiang, C.; Cheng, C. B cell infiltration is highly associated with prognosis and an immune-infiltrated tumor microenvironment in neuroblastoma. J. Cancer Metastasis Treat. 2021, 7, 34. [Google Scholar] [CrossRef]
- Wouters, M.C.A.; Nelson, B.H. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef]
- Tobias, J.; Jasinska, J.; Baier, K.; Kundi, M.; Ede, N.; Zielinski, C.; Wiedermann, U. Enhanced and long term immunogenicity of a Her-2/neu multi-epitope vaccine conjugated to the carrier CRM197 in conjunction with the adjuvant Montanide. BMC Cancer 2017, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, U.; Garner-Spitzer, E.; Chao, Y.; Bulat, I.; Dechaphunkul, A.; Arpornwirat, W.; Charoentum, C.; Yen, C.J.; Yau, T.; Maglakelidzde, M.; et al. Comprehensive results of a phase Ib study with a HER2/neu B-cell peptide vaccine administered with cisplatin and 5-fluorouracil or capecitabine chemotherapy show safety, immunogenicity and clinical response in patients with HER2/Neu overexpressing advanced gastric cancer. Ann. Oncol. 2019, 30, v495–v496. [Google Scholar]
- Michaud, D.; Steward, C.R.; Mirlekar, B.; Pylayeva-Gupta, Y. Regulatory B cells in cancer. Immunol. Rev. 2021, 299, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xia, L.; Jia, D.; Zou, H.; Jin, G.; Qian, W.; Xu, H.; Li, T. PD-L1(+) regulatory B cells act as a T cell suppressor in a PD-L1-dependent manner in melanoma patients with bone metastasis. Mol. Immunol. 2020, 119, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mao, Y.; Lv, Y.; Tang, W.; Xu, J. B cells in tumor metastasis: Friend or foe? Int. J. Biol. Sci. 2023, 19, 2382–2393. [Google Scholar] [CrossRef]
- Palle, J.; Rochand, A.; Pernot, S.; Gallois, C.; Taieb, J.; Zaanan, A. Human Epidermal Growth Factor Receptor 2 (HER2) in Advanced Gastric Cancer: Current Knowledge and Future Perspectives. Drugs 2020, 80, 401–415. [Google Scholar] [CrossRef]
- Borgeaud, M.; Sandoval, J.; Obeid, M.; Banna, G.; Michielin, O.; Addeo, A.; Friedlaender, A. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treat. Rev. 2023, 120, 102614. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Correction: Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 105. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Wan, L.; Ling, W.; Chen, H.; Wang, J. New developments in the mechanism and application of immune checkpoint inhibitors in cancer therapy (Review). Int. J. Oncol. 2023, 63, 86. [Google Scholar] [CrossRef]
- Brossart, P. Antitumor immunity and T-cell avidity. Blood 2020, 136, 378–380. [Google Scholar] [CrossRef]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune Checkpoint Inhibitors Combined with Targeted Therapy: The Recent Advances and Future Potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xue, R.; Zhu, Z.; Farrukh, H.; Song, W.; Li, T.; Zheng, L.; Pan, C.X. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp. Hematol. Oncol. 2023, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Kang, L.; Li, D.; Song, Y. Immunotherapy for HER-2 positive breast cancer. Front. Oncol. 2023, 13, 1097983. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.J.; De Vita, F.; Landers, G.; Yen, C.J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef]
- Tobias, J.; Battin, C.; De Sousa Linhares, A.; Lebens, M.; Baier, K.; Ambroz, K.; Drinic, M.; Hogler, S.; Inic-Kanada, A.; Garner-Spitzer, E.; et al. A New Strategy Toward B Cell-Based Cancer Vaccines by Active Immunization With Mimotopes of Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobias, J.; Högler, S.; Raigel, M.; Lin, D.S.-C.; Chao, Y.; Kenner, L.; Garner-Spitzer, E.; Yavrom, S.; Ede, N.J.; Zielinski, C.C.; et al. Preclinical and Clinical Observations Implying Combination Therapy to Enhance the Efficacy of the Her-2/neu B-Cell Peptide-Based Vaccine HER-Vaxx and to Prevent Immune Evasion. Int. J. Mol. Sci. 2024, 25, 287. https://doi.org/10.3390/ijms25010287
Tobias J, Högler S, Raigel M, Lin DS-C, Chao Y, Kenner L, Garner-Spitzer E, Yavrom S, Ede NJ, Zielinski CC, et al. Preclinical and Clinical Observations Implying Combination Therapy to Enhance the Efficacy of the Her-2/neu B-Cell Peptide-Based Vaccine HER-Vaxx and to Prevent Immune Evasion. International Journal of Molecular Sciences. 2024; 25(1):287. https://doi.org/10.3390/ijms25010287
Chicago/Turabian StyleTobias, Joshua, Sandra Högler, Martin Raigel, Diego Shih-Chieh Lin, Yee Chao, Lukas Kenner, Erika Garner-Spitzer, Sharon Yavrom, Nicholas J. Ede, Christoph C. Zielinski, and et al. 2024. "Preclinical and Clinical Observations Implying Combination Therapy to Enhance the Efficacy of the Her-2/neu B-Cell Peptide-Based Vaccine HER-Vaxx and to Prevent Immune Evasion" International Journal of Molecular Sciences 25, no. 1: 287. https://doi.org/10.3390/ijms25010287
APA StyleTobias, J., Högler, S., Raigel, M., Lin, D. S.-C., Chao, Y., Kenner, L., Garner-Spitzer, E., Yavrom, S., Ede, N. J., Zielinski, C. C., Kundi, M., & Wiedermann, U. (2024). Preclinical and Clinical Observations Implying Combination Therapy to Enhance the Efficacy of the Her-2/neu B-Cell Peptide-Based Vaccine HER-Vaxx and to Prevent Immune Evasion. International Journal of Molecular Sciences, 25(1), 287. https://doi.org/10.3390/ijms25010287







