Skin Anti-Inflammatory Potential with Reduced Side Effects of Novel Glucocorticoid Receptor Agonists
Abstract
1. Introduction
2. Results
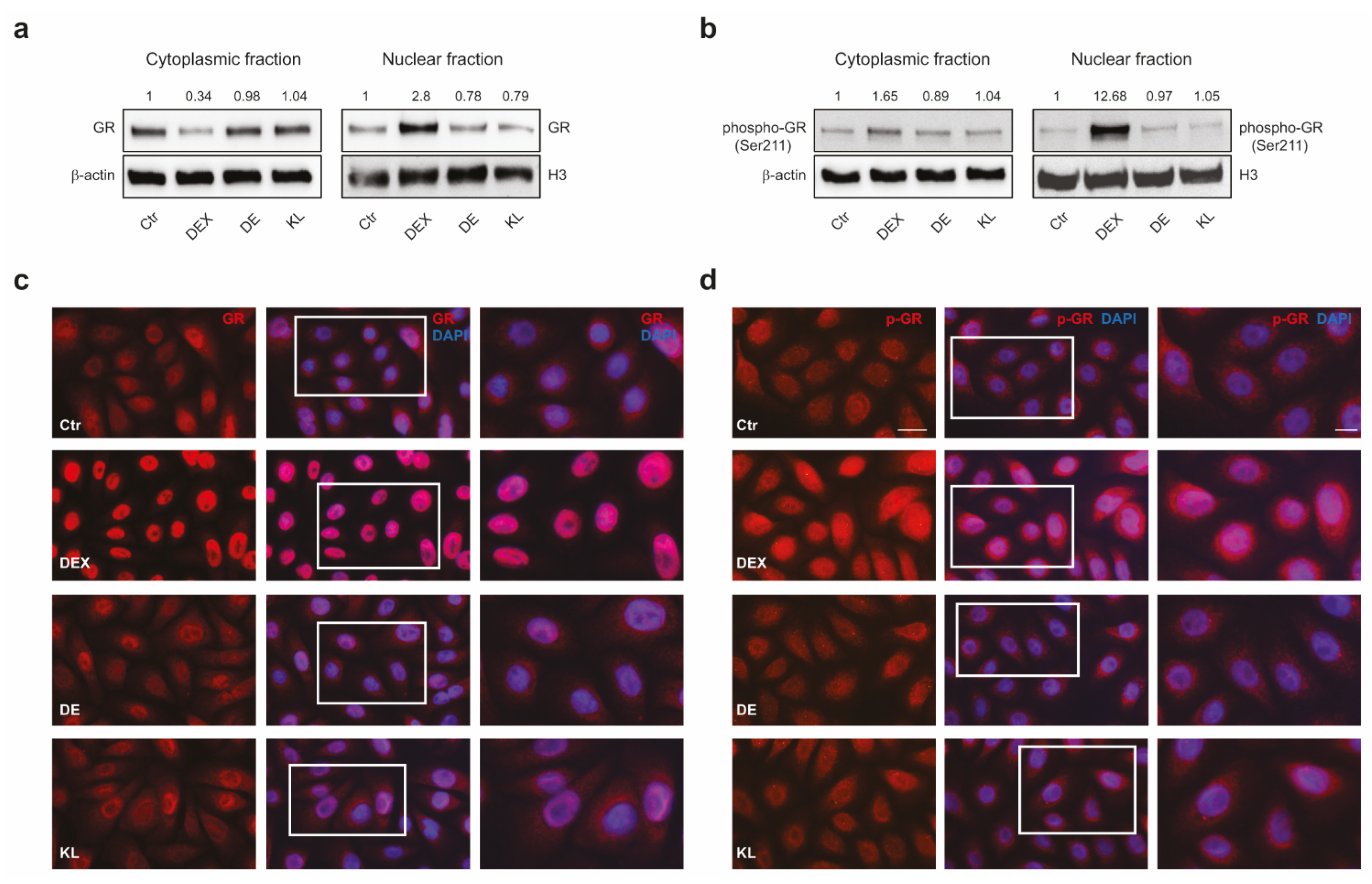
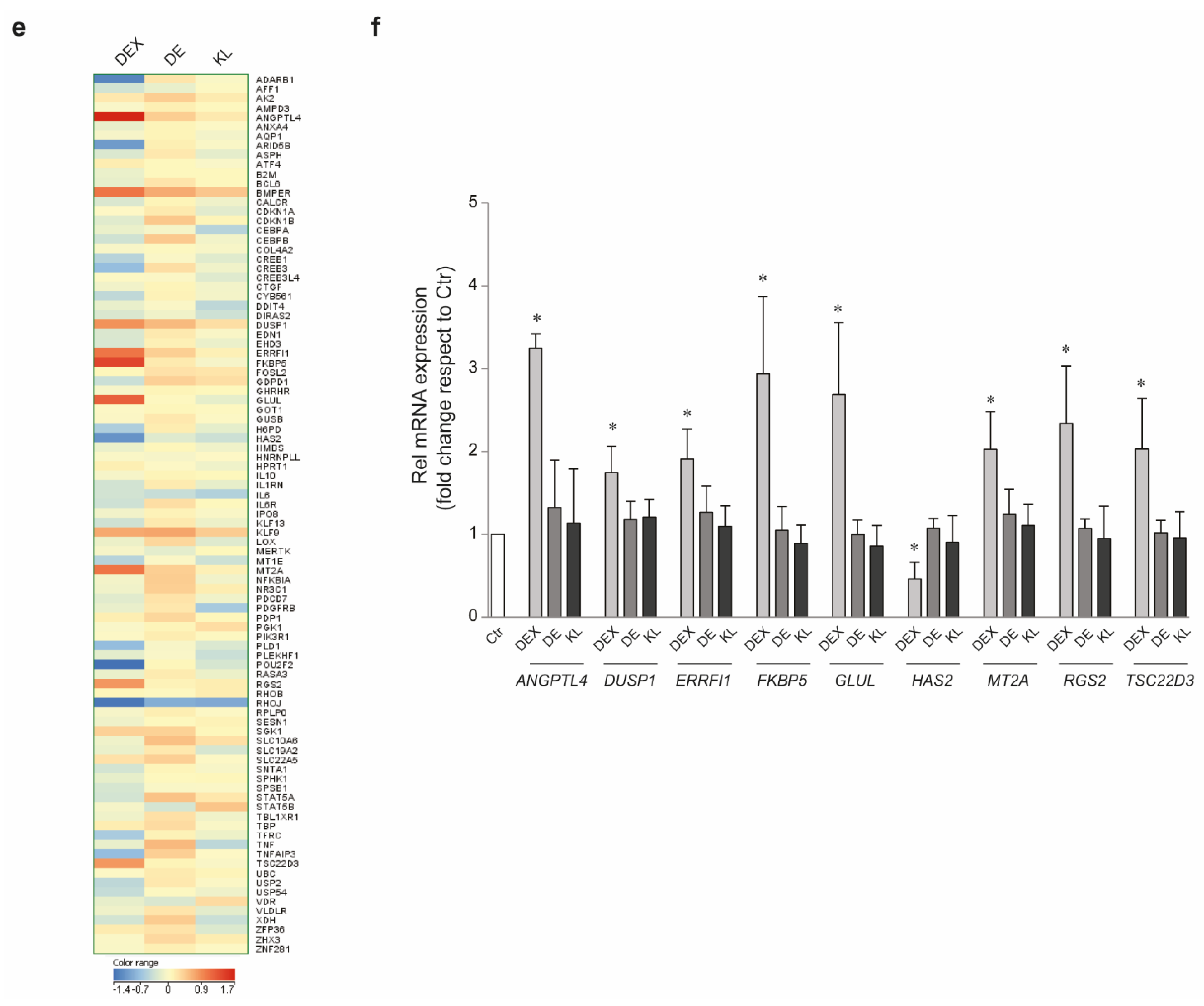
2.1. DE and KL Did Not Induce GR Transcriptional Activation
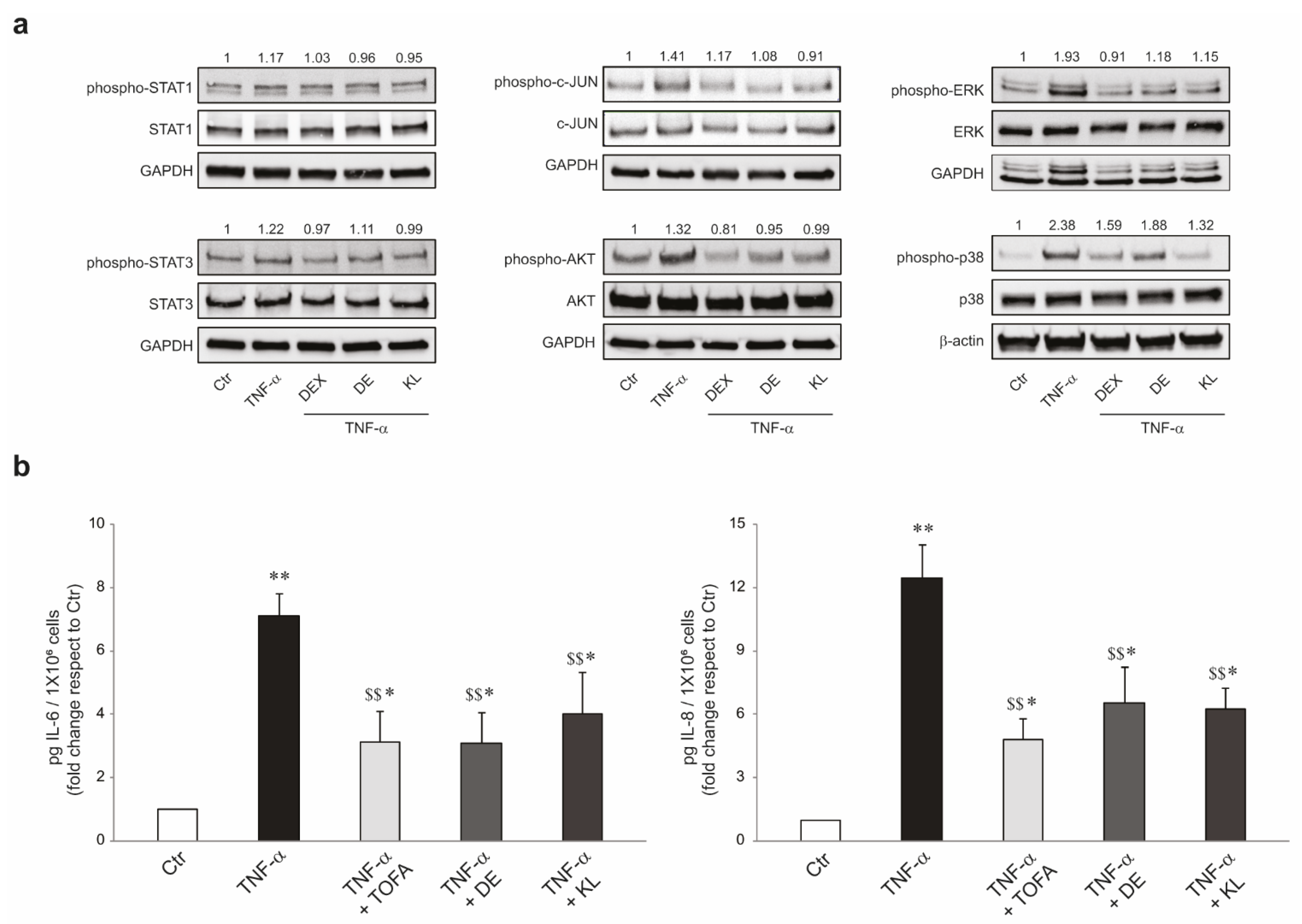
2.2. DE and KL Inhibited TNF-α-Induced Inflammatory Cytokines
2.3. DE and KL Counteract the Pro-Inflammatory Effects Induced by TNF-α
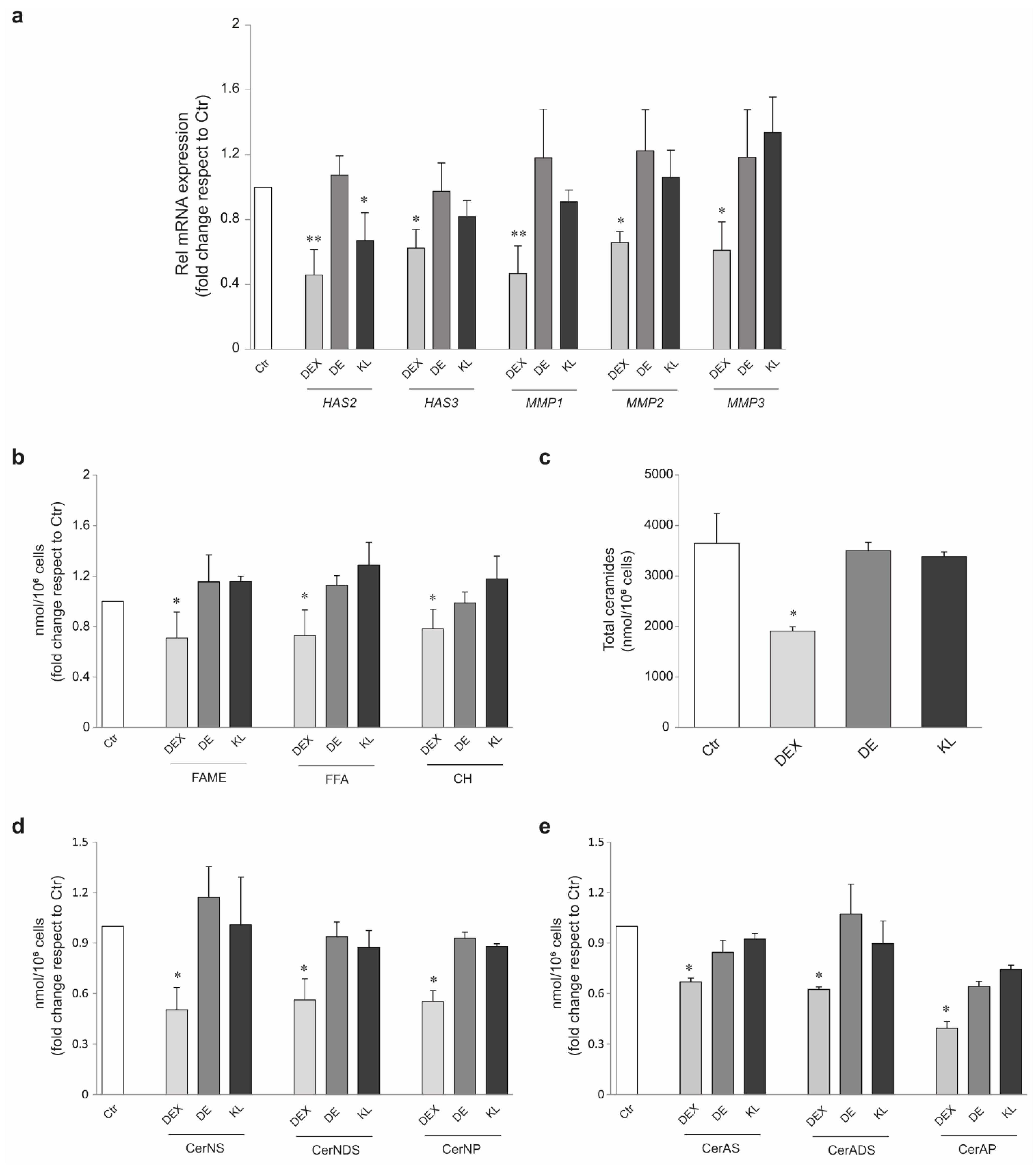
2.4. DE and KL Safety Profile in Different Skin Cell Populations
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Materials
4.2. New Chemical Ligands
4.3. Cell Cultures
4.4. MTT Assay
4.5. RNA Extraction, Gene Expression Array Card Analysis, and Real-Time RT-PCR
4.6. Western Blot Analysis
4.7. Protein Determination by Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Cell Number Analysis
4.9. Cell Size Measurement
4.10. RNA Interference Experiments
4.11. Immunofluorescence Analysis
4.12. Lipid Extraction
4.13. GC-MS Analysis of FAMEs
4.14. GC-MS Analysis of FFAs and CH
4.15. LC-MS Analysis of Ceramides
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, L.M.; Pérez, P. Roles of the Glucocorticoid and Mineralocorticoid Receptors in Skin Pathophysiology. Int. J. Mol. Sci. 2018, 19, 1906. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Vandewalle, J.; Libert, C. Dimerization of the Glucocorticoid Receptor and its Importance in (Patho)Physiology: A Primer. Cells 2022, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Tuckermann, J.; Libert, C. New Insights into the Anti-Inflammatory Mechanisms of Glucocorticoids: An Emerging Role for Glucocorticoid-Receptor-Mediated Transactivation. Endocrinology 2013, 154, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Bekhbat, M.; Rowson, S.A.; Neigh, G.N. Checks and Balances: The Glucocorticoid Receptor and NFĸB in Good Times and Bad. Front. Neuroendocrinol. 2017, 46, 15–31. [Google Scholar] [CrossRef]
- Löwenberg, M.; Tuynman, J.; Scheffer, M.; Verhaar, A.; Vermeulen, L.; van Deventer, S.; Hommes, D.; Peppelenbosch, M. Kinome Analysis Reveals Nongenomic Glucocorticoid Receptor-Dependent Inhibition of Insulin Signaling. Endocrinology 2006, 147, 3555–3562. [Google Scholar] [CrossRef][Green Version]
- Löwenberg, M.; Verhaar, A.P.; Bilderbeek, J.; van Marle, J.; Buttgereit, F.; Peppelenbosch, M.P.; van Deventer, S.J.; Hommes, D.W. Glucocorticoids Cause Rapid Dissociation of a T-Cell-Receptor-Associated Protein Complex Containing LCK and FYN. EMBO Rep. 2006, 7, 1023–1029. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Schaafsma, D.; Amrani, Y.; Koziol-White, C.; Ostrom, R.; Tliba, O. Non-Genomic Effects of Glucocorticoids: An Updated View. Trends Pharmacol. Sci. 2019, 40, 38–49. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109. [Google Scholar] [CrossRef]
- Boehncke, W.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, L.M.; Pérez, P. Glucocorticoids and Glucocorticoid-Induced-Leucine-Zipper (GILZ) in Psoriasis. Front. Immunol. 2019, 10, 2220. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Martora, L.; Fabbrocini, G.; Marasca, C. A Case of Pemphigus Vulgaris and Hidradenitis Suppurativa: May Systemic Steroids Be Considered in the Standard Management of Hidradenitis Suppurativa? Skin Appendage Disord. 2022, 8, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Niculet, E.; Bobeica, C.; Tatu, A.L. Glucocorticoid-Induced Skin Atrophy: The Old and the New. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Røpke, M.A.; Alonso, C.; Jung, S.; Norsgaard, H.; Richter, C.; Darvin, M.E.; Litman, T.; Vogt, A.; Lademann, J.; Blume-Peytavi, U.; et al. Effects of Glucocorticoids on Stratum Corneum Lipids and Function in Human skin—A Detailed Lipidomic Analysis. J. Dermatol. Sci. 2017, 88, 330–338. [Google Scholar] [CrossRef]
- Norsgaard, H.; Kurdykowski, S.; Descargues, P.; Gonzalez, T.; Marstrand, T.; Dünstl, G.; Røpke, M. Calcipotriol Counteracts Betamethasone-Induced Decrease in Extracellular Matrix Components Related to Skin Atrophy. Arch. Dermatol. Res. 2014, 306, 719–729. [Google Scholar] [CrossRef]
- Averbeck, M.; Gebhardt, C.; Anderegg, U.; Simon, J.C. Suppression of Hyaluronan Synthase 2 Expression Reflects the Atrophogenic Potential of Glucocorticoids. Exp. Dermatol. 2010, 19, 757–759. [Google Scholar] [CrossRef]
- Souffriau, J.; Eggermont, M.; Van Ryckeghem, S.; Van Looveren, K.; Van Wyngene, L.; Van Hamme, E.; Vuylsteke, M.; Beyaert, R.; De Bosscher, K.; Libert, C. A Screening Assay for Selective Dimerizing Glucocorticoid Receptor Agonists and Modulators (SEDIGRAM) that are Effective Against Acute Inflammation. Sci. Rep. 2018, 8, 12894. [Google Scholar] [CrossRef]
- Gebhardt, C.; Averbeck, M.; Diedenhofen, N.; Willenberg, A.; Anderegg, U.; Sleeman, J.P.; Simon, J.C. Dermal Hyaluronan is Rapidly Reduced by Topical Treatment with Glucocorticoids. J. Investig. Dermatol. 2010, 130, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Fu, Z.W.; Ohnuki, Y.; Kato, H.; Noguchi, T. Molecular Basis of the Alteration in Skin Collagen Metabolism in Response to in Vivo Dexamethasone Treatment: Effects on the Synthesis of Collagen Type I and III, Collagenase, and Tissue Inhibitors of Metalloproteinases. Br. J. Dermatol. 2002, 147, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Gossye, V.; Elewaut, D.; Van Beneden, K.; Dewint, P.; Haegeman, G.; De Bosscher, K. A Plant-Derived Glucocorticoid Receptor Modulator Attenuates Inflammation without Provoking Ligand-Induced Resistance. Ann. Rheum. Dis. 2010, 69, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Yemelyanov, A.; Swart, A.C.; Swart, P.; Haegeman, G.; Budunova, I. Discovery of Compound A--a Selective Activator of the Glucocorticoid Receptor with Anti-Inflammatory and Anti-Cancer Activity. Oncotarget 2015, 6, 30730–30744. [Google Scholar] [CrossRef] [PubMed]
- Schäcke, H.; Zollner, T.M.; Döcke, W.D.; Rehwinkel, H.; Jaroch, S.; Skuballa, W.; Neuhaus, R.; May, E.; Zügel, U.; Asadullah, K. Characterization of ZK 245186, a Novel, Selective Glucocorticoid Receptor Agonist for the Topical Treatment of Inflammatory Skin Diseases. Br. J. Pharmacol. 2009, 158, 1088–1103. [Google Scholar] [CrossRef]
- Sundahl, N.; Bridelance, J.; Libert, C.; De Bosscher, K.; Beck, I.M. Selective Glucocorticoid Receptor Modulation: New Directions with Non-Steroidal Scaffolds. Pharmacol. Ther. 2015, 152, 28–41. [Google Scholar] [CrossRef]
- Dendoncker, K.; Timmermans, S.; Vandewalle, J.; Eggermont, M.; Lempiäinen, J.; Paakinaho, V.; Van Hamme, E.; Dewaele, S.; Vandevyver, S.; Ballegeer, M.; et al. TNF-A Inhibits Glucocorticoid Receptor-Induced Gene Expression by Reshaping the GR Nuclear Cofactor Profile. Proc. Natl. Acad. Sci. USA 2019, 116, 12942–12951. [Google Scholar] [CrossRef]
- Rauch, A.; Gossye, V.; Bracke, D.; Gevaert, E.; Jacques, P.; Van Beneden, K.; Vandooren, B.; Rauner, M.; Hofbauer, L.C.; Haegeman, G.; et al. An Anti-Inflammatory Selective Glucocorticoid Receptor Modulator Preserves Osteoblast Differentiation. FASEB J. 2011, 25, 1323–1332. [Google Scholar] [CrossRef]
- Sung, J.; An, H.; Jeong, J.; Shin, S.; Song, S.Y. Megestrol Acetate Increases the Proliferation, Migration, and Adipogenic Differentiation of Adipose-Derived Stem Cells Via Glucocorticoid Receptor. Stem Cells Transl. Med. 2015, 4, 789–799. [Google Scholar] [CrossRef]
- Su, Q.; Pfalzgraff, A.; Weindl, G. Cell Type-Specific Regulatory Effects of Glucocorticoids on Cutaneous TLR2 Expression and Signalling. J. Steroid Biochem. Mol. Biol. 2017, 171, 201–208. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Yu, Y.; Zhou, L.; Wang, M.; Xue, W.; Liu, B.; Wu, X.; Wu, X.; Gao, H.; et al. A Plant-Derived Glucocorticoid Receptor Modulator with Potency to Attenuate the Side Effects of Glucocorticoid Therapy. Br. J. Pharmacol. 2023, 180, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Lee, B.; Vouthounis, C.; Vukelic, S.; Pastar, I.; Blumenberg, M.; Brem, H.; Tomic-Canic, M. Novel Genomic Effects of Glucocorticoids in Epidermal Keratinocytes. J. Biol. Chem. 2007, 282, 4021–4034. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Vanden Berghe, W.; Beck, I.M.E.; Van Molle, W.; Hennuyer, N.; Hapgood, J.; Libert, C.; Staels, B.; Louw, A.; Haegeman, G. A Fully Dissociated Compound of Plant Origin for Inflammatory Gene Repression. Proc. Natl. Acad. Sci. USA 2005, 102, 15827–15832. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB Signaling in Inflammation. Sig. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF- B Family of Transcription Factors and its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK Inhibition as a Therapeutic Strategy for Immune and Inflammatory Diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef] [PubMed]
- Kogame, T.; Egawa, G.; Kabashima, K. Exploring the Role of Janus Kinase (JAK) in Atopic Dermatitis: A Review of Molecular Mechanisms and Therapeutic Strategies. Immunol. Med. 2023, 46, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.; Li, C.; Yong, S. Emerging Trends in Clinical Research on Janus Kinase Inhibitors for Atopic Dermatitis Treatment. Int. Immunopharmacol. 2023, 124, 111029. [Google Scholar] [CrossRef]
- Klopot, A.; Baida, G.; Bhalla, P.; Haegeman, G.; Budunova, I.; Budunova, I. Selective Activator of the Glucocorticoid Receptor Compound A Dissociates Therapeutic and Atrophogenic Effects of Glucocorticoid Receptor Signaling in Skin Original Article. J. Cancer Prev. 2015, 20, 250. [Google Scholar] [CrossRef]
- Weindl, G.; Castello, F.; Schäfer-Korting, M. Evaluation of Anti-Inflammatory and Atrophogenic Effects of Glucocorticoids on Reconstructed Human Skin. Altern. Lab. Anim. 2011, 39, 173–187. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse Effects of Topical Glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Samarasekera, E.J.; Sawyer, L.; Wonderling, D.; Tucker, R.; Smith, C.H. Topical Therapies for the Treatment of Plaque Psoriasis: Systematic Review and Network Meta-Analyses. Br. J. Dermatol. 2013, 168, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Baida, G.; Bhalla, P.; Yemelyanov, A.; Stechschulte, L.A.; Shou, W.; Readhead, B.; Dudley, J.T.; Sánchez, E.R.; Budunova, I. Deletion of the Glucocorticoid Receptor Chaperone FKBP51 Prevents Glucocorticoid-Induced Skin Atrophy. Oncotarget 2018, 9, 34772. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; De Leo, S.A.; Mazaira, G.I.; Molinari, A.M.; Camisay, M.F.; Fontana, V.; Cox, M.B.; Piwien-Pilipuk, G.; Galigniana, M.D. NF-κB Transcriptional Activity is Modulated by FK506-Binding Proteins FKBP51 and FKBP52. J. Biol. Chem. 2014, 289, 26263–26276. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Lasa, M. Crosstalk between Glucocorticoids and Mitogen-Activated Protein Kinase Signalling Pathways. Curr. Opin. Pharmacol. 2003, 3, 404. [Google Scholar] [CrossRef]
- Haftcheshmeh, S.M.; Abedi, M.; Mashayekhi, K.; Mousavi, M.J.; Navashenaq, J.G.; Mohammadi, A.; Momtazi-borojeni, A.A. Berberine as a Natural Modulator of Inflammatory Signaling Pathways in the Immune System: Focus on NF-κB, JAK/STAT, and MAPK Signaling Pathways. Phytother. Res. 2022, 36, 1216. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; McInnes, I.B.; Siebert, S. JAK-Inhibitors. New Players in the Field of Immune-Mediated Diseases, Beyond Rheumatoid Arthritis. Rheumatology 2019, 58, i43–i54. [Google Scholar] [CrossRef]
- Merola, J.F.; Papp, K.A.; Nash, P.; Gratacós, J.; Boehncke, W.H.; Thaçi, D.; Graham, D.; Hsu, M.; Wang, C.; Wu, J.; et al. Tofacitinib in Psoriatic Arthritis Patients: Skin Signs and Symptoms and Health-related Quality of Life from Two Randomized Phase 3 Studies. Acad. Dermatol. Venereol. 2020, 34, 2809. [Google Scholar] [CrossRef]
- Alharthi, S.; Turkmani, M.; AlJasser, M.I. Acne Exacerbation After Tofacitinib Treatment for Alopecia Areata. Dermatol. Rep. 2022, 14, 9396. [Google Scholar] [CrossRef]
- Chen, T.; Lee, L.; Huang, H.; Chen, L.; Loh, C.; Chi, C. Association of Risk of Incident Venous Thromboembolism with Atopic Dermatitis and Treatment with Janus Kinase Inhibitors. JAMA Dermatol. 2022, 158, 1254–1261. [Google Scholar] [CrossRef]
- Dong, Z.; Ye, X.X.; Chen, C.C.; Wang, R.; Liu, D.; Xu, X.; Zhou, X.X.; He, J. Thromboembolic Events in Janus Kinase Inhibitors: A Pharmacovigilance Study from 2012 to 2021 Based on the Food and Drug Administration’s Adverse Event Reporting System. Br. J. Clin. Pharmacol. 2022, 88, 4180. [Google Scholar] [CrossRef] [PubMed]
- Makara, G.B.; Haller, J. Non-Genomic Effects of Glucocorticoids in the Neural System. Prog. Neurobiol. 2001, 65, 367. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Buttgereit, F. Non-Genomic Glucocorticoid Effects to Provide the Basis for New Drug Developments. Mol. Cell. Endocrinol. 2006, 246, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Veleiro, A.; Alvarez, L.; Eduardo, S.; Burton, G. Structure of the Glucocorticoid Receptor, a Flexible Protein that can Adapt to Different Ligands. ChemMedChem 2010, 5, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Dack, K.N.; Johnson, P.S.; Henriksson, K.; Eirefelt, S.; Carnerup, M.A.; Stahlhut, M.; Ollerstam, A.K. Topical ‘dual-Soft’ Glucocorticoid Receptor Agonist for Dermatology. Bioorganic Med. Chem. Lett. 2020, 30, 127402. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Agarwal, S.; Readhead, B.; Vinokour, E.; Baida, G.; Bhalla, P.; Kirsanov, K.; Yakubovskaya, M.; Platanias, L.C.; Dudley, J.T.; et al. Rapamycin Modulates Glucocorticoid Receptor Function, Blocks Atrophogene REDD1, and Protects Skin from Steroid Atrophy. J. Investig. Dermatol. 2018, 138, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Eirefelt, S.; Stahlhut, M.; Svitacheva, N.; Carnerup, M.A.; Da Rosa, J.M.C.; Ewald, D.A.; Marstrand, T.T.; Krogh-Madsen, M.; Dünstl, G.; Dack, K.N.; et al. Characterization of a Novel Non-Steroidal Glucocorticoid Receptor Agonist Optimized for Topical Treatment. Sci. Rep. 2022, 12, 1501. [Google Scholar] [CrossRef]
- Schoepe, S.; Schäcke, H.; Asadullah, K. Test Systems for the Determination of Glucocorticoid Receptor Ligand Induced Skin Atrophy. Dermatoendocrinology 2011, 3, 175–179. [Google Scholar] [CrossRef]
- Bogarin, T.; Saraswathy, S.; Akiyama, G.; Xie, X.; Weinreb, R.N.; Zheng, J.; Huang, A.S. Cellular and Cytoskeletal Alterations of Scleral Fibroblasts in Response to Glucocorticoid Steroids. Exp. Eye Res. 2019, 187, 107774. [Google Scholar] [CrossRef]
- Durant, S.; Duval, D.; Homo-Delarche, F. Factors Involved in the Control of Fibroblast Proliferation by Glucocorticoids: A Review. Endocr. Rev. 1986, 7, 254–269. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular Senescence: The Good, the Bad and the Unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Hamsanathan, S.; Gurkar, A.U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [Google Scholar] [CrossRef] [PubMed]
- Fafián-Labora, J.; Carpintero-Fernández, P.; Jordan, S.J.D.; Shikh-Bahaei, T.; Abdullah, S.M.; Mahenthiran, M.; Rodríguez-Navarro, J.A.; Niklison-Chirou, M.V.; O’Loghlen, A. FASN Activity is Important for the Initial Stages of the Induction of Senescence. Cell Death Dis. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Naru, E.; Takanezawa, Y.; Kobayashi, M.; Misaki, Y.; Kaji, K.; Arakane, K. Increased Levels of a Particular Phosphatidylcholine Species in Senescent Human Dermal Fibroblasts in Vitro. Hum. Cell 2008, 21, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Alicea, G.M.; Rebecca, V.W.; Goldman, A.R.; Fane, M.E.; Douglass, S.M.; Behera, R.; Webster, M.R.; Kugel, C.H.; Ecker, B.L.; Caino, M.C.; et al. Changes in Aged Fibroblast Lipid Metabolism Induce Age-Dependent Melanoma Cell Resistance to Targeted Therapy Via the Fatty Acid Transporter FATP2. Cancer Discov. 2020, 10, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a Protein-Small Molecule Docking Web Service Based on EADock DSS. Nucleic Acids Res. 2011, 39, 270. [Google Scholar] [CrossRef] [PubMed]
- Petterson, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Briganti, S.; Flori, E.; Bellei, B.; Picardo, M. Modulation of PPARgamma Provides New Insights in a Stress Induced Premature Senescence Model. PLoS ONE 2014, 9, e104045. [Google Scholar] [CrossRef]
- Kovacs, D.; Flori, E.; Maresca, V.; Ottaviani, M.; Aspite, N.; Dell’Anna, M.L.; Panzella, L.; Napolitano, A.; Picardo, M.; d’Ischia, M. The Eumelanin Intermediate 5,6-Dihydroxyindole-2-Carboxylic Acid is a Messenger in the Cross-Talk among Epidermal Cells. J. Investig. Dermatol. 2012, 132, 1196–1205. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Seltmann, H.; Neitzel, H.; Orfanos, C.E. Establishment and Characterization of an Immortalized Human Sebaceous Gland Cell Line (SZ95). J. Investig. Dermatol. 1999, 113, 1011–1020. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, M.E.; Maugeri-Saccà, M.; Fattore, L.; Bruschini, S.; De Vitis, C.; Tabbì, E.; Bellei, B.; Migliano, E.; Kovacs, D.; Camera, E.; et al. Inhibition of Stearoyl-CoA desaturase 1 reverts BRAF and MEK inhibition-induced selection of cancer stem cells in BRAF-mutated melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 318. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flori, E.; Mosca, S.; Kovacs, D.; Briganti, S.; Ottaviani, M.; Mastrofrancesco, A.; Truglio, M.; Picardo, M. Skin Anti-Inflammatory Potential with Reduced Side Effects of Novel Glucocorticoid Receptor Agonists. Int. J. Mol. Sci. 2024, 25, 267. https://doi.org/10.3390/ijms25010267
Flori E, Mosca S, Kovacs D, Briganti S, Ottaviani M, Mastrofrancesco A, Truglio M, Picardo M. Skin Anti-Inflammatory Potential with Reduced Side Effects of Novel Glucocorticoid Receptor Agonists. International Journal of Molecular Sciences. 2024; 25(1):267. https://doi.org/10.3390/ijms25010267
Chicago/Turabian StyleFlori, Enrica, Sarah Mosca, Daniela Kovacs, Stefania Briganti, Monica Ottaviani, Arianna Mastrofrancesco, Mauro Truglio, and Mauro Picardo. 2024. "Skin Anti-Inflammatory Potential with Reduced Side Effects of Novel Glucocorticoid Receptor Agonists" International Journal of Molecular Sciences 25, no. 1: 267. https://doi.org/10.3390/ijms25010267
APA StyleFlori, E., Mosca, S., Kovacs, D., Briganti, S., Ottaviani, M., Mastrofrancesco, A., Truglio, M., & Picardo, M. (2024). Skin Anti-Inflammatory Potential with Reduced Side Effects of Novel Glucocorticoid Receptor Agonists. International Journal of Molecular Sciences, 25(1), 267. https://doi.org/10.3390/ijms25010267




