Palmitate Stimulates Expression of the von Willebrand Factor and Modulates Toll-like Receptors Level and Activity in Human Umbilical Vein Endothelial Cells (HUVECs)
Abstract
:1. Introduction
2. Results
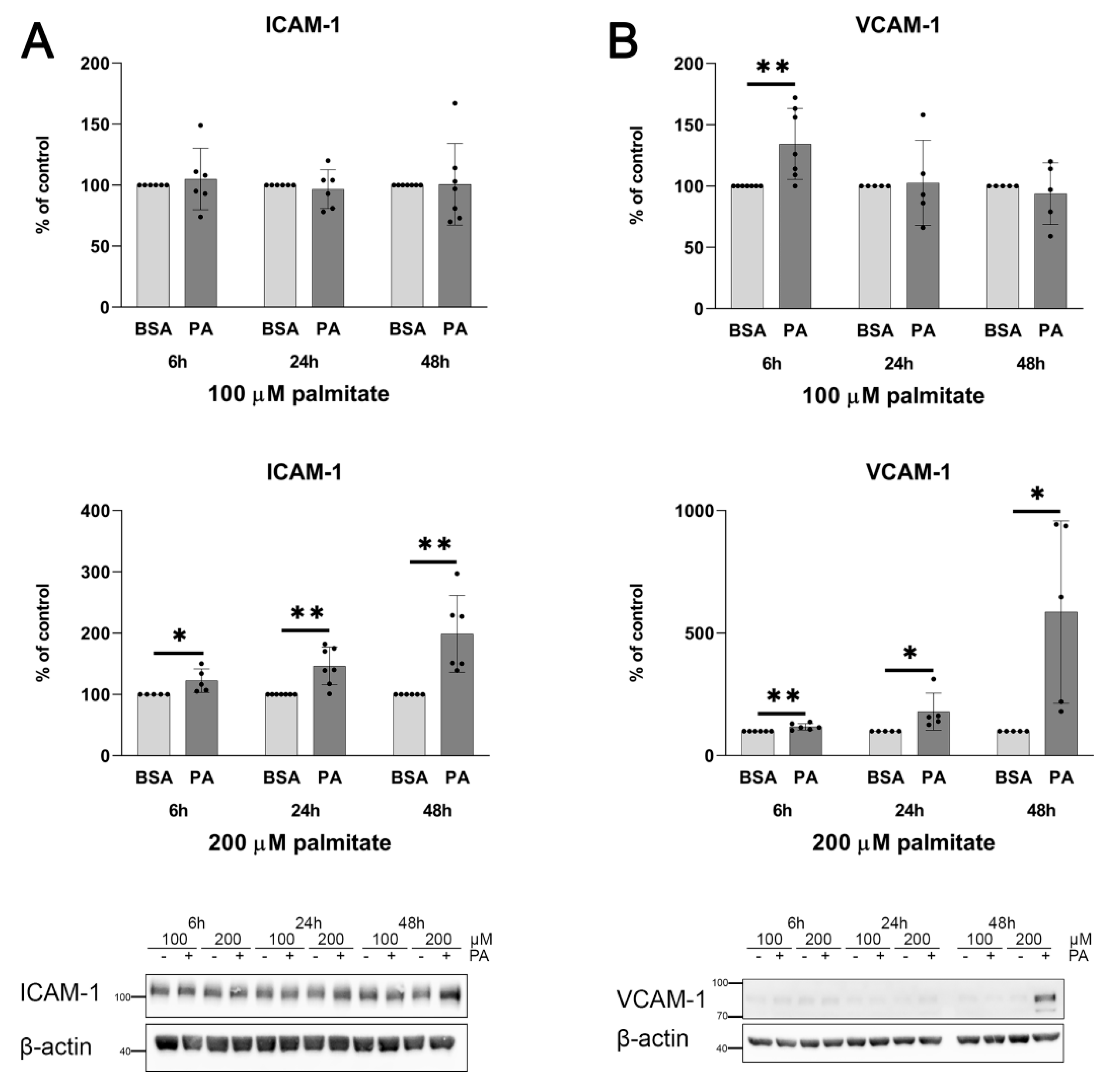
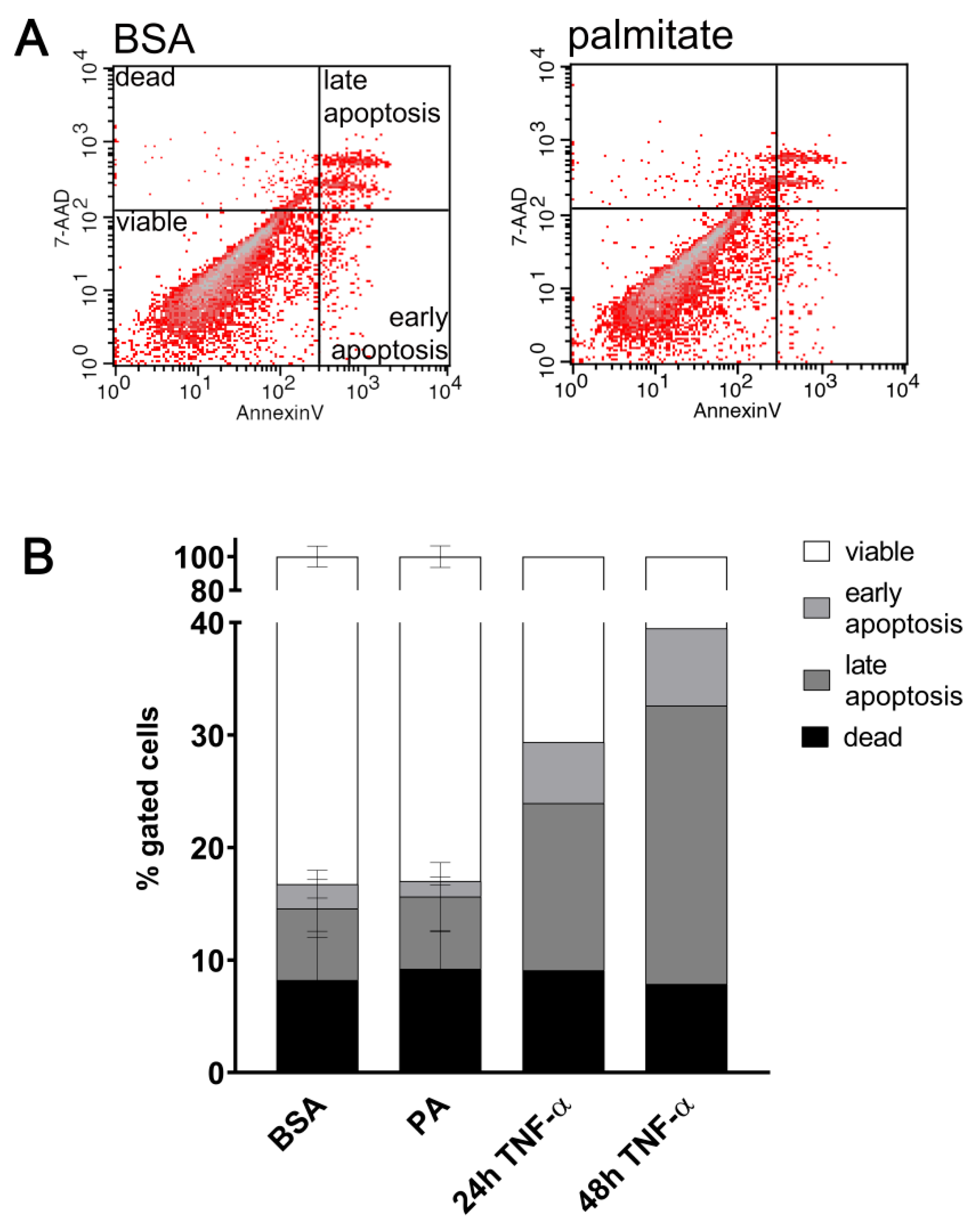
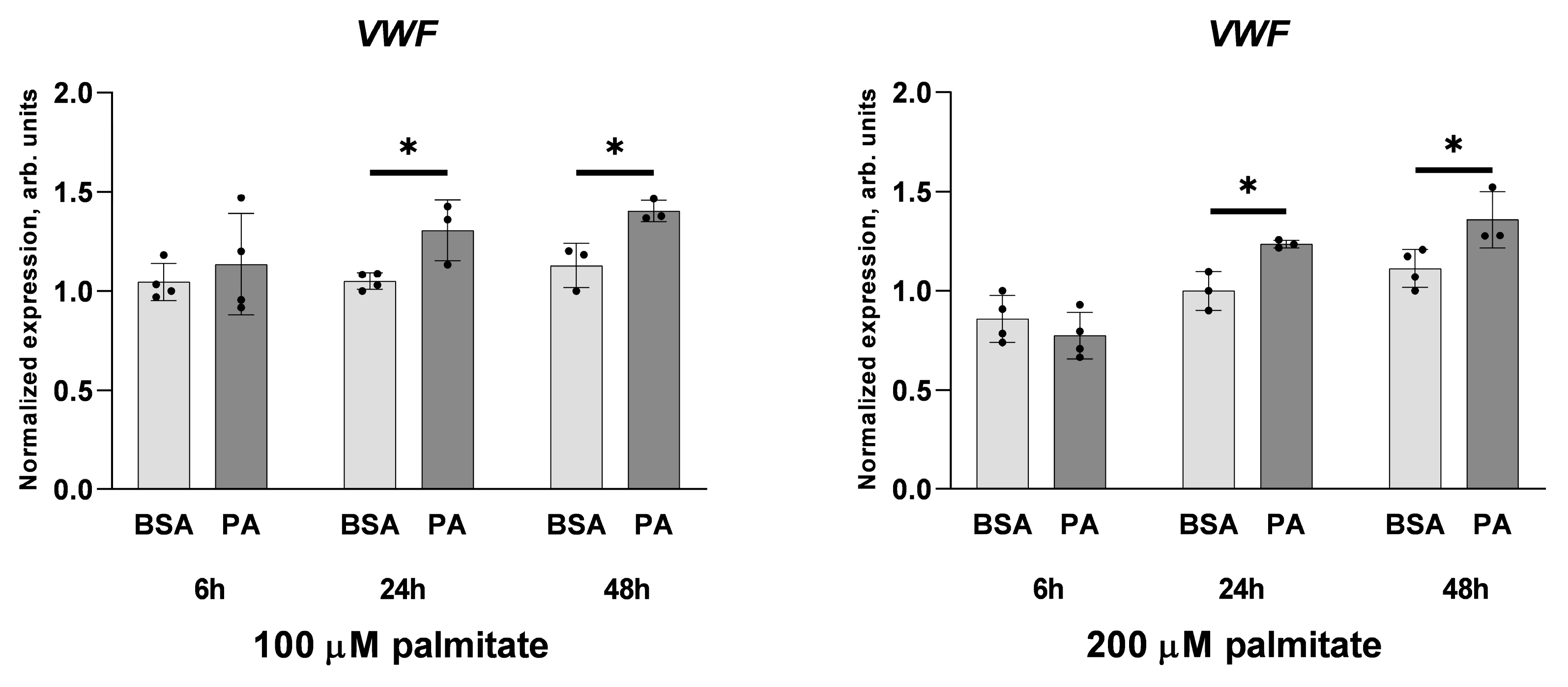
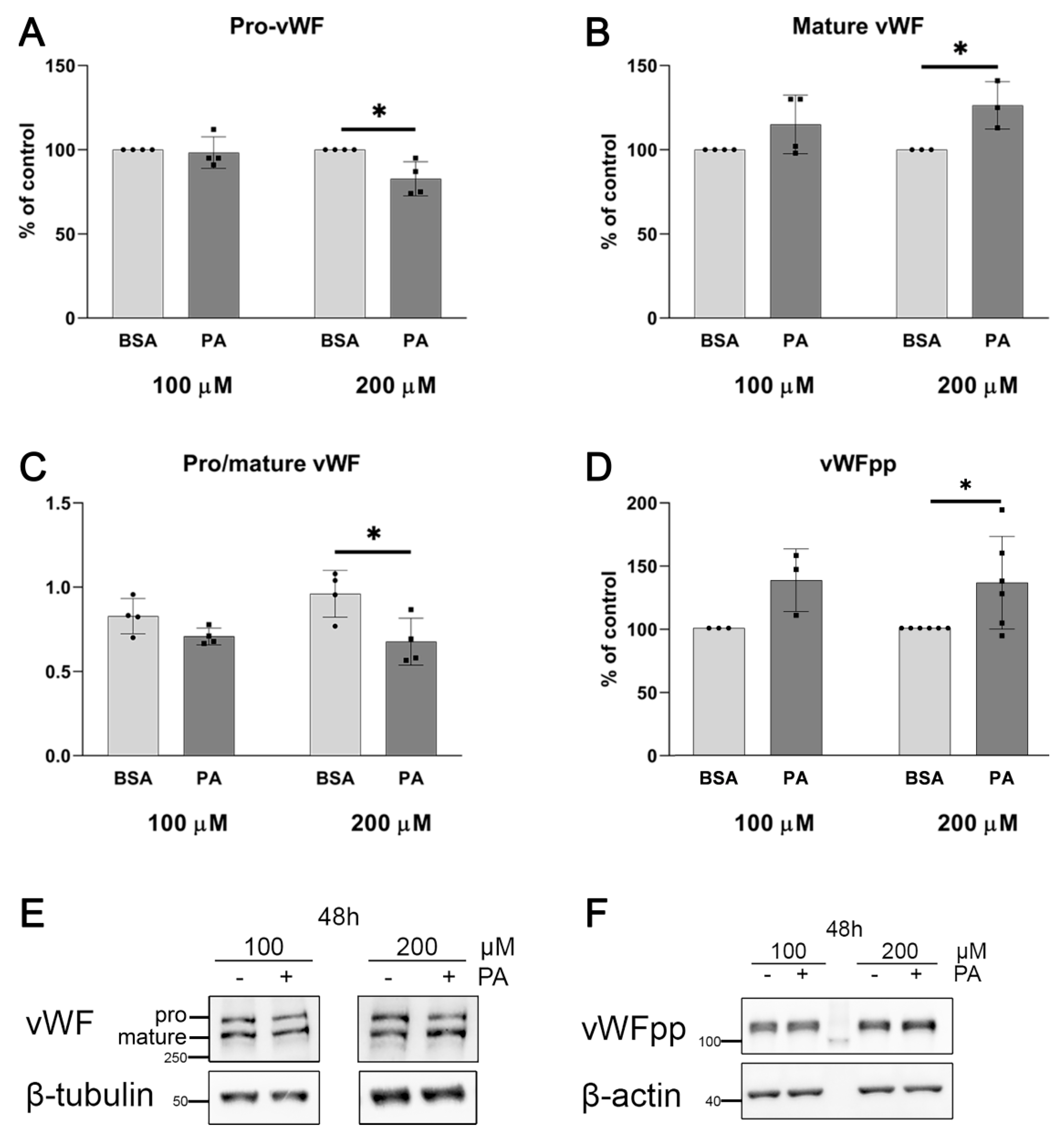
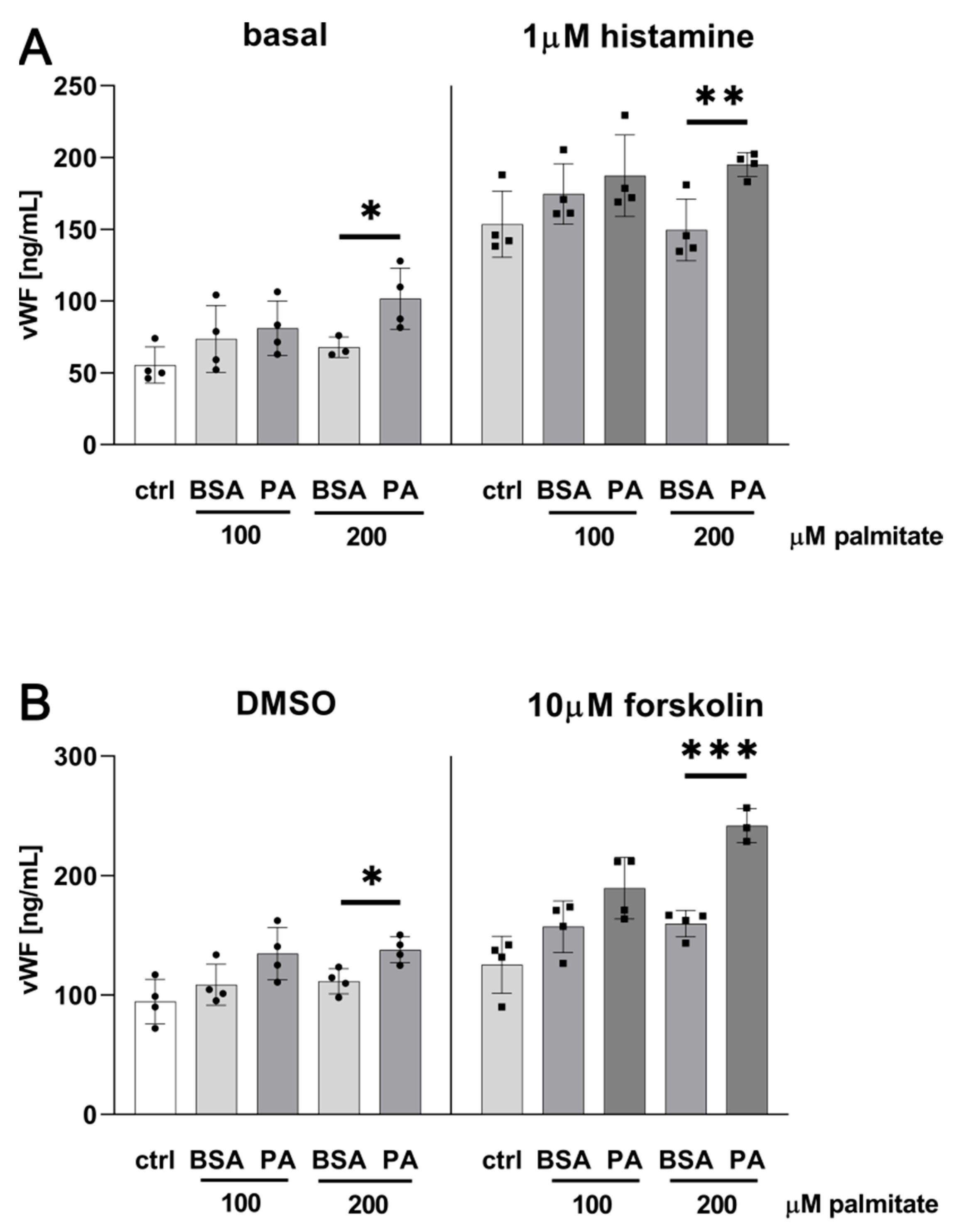
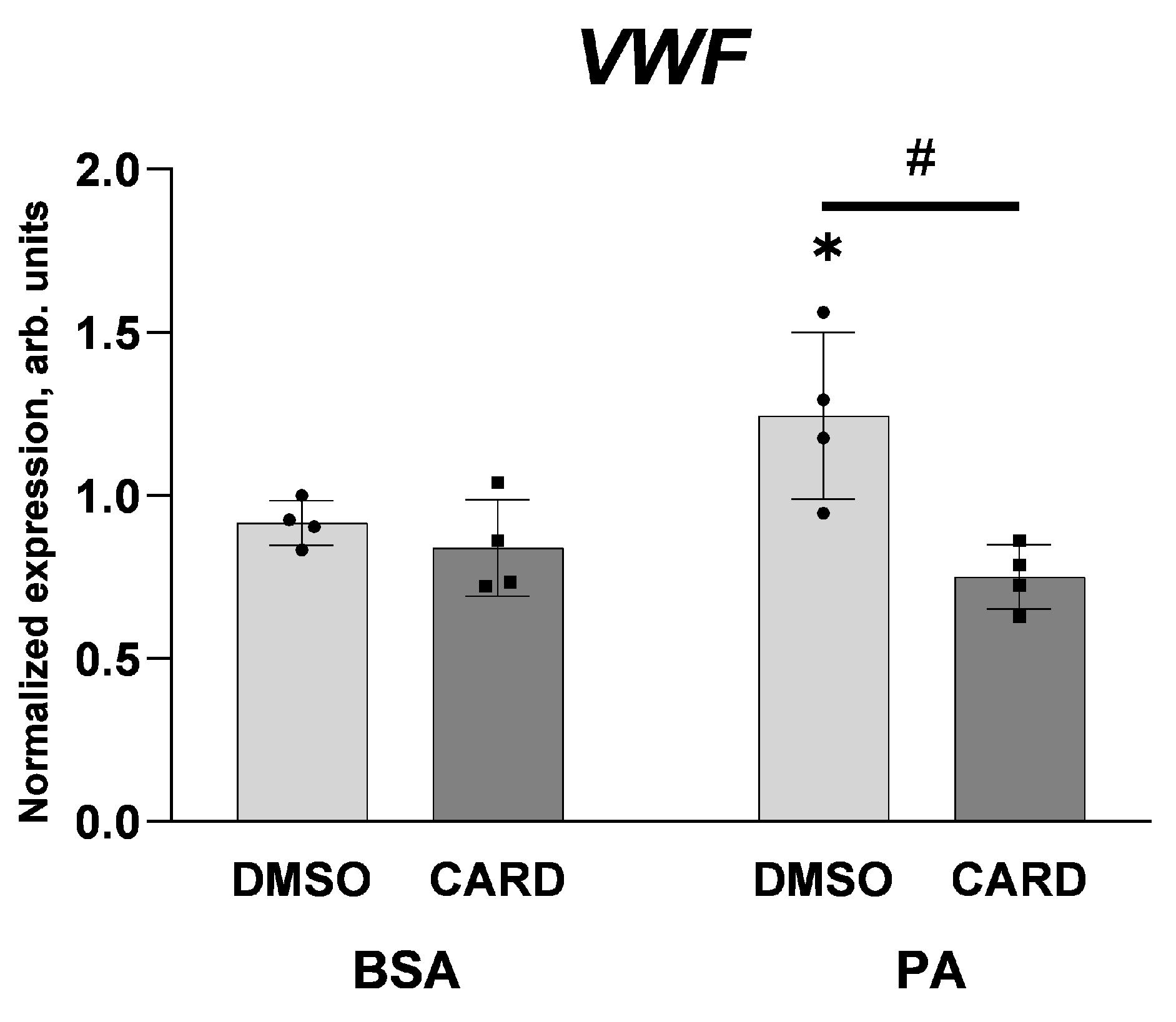
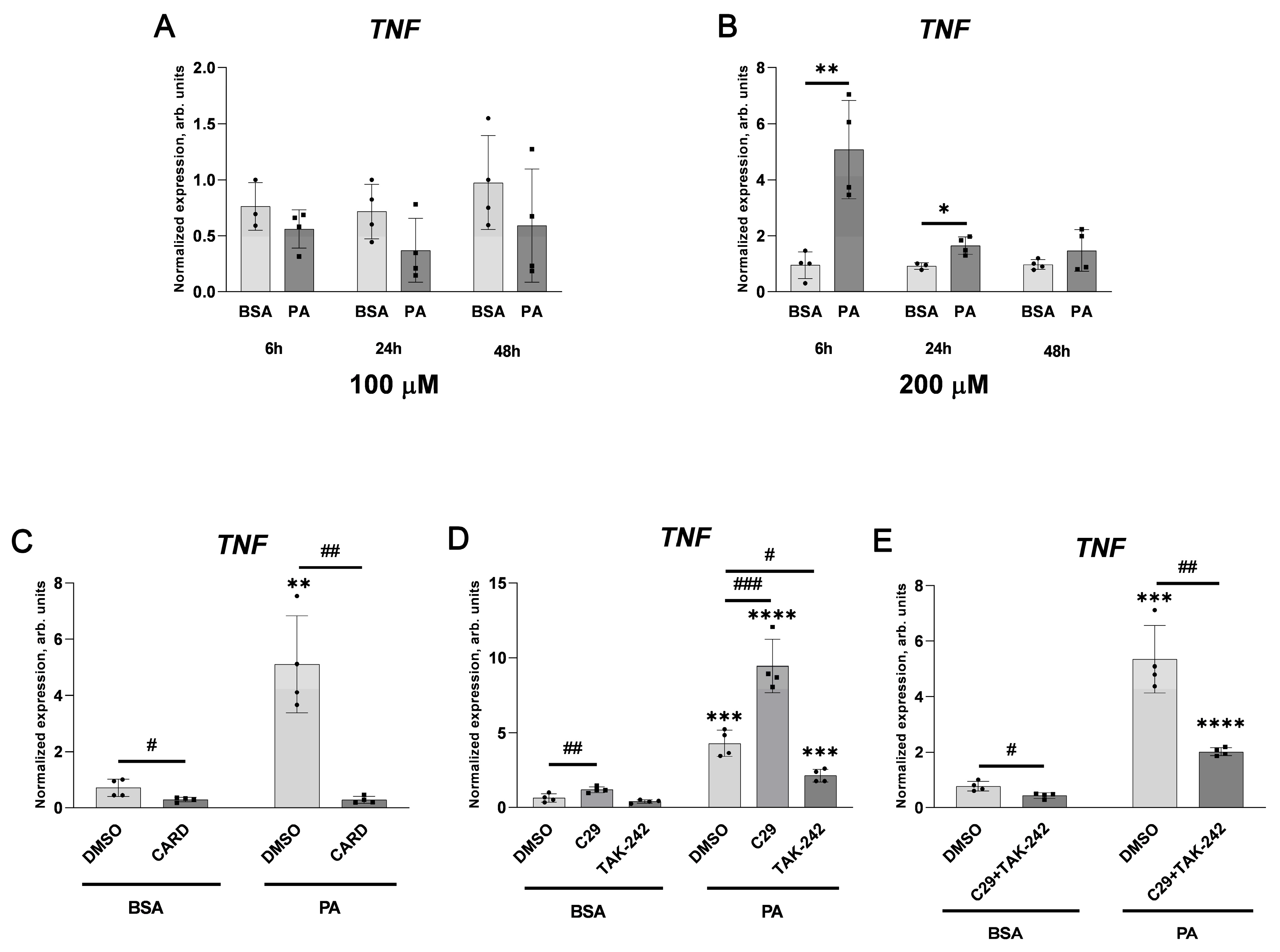
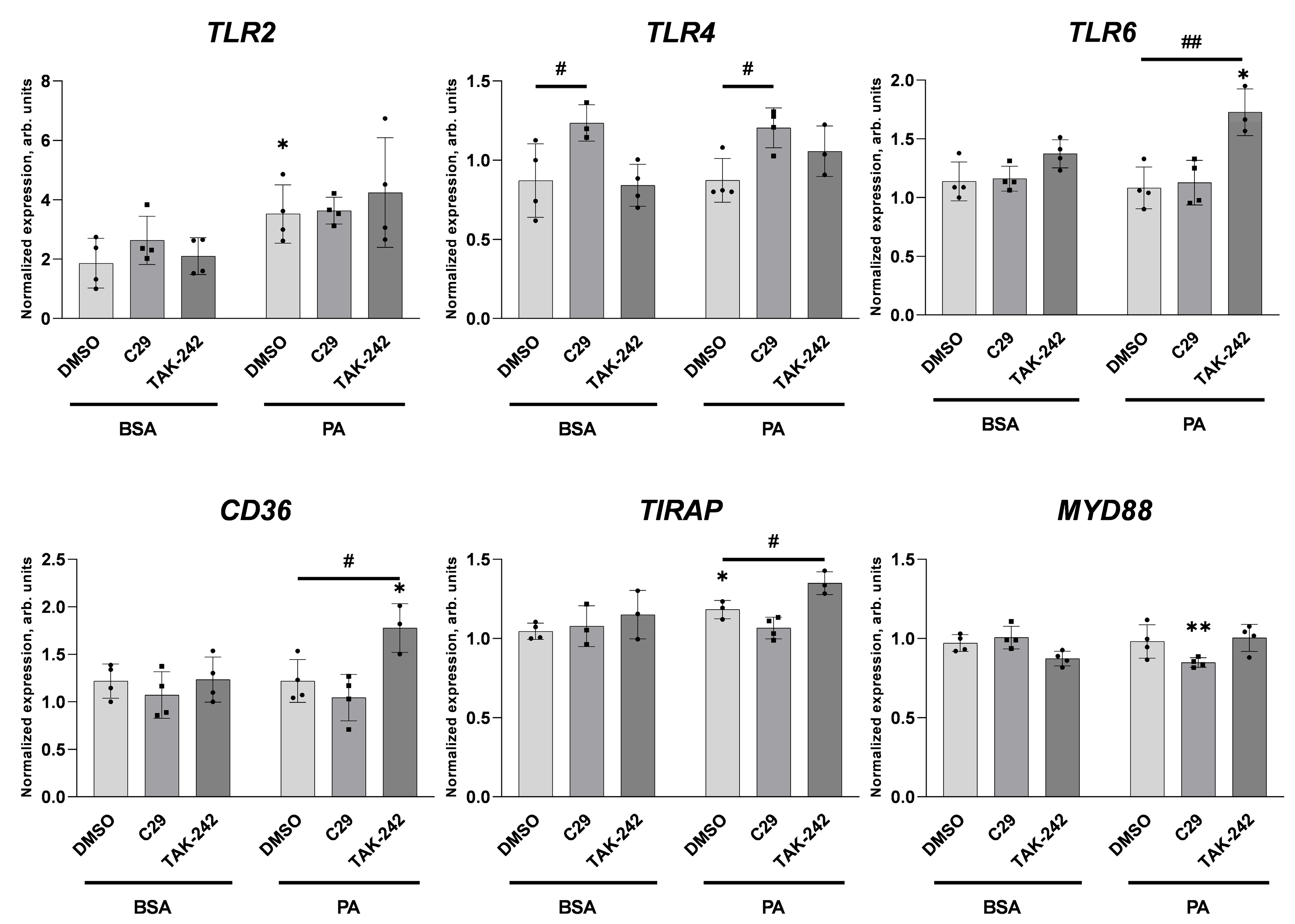
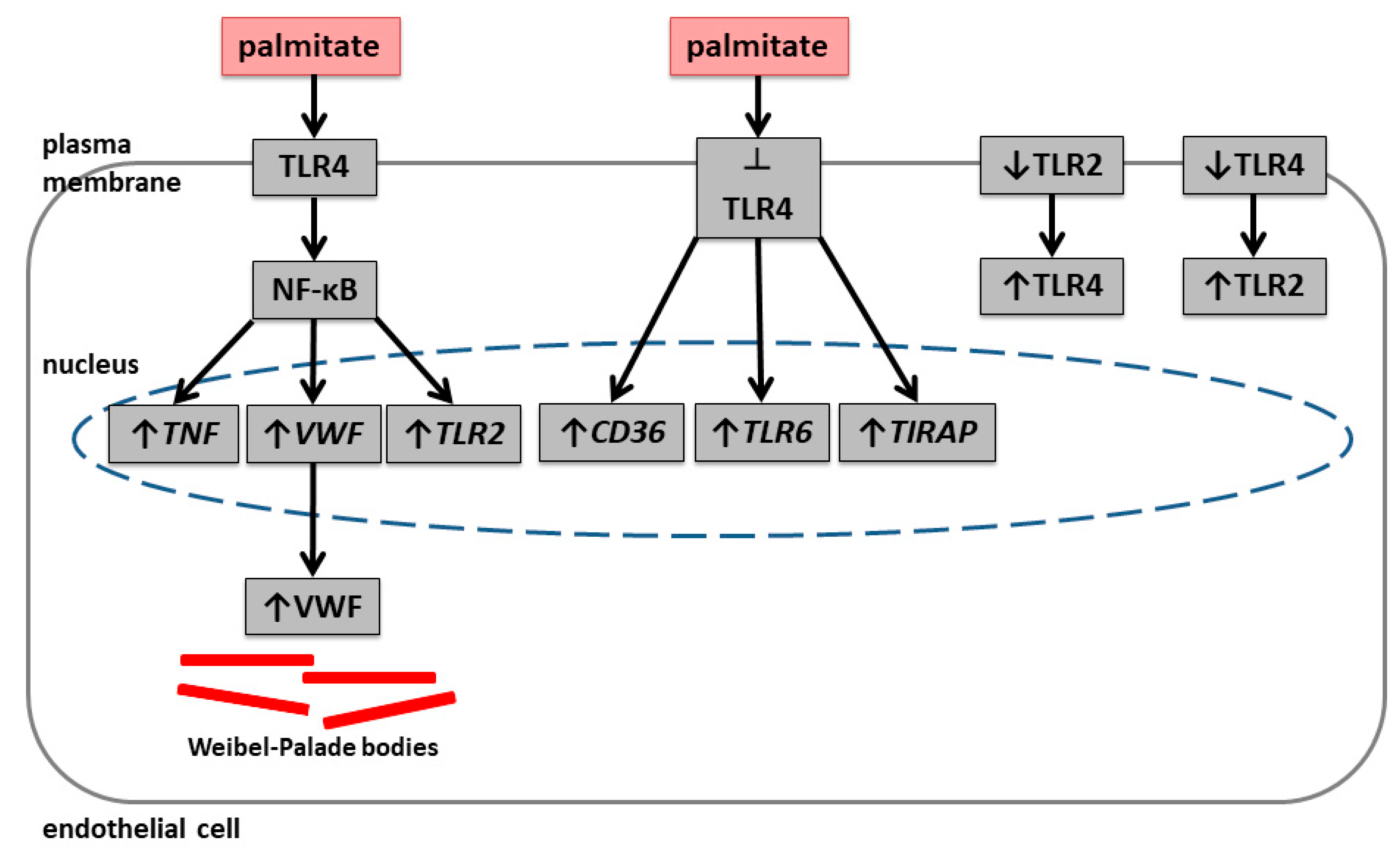
2.1. Palmitate Induces Inflammatory Response and Elevates vWF-Encoding Gene Expression and Release
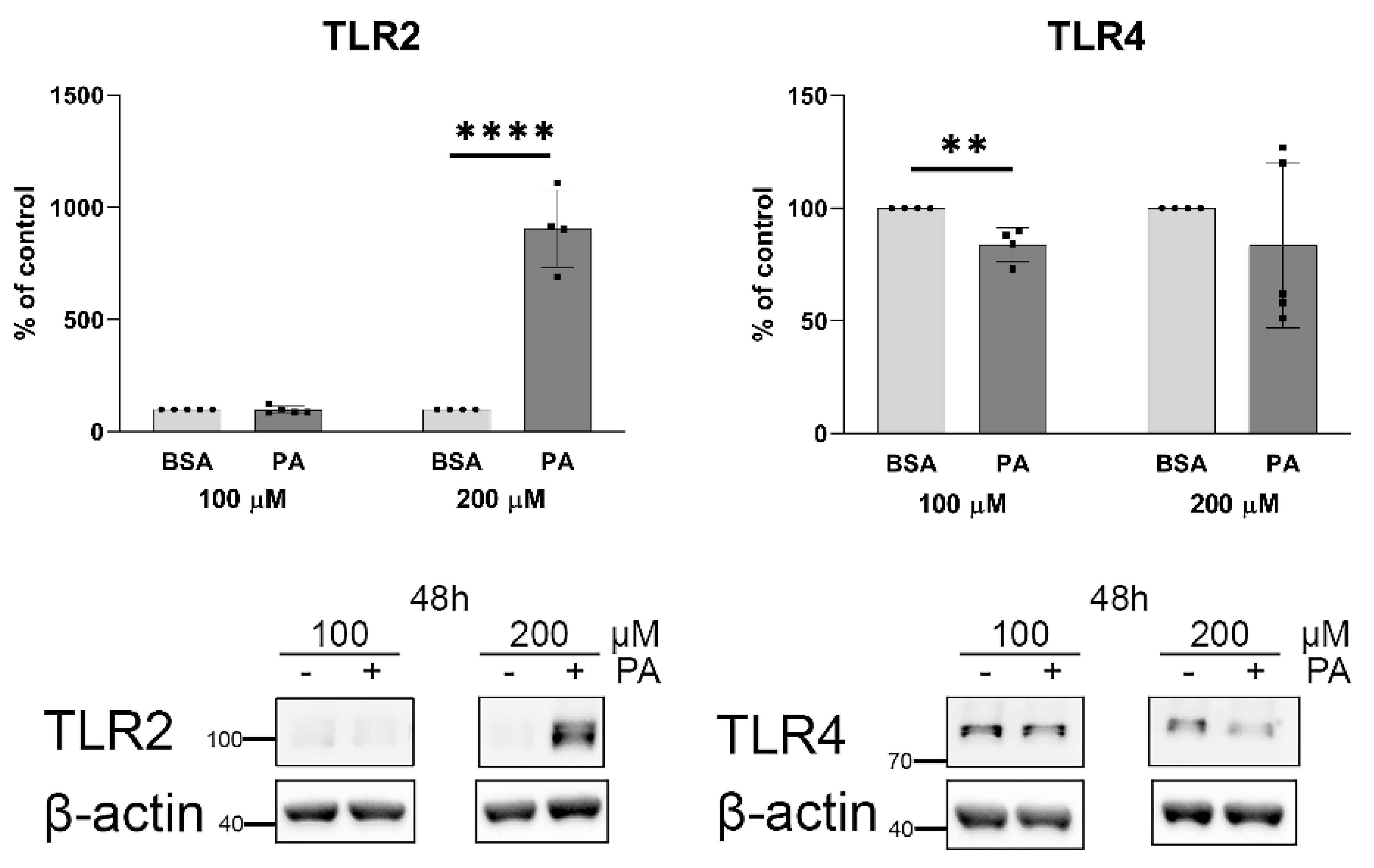
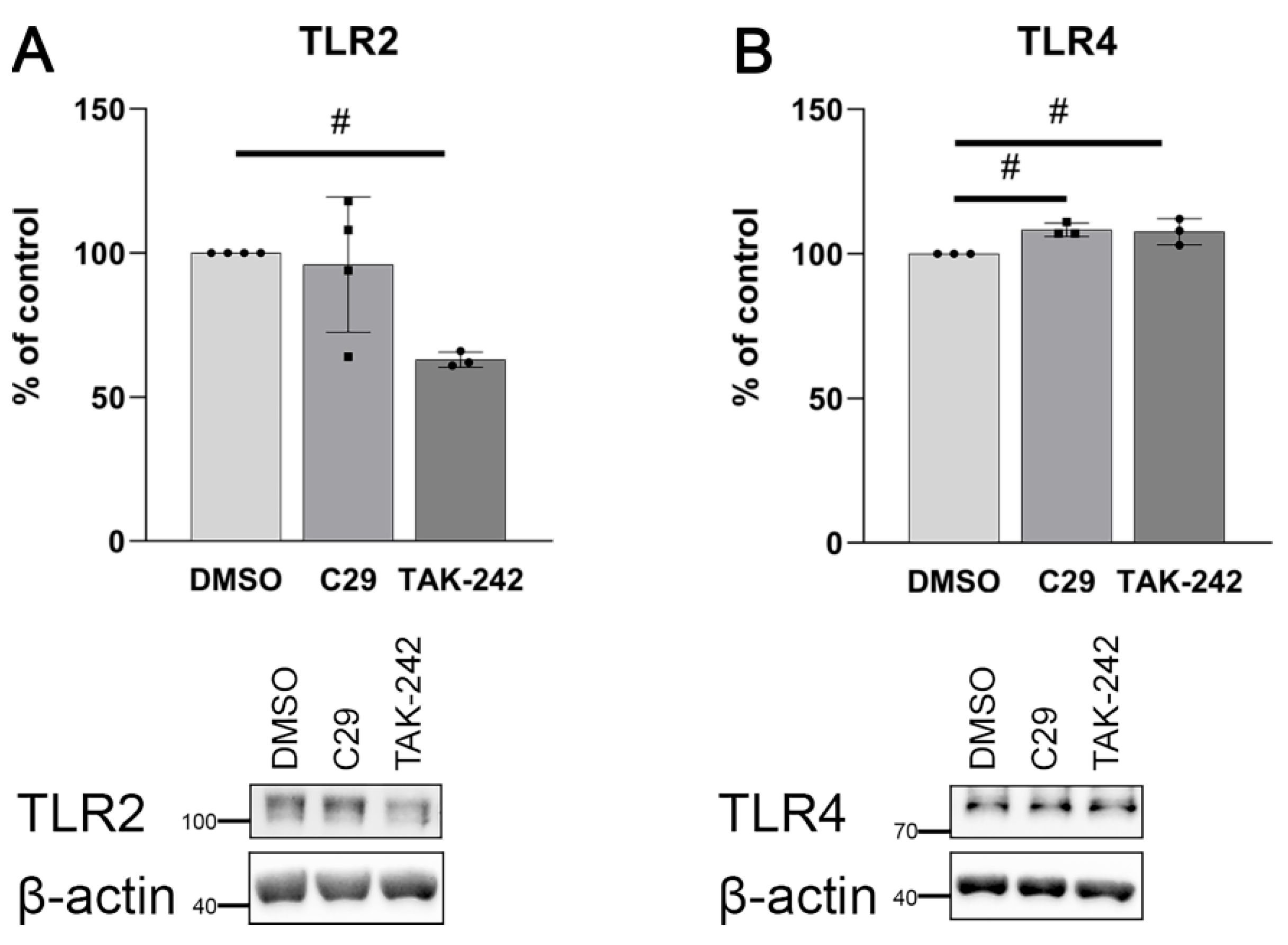
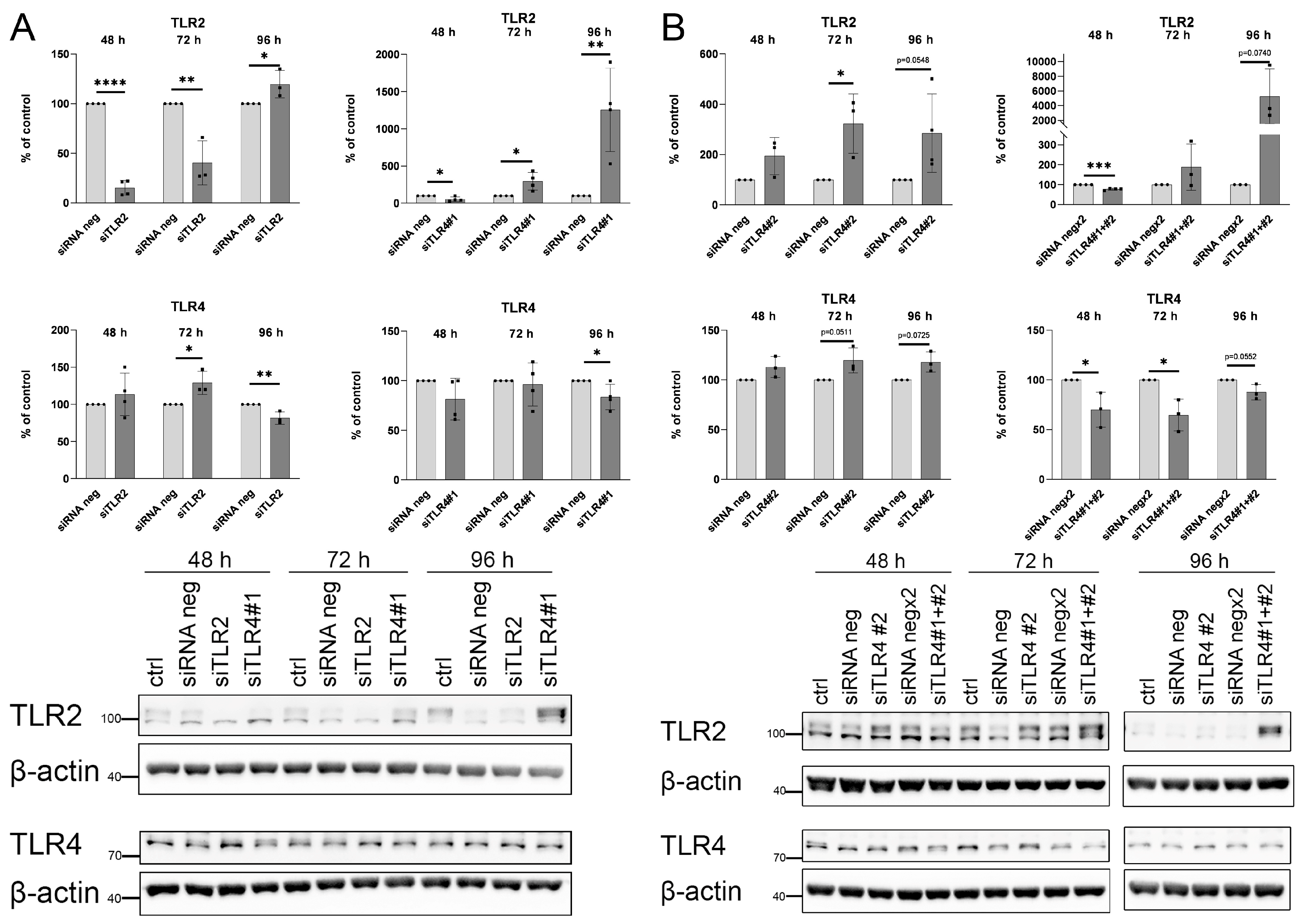
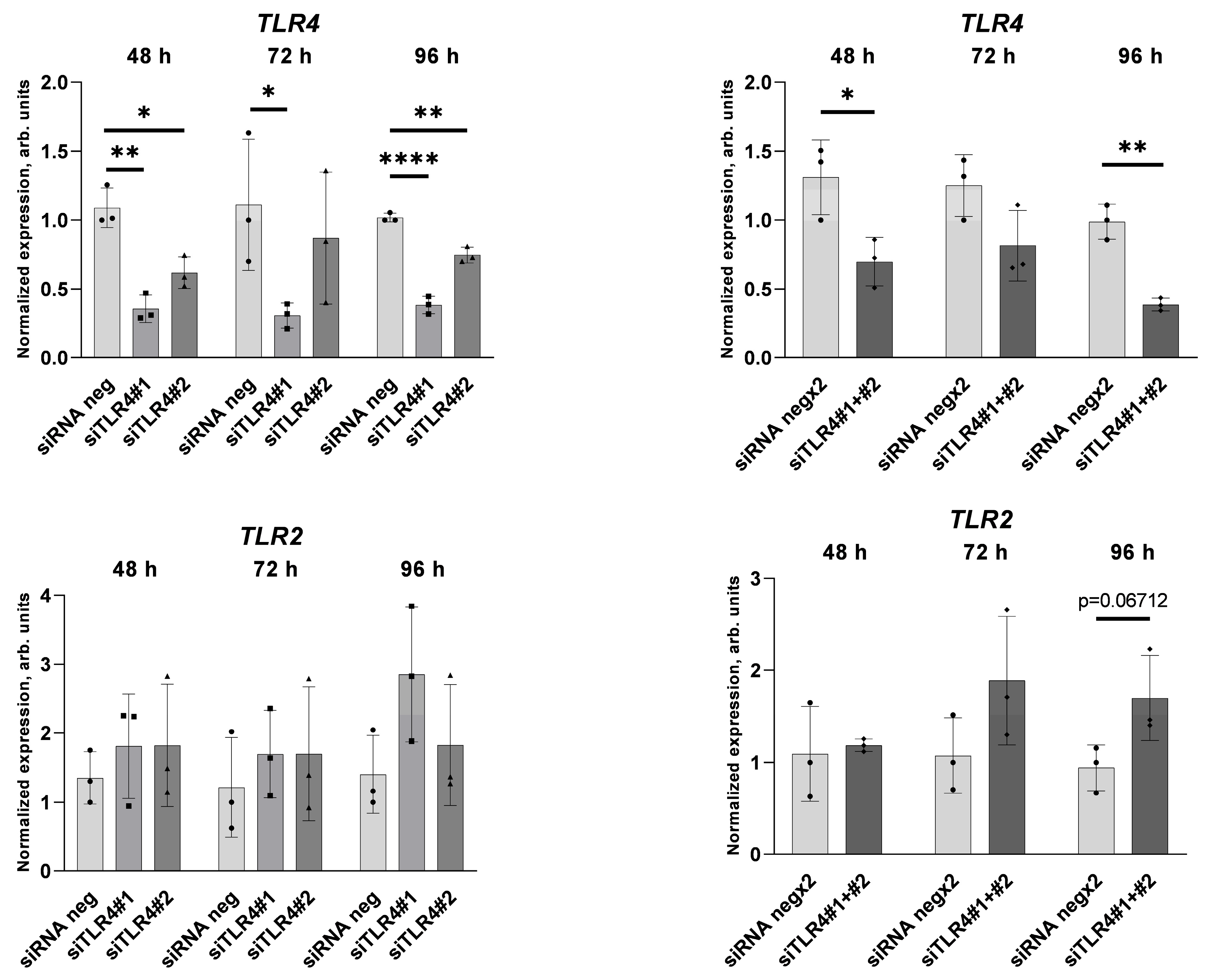
2.2. Palmitate Affects TLR Levels and Activity
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. siRNA Transfection
4.3. Cell Lysis and Western Blot Analysis
4.4. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
4.5. von Willebrand Factor Level
4.6. Apoptosis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Pablo-Moreno, J.A.D.; Serrano, L.J.; Revuelta, L.; Sánchez, M.J.; Liras, A. The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V. Int. J. Mol. Sci. 2022, 23, 8283. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Tay, E.; Cusi, K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc. Diabetol. 2010, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Fryk, E.; Olausson, J.; Mossberg, K.; Strindberg, L.; Schmelz, M.; Brogren, H.; Gan, L.-M.; Piazza, S.; Provenzani, A.; Becattini, B.; et al. Hyperinsulinemia and insulin resistance in the obese may develop as part of a homeostatic response to elevated free fatty acids: A mechanistic case-control and a population-based cohort study. EBioMedicine 2021, 65, 103264. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Rydén, M. Fatty Acids, Obesity and Insulin Resistance. Obes. Facts 2015, 8, 147–155. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Tarshoby, M.; Monestel, R.; Hook, G.; Cronin, J.; Johnson, A.; Bayazeed, B.; Baron, A.D. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Investig. 1997, 100, 1230–1239. [Google Scholar] [CrossRef]
- Pilz, S.; Scharnagl, H.; Tiran, B.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; Schaefer, J.R.; März, W. Free Fatty Acids Are Independently Associated with All-Cause and Cardiovascular Mortality in Subjects with Coronary Artery Disease. J. Clin. Endocrinol. Metab. 2006, 91, 2542–2547. [Google Scholar] [CrossRef]
- Lee, D.M.; Sevits, K.J.; Battson, M.L.; Wei, Y.; Cox-York, K.A.; Gentile, C.L. Monounsaturated fatty acids protect against palmitate-induced lipoapoptosis in human umbilical vein endothelial cells. PLoS ONE 2019, 14, e0226940. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Staiger, H.; Staiger, K.; Stefan, N.; Wahl, H.G.; Machicao, F.; Kellerer, M.; Häring, H.-U. Palmitate-Induced Interleukin-6 Expression in Human Coronary Artery Endothelial Cells. Diabetes 2004, 53, 3209–3216. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic Acid, but Not Lauric Acid, Induces Metabolic Inflammation, Mitochondrial Fragmentation, and a Drop in Mitochondrial Membrane Potential in Human Primary Myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef] [PubMed]
- Dymkowska, D.; Kawalec, M.; Wyszomirski, T.; Zabłocki, K. Mild palmitate treatment increases mitochondrial mass but does not affect EA.hy926 endothelial cells viability. Arch. Biochem. Biophys. 2017, 634, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chung, D.W. Inflammation, von Willebrand factor, and ADAMTS13. Blood 2018, 132, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Raparelli, V.; Nocella, C.; Bartimoccia, S.; Novo, M.; Severino, A.; De Falco, E.; Cammisotto, V.; Pasquale, C.; Crescioli, C.; et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J. Hepatol. 2017, 67, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Dong, F.; Guo, L.; Hou, Y.; Hu, H.; Yan, S.; Zhou, X.; Liao, L.; Allen, T.D.; et al. Plasma von Willebrand factor level is transiently elevated in a rat model of acute myocardial infarction. Exp. Ther. Med. 2015, 10, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Urazgildeeva, S.; Shugurova, I.; Vasina, L.; Muzalevskaya, M.; Baranova, E.; Shurygina, V.; Gurevich, V.; Perepech, N. Obesity and elevated levels of von Willebrand factor in patients with hypertension and early coronary atherosclerosis. J. Hypertens. 2021, 39, e336. [Google Scholar] [CrossRef]
- Blann, A.D.; Bushell, D.; Davies, A.; Faragher, E.B.; Miller, J.P.; McCollum, C.N. von Willebrand factor, the endothelium and obesity. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1993, 17, 723–725. [Google Scholar]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood 2015, 125, 2019–2028. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol. Rev. 2016, 68, 142–167. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like Receptor Status in Obesity and Metabolic Syndrome: A Translational Perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Senn, J.J. Toll-like Receptor-2 Is Essential for the Development of Palmitate-induced Insulin Resistance in Myotubes. J. Biol. Chem. 2006, 281, 26865–26875. [Google Scholar] [CrossRef]
- Suganami, T.; Mieda, T.; Itoh, M.; Shimoda, Y.; Kamei, Y.; Ogawa, Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem. Biophys. Res. Commun. 2007, 354, 45–49. [Google Scholar] [CrossRef]
- Caricilli, A.M.; Nascimento, P.H.; Pauli, J.R.; Tsukumo, D.M.L.; Velloso, L.A.; Carvalheira, J.B.; Saad, M.J.A. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J. Endocrinol. 2008, 199, 399–406. [Google Scholar] [CrossRef]
- Kim, F.; Pham, M.; Luttrell, I.; Bannerman, D.D.; Tupper, J.; Thaler, J.; Hawn, T.R.; Raines, E.W.; Schwartz, M.W. Toll-Like Receptor-4 Mediates Vascular Inflammation and Insulin Resistance in Diet-Induced Obesity. Circ. Res. 2007, 100, 1589–1596. [Google Scholar] [CrossRef]
- Davis, J.E.; Gabler, N.K.; Walker-Daniels, J.; Spurlock, M.E. Tlr-4 Deficiency Selectively Protects Against Obesity Induced by Diets High in Saturated Fat. Obesity 2008, 16, 1248–1255. [Google Scholar] [CrossRef]
- Ehses, J.A.; Meier, D.T.; Wueest, S.; Rytka, J.; Boller, S.; Wielinga, P.Y.; Schraenen, A.; Lemaire, K.; Debray, S.; Van Lommel, L.; et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 2010, 53, 1795–1806. [Google Scholar] [CrossRef]
- Himes, R.W.; Smith, C.W. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010, 24, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knight, A.G.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J. Neurochem. 2012, 120, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.; Wang, F.; Liu, S.; Zhao, S.; Ling, E.-A.; Hao, A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2012, 107, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Kim, H.-S.; Hwang, D.H.; Quon, M.J.; Kim, J. Toll-like receptor 2 mediates high-fat diet-induced impairment of vasodilator actions of insulin. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, E1077–E1088. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Huang, S.; Choi, I.-W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-Mediated Secretion of IL-1β in Human Monocytes through TLR2 Activation; Modulation by Dietary Fatty Acids. J. Immunol. 2013, 191, 4337–4347. [Google Scholar] [CrossRef]
- Quan, J.; Liu, J.; Gao, X.; Liu, J.; Yang, H.; Chen, W.; Li, W.; Li, Y.; Yang, W.; Wang, B. Palmitate induces interleukin-8 expression in human aortic vascular smooth muscle cells via T oll-like receptor 4/nuclear factor-κB pathway. J. Diabetes 2014, 6, 33–41. [Google Scholar] [CrossRef]
- Brehm, M. Von Willebrand factor processing. Hämostaseologie 2017, 37, 59–72. [Google Scholar] [CrossRef]
- Haberichter, S.L. von Willebrand factor propeptide: Biology and clinical utility. Blood 2015, 126, 1753–1761. [Google Scholar] [CrossRef]
- Maloney, E.; Sweet, I.R.; Hockenbery, D.M.; Pham, M.; Rizzo, N.O.; Tateya, S.; Handa, P.; Schwartz, M.W.; Kim, F. Activation of NF-κB by Palmitate in Endothelial Cells: A Key Role for NADPH Oxidase-Derived Superoxide in Response to TLR4 Activation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1370–1375. [Google Scholar] [CrossRef]
- Ajuwon, K.M.; Spurlock, M.E. Palmitate Activates the NF-κB Transcription Factor and Induces IL-6 and TNFα Expression in 3T3-L1 Adipocytes. J. Nutr. 2005, 135, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Nisr, R.B.; Shah, D.S.; Ganley, I.G.; Hundal, H.S. Proinflammatory NFkB signalling promotes mitochondrial dysfunction in skeletal muscle in response to cellular fuel overloading. Cell. Mol. Life Sci. 2019, 76, 4887–4904. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Langley, K.G.; Berglund, N.A.; Kammoun, H.L.; Reibe, S.; Estevez, E.; Weir, J.; Mellett, N.A.; Pernes, G.; Conway, J.R.W.; et al. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018, 27, 1096–1110.e5. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.; Dwyer, C.N.; Mewburn, J.; Nesbitt, K.; Kawecki, C.; Lenting, P.; Swystun, L.L.; Lillicrap, D. von Willebrand Factor Is a Critical Mediator of Deep Vein Thrombosis in a Mouse Model of Diet-Induced Obesity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2860–2874. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Katneni, U.; Akpan, I.J.; Tanaka, K.; Thomas, T.; Setua, S.; Reisz, J.A.; Cendali, F.; Gamboni, F.; Nemkov, T.; et al. The Impact of Age and BMI on the VWF/ADAMTS13 Axis and Simultaneous Thrombin and Plasmin Generation in Hospitalized COVID-19 Patients. Front. Med. 2022, 8, 817305. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, S.; Baxter, B.A.; Dooley, G.; LaVergne, S.M.; Gallichotte, E.; Dutt, T.; Tipton, M.; Berry, K.; Haberman, J.; Natter, N.; et al. Relationships between plasma fatty acids in adults with mild, moderate, or severe COVID-19 and the development of post-acute sequelae. Front. Nutr. 2022, 9, 960409. [Google Scholar] [CrossRef]
- Huisman, A.; Beun, R.; Sikma, M.; Westerink, J.; Kusadasi, N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020, 42, e211–e212. [Google Scholar] [CrossRef]
- Dymkowska, D.; Drabarek, B.; Michalik, A.; Nowak, N.; Zabłocki, K. TNFα stimulates NO release in EA.hy926 cells by activating the CaMKKβ-AMPK-eNOS pathway. Int. J. Biochem. Cell Biol. 2019, 106, 57–67. [Google Scholar] [CrossRef]
- Rondaij, M.G.; Bierings, R.; Kragt, A.; Van Mourik, J.A.; Voorberg, J. Dynamics and Plasticity of Weibel-Palade Bodies in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1002–1007. [Google Scholar] [CrossRef]
- Poeter, M.; Brandherm, I.; Rossaint, J.; Rosso, G.; Shahin, V.; Skryabin, B.V.; Zarbock, A.; Gerke, V.; Rescher, U. Annexin A8 controls leukocyte recruitment to activated endothelial cells via cell surface delivery of CD63. Nat. Commun. 2014, 5, 3738. [Google Scholar] [CrossRef]
- Doyle, E.L.; Ridger, V.; Ferraro, F.; Turmaine, M.; Saftig, P.; Cutler, D.F. CD63 is an essential cofactor to leukocyte recruitment by endothelial P-selectin. Blood 2011, 118, 4265–4273. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.; Aareskjold, E.; Bode, G.; Gerke, V. Rab3D and annexin A2 play a role in regulated secretion of vWF, but not tPA, from endothelial cells. EMBO J. 2004, 23, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Chehab, T.; Santos, N.C.; Holthenrich, A.; Koerdt, S.N.; Disse, J.; Schuberth, C.; Nazmi, A.R.; Neeft, M.; Koch, H.; Man, K.N.M.; et al. A novel Munc13-4/S100A10/annexin A2 complex promotes Weibel–Palade body exocytosis in endothelial cells. Mol. Biol. Cell 2017, 28, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, M.A.H.; Franco, O.H.; Ikram, M.A.; Hofman, A.; Kavousi, M.; De Maat, M.P.M.; Leebeek, F.W.G. Von Willebrand Factor, ADAMTS13, and the Risk of Mortality: The Rotterdam Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Manz, X.D.; Bogaard, H.J.; Aman, J. Regulation of VWF (Von Willebrand Factor) in Inflammatory Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Manz, X.D.; Szulcek, R.; Pan, X.; Symersky, P.; Dickhoff, C.; Majolée, J.; Kremer, V.; Michielon, E.; Jordanova, E.S.; Radonic, T.; et al. Epigenetic Modification of the von Willebrand Factor Promoter Drives Platelet Aggregation on the Pulmonary Endothelium in Chronic Thromboembolic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2022, 205, 806–818. [Google Scholar] [CrossRef]
- Jové, M.; Planavila, A.; Sánchez, R.M.; Merlos, M.; Laguna, J.C.; Vázquez-Carrera, M. Palmitate Induces Tumor Necrosis Factor-α Expression in C2C12 Skeletal Muscle Cells by a Mechanism Involving Protein Kinase C and Nuclear Factor-κB Activation. Endocrinology 2006, 147, 552–561. [Google Scholar] [CrossRef]
- Harvey, P.J.; Keightley, A.M.; Lam, Y.M.; Cameron, C.; Lillicrap, D. A single nucleotide polymorphism at nucleotide −1793 in the von Willebrand factor (VWF) regulatory region is associated with plasma VWF:Ag levels: VWF Promoter Polymorphism and Plasma VWF Levels. Br. J. Haematol. 2000, 109, 349–353. [Google Scholar] [CrossRef]
- Schlegel, N.; Leweke, R.; Meir, M.; Germer, C.-T.; Waschke, J. Role of NF-κB activation in LPS-induced endothelial barrier breakdown. Histochem. Cell Biol. 2012, 138, 627–641. [Google Scholar] [CrossRef]
- Fusco, A.J.; Huang, D.; Miller, D.; Wang, V.Y.; Vu, D.; Ghosh, G. NF-κB p52:RelB heterodimer recognizes two classes of κB sites with two distinct modes. EMBO Rep. 2009, 10, 152–159. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An Overview on Cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Shih, V.F.-S.; Tsui, R.; Caldwell, A.; Hoffmann, A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011, 21, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; McIlwraith, E.K.; Chalmers, J.A.; Belsham, D.D. Palmitate Induces an Anti-Inflammatory Response in Immortalized Microglial BV-2 and IMG Cell Lines that Decreases TNFα Levels in mHypoE-46 Hypothalamic Neurons in Co-Culture. Neuroendocrinology 2018, 107, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Seixas Da Silva, G.D.S.; Sant’Ana, M.R.; Teixeira, C.V.L.; Clarke, J.R.; Miya Coreixas, V.S.; De Melo, B.C.; Fortuna, J.T.S.; Forny-Germano, L.; Ledo, J.H.; et al. Palmitate Is Increased in the Cerebrospinal Fluid of Humans with Obesity and Induces Memory Impairment in Mice via Pro-inflammatory TNF-α. Cell Rep. 2020, 30, 2180–2194.e8. [Google Scholar] [CrossRef]
- Pillon, N.J.; Azizi, P.M.; Li, Y.E.; Liu, J.; Wang, C.; Chan, K.L.; Hopperton, K.E.; Bazinet, R.P.; Heit, B.; Bilan, P.J.; et al. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E35–E44. [Google Scholar] [CrossRef]
- Belinky, F.; Nativ, N.; Stelzer, G.; Zimmerman, S.; Iny Stein, T.; Safran, M.; Lancet, D. PathCards: Multi-source consolidation of human biological pathways. Database 2015, 2015, bav006. [Google Scholar] [CrossRef]
- Husebye, H.; Halaas, Ø.; Stenmark, H.; Tunheim, G.; Sandanger, Ø.; Bogen, B.; Brech, A.; Latz, E.; Espevik, T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006, 25, 683–692. [Google Scholar] [CrossRef]
- Jung, Y.O.; Cho, M.-L.; Lee, S.-Y.; Oh, H.-J.; Park, J.-S.; Park, M.-K.; Park, M.-J.; Ju, J.-H.; Kim, S.-I.; Park, S.-H.; et al. Synergism of toll-like receptor 2 (TLR2), TLR4, and TLR6 ligation on the production of tumor necrosis factor (TNF)-α in a spontaneous arthritis animal model of interleukin (IL)-1 receptor antagonist-deficient mice. Immunol. Lett. 2009, 123, 138–143. [Google Scholar] [CrossRef]
- Latorre, E.; Layunta, E.; Grasa, L.; Pardo, J.; García, S.; Alcalde, A.I.; Mesonero, J.E. Toll-like receptors 2 and 4 modulate intestinal IL-10 differently in ileum and colon. United Eur. Gastroenterol. J. 2018, 6, 446–453. [Google Scholar] [CrossRef]
- Cao, D.; Wang, W.; Li, S.; Lai, W.; Huang, X.; Zhou, J.; Chen, X.; Li, X. TLR2-Deficiency Promotes Prenatal LPS Exposure-Induced Offspring Hyperlipidemia. Front. Physiol. 2019, 10, 1102. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-Like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; Van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Shimamura, M.; Jackman, K.; Kurinami, H.; Anrather, J.; Zhou, P.; Iadecola, C. Key Role of CD36 in Toll-Like Receptor 2 Signaling in Cerebral Ischemia. Stroke 2010, 41, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Brinson, C.; Kirkwood, K.; Lopes-Virella, M.; Huang, Y. CD36 is upregulated in mice with periodontitis and metabolic syndrome and involved in macrophage gene upregulation by palmitate. Oral Dis. 2017, 23, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, N.G.; Kocan, M.; Schofield, L.; Pfleger, K.D.G.; Eriksson, E.M. Investigation of interactions between TLR2, MyD88 and TIRAP by bioluminescence resonance energy transfer is hampered by artefacts of protein overexpression. PLoS ONE 2018, 13, e0202408. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Ii, M.; Kitazaki, T.; Iizawa, Y.; Kimura, H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 2008, 584, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Maejima, Y.; Saito, M.; Sakamoto, K.; Horita, S.; Shimomura, K.; Inoue, S.; Kotani, J. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci. Rep. 2020, 10, 694. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.-L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis*. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef]
- He, X.; Jing, Z.; Cheng, G. MicroRNAs: New Regulators of Toll-Like Receptor Signalling Pathways. BioMed Res. Int. 2014, 2014, 945169. [Google Scholar] [CrossRef]
- LoMonaco, M.B.; Lowenstein, C.J. Enhanced assay of endothelial exocytosis using extracellular matrix components. Anal. Biochem. 2014, 452, 19–24. [Google Scholar] [CrossRef]
- Dymkowska, D.; Drabarek, B.; Jakubczyk, J.; Wojciechowska, S.; Zabłocki, K. Potassium channel openers prevent palmitate-induced insulin resistance in C2C12 myotubes. Arch. Biochem. Biophys. 2014, 541, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Drabarek, B.; Dymkowska, D.; Szczepanowska, J.; Zabłocki, K. TNFα affects energy metabolism and stimulates biogenesis of mitochondria in EA.hy926 endothelial cells. Int. J. Biochem. Cell Biol. 2012, 44, 1390–1397. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seliga, A.K.; Zabłocki, K.; Bandorowicz-Pikuła, J. Palmitate Stimulates Expression of the von Willebrand Factor and Modulates Toll-like Receptors Level and Activity in Human Umbilical Vein Endothelial Cells (HUVECs). Int. J. Mol. Sci. 2024, 25, 254. https://doi.org/10.3390/ijms25010254
Seliga AK, Zabłocki K, Bandorowicz-Pikuła J. Palmitate Stimulates Expression of the von Willebrand Factor and Modulates Toll-like Receptors Level and Activity in Human Umbilical Vein Endothelial Cells (HUVECs). International Journal of Molecular Sciences. 2024; 25(1):254. https://doi.org/10.3390/ijms25010254
Chicago/Turabian StyleSeliga, Agnieszka K., Krzysztof Zabłocki, and Joanna Bandorowicz-Pikuła. 2024. "Palmitate Stimulates Expression of the von Willebrand Factor and Modulates Toll-like Receptors Level and Activity in Human Umbilical Vein Endothelial Cells (HUVECs)" International Journal of Molecular Sciences 25, no. 1: 254. https://doi.org/10.3390/ijms25010254
APA StyleSeliga, A. K., Zabłocki, K., & Bandorowicz-Pikuła, J. (2024). Palmitate Stimulates Expression of the von Willebrand Factor and Modulates Toll-like Receptors Level and Activity in Human Umbilical Vein Endothelial Cells (HUVECs). International Journal of Molecular Sciences, 25(1), 254. https://doi.org/10.3390/ijms25010254






