The Roles of Mepiquate Chloride and Melatonin in the Morpho-Physiological Activity of Cotton under Abiotic Stress
Abstract
:1. Introduction
2. Biosynthesis, Signaling, and Transduction Pathways of MC and MT
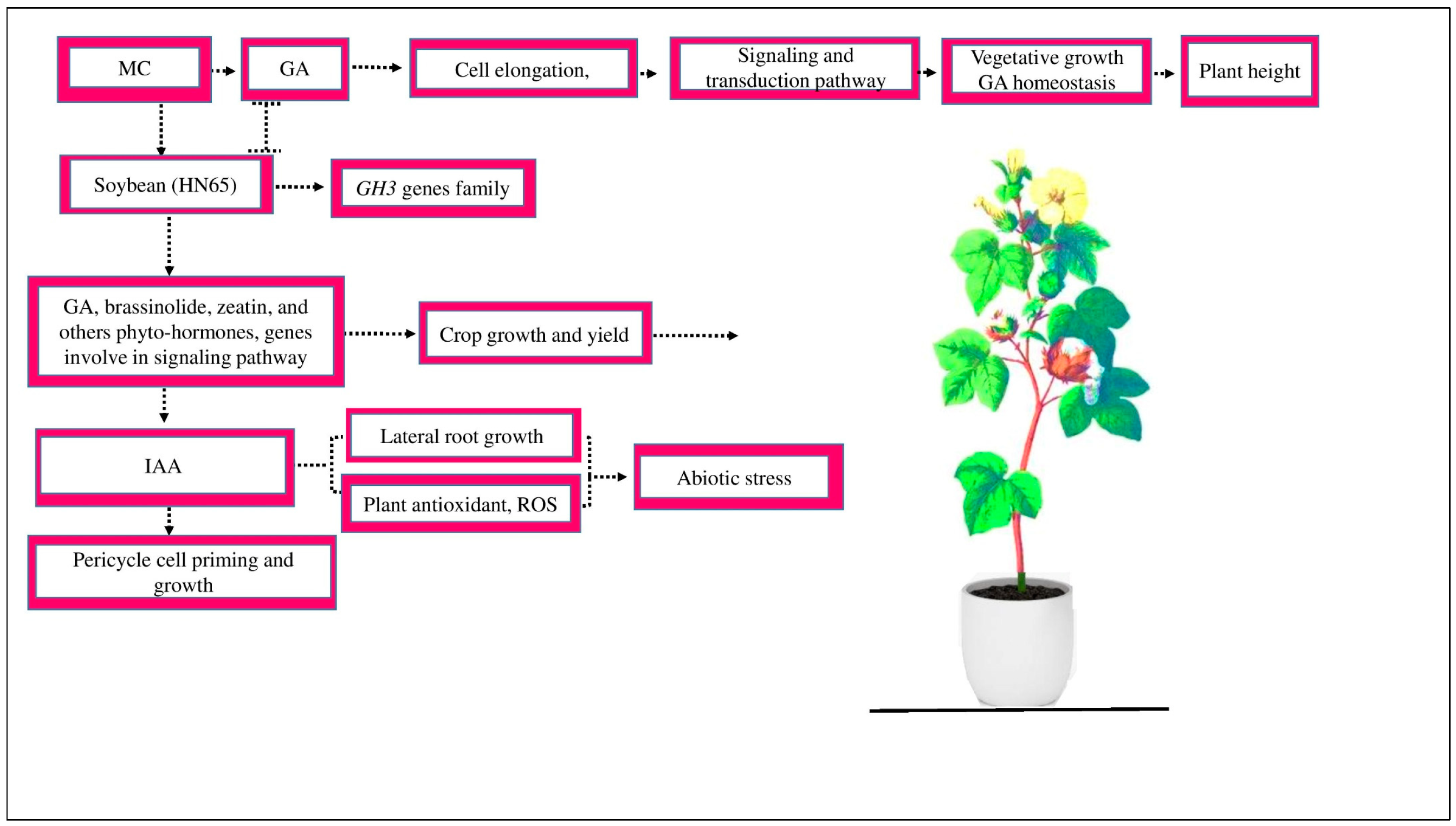
2.1. Synthetic Pathway of MC
2.2. The Signaling and Transduction Process of MC
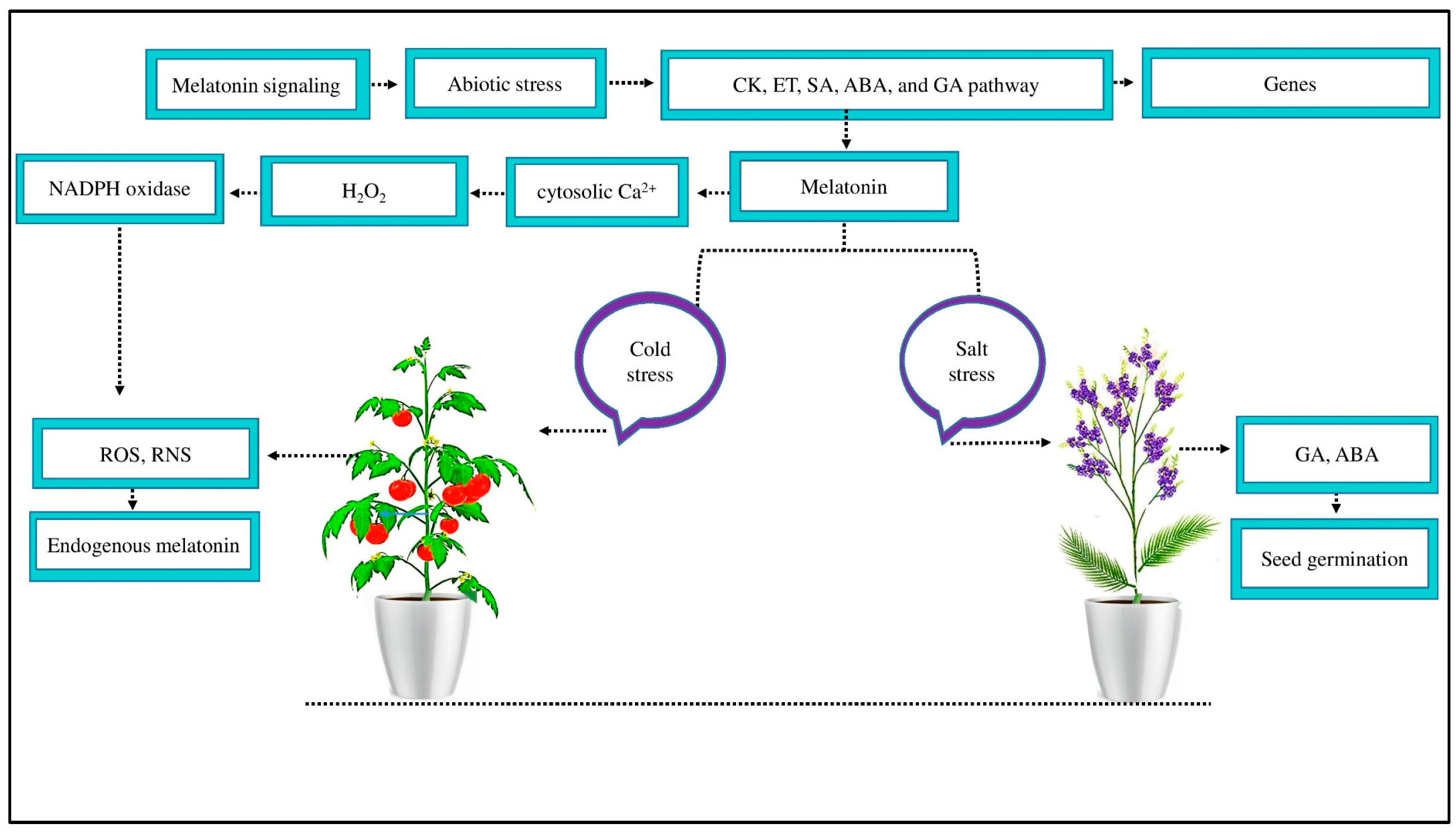
2.3. The Biosynthetic Pathway of MT
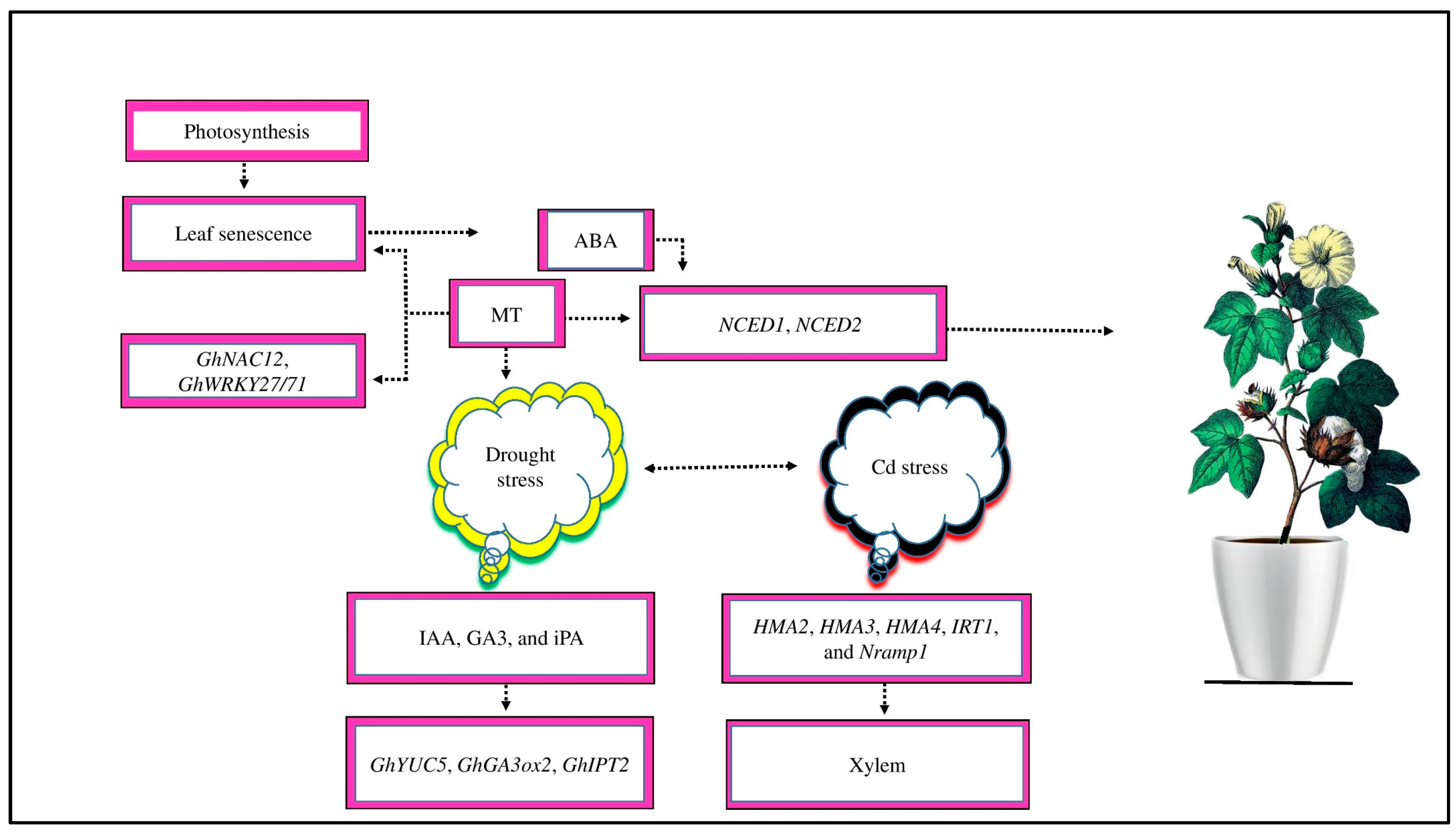
2.4. The Signaling and Transduction Pathway of MT
3. Role of MC and MT in Plant Growth, Biochemistry, and Yield under Abiotic Stress in Cotton
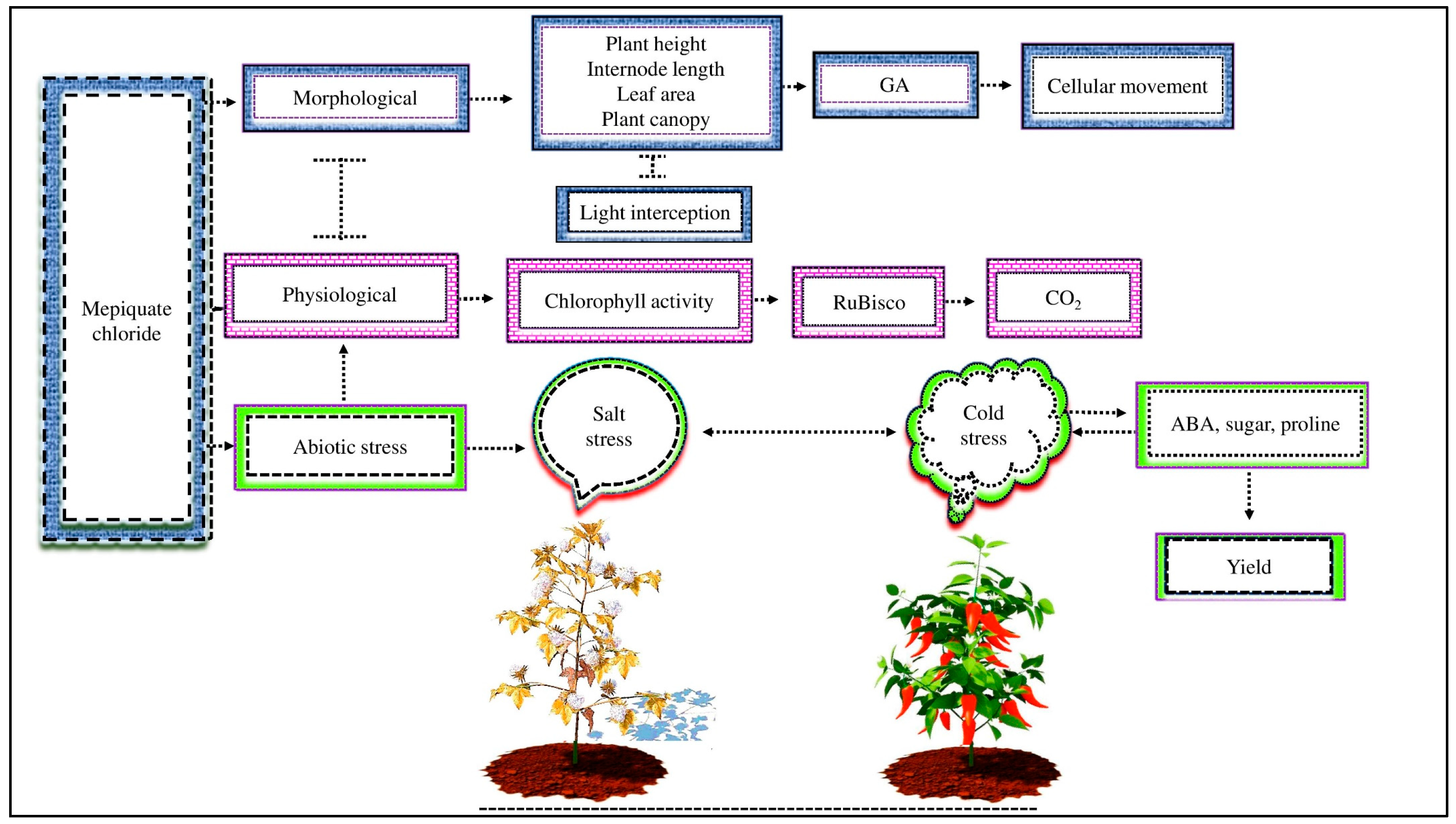
3.1. Role of MC in Cotton Growth, Physiology, and Yield
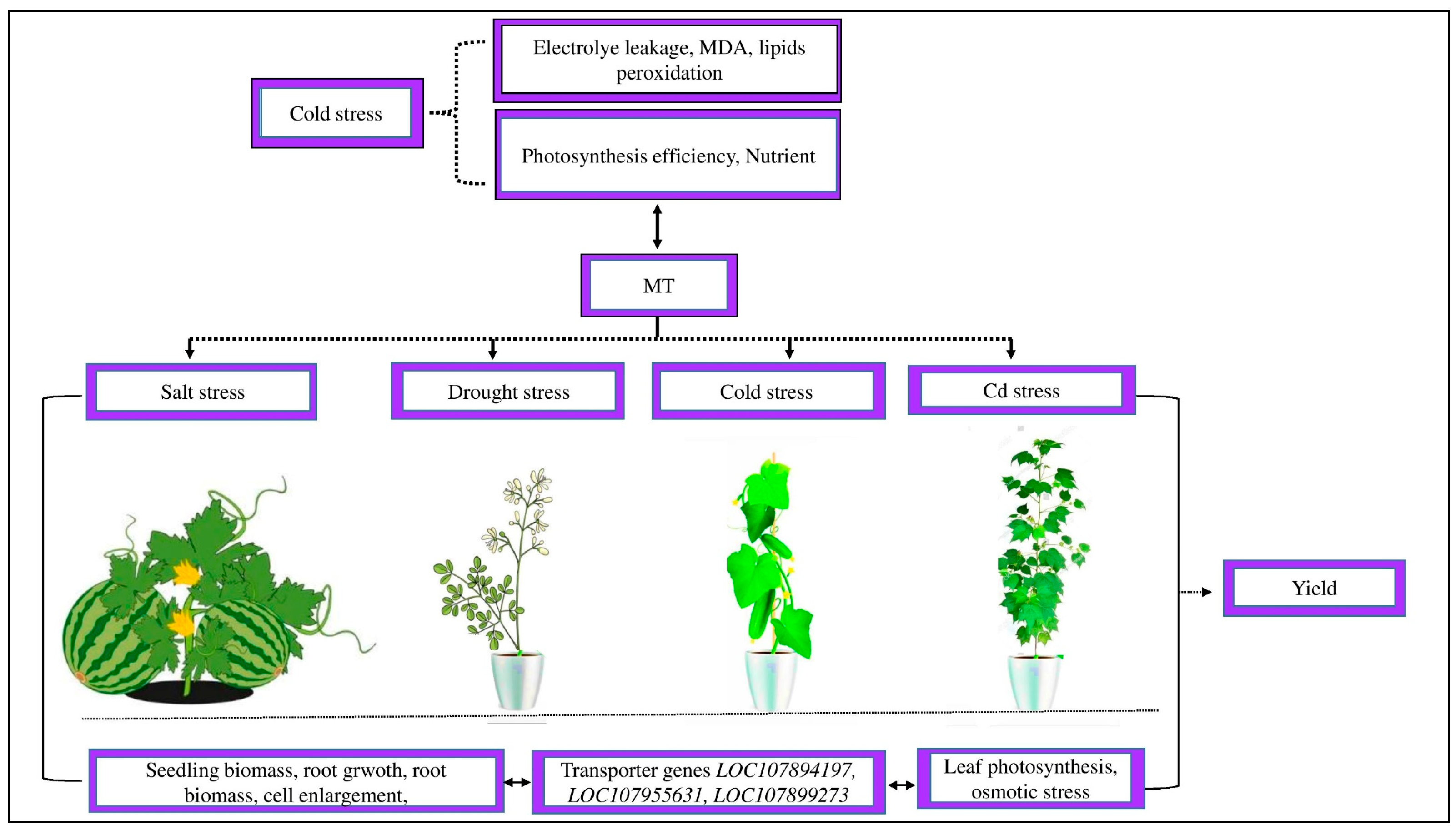
3.2. Role of MT in Cotton Growth, Physiology, and Yield under Abiotic Stress
3.3. Role of MC in Plant Defense Systems
3.4. Role of MT in Plant Defense Systems
4. MC and MT Relevant Gene Involved in Cotton Growth
4.1. MC Relevant Genes Involved in Cotton Growth
4.2. MT Relevant Genes Involve in Cotton Growth
5. Conclusions and Future Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Hussein Ibrahim, M.E.; Ibrahim Salih, E.G.; Hussain, S.; Younas, M.U. Pivotal role of phytohormones and their responsive genes in plant growth and their signaling and transduction pathway under salt stress in cotton. Int. J. Mol. Sci. 2022, 23, 7339. [Google Scholar] [CrossRef] [PubMed]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Improved yield potential with an early planting cotton production system. J. Agron. 2002, 94, 997–1003. [Google Scholar] [CrossRef]
- Hussain, M.; Gao, X.; Qin, D.; Qin, X.; Wu, G. Role of Biotic and Abiotic Factors for Sustainable Cotton Production; Intechopen: London, UK, 2023. [Google Scholar]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Li, X.; He, Q.; Yuan, Y.; Tang, F. Cold disasters: The most serious meteorological disasters to the cotton production in Xinjiang, China. In Proceedings of the Ecosystems Dynamics, Ecosystem-Society Interactions, and Remote Sensing Applications for Semi-Arid and Arid Land, Hangzhou, China, 24–27 October 2002; pp. 406–411. [Google Scholar]
- Cao, Z.; Zhao, T.; Wang, L.; Han, J.; Chen, J.; Hao, Y.; Guan, X. The lincRNA XH123 is involved in cotton cold-stress regulation. Plant Mol. Biol. 2021, 106, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.K.; Wardhan, V.; Singh, D.; Chakraborty, S.; Chakraborty, N. Genome-wide identification of the Alba gene family in plants and stress-responsive expression of the rice Alba genes. Genes 2018, 9, 183. [Google Scholar] [CrossRef]
- Wang, D.Z.; Jin, Y.N.; Ding, X.H.; Wang, W.-J.; Zhai, S.S.; Bai, L.P.; Guo, Z.F. Gene regulation and signal transduction in the ICE–CBF–COR signaling pathway during cold stress in plants. Biochemistry 2017, 82, 1103–1117. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef]
- Lu, P.; Magwanga, R.O.; Guo, X.; Kirungu, J.N.; Lu, H.; Cai, X.; Zhou, Z.; Wei, Y.; Wang, X.; Zhang, Z. Genome-wide analysis of multidrug and toxic compound extrusion (MATE) family in Gossypium raimondii and Gossypium arboreum and its expression analysis under salt, cadmium, and drought stress. G3 Genes Genomes Genet. 2018, 8, 2483–2500. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Luo, H.; Tang, F. Mepiquat chloride application combined with high plant population density promotes carbon remobilization in the roots of upland cotton. Plant Physiol. Biochem. 2023, 194, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, X.; Tian, Y.; Zhou, X.; Qu, Z.; Liu, J.; Dong, S. Physiology and proteomics analyses reveal the regulatory mechanism of mepiquat chloride in soybean. Front. Sustain. Food Syst. 2023, 7, 1188159. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Wang, L.-F.; Wang, M.; Zhao, M.; Tian, Y.; Li, Y.-F. Transcriptome profiling of the elongating internode of cotton (Gossypium hirsutum L.) seedlings in response to mepiquat chloride. Front. Plant Sci. 2020, 10, 1751. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, J.; Tian, J.; Du, M.; Gou, L.; Zhang, Y.; Zhang, W. Different Concentrations of Chemical Topping Agents Affect Cotton Yield and Quality by Regulating Plant Architecture. Agronomy 2023, 13, 1741. [Google Scholar] [CrossRef]
- Tung, S.A.; Huang, Y.; Ali, S.; Hafeez, A.; Shah, A.N.; Ma, X.; Ahmad, S.; Chattha, M.S.; Liu, A.; Liu, J. Mepiquat chloride effects on potassium acquisition and functional leaf physiology as well as lint yield in highly dense late-sown cotton. Ind. Crops Prod. 2019, 129, 142–155. [Google Scholar] [CrossRef]
- Reddy, A.R.; Reddy, K.R.; Hodges, H. Mepiquat chloride (PIX)-induced changes in photosynthesis and growth of cotton. J. Plant Growth Regul. 1996, 20, 179–183. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M. Pix plus and mepiquat chloride effects on physiology, growth, and yield of field-grown cotton. J. Plant Growth Regul. 2000, 19, 415–422. [Google Scholar] [CrossRef]
- Meng, L.; Yu, K.; Wei, Z.; Li, K.; Dai, J.; Li, F.; Qi, H.; Sun, L.; Zhang, L.; Dong, H. High dosage of mepiquat chloride delays defoliation of harvest aids in cotton. Ind. Crops Prod. 2023, 202, 116998. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Chen, L.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H. Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism. PeerJ 2020, 8, e10486. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, I.; Kaźmierczak, A.; Posmyk, M.M. Melatonin application modifies antioxidant defense and induces endoreplication in maize seeds exposed to chilling stress. Int. J. Mol. Sci. 2021, 22, 8628. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2023, 42, 2408–2432. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Gohari, G.; Esmaielpour, B.; Panahirad, S.; Milani, M.H.; Kulak, M.; Janda, T. Melatonin and tio2 nps application-induced changes in growth, photosynthesis, antioxidant enzymes activities and secondary metabolites in stevia (Stevia rebaudiana bertoni) under drought stress conditions. J. Plant Growth Regul. 2023, 42, 2023–2040. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, Z.W.; Chen, Y.E.; Ding, C.B.; Yuan, S.; Reiter, R.J.; Yuan, M. Melatonin: A potential agent in delaying leaf senescence. Crit. Rev. Plant Sci. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Wang, Z.; Feng, G.; Gao, Q.; Li, X. Induction of low temperature tolerance in wheat by pre-soaking and parental treatment with melatonin. Molecules 2021, 26, 1192. [Google Scholar] [CrossRef]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.-P.; Scott, E.R.; Liu, J.-W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.-Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef]
- Li, X.; Brestic, M.; Tan, D.X.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin alleviates low PS I-limited carbon assimilation under elevated CO 2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef]
- Qari, S.H.; Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Naqve, M.; Nawaz, M.; Barbanti, L.; Alahdal, M.A.; Aljabri, M. Melatonin induced cold tolerance in plants: Physiological and molecular responses. Front. Plant Sci. 2022, 13, 843071. [Google Scholar] [CrossRef]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 2013, 55, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, X.; Qi, Q.; Iqbal, A.; Zhang, H.; Shi, J.; Dong, Q.; Xu, Q.; Liu, X.; Gui, H. Analysis of the effects of mepiquat chloride priming on the seedling growth-promoting in cotton under salt stress by multi-omics. Ind. Crops Prod. 2022, 186, 115296. [Google Scholar] [CrossRef]
- Luo, L.-J.; Xu, F.-L.; Hong, S.-Z.; Weng, H.-Q.; Duan, L.-S.; Li, Z.-H. Inducing effects of mepiquat chloride on the chilling resistanceof sweet pepperseedlings. Chin. J. Pestic. Sci. 2010, 12, 142–148. [Google Scholar]
- Dian, J.; Liu, Y.T.; Liu, Z.Y.; Dai, Y.Y.; Du, J.N.; Run, H.; Wu, T.F.; Yuan, C.; Chen, D.H.; Zhang, X. Mepiquat chloride increases Cry1Ac protein content by regulating the carbon and amino acid metabolism of Bt cotton under high temperature and drought stress. J. Integr. Agric. 2023, in press. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Qu, Z.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Regulation of soybean drought response by mepiquat chloride pretreatment. Front. Plant Sci. 2023, 14, 1149114. [Google Scholar] [CrossRef]
- Fu, Y.; Hamani, A.K.M.; Sun, W.; Wang, H.; Amin, A.S.; Wang, X.; Qin, A.; Gao, Y. Exogenous Foliar Melatonin Improves Cotton (Gossypium hirsutum L.) Physio-Biochemical Characteristics Under the Synergetic Effects of Low Temperature and Salinity Stress. Res. Square, 2021; preprint. [Google Scholar] [CrossRef]
- Khan, A.; Jie, Z.; Xiangjun, K.; Ullah, N.; Short, A.W.; Diao, Y.; Zhou, R.; Xiong, Y.C. Pre treatment of melatonin rescues cotton seedlings from cadmium toxicity by regulating key physio-biochemical and molecular pathways. J. Hazard. Mater. 2023, 445, 130530. [Google Scholar] [CrossRef]
- Li, L.; Yan, X.; Li, J.; Wu, X.; Wang, X. Metabolome and transcriptome association analysis revealed key factors involved in melatonin mediated cadmium-stress tolerance in cotton. Front. Plant Sci. 2022, 13, 995205. [Google Scholar] [CrossRef]
- Li, X.; Tan, D.X.; Jiang, D.; Liu, F. Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J. Pineal Res. 2016, 61, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, J.; Wu, Z.; Loka, D.A.; Zhao, W.; Chen, B.; Wang, Y.; Meng, Y.; Zhou, Z.; Gao, L. Effects of single and combined exogenous application of abscisic acid and melatonin on cotton carbohydrate metabolism and yield under drought stress. Ind. Crops Prod. 2022, 176, 114302. [Google Scholar] [CrossRef]
- Zhang, Y.; Rui, C.; Fan, Y.; Xu, N.; Zhang, H.; Wang, J.; Sun, L.; Dai, M.; Ni, K.; Chen, X. Identification of SNAT family genes suggests GhSNAT3D functional reponse to melatonin synthesis under salinity stress in cotton. Front. Mol. Biosci. 2022, 9, 843814. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 1–19. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Liu, J.; Younas, M.U.; Zhu, Y. Melatonin Role in Plant Growth and Physiology under Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 8759. [Google Scholar] [CrossRef]
- Tang, G.; Niu, J.; Tang, J.; Yang, J.; Zhou, Z.; Gao, Y.; Chen, X.; Tang, R.; Tian, Y.; Li, Y. Development of poly (ionic liquids) based on mepiquat chloride with improved rainfastness and long-lasting activity on growth regulation of cotton plant. ACS Sustain. Chem. Eng. 2020, 8, 14996–15004. [Google Scholar] [CrossRef]
- Wu, Q.; Du, M.; Wu, J.; Wang, N.; Wang, B.; Li, F.; Tian, X.; Li, Z. Mepiquat chloride promotes cotton lateral root formation by modulating plant hormone homeostasis. BMC Plant Biol. 2019, 19, 573. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, N.; Li, S.; Wang, Y.; Xiao, S.; Zhang, Y.; Egrinya Eneji, A.; Zhang, M.; Wang, B.; Duan, L. Gibberellin biosynthesis inhibitor mepiquat chloride enhances root K+ uptake in cotton by modulating plasma membrane H+-ATPase. J. Exp. Bot. 2021, 72, 6659–6671. [Google Scholar] [CrossRef]
- Qiu, L.H.; Chen, R.F.; Luo, H.M.; Fan, Y.G.; Huang, X.; Liu, J.X.; Xiong, F.Q.; Zhou, H.W.; Gan, C.K.; Wu, J.M. Effects of exogenous GA3 and DPC treatments on levels of endogenous hormone and expression of key gibberellin biosynthesis pathway genes during stem elongation in sugarcane. Sugar Tech 2019, 21, 936–948. [Google Scholar] [CrossRef]
- Tung, S.A.; Huang, Y.; Hafeez, A.; Ali, S.; Liu, A.; Chattha, M.S.; Ahmad, S.; Yang, G. Morpho-physiological effects and molecular mode of action of mepiquat chloride application in cotton: A review. J. Soil Sci. Plant Nutr. 2020, 20, 2073–2086. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mu, C.; Du, M.; Chen, Y.; Tian, X.; Zhang, M.; Li, Z. The effect of mepiquat chloride on elongation of cotton (Gossypium hirsutum L.) internode is associated with low concentration of gibberellic acid. Plant Sci. 2014, 225, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, X.; Li, X.; Tian, Y.; Zhou, X.; Qu, Z.; Wang, X.; Liu, L. Mechanism of Mepiquat Chloride Regulating Soybean Response to Drought Stress Revealed by Proteomics. Plants 2023, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Shahrbano, G.; Maryam, N.; Omran, A.; Reza, Z.M. Drought tolerance enhancment in cotton (Gossypium hirsutum L.) by mepiquate chloride seed priming. Pak. J. Agric. Sci. 2022, 59, 923–934. [Google Scholar]
- Chalise, D.P.; Snider, J.L.; Hand, L.C.; Roberts, P.; Vellidis, G.; Ermanis, A.; Collins, G.D.; Lacerda, L.N.; Cohen, Y.; Pokhrel, A. Cultivar, irrigation management, and mepiquat chloride strategy: Effects on cotton growth, maturity, yield, and fiber quality. Field Crops Res. 2022, 286, 108633. [Google Scholar] [CrossRef]
- Kang, W.; Zhu, X.; Wang, Y.; Chen, L.; Duan, Y. Transcriptomic and metabolomic analyses reveal that bacteria promote plant defense during infection of soybean cyst nematode in soybean. BMC Plant Biol. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Du, M.; Evers, J.B.; van der Werf, W.; Tian, X.; Li, Z. Managing mepiquat chloride and plant density for optimal yield and quality of cotton. Field Crops Res. 2013, 149, 1–10. [Google Scholar] [CrossRef]
- Siebert, J.D.; Stewart, A.M. Influence of plant density on cotton response to mepiquat chloride application. Agron. J. 2006, 98, 1634–1639. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Q.; Wang, X.; Song, S.; Liu, J.; Dong, S. Mepiquat chloride inhibits soybean growth but improves drought resistance. Front. Plant Sci. 2022, 13, 982415. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, M.; Wang, M.; Tan, G.; Zhang, M.; Hou, Y.X.; Wang, B.; Li, Z. The effects of mepiquat chloride on the lateral root initiation of cotton seedlings are associated with auxin and auxin-conjugate homeostasis. BMC Plant Biol. 2018, 18, 361. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Feng, Z.-Q.; Zhang, T.-T.; You, C.-X.; Chao, Z.; Wang, X.-F. The nitrate-responsive transcription factor MdNLP7 regulates callus formation by modulating auxin response. J. Integr. Agric. 2023, 22, 3022–3033. [Google Scholar]
- Zhao, C.Y.; Si, J.H.; Feng, Q.; Yu, T.F.; Li, P.D. Comparative study of daytime and nighttime sap flow of Populus euphratica. J. Plant Growth Regul. 2017, 82, 353–362. [Google Scholar] [CrossRef]
- Yan-tao, L.; An-yang, X.; Wei, D.; Peng, W.; Sheng-li, L. Effects of mepiquat chloride, paclobutrazol and chlorocholine chloride on physiological characteristics of sunflower. Chin. J. Oil Crop Sci. 2018, 40, 241. [Google Scholar]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 2004, 25, 951–960. [Google Scholar] [PubMed]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.Q.; Kuang, J.F.; Lu, W.J.; Reiter, R.J.; Lakshmanan, P.; Su, X.G.; Zhou, J.; Chen, J.Y.; Shan, W. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.; Kim, Y.S.; Back, K. Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J. Pineal Res. 2012, 52, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Yang, F.; Du, M.; Tian, X.; Eneji, A.E.; Duan, L.; Li, Z. Plant growth regulation enhanced potassium uptake and use efficiency in cotton. Field Crops Res. 2014, 163, 109–118. [Google Scholar] [CrossRef]
- Yang, T.; Davies, P.J.; Reid, J.B. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 1996, 110, 1029–1034. [Google Scholar] [CrossRef]
- Abbas, H.; Wahid, M.A.; Sattar, A.; Tung, S.A.; Saleem, M.F.; Irshad, S.; Alkahtani, J.; Elshikh, M.S.; Cheema, M.; Li, Y. Foliar application of mepiquat chloride and nitrogen improves yield and fiber quality traits of cotton (Gossypium hirsutum L.). PLoS ONE 2022, 17, e0268907. [Google Scholar] [CrossRef]
- Gao, H.; Ma, H.; Khan, A.; Xia, J.; Hao, X.; Wang, F.; Luo, H. Moderate drip irrigation level with low mepiquat chloride application increases cotton lint yield by improving leaf photosynthetic rate and reproductive organ biomass accumulation in arid region. Agronomy 2019, 9, 834. [Google Scholar] [CrossRef]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Guo, H.; Baskin, C.C.; Xiong, W.; Yang, C.; Li, Z.; Song, H.; Wang, T.; Yin, J.; Wu, X. Effect of light intensity on morphology, photosynthesis and carbon metabolism of alfalfa (Medicago sativa) seedlings. Plants 2022, 11, 1688. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jiang, N.; Yang, J.; Meng, Y.; Wang, Y.; Chen, B.; Zhao, W.; Oosterhuis, D.M.; Zhou, Z. Potassium (K) supply affects K accumulation and photosynthetic physiology in two cotton (Gossypium hirsutum L.) cultivars with different K sensitivities. Field Crops Res. 2016, 196, 51–63. [Google Scholar] [CrossRef]
- Thomas, R.O. Cotton Flowering and Fruiting Responses to Application Timing of Chemical Growth Retardants 1. Crop Sci. 1975, 15, 87–90. [Google Scholar] [CrossRef]
- Tung, S.A.; Huang, Y.; Hafeez, A.; Ali, S.; Khan, A.; Souliyanonh, B.; Song, X.; Liu, A.; Yang, G. Mepiquat chloride effects on cotton yield and biomass accumulation under late sowing and high density. Field Crops Res. 2018, 215, 59–65. [Google Scholar] [CrossRef]
- Cook, D.R.; Kennedy, C.W. Early flower bud loss and mepiquat chloride effects on cotton yield distribution. Crop Sci. 2000, 40, 1678–1684. [Google Scholar] [CrossRef]
- Heilman, M.; Brown, J. Interactions of nitrogen with Pix on the growth and yield of cotton. In Proceedings of the Beltwide Cotton Production Research Conference, New Orleans, LA, USA, 4–8 January 1981; pp. 4–8. [Google Scholar]
- Stuart, B.; Isbell, V.; Wendt, C.; Abernathy, J. Modification of cotton water relations and growth with mepiquat chloride 1. J. Agron. 1984, 76, 651–655. [Google Scholar] [CrossRef]
- Turk, H.; Genisel, M. Melatonin-related mitochondrial respiration responses are associated with growth promotion and cold tolerance in plants. Cryobiology 2020, 92, 76–85. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, J.; Xie, Y.; Amombo, E.; Liu, A.; Gitau, M.M.; Khaldun, A.; Chen, L.; Fu, J. Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin. Plant Physiol. Biochem. 2016, 100, 94–104. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Naeem, M.; Ali, H.; Alabbosh, K.F.; Hussain, H.; Khan, I.; Siddiqui, S.A.; Khan, A.A.; Iqbal, B. From challenges to solutions: The impact of melatonin on abiotic stress synergies in horticultural plants via redox regulation and epigenetic signaling. Sci. Hortic. 2023, 321, 112369. [Google Scholar] [CrossRef]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Atif, M.J.; Meng, H.; Ali, M.; Li, S.; Alharby, H.F.; Majrashi, A.; Hakeem, K.R.; Cheng, Z. Melatonin rescues photosynthesis and triggers antioxidant defense response in Cucumis sativus plants challenged by low temperature and high humidity. Front. Plant Sci. 2022, 13, 855900. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.; Tang, M.; Wang, Y.; Dong, J.; Ying, J.; Chen, Y.; Hu, B.; Li, C.; Liu, L. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.P.; Wang, Z.Q.; Qi, M.F.; Meng, S.D.; Li, T.-L. Effects of melatonin application on photosynthetic function in tomato seedlings under salt stress. Chin. J. Ecol. 2019, 38, 467. [Google Scholar]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Hassan, M.U.; Nawaz, M.; Shah, A.N.; Raza, A.; Barbanti, L.; Skalicky, M.; Hashem, M.; Brestic, M.; Pandey, S.; Alamri, S. Trehalose: A key player in plant growth regulation and tolerance to abiotic stresses. J. Plant Growth Regul. 2023, 42, 4935–4957. [Google Scholar] [CrossRef]
- Liu, L.; Cao, X.; Zhai, Z.; Ma, S.; Tian, Y.; Cheng, J. Direct evidence of drought stress memory in mulberry from a physiological perspective: Antioxidative, osmotic and phytohormonal regulations. Plant Physiol. Biochem. 2022, 186, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Hou, S.; Chen, J.; Guo, J.; Wang, F.; He, C.; Zou, C.; Xie, X. The physiological response of different tobacco varieties to chilling stress during the vigorous growing period. Sci. Rep. 2021, 11, 22136. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Dall’Osto, L. Dissipation of light energy absorbed in excess: The molecular mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef]
- Ren, M.; Mao, G.; Zheng, H.; Wang, W.; Tang, Q. Growth changes of tomato seedlings responding to sodium salt of α-naphthalene acetic acid and potassium salt of fulvic acid. Sci. Rep. 2023, 13, 4024. [Google Scholar] [CrossRef]
- Menhas, S.; Yang, X.; Hayat, K.; Bundschuh, J.; Chen, X.; Hui, N.; Zhang, D.; Chu, S.; Zhou, Y.; Ali, E.F. Pleiotropic melatonin-mediated responses on grsowth and cadmium phytoextraction of Brassica napus: A bioecological trial for enhancing phytoremediation of soil cadmium. J. Hazard. Mater. 2023, 457, 131862. [Google Scholar] [CrossRef]
- Pieri, C.; Marra, M.; Moroni, F.; Recchioni, R.; Marcheselli, F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994, 55, PL271–PL276. [Google Scholar] [CrossRef]
- Khattak, W.A.; He, J.; Abdalmegeed, D.; Hu, W.; Wang, Y.; Zhou, Z. Foliar melatonin stimulates cotton boll distribution characteristics by modifying leaf sugar metabolism and antioxidant activities during drought conditions. Physiol. Plant. 2022, 174, e13526. [Google Scholar] [CrossRef]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Tian, X.; Li, Z. GhCPS and GhKS encoding gibberellin biosynthesis enzymes involve in inhibition of leaf growth by mepiquat chloride in cotton (Gossypium hirsutum L.). Acta Agron. Sin. 2014, 40, 1350–1355. [Google Scholar] [CrossRef]
- Van De Velde, K.; Ruelens, P.; Geuten, K.; Rohde, A.; Van Der Straeten, D. Exploiting DELLA signaling in cereals. Trends Plant Sci. 2017, 22, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Lantzouni, O.; Alkofer, A.; Falter-Braun, P.; Schwechheimer, C. Growth-regulating factors interact with DELLAs and regulate growth in cold stress. Plant Cell 2020, 32, 1018–1034. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Mori, M.; Watanabe, S.; Miyamoto, C.; Ito, T.; Takahashi, Y. DELLA-GAF1 complex is a main component in gibberellin feedback regulation of GA20 oxidase 2. Plant Physiol. 2017, 175, 1395–1406. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. The regulatory signaling of gibberellin metabolism and its crosstalk with phytohormones in response to plant abiotic stresses. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 333–339. [Google Scholar]
- Ariizumi, T.; Hauvermale, A.L.; Nelson, S.K.; Hanada, A.; Yamaguchi, S.; Steber, C.M. Lifting DELLA repression of Arabidopsis seed germination by nonproteolytic gibberellin signaling. Plant Physiol. 2013, 162, 2125–2139. [Google Scholar] [CrossRef]
- Plaza-Wüthrich, S.; Blösch, R.; Rindisbacher, A.; Cannarozzi, G.; Tadele, Z. Gibberellin deficiency confers both lodging and drought tolerance in small cereals. Front. Plant Sci. 2016, 7, 643. [Google Scholar] [CrossRef]
- Upreti, K.; Sharma, M. Role of plant growth regulators in abiotic stress tolerance. In Abiotic Stress Physiology of Horticultural Crops; Springer: Berlin/Heidelberg, Germany, 2016; pp. 19–46. [Google Scholar]
- Sun, J.; Chen, Q.; Qi, L.; Jiang, H.; Li, S.; Xu, Y.; Liu, F.; Zhou, W.; Pan, J.; Li, X. Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein. New Phytol. 2011, 191, 360–375. [Google Scholar] [CrossRef]
- Desingh, R.; Kanagaraj, G. Influence of salinity stress on photosynthesis and antioxidative systems in two cotton varieties. Gen. Appl. Plant Physiol. 2007, 33, 221–234. [Google Scholar]
- Yang, K.; Sun, H.; Liu, M.; Zhu, L.; Zhang, K.; Zhang, Y.; Li, A.; Zhang, H.; Zhu, J.; Liu, X. Morphological and Physiological Mechanisms of Melatonin on Delaying Drought-Induced Leaf Senescence in Cotton. Int. J. Mol. Sci. 2023, 24, 7269. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Azam, S.; Shahid, N.; Javed, M.R.; Haider, Z.; Yasmeen, A.; Sadaqat, S.; Shad, M.; Husnain, T.; Rao, A.Q. Overexpression of the AGL42 gene in cotton delayed leaf senescence through downregulation of NAC transcription factors. Sci. Rep. 2022, 12, 21093. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA-and GA-mediated pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef]

| Hormones | Dose of MC and MT | Crops | Abiotic Stress | References |
|---|---|---|---|---|
| MC | 50–150 g ha−1 | Cotton | Enhanced salt tolerance | [37] |
| Enhanced cold tolerance | [38] | |||
| Heat stress | [39] | |||
| Drought stress | [39] | |||
| Enhanced drought tolerance | [40] | |||
| MT | 50 μM to 100 μM | Cotton | Enhanced cold tolerance | [28,41] |
| Enhanced Cd tolerance | [42] | |||
| Enhanced Cd tolerance | [43] | |||
| Enhanced cold tolerance | [44] | |||
| Enhanced drought tolerance | [44,45] | |||
| Enhanced salt tolerance | [41,46,47] | |||
| Enhanced salt tolerance | [30,48] | |||
| Ultraviolet stress | [49] | |||
| Heavy metal stress | [49,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liu, J.; Wu, H.; Zhu, Y.; Ahmad, I.; Zhou, G. The Roles of Mepiquate Chloride and Melatonin in the Morpho-Physiological Activity of Cotton under Abiotic Stress. Int. J. Mol. Sci. 2024, 25, 235. https://doi.org/10.3390/ijms25010235
Wu Y, Liu J, Wu H, Zhu Y, Ahmad I, Zhou G. The Roles of Mepiquate Chloride and Melatonin in the Morpho-Physiological Activity of Cotton under Abiotic Stress. International Journal of Molecular Sciences. 2024; 25(1):235. https://doi.org/10.3390/ijms25010235
Chicago/Turabian StyleWu, Yanqing, Jiao Liu, Hao Wu, Yiming Zhu, Irshad Ahmad, and Guisheng Zhou. 2024. "The Roles of Mepiquate Chloride and Melatonin in the Morpho-Physiological Activity of Cotton under Abiotic Stress" International Journal of Molecular Sciences 25, no. 1: 235. https://doi.org/10.3390/ijms25010235
APA StyleWu, Y., Liu, J., Wu, H., Zhu, Y., Ahmad, I., & Zhou, G. (2024). The Roles of Mepiquate Chloride and Melatonin in the Morpho-Physiological Activity of Cotton under Abiotic Stress. International Journal of Molecular Sciences, 25(1), 235. https://doi.org/10.3390/ijms25010235








