Molecular Dynamics Investigations of Human DNA-Topoisomerase I Interacting with Novel Dewar Valence Photo-Adducts: Insights into Inhibitory Activity
Abstract
1. Introduction
2. Results and Discussion
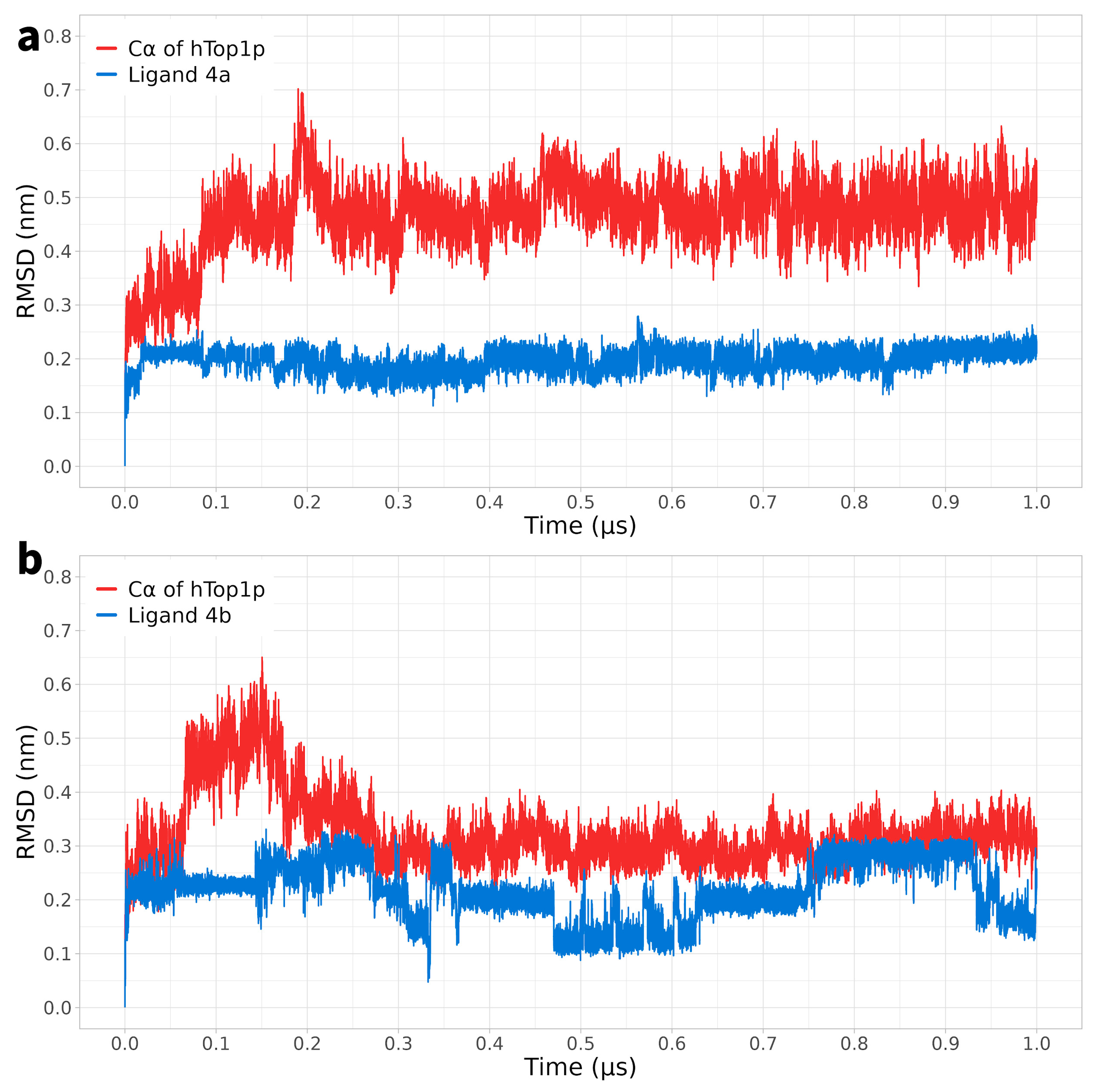
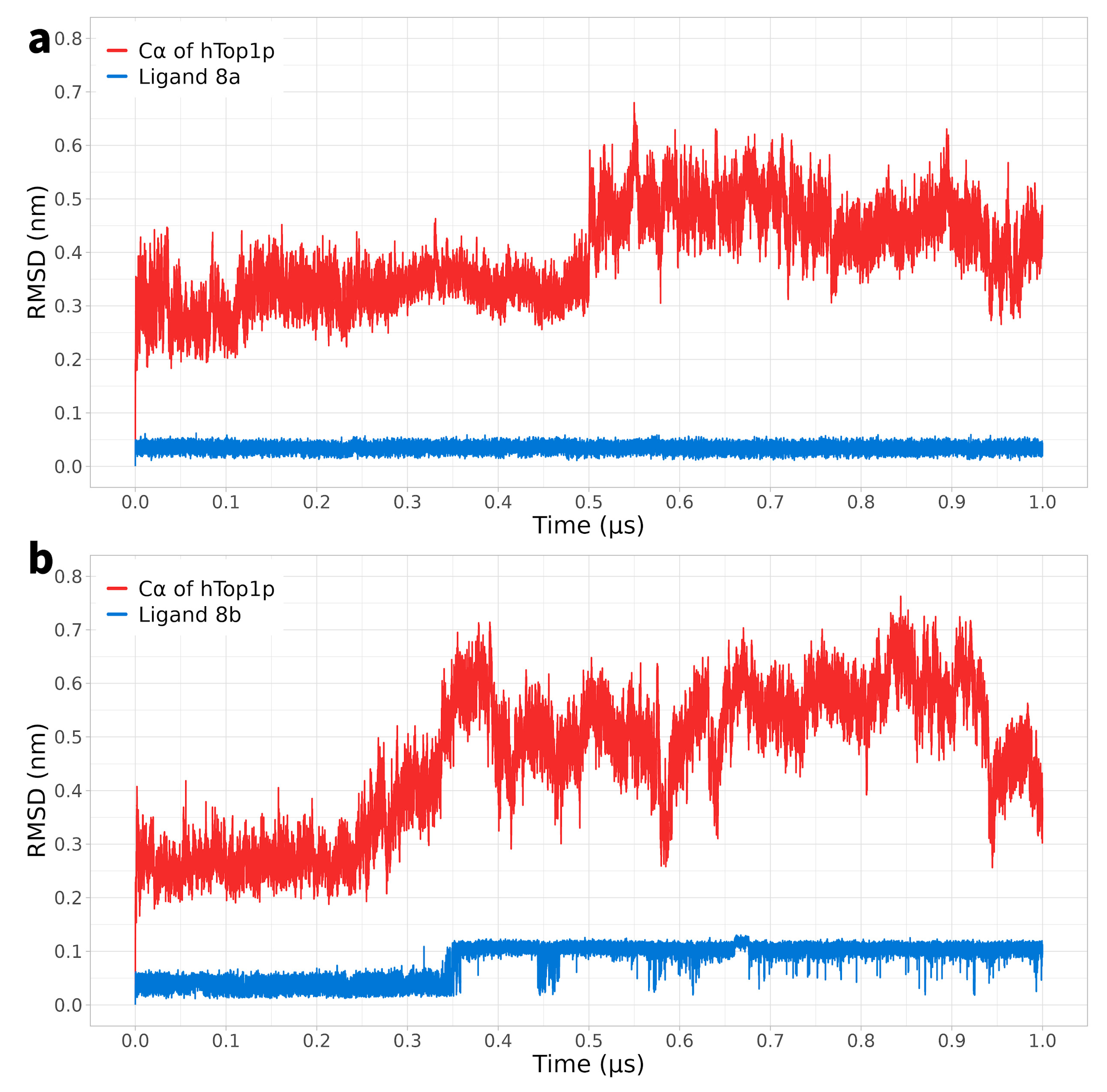
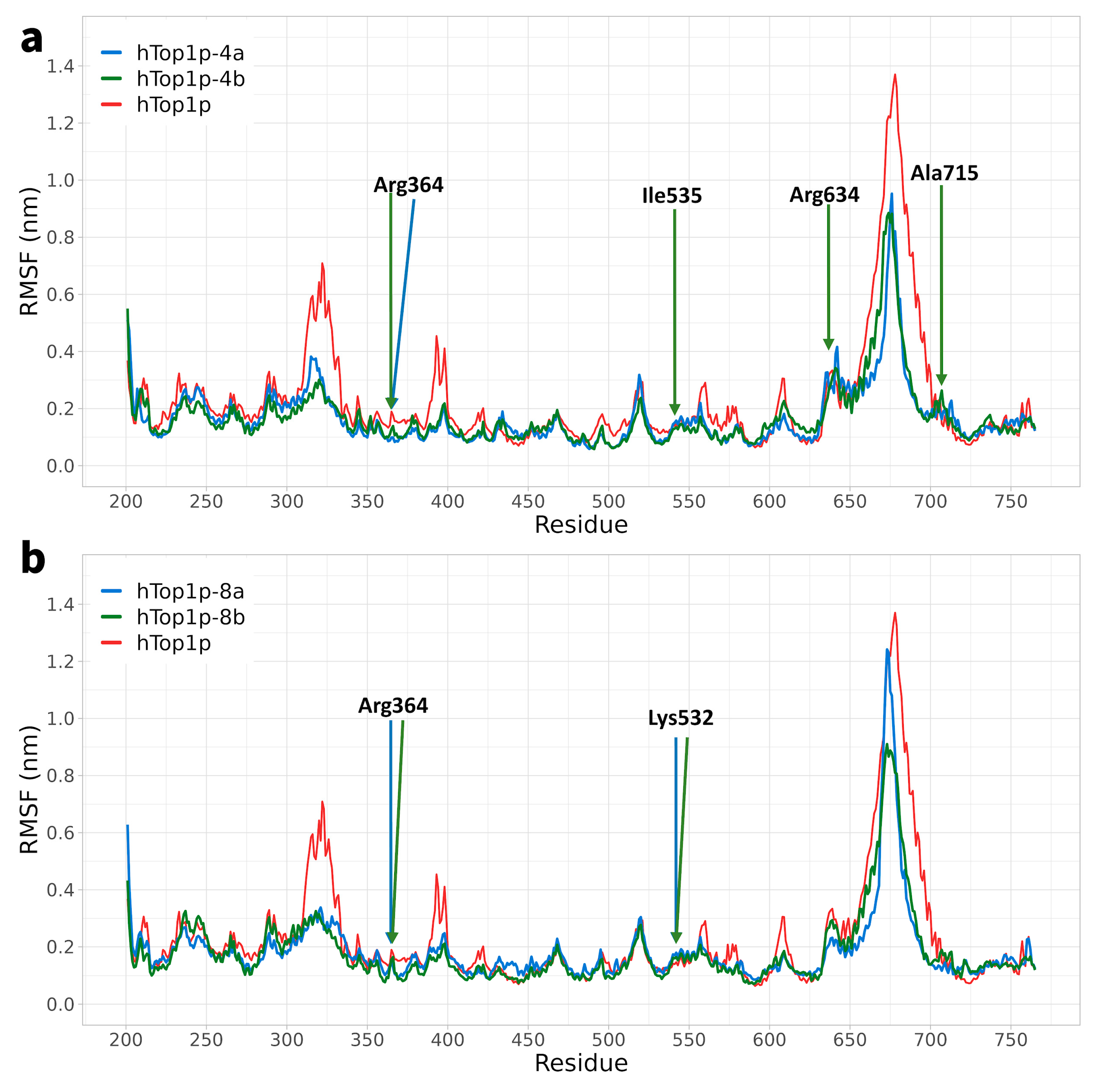
2.1. Classical Molecular Dynamics Simulation
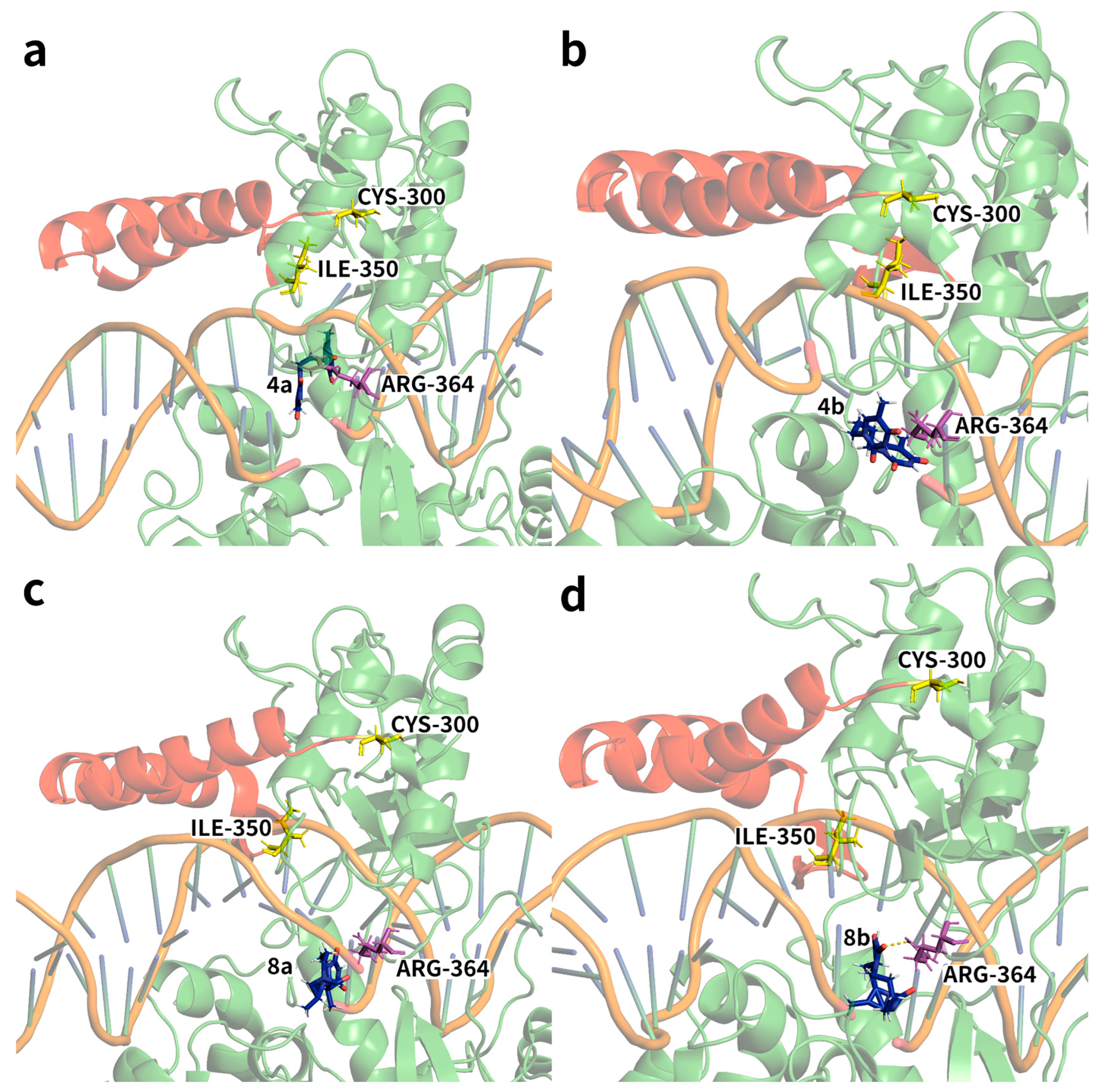
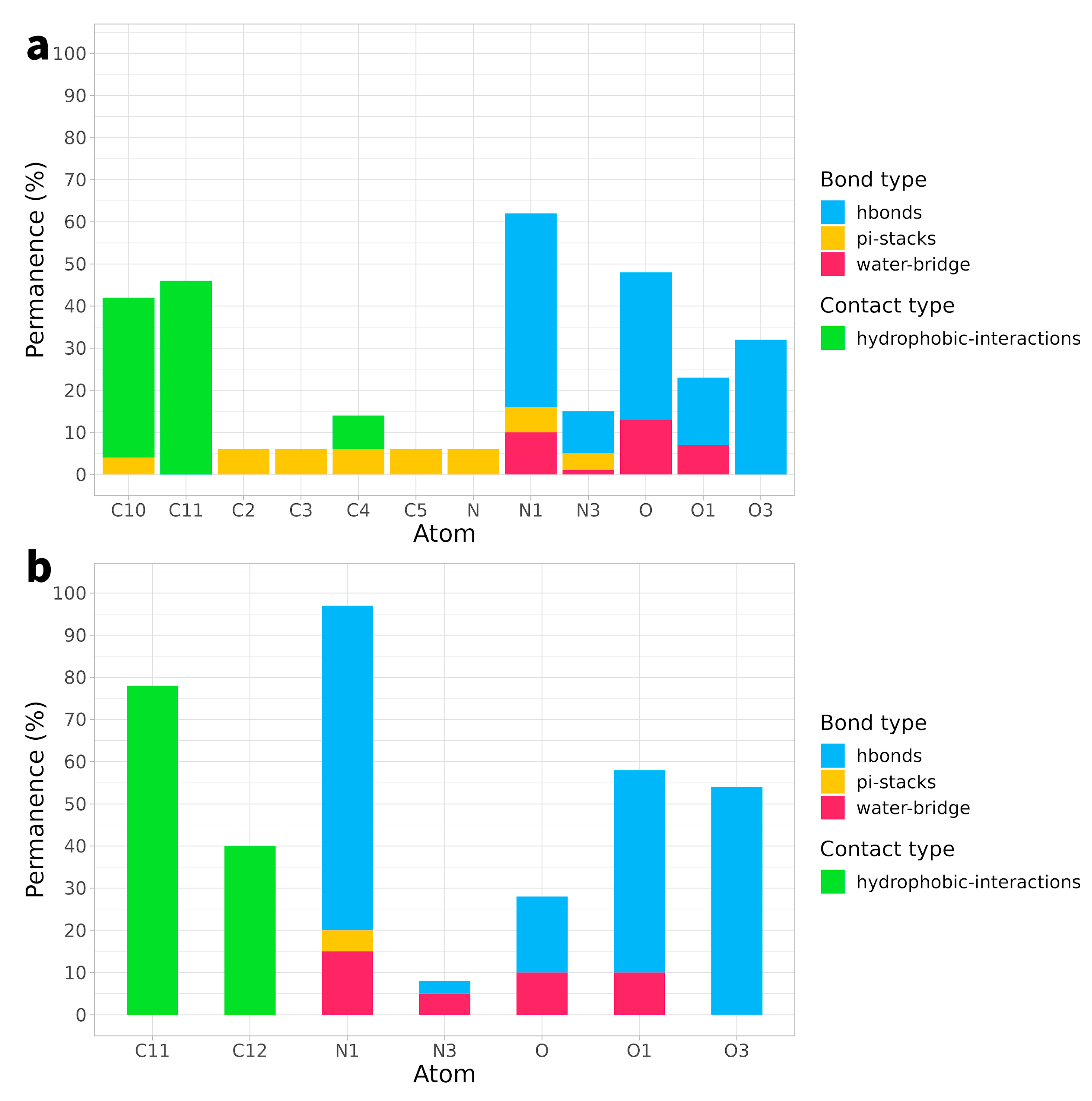
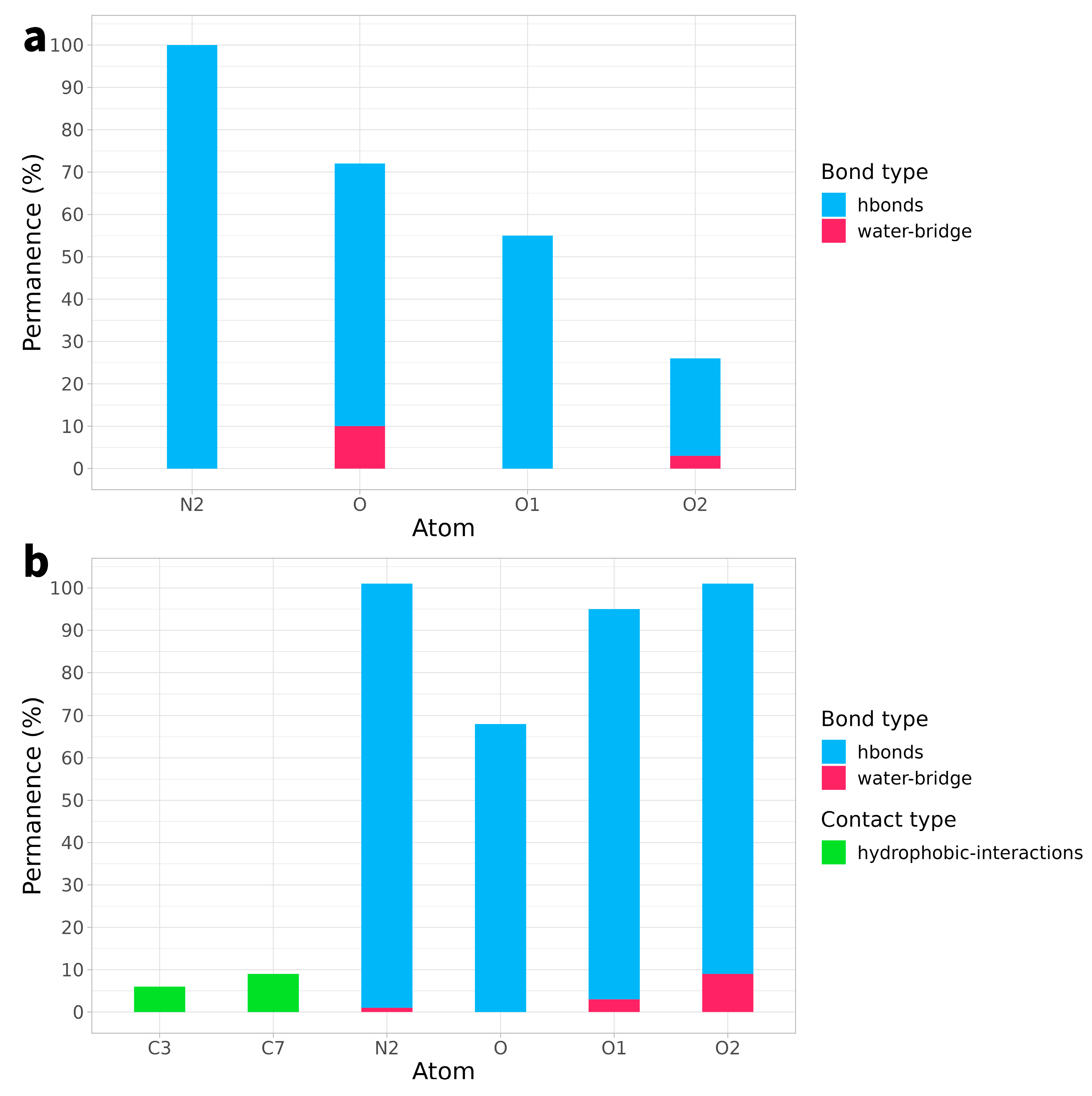
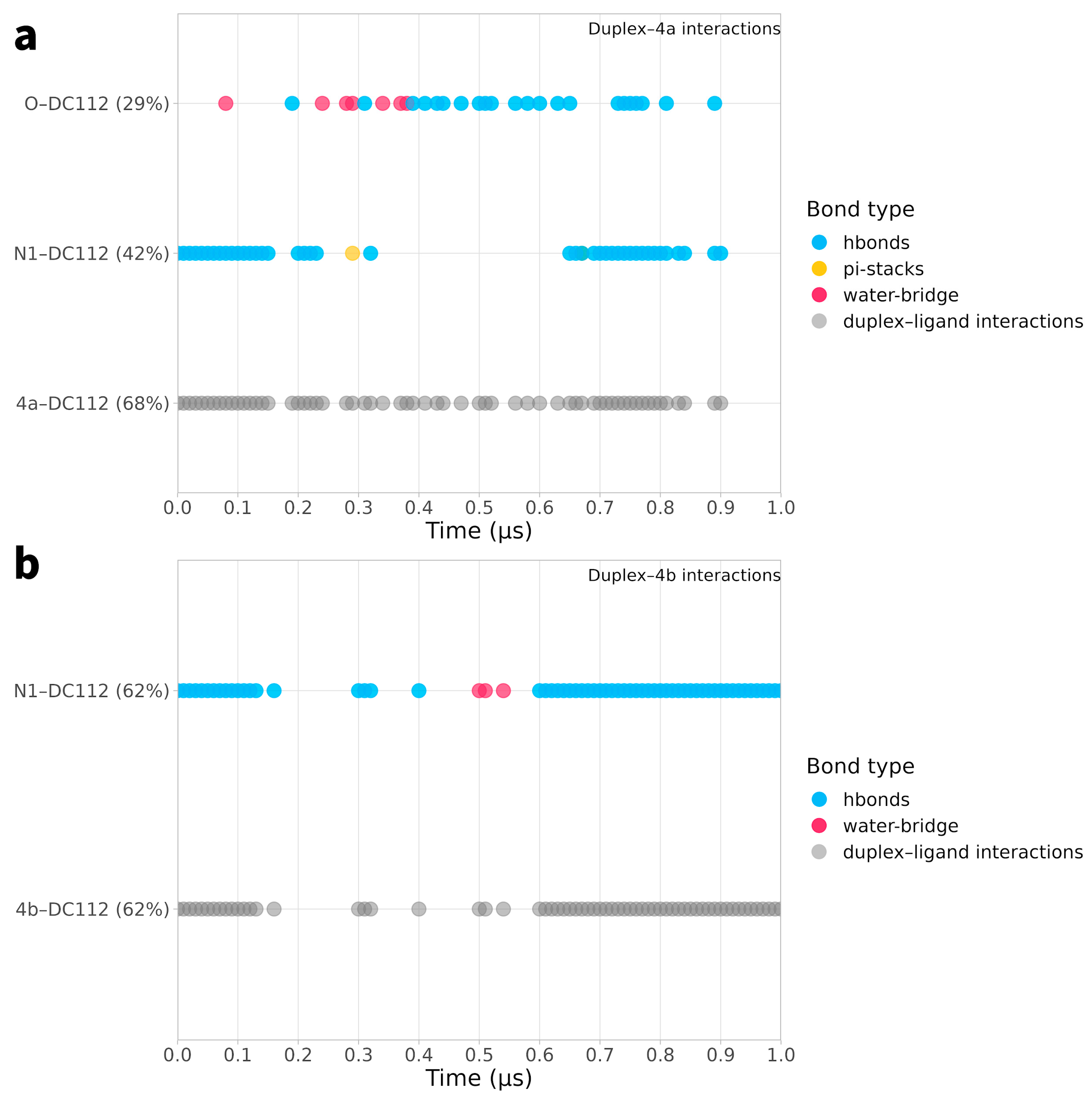
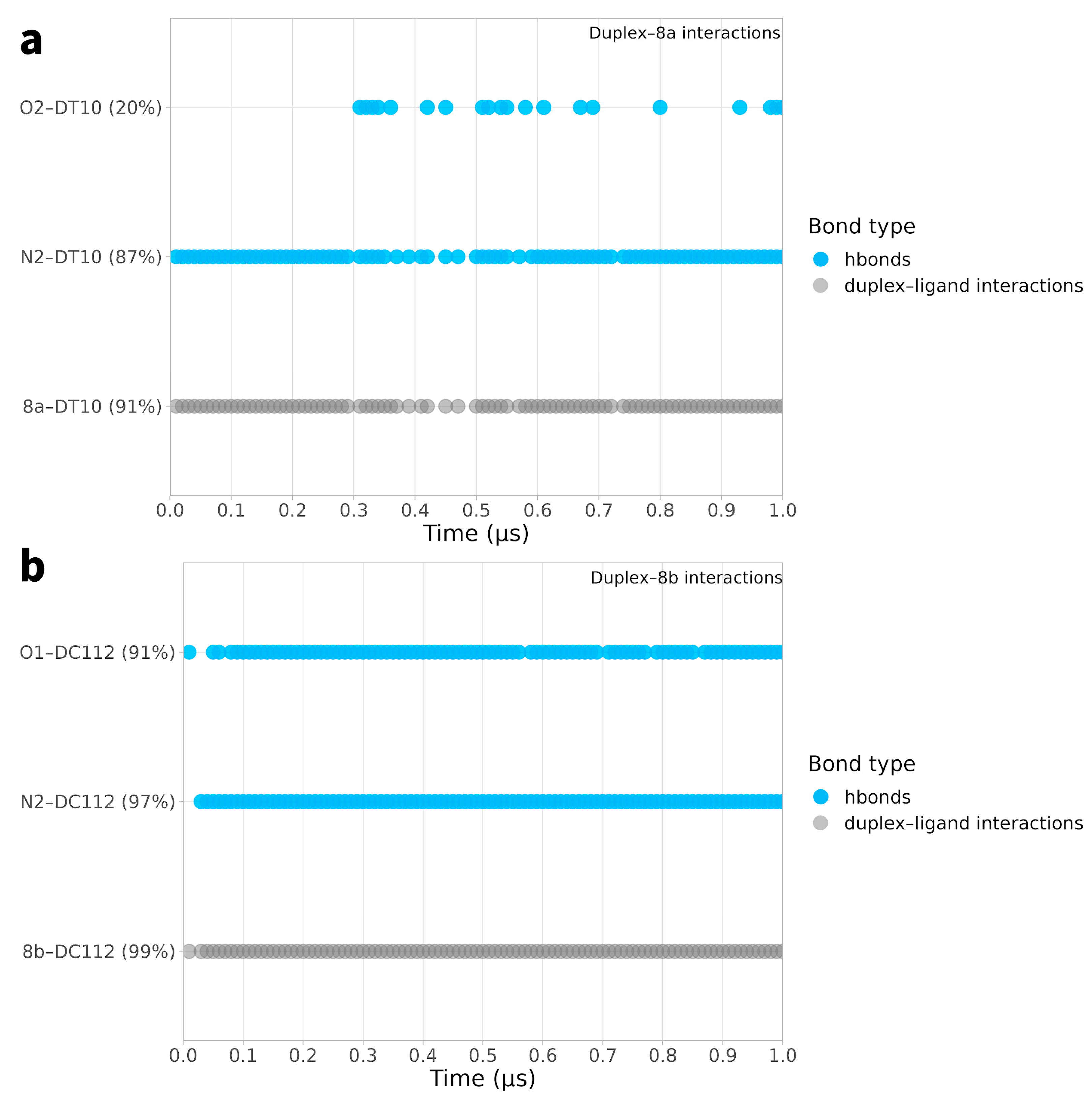
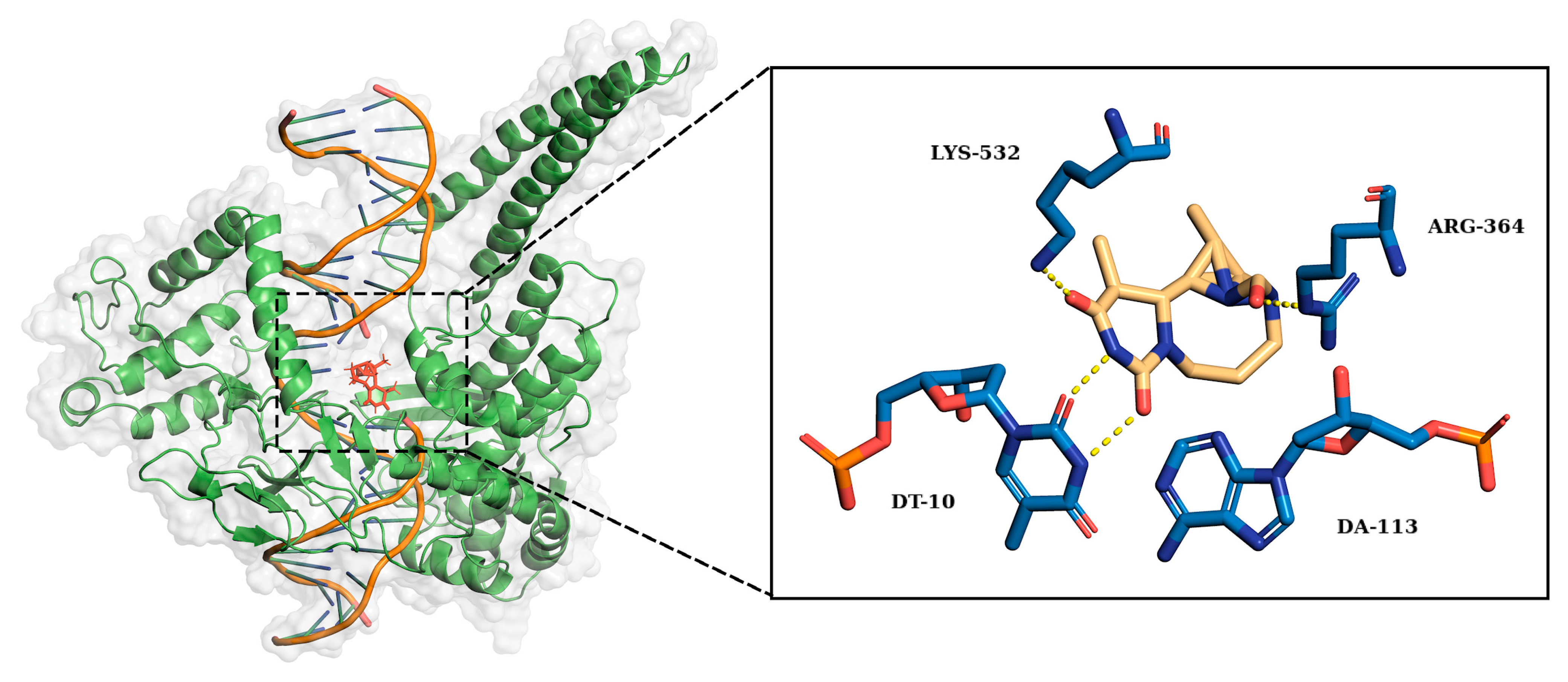
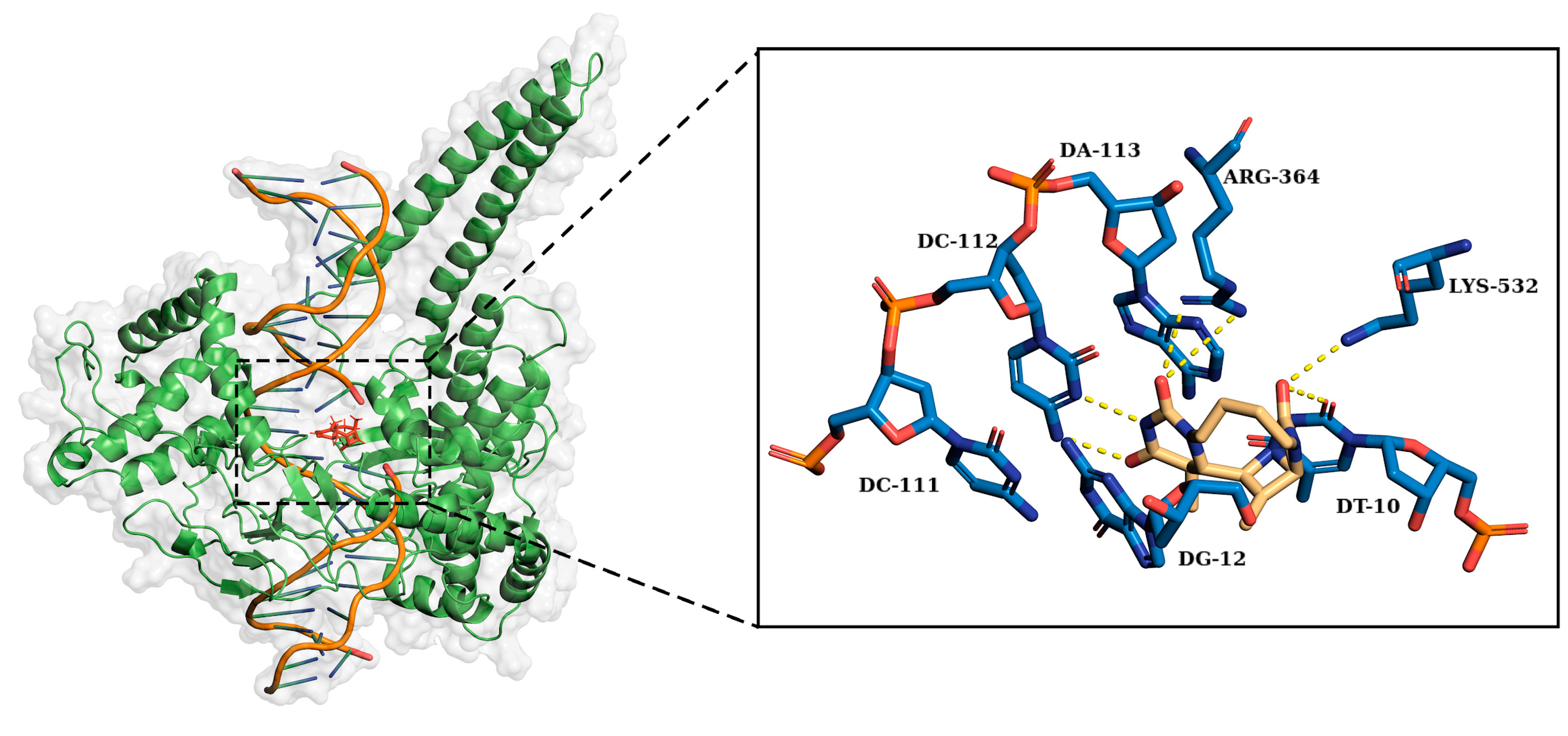
2.2. Ligand Receptor Binding Interactions as a Function of Time
2.3. PCA and Free-energy Landscape Analysis
3. Materials and Methods
3.1. Data Preparation (In Silico Molecular Docking)
3.2. Classical Molecular Dynamics Simulation
3.3. Analysis of Ligand–Receptor Interactions
3.4. PCA Analysis and Free-energy Calculation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; Stanislauskas, M.; Li, G.; Zheng, D.; Liu, L. Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci. Rep. 2017, 7, 42646. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Mouret, S.; Ravanat, J.L.; Douki, T. Photoinduced damage to cellular DNA: Direct and photosensitized reactions. Photochem. Photobiol. 2012, 88, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Saha, L.K.; Wakasugi, M.; Akter, S.; Akter, S.; Prasad, R.; Wilson, S.H.; Shimizu, N.; Sasanuma, H.; Huang, S.N.; Agama, K.; et al. Topoisomerase I-driven repair of UV-induced damage in NER-deficient cells. Proc. Natl. Acad. Sci. USA 2020, 117, 14412–14420. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidising agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Panzella, L.; Monfrecola, G.; d’Ischia, M. Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment. Cell Melanoma Res. 2014, 27, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Giglia-Mari, G.; Zotter, A.; Vermeulen, W. DNA damage response. Cold Spring Harb. Perspect. Biol. 2011, 3, a000745. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Kim, A.L.; Ahmad, N.; Mukhtar, H.; Gautier, J.; Bickers, D.R. Mechanism of ultraviolet B-induced cell cycle arrest in G2/M phase in immortalised skin keratinocytes with defective p53. Biochem. Biophys. Res. Commun. 2000, 277, 107–111. [Google Scholar] [CrossRef]
- Cooke, M.S.; Harry, E.L.; Liljendahl, T.S.; Segerbäck, D. DNA nucleotide excision repair, where do all the cyclobutane pyrimidine dimers go? Cell Cycle 2013, 12, 1642. [Google Scholar] [CrossRef][Green Version]
- Kemp, M.G.; Hu, J. PostExcision Events in Human Nucleotide Excision Repair. Photochem. Photobiol. 2016, 93, 178–191. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef]
- Lanza, A.; Tornaletti, S.; Rodolfo, C.; Scanavini, M.C.; Pedrini, A.M. Human DNA topoisomerase I-mediated cleavages stimulated by ultraviolet light-induced DNA damage. J. Biol. Chem. 1996, 271, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, D.; Rosenstein, B.S.; Muller, M.T. Ultraviolet-induced DNA damage stimulates topoisomerase I-DNA complex formation in vivo: Possible relationship with DNA repair. Cancer Res. 1998, 58, 976–984. [Google Scholar] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer. 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Barcelo, J.M.; Rao, V.A.; Sordet, O.; Jobson, A.G.; Thibaut, L.; Miao, Z.H.; Seiler, J.A.; Zhang, H.; Marchand, C.; et al. Repair of topoisomerase I-mediated DNA damage. Prog. Nucleic Acid Res. Mol. Biol. 2006, 81, 179–229. [Google Scholar] [PubMed]
- Beiu, C.; Giurcaneanu, C.; Grumezescu, A.M. Nanosystems for Improved Targeted Therapies in Melanoma. J. Clin. Med. 2020, 9, 318. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Victor, L.; Maricica, H.; Raluca, T.; Vlad, O.; Gheorghe, I.; Bolocan, A.; Grumezescu, A.M.; Holban, A.M. Nanostructures for cancer therapy: From targeting to selective toxicology. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 831–847. [Google Scholar]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Madeddu, F.; Di Martino, J.; Pieroni, M.; Del Buono, D.; Bottoni, P.; Botta, L.; Saladino, R. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of New Drugs against Human Topoisomerase I Receptor. Int. J. Mol. Sci. 2022, 23, 14652. [Google Scholar] [CrossRef]
- Gabellone, S.; Piccinino, D.; Filippi, S.; Castrignanò, T.; Zippilli, C.; Del Buono, D.; Saladino, R. Lignin nanoparticles provide novel biomimetic thymine photoadducts with anti-melanoma activity. Int. J. Mol. Sci. 2022, 23, 915. [Google Scholar] [CrossRef]
- Castrignanò, T.; Gioiosa, S.; Flati, T.; Cestari, M.; Picardi, E.; Chiara, M.; Zambelli, F. ELIXIR-IT HPC@ CINECA: High performance computing resources for the bioinformatics community. BMC Bioinform. 2020, 21, 352. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Pieroni, M.; Madeddu, F.; Di Martino, J.; Arcieri, M.; Parisi, V.; Bottoni, P.; Castrignanò, T. MD–Ligand–Receptor: A High-Performance Computing Tool for Characterizing Ligand–Receptor Binding Interactions in Molecular Dynamics Trajectories. Int. J. Mol. Sci. 2023, 24, 11671. [Google Scholar] [CrossRef] [PubMed]
- Mansi, L.; Tangaro, M.A.; Lo Giudice, C.; Flati, T.; Kopel, E.; Schaffer, A.A.; Picardi, E. REDIportal: Millions of novel A-to-I RNA editing events from thousands of RNAseq experiments. Nucleic Acids Res. 2021, 49, D1012–D1019. [Google Scholar] [CrossRef]
- Petrini, A.; Mesiti, M.; Schubach, M.; Frasca, M.; Danis, D.; Re, M.; Valentini, G. parSMURF, a high-performance computing tool for the genome-wide detection of pathogenic variants. GigaScience 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Castrignano, T.; Chillemi, G.; Desideri, A. Structure and hydration of BamHI DNA recognition site: A molecular dynamics investigation. Biophys. J. 2000, 79, 1263–1272. [Google Scholar] [CrossRef]
- Chillemi, G.; Castrignano, T.; Desideri, A. Structure and hydration of the DNA-human topoisomerase I covalent complex. Biophys. J. 2001, 81, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Castrignanò, T.; Chillemi, G.; Varani, G.; Desideri, A. Molecular dynamics simulation of the RNA complex of a double-stranded RNA-binding domain reveals dynamic features of the intermolecular interface and its hydration. Biophys. J. 2002, 83, 3542–3552. [Google Scholar] [CrossRef]
- Komatsu, T.S.; Okimoto, N.; Koyama, Y.M.; Hirano, Y.; Morimoto, G.; Ohno, Y.; Taiji, M. Drug binding dynamics of the dimeric SARS-CoV-2 main protease, determined by molecular dynamics simulation. Sci. Rep. 2020, 10, 16986. [Google Scholar] [CrossRef]
- Papaleo, E.; Mereghetti, P.; Fantucci, P.; Grandori, R.; De Gioia, L. Free-energy landscape, principal component analysis, and structural clustering to identify representative conformations from molecular dynamics simulations: The myoglobin case. J. Mol. Graph. Model. 2009, 27, 889–899. [Google Scholar] [CrossRef]
- Righino, B.; Galisson, F.; Pirolli, D.; Vitale, S.; Réty, S.; Gouet, P.; De Rosa, M.C. Structural model of the full-length Ser/Thr protein kinase StkP from S. pneumoniae and its recognition of peptidoglycan fragments. J. Biomol. Struct. Dyn. 2018, 36, 3666–3679. [Google Scholar] [CrossRef]
- Stewart, L.; Redinbo, M.R.; Qiu, X.; Hol, W.G.; Champoux, J.J. A model for the mechanism of human topoisomerase I. Science 1998, 279, 1534–1541. [Google Scholar] [CrossRef]
- Pirolli, D.; Alinovi, C.C.; Capoluongo, E.; Satta, M.A.; Concolino, P.; Giardina, B.; De Rosa, M.C. Insight into a novel p53 single point mutation (G389E) by molecular dynamics simulations. Int. J. Mol. Sci. 2010, 12, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Staker, B.L.; Feese, M.D.; Cushman, M.; Pommier, Y.; Zembower, D.; Stewart, L.; Burgin, A.B. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J. Med. Chem. 2005, 48, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Loschwitz, J.; Jäckering, A.; Keutmann, M.; Olagunju, M.; Olubiyi, O.O.; Strodel, B. Dataset of AMBER force field parameters of drugs, natural products and steroids for simulations using GROMACS. Data Brief 2021, 35, 106948. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.; Alan, W.; Vranken, W.F. Acpype-Antechamber python parser interface. BMC Res. Notes 2012, 5, 367. [Google Scholar]
- Mark, P.; Lennart, N. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]


| Compound Name | SMILES Strings | 2D Structure | Binding Affinity (Kcal/mol) a |
|---|---|---|---|
| 4a | C1(C(=CN(C(N1)=O)CCCN2C=C(C(NC2=O)=O)C)C)=O | 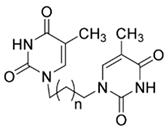 n = 1 | −7.1 |
| 4b | C(CCCN1C=C(C(NC1=O)=O)C)N2C(NC(C(=C2)C)=O)=O | 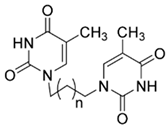 n = 2 | −7.3 |
| 8a | O=C(N1)N2CCCN3C(N4C3=O)C(C)=C4C2C(C)(O)C1=O | 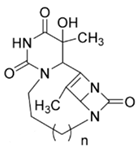 n = 1 | −7.9 |
| 8b | O=C(N1)N2CCCCN3C(N4C3=O)C(C)=C4C2C(C)(O)C1=O | 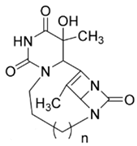 n = 2 | −8.8 |
| Arg364 | Thr718 | Leu721 | Lys751 | DC111 | DC112 | DG12 | DT10 | |
|---|---|---|---|---|---|---|---|---|
| O | 18% | 29% | ||||||
| O1 | 13% | 14% | ||||||
| O3 | 8% | 24% | ||||||
| N | 5% | |||||||
| N1 | 9% | 12% | 42% | 8% | ||||
| N3 | 5% | |||||||
| C2 | 5% | |||||||
| C3 | 5% | |||||||
| C4 | 5% + 8% * | |||||||
| C5 | 5% | |||||||
| C10 | 4% + 35% * | |||||||
| C11 | 10% * | 39% * |
| Arg364 | Ile535 | Arg634 | Ala715 | DC112 | DG12 | |
|---|---|---|---|---|---|---|
| O | 23% | |||||
| O1 | 11% | 51% | ||||
| O3 | 50% | |||||
| N | 5% | |||||
| N1 | 42% | 62% | 9% | |||
| N3 | 5% | |||||
| C2 | 5% | |||||
| C3 | 5% | |||||
| C4 | 5% | |||||
| C5 | 5% | |||||
| C11 | 77% * | |||||
| C12 | 13% * | 25% * |
| Arg364 | Lys532 | DA113 | DT10 | |
|---|---|---|---|---|
| O | 50% | 17% | ||
| O1 | 54% | |||
| O2 | 20% | |||
| N2 | 19% | 87% |
| Arg364 | Lys532 | DA113 | DC111 | DC112 | DG12 | DT10 | |
|---|---|---|---|---|---|---|---|
| O | 18% | 55% | 17% | ||||
| O1 | 6% | 5% | 91% | ||||
| O2 | 92% | 9% | |||||
| C7 | 9% * | ||||||
| N2 | 97% |
| Label | Name of Data File | File Type | Identifier in Data Repository (Figshare) |
|---|---|---|---|
| Data file 1 | htop1p-8a | Best pose (.pdb) | https://figshare.com/ndownloader/files/42792178 (accessed on 18 October 2023) |
| Data file 2 | htop1p-8b | Best pose (.pdb) | https://figshare.com/ndownloader/files/42792181 (accessed on 18 October 2023) |
| Data file 3 | htop1p-4a | Best pose (.pdb) | https://figshare.com/ndownloader/files/42792172 (accessed on 18 October 2023) |
| Data file 4 | htop1p-4b | Best pose (.pdb) | https://figshare.com/ndownloader/files/42792175 (accessed on 18 October 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Martino, J.; Arcieri, M.; Madeddu, F.; Pieroni, M.; Carotenuto, G.; Bottoni, P.; Botta, L.; Castrignanò, T.; Gabellone, S.; Saladino, R. Molecular Dynamics Investigations of Human DNA-Topoisomerase I Interacting with Novel Dewar Valence Photo-Adducts: Insights into Inhibitory Activity. Int. J. Mol. Sci. 2024, 25, 234. https://doi.org/10.3390/ijms25010234
Di Martino J, Arcieri M, Madeddu F, Pieroni M, Carotenuto G, Bottoni P, Botta L, Castrignanò T, Gabellone S, Saladino R. Molecular Dynamics Investigations of Human DNA-Topoisomerase I Interacting with Novel Dewar Valence Photo-Adducts: Insights into Inhibitory Activity. International Journal of Molecular Sciences. 2024; 25(1):234. https://doi.org/10.3390/ijms25010234
Chicago/Turabian StyleDi Martino, Jessica, Manuel Arcieri, Francesco Madeddu, Michele Pieroni, Giovanni Carotenuto, Paolo Bottoni, Lorenzo Botta, Tiziana Castrignanò, Sofia Gabellone, and Raffaele Saladino. 2024. "Molecular Dynamics Investigations of Human DNA-Topoisomerase I Interacting with Novel Dewar Valence Photo-Adducts: Insights into Inhibitory Activity" International Journal of Molecular Sciences 25, no. 1: 234. https://doi.org/10.3390/ijms25010234
APA StyleDi Martino, J., Arcieri, M., Madeddu, F., Pieroni, M., Carotenuto, G., Bottoni, P., Botta, L., Castrignanò, T., Gabellone, S., & Saladino, R. (2024). Molecular Dynamics Investigations of Human DNA-Topoisomerase I Interacting with Novel Dewar Valence Photo-Adducts: Insights into Inhibitory Activity. International Journal of Molecular Sciences, 25(1), 234. https://doi.org/10.3390/ijms25010234








