Intratumoral Influenza Vaccine Administration Attenuates Breast Cancer Growth and Restructures the Tumor Microenvironment through Sialic Acid Binding of Vaccine Hemagglutinin
Abstract
1. Introduction
2. Results
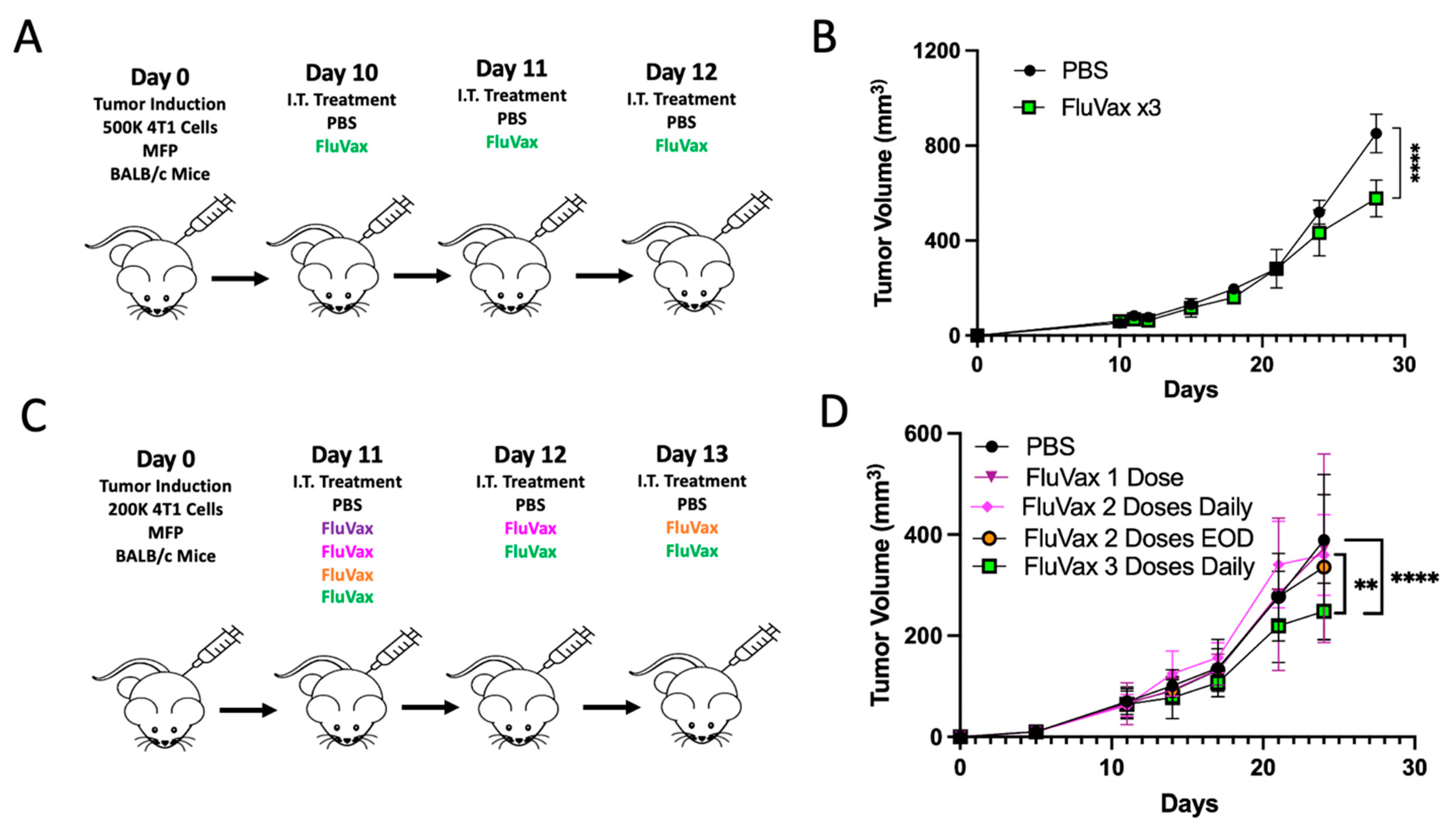
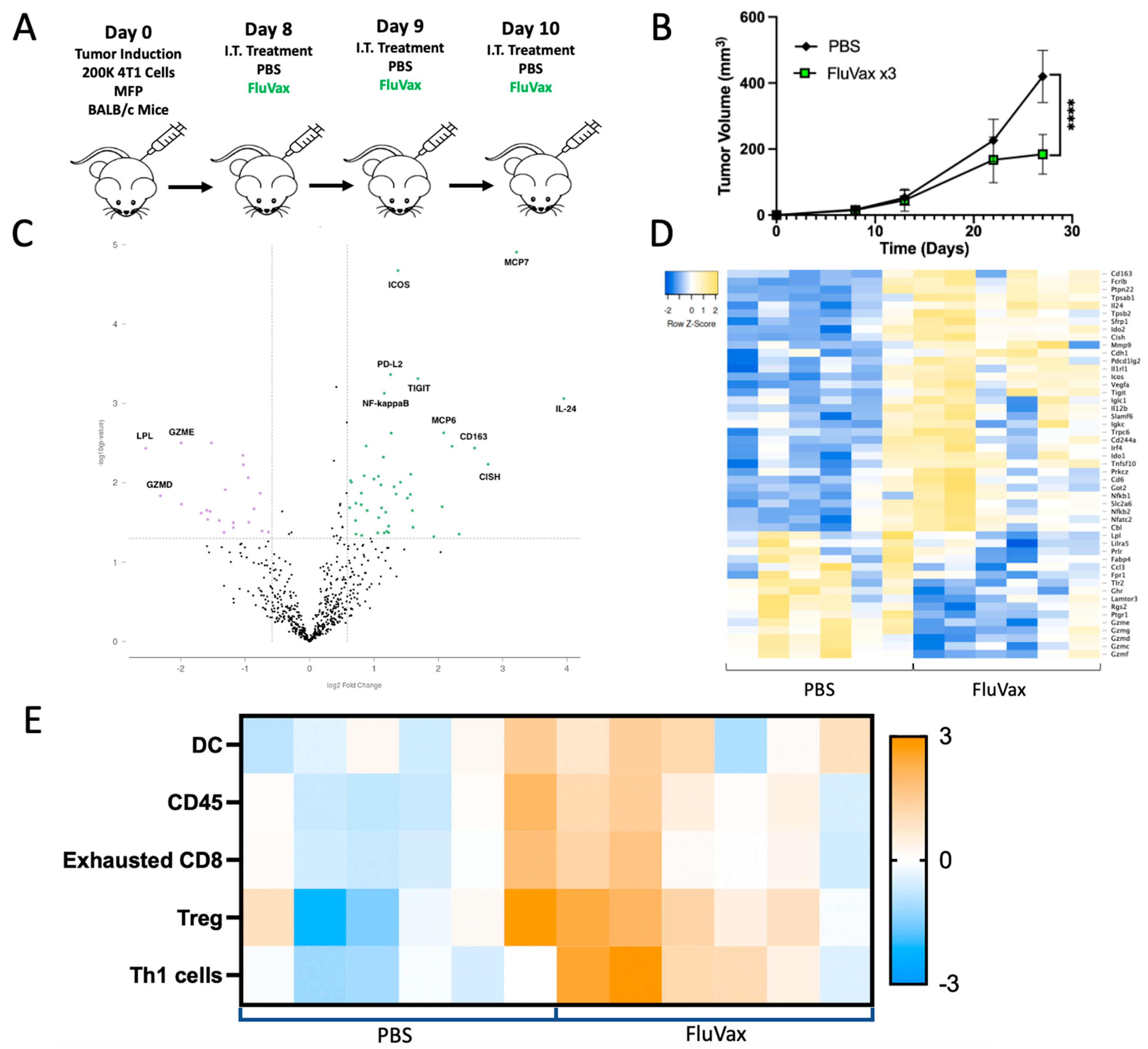
2.1. Intratumoral Administration of Flu Vaccine Reduces 4T1 Breast Tumor Growth
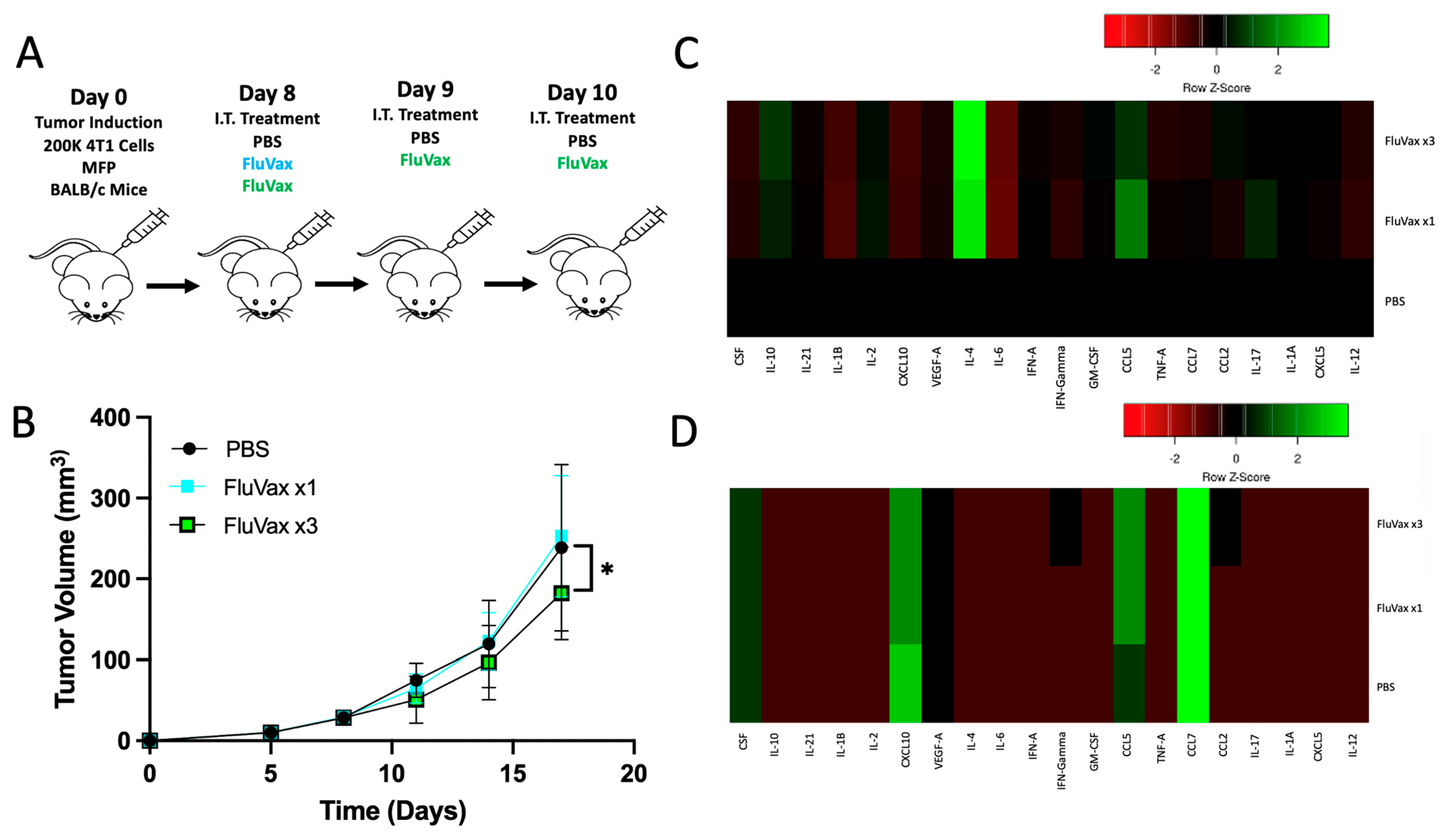
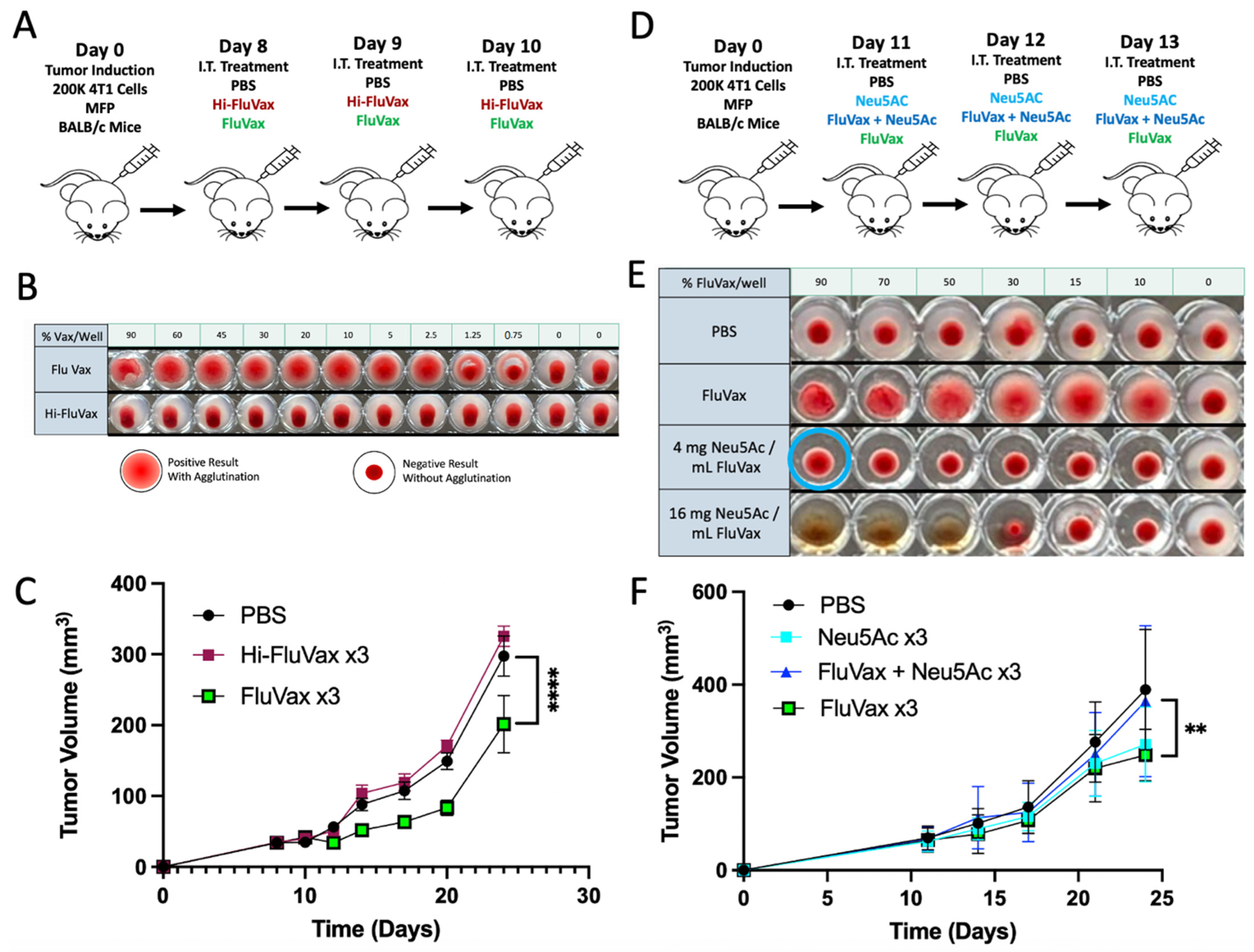
2.2. Pre-Existing Vaccination Does Not Hinder Efficacy of Subsequent Intratumoral Influenza Vaccine Administrations
2.3. Intratumoral Influenza Vaccine Administration Reshapes the 4T1 Tumor Microenvironment
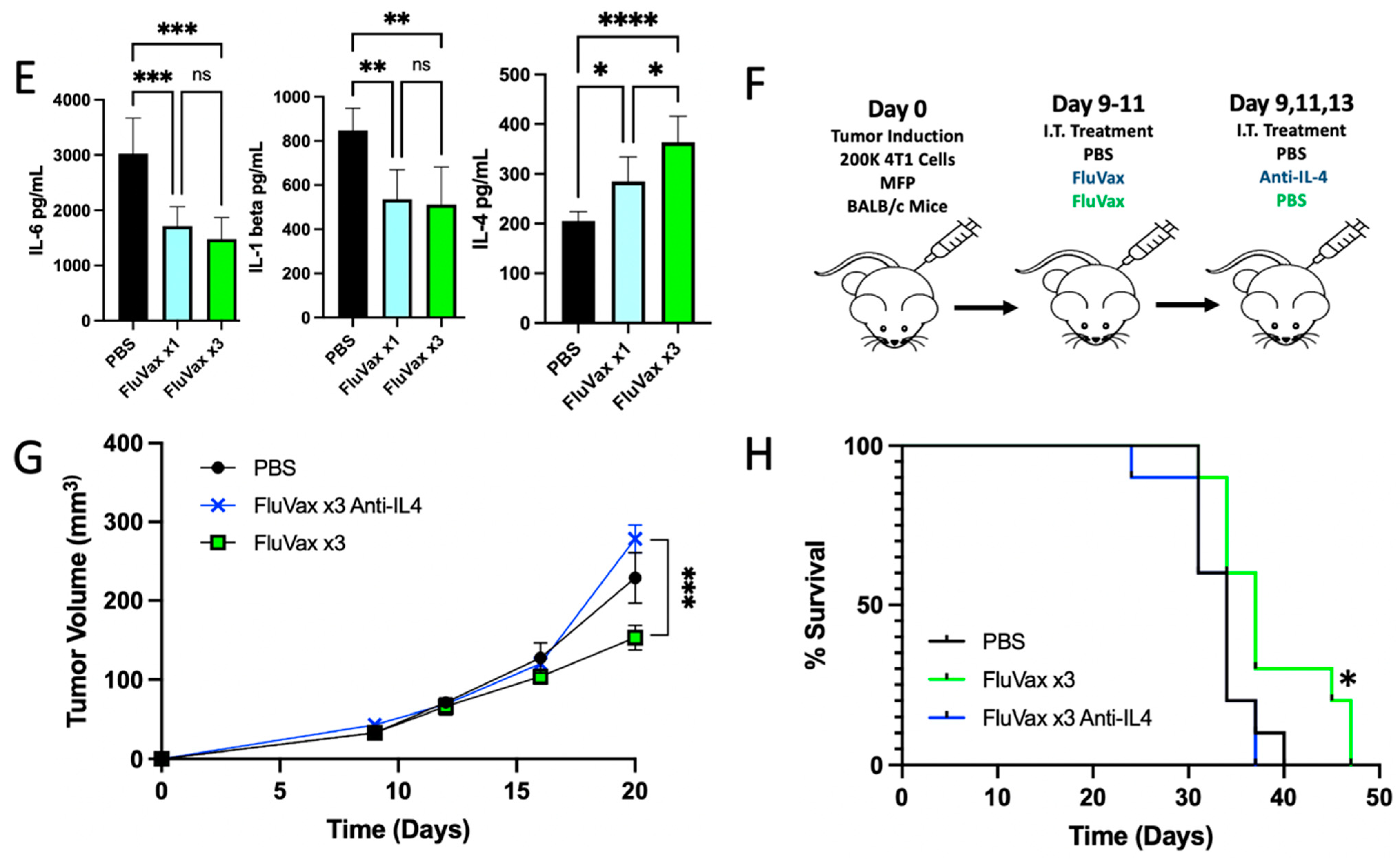
2.4. Intratumoral Influenza Vaccine Upregulates IL-4, Which in Part Mediates Anti-Tumor Effects
2.5. Sialic Acid Binding Ability Mediates the Anti-Tumor Activity of the Influenza Vaccine
2.6. Tumoral Sialic Acid Expression Modulates Influenza Hemagglutinin Binding
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Animal Models
4.3. Tumor Challenge and Vaccine Treatment
4.4. RNA Expression Analysis with NanoString Exhaustion Panel
4.5. Hemagglutination Assay
4.6. Cytokine Profiling
4.7. Sialic Acid–Hemagglutinin Binding Assay by Flow Cytometry
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joensuu, H.; Kellokumpu-Lehtinen, P.L.; Huovinen, R.; Jukkola, A.; Tanner, M.; Ahlgren, J.; Auvinen, P.; Lahdenperä, O.; Villman, K.; Nyandoto, P.; et al. Adjuvant Capecitabine for Early Breast Cancer: 15-Year Overall Survival Results from a Randomized Trial. J. Clin. Oncol. 2022, 40, 1051–1058. [Google Scholar] [CrossRef]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated with Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 1175, Correction in Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- von der Lippe Gythfeldt, H.; Lien, T.; Tekpli, X.; Silwal-Pandit, L.; Borgen, E.; Garred, Ø.; Skjerven, H.; Schlichting, E.; Lundgren, S.; Wist, E.; et al. Immune phenotype of tumor microenvironment predicts response to bevacizumab in neoadjuvant treatment of ER-positive breast cancer. Int. J. Cancer 2020, 147, 2515–2525, Correction in Int. J. Cancer 2022, 150, E9. [Google Scholar] [CrossRef]
- Wagner, J.; Rapsomaniki, M.A.; Chevrier, S.; Anzeneder, T.; Langwieder, C.; Dykgers, A.; Rees, M.; Ramaswamy, A.; Muenst, S.; Soysal, S.D.; et al. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell 2019, 177, 1330–1345.e18. [Google Scholar] [CrossRef]
- Rous, P.; Murphy, J.B. ON the Causation by Filterable Agents of Three Distinct Chicken Tumors. J. Exp. Med. 1914, 19, 52–68. [Google Scholar] [CrossRef][Green Version]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus Particles in Cultured Lymphoblasts from Burkitt’s lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Karbach, J.; Neumann, A.; Brand, K.; Wahle, C.; Siegel, E.; Maeurer, M.; Ritter, E.; Tsuji, T.; Gnjatic, S.; Old, L.J.; et al. I clinical trial of mixed bacterial vaccine (Coley’s toxins) in patients with NY-ESO-1 expressing cancers: Immunological effects and clinical activity. Clin. Cancer Res. 2012, 18, 5449–5459. [Google Scholar] [CrossRef]
- Adams, S.; Kozhaya, L.; Martiniuk, F.; Meng, T.C.; Chiriboga, L.; Liebes, L.; Hochman, T.; Shuman, N.; Axelrod, D.; Speyer, J.; et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin. Cancer Res. 2012, 18, 6748–6757. [Google Scholar] [CrossRef]
- Adams, S.; Kozhaya, L.; Martiniuk, F.; Meng, T.C.; Chiriboga, L.; Liebes, L.; Hochman, T.; Shuman, N.; Axelrod, D.; Speyer, J.; et al. Topical Imiquimod Plus Nab-paclitaxel for Breast Cancer Cutaneous Metastases: A Phase 2 Clinical Trial. JAMA Oncol. 2017, 3, 969–973. [Google Scholar]
- Newman, J.H.; Chesson, C.B.; Herzog, N.L.; Bommareddy, P.K.; Aspromonte, S.M.; Pepe, R.; Estupinian, R.; Aboelatta, M.M.; Buddhadev, S.; Tarabichi, S.; et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 1119–1128. [Google Scholar] [CrossRef]
- Aznar, M.A.; Molina, C.; Teijeira, A.; Rodriguez, I.; Azpilikueta, A.; Garasa, S.; Sanchez-Paulete, A.R.; Cordeiro, L.; Etxeberria, I.; Alvarez, M.; et al. Repurposing the yellow fever vaccine for intratumoral immunotherapy. EMBO Mol. Med. 2020, 12, e10375. [Google Scholar] [CrossRef]
- Dai, P.; Wang, W.; Yang, N.; Serna-Tamayo, C.; Ricca, J.M.; Zamarin, D.; Shuman, S.; Merghoub, T.; Wolchok, J.D.; Deng, L. Intratumoral delivery of inactivated modified vaccinia virus Ankara (iMVA) induces systemic anti-tumor immunity via STING and Batf3-dependent dendritic cells. Sci. Immunol. 2017, 2, eaal1713. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, X.; Liu, X.; He, Y.; Hu, Z.; Xu, W.; Cao, G.; He, W. Remodels the Immunosuppressive Tumor Microenvironment by Combination of Bacillus Calmette-Guérin and Anti-PD-L1 in an Orthotopic Triple-Negative Breast Cancer Mouse Model. Onco. Targets Ther. 2021, 14, 2247–2258. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.L.; Morales, A. A BCG success story: From prevention of tuberculosis to optimal bladder cancer treatment. Vaccine 2021, 39, 7308–7318. [Google Scholar] [CrossRef] [PubMed]
- Hock, K.; Laengle, J.; Kuznetsova, I.; Egorov, A.; Hegedus, B.; Dome, B.; Wekerle, T.; Sachet, M.; Bergmann, M. Oncolytic influenza A virus expressing interleukin-15 decreases tumor growth in vivo. Surgery 2017, 161, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Gögenur, M.; Balsevicius, L.; Bulut, M.; Colak, N.; Justesen, T.F.; Fiehn, A.K.; Jensen, M.B.; Høst-Rasmussen, K.; Cappelen, B.; Gaggar, S.; et al. Neoadjuvant intratumoral influenza vaccine treatment in patients with proficient mismatch repair colorectal cancer leads to increased tumor infiltration of CD8+ T cells and upregulation of PD-L1: A phase 1/2 clinical trial. J. Immunother. Cancer 2023, 11, e006774. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Sieben, C.; Sezgin, E.; Eggeling, C.; Manley, S. Influenza A viruses use multivalent sialic acid clusters for cell binding and receptor activation. PLoS Pathog. 2020, 16, e1008656. [Google Scholar] [CrossRef] [PubMed]
- Pally, D.; Pramanik, D.; Hussain, S.; Verma, S.; Srinivas, A.; Kumar, R.V.; Everest-Dass, A.; Bhat, R. Heterogeneity in 2, 6-Linked Sialic Acids Potentiates Invasion of Breast Cancer Epithelia. ACS Cent Sci. 2021, 7, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lin, Y.; Yue, L.; Zhao, X.; Liu, J. Differential expression of the α2, 3-sialic acid residues in breast cancer is associated with metastatic potential. Oncol. Rep. 2011, 25, 1365–1371. [Google Scholar] [PubMed]
- Teoh, S.T.; Ogrodzinski, M.P.; Ross, C.; Hunter, K.W.; Lunt, S.Y. Sialic Acid Metabolism: A Key Player in Breast Cancer Metastasis Revealed by Metabolomics. Front. Oncol. 2018, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Maiti, A.; Wu, R.; Andersen, V.L.; Hsu, C.C.; Wu, Y.; Chapla, D.G.; Takabe, K.; Rusiniak, M.E.; Bshara, W.; et al. Extracellular sialyltransferase st6gal1 in breast tumor cell growth and invasiveness. Cancer Gene Ther. 2022, 29, 1662–1675. [Google Scholar] [CrossRef]
- Gray, M.A.; Stanczak, M.A.; Mantuano, N.R.; Xiao, H.; Pijnenborg, J.F.A.; Malaker, S.A.; Miller, C.L.; Weidenbacher, P.A.; Tanzo, J.T.; Ahn, G.; et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 2020, 16, 1376–1384. [Google Scholar] [CrossRef]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef]
- Yamaji, T.; Mitsuki, M.; Teranishi, T.; Hashimoto, Y. Characterization of inhibitory signaling motifs of the natural killer cell receptor Siglec-7: Attenuated recruitment of phosphatases by the receptor is attributed to two amino acids in the motifs. Glycobiology 2005, 15, 667–676. [Google Scholar] [CrossRef]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 124, Correction in Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viswanathan, K.; Raman, R.; Chandrasekaran, A.; Raguram, S.; Tumpey, T.M.; Sasisekharan, V.; Sasisekharan, R. Quantitative biochemical rationale for differences in transmissibility of 1918 pandemic influenza A viruses. Proc. Natl. Acad. Sci. USA 2008, 105, 2800–2805. [Google Scholar] [CrossRef]
- van Riel, D.; Munster, V.J.; de Wit, E.; Rimmelzwaan, G.F.; Fouchier, R.A.; Osterhaus, A.D.; Kuiken, T. H5N1 Virus Attachment to Lower Respiratory Tract. Science. 2006, 312, 399. [Google Scholar] [CrossRef]
- Grimm, M.O.; van der Heijden, A.G.; Colombel, M.; Muilwijk, T.; Martínez-Piñeiro, L.; Babjuk, M.M.; Türkeri, L.N.; Palou, J.; Patel, A.; Bjartell, A.S.; et al. Treatment of High-grade Non-muscle-invasive Bladder Carcinoma by Standard Number and Dose of BCG Instillations Versus Reduced Number and Standard Dose of BCG Instillations: Results of the European Association of Urology Research Foundation Randomised Phase III Clinical Trial “NIMBUS”. Eur. Urol. 2020, 78, 690–698. [Google Scholar] [PubMed]
- Wu, C.; Zanker, D.; Valkenburg, S.; Tan, B.; Kedzierska, K.; Zou, Q.M.; Doherty, P.C.; Chen, W. Systematic identification of immunodominant CD8+ T-cell responses to influenza A virus in HLA-A2 individuals. Proc. Natl. Acad. Sci. USA 2011, 108, 9178–9183. [Google Scholar] [CrossRef] [PubMed]
- Mettelman, R.C.; Souquette, A.; Van de Velde, L.A.; Vegesana, K.; Allen, E.K.; Kackos, C.M.; Trifkovic, S.; DeBeauchamp, J.; Wilson, T.L.; St James, D.G.; et al. Baseline innate and T cell populations are correlates of protection against symptomatic influenza virus infection independent of serology. Nat. Immunol. 2023, 24, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Bocangel, D.; Doneske, B.; Mhashilkar, A.; Ramesh, R.; Hunt, K.K.; Ekmekcioglu, S.; Sutton, R.B.; Poindexter, N.; Grimm, E.A.; et al. Human interleukin 24 (MDA-7/IL-24) protein kills breast cancer cells via the IL-20 receptor and is antagonized by IL-10. Cancer Immunol. Immunother. 2007, 56, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Persaud, L.; Mighty, J.; Zhong, X.; Francis, A.; Mendez, M.; Muharam, H.; Redenti, S.M.; Das, D.; Aktas, B.H.; Sauane, M. IL-24 Promotes Apoptosis through cAMP-Dependent PKA Pathways in Human Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3561. [Google Scholar] [CrossRef]
- Niwa, Y.; Adachi, K.; Tabata, K.; Ishida, R.; Hotta, K.; Ishida, T.; Mano, Y.; Ozawa, Y.; Minoshima, Y.; Funahashi, Y.; et al. Liposome-Encapsulated Eribulin Shows Enhanced Anti-tumor Activity over Eribulin for Combination Therapy with Anti-PD-1 Antibody. Mol. Cancer Ther. 2023, 22, 499–510. [Google Scholar] [CrossRef]
- Manupati, K.; Yeeravalli, R.; Kaushik, K.; Singh, D.; Mehra, B.; Gangane, N.; Gupta, A.; Goswami, K.; Das, A. Activation of CD44-Lipoprotein lipase axis in breast cancer stem cells promotes tumorigenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166228. [Google Scholar] [CrossRef]
- Holen, I.; Lefley, D.V.; Francis, S.E.; Rennicks, S.; Bradbury, S.; Coleman, R.E.; Ottewell, P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016, 7, 75571–75584. [Google Scholar] [CrossRef]
- Radharani, N.N.V.; Yadav, A.S.; Nimma, R.; Kumar, T.V.S.; Bulbule, A.; Chanukuppa, V.; Kumar, D.; Patnaik, S.; Rapole, S.; Kundu, G.C. Tumor-associated macrophage derived IL-6 enriches cancer stem cell population and promotes breast tumor progression via Stat-3 pathway. Cancer Cell Int. 2022, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, H.; Mohan, G.C.; Shen, K.; Chan, L.S. Differential expression of inflammation-related genes in IL-4 transgenic mice before and after the onset of atopic dermatitis skin lesions. Mol. Cell Probes. 2016, 30, 30–38. [Google Scholar] [CrossRef]
- Dabitao, D.; Hedrich, C.M.; Wang, F.; Vacharathit, V.; Bream, J.H. Cell-Specific Requirements for STAT Proteins and Type I IFN Receptor Signaling Discretely Regulate IL-24 and IL-10 Expression in NK Cells and Macrophages. J. Immunol. 2018, 200, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Zissler, U.M.; Chaker, A.M.; Effner, R.; Ulrich, M.; Guerth, F.; Piontek, G.; Dietz, K.; Regn, M.; Knapp, B.; Theis, F.J.; et al. Interleukin-4 and interferon-γ orchestrate an epithelial polarization in the airways. Mucosal. Immunol. 2016, 9, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Boltje, T.J.; van Dinther, E.A.; Peters, T.; de Graaf, A.M.; Leusen, J.H.; Kreutz, M.; Figdor, C.G.; den Brok, M.H.; Adema, G.J. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, S.M.; Nguyen, H.M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, W.; Zhang, Y.; Dong, X.; Liu, C.; Yi, J.; Zhang, S.; Wen, C.; Zheng, L.; Wang, H. Oncolytic adenoviruses synergistically enhance anti-PD-L1 and anti-CTLA-4 immunotherapy by modulating the tumour microenvironment in a 4T1 orthotopic mouse model. Cancer Gene Ther. 2022, 29, 456–465. [Google Scholar] [CrossRef]
- Nahar, S.; Huang, Y.; Nagy, B.A.; Zebala, J.A.; Maeda, D.Y.; Rudloff, U.; Oppenheim, J.J.; Yang, D. Regression and Eradication of Triple-Negative Breast Carcinoma in 4T1 Mouse Model by Combination Immunotherapies. Cancers 2023, 15, 2366. [Google Scholar] [CrossRef]
- Büll, C.; Nason, R.; Sun, L.; Van Coillie, J.; Madriz Sørensen, D.; Moons, S.J.; Yang, Z.; Arbitman, S.; Fernandes, S.M.; Furukawa, S.; et al. Probing the binding specificities of human Siglecs by cell-based glycan arrays. Proc. Natl. Acad. Sci. USA 2021, 118, e2026102118. [Google Scholar] [CrossRef]
- Yu, H.; Gonzalez-Gil, A.; Wei, Y.; Fernandes, S.M.; Porell, R.N.; Vajn, K.; Paulson, J.C.; Nycholat, C.M.; Schnaar, R.L. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology 2017, 27, 657–668. [Google Scholar] [CrossRef]
- Mitic, N.; Milutinovic, B.; Jankovic, M. Assessment of sialic acid diversity in cancer- and non-cancer related CA125 antigen using sialic acid-binding Ig-like lectins (Siglecs). Dis. Markers 2012, 32, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, J.; Kuwashima, N.; Hatano, M.; Nishimura, F.; Dusak, J.E.; Storkus, W.J.; Okada, H. IL-4-transfected tumor cell vaccines activate tumor-infiltrating dendritic cells and promote type-1 immunity. J. Immunol. 2005, 174, 7194–7201. [Google Scholar] [CrossRef]
- Tepper, R.I.; Pattengale, P.K.; Leder, P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell 1989, 57, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Pippin, B.A.; Rosenstein, M.; Jacob, W.F.; Chiang, Y.; Lotze, M.T. Local IL-4 delivery enhances immune reactivity to murine tumors: Gene therapy in combination with IL-2. Cancer Gene Ther. 1994, 1, 35–42. [Google Scholar] [PubMed]
- Pericle, F.; Giovarelli, M.; Colombo, M.P.; Ferrari, G.; Musiani, P.; Modesti, A.; Cavallo, F.; Di Pierro, F.; Novelli, F.; Forni, G. An efficient Th2-type memory follows CD8+ lymphocyte-driven and eosinophil-mediated rejection of a spontaneous mouse mammary adenocarcinoma engineered to release IL-4. J. Immunol. 1994, 153, 5659–5673. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, Y. Interleukin-24 Regulates T Cell Activity in Patients with Colorectal Adenocarcinoma. Front Oncol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Nishikawa, T.; Ramesh, R.; Munshi, A.; Chada, S.; Meyn, R.E. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol. Ther. 2004, 9, 818–828. [Google Scholar] [CrossRef]
- Tong, A.W.; Nemunaitis, J.; Su, D.; Zhang, Y.; Cunningham, C.; Senzer, N.; Netto, G.; Rich, D.; Mhashilkar, A.; Parker, K.; et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients. Mol. Ther. 2005, 11, 160–172. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, P.; Cassoday, S.; Gupta, K.; Giurini, E.; Leifheit, M.E.; Zloza, A.; Marzo, A.L. Intratumoral Influenza Vaccine Administration Attenuates Breast Cancer Growth and Restructures the Tumor Microenvironment through Sialic Acid Binding of Vaccine Hemagglutinin. Int. J. Mol. Sci. 2024, 25, 225. https://doi.org/10.3390/ijms25010225
Daniels P, Cassoday S, Gupta K, Giurini E, Leifheit ME, Zloza A, Marzo AL. Intratumoral Influenza Vaccine Administration Attenuates Breast Cancer Growth and Restructures the Tumor Microenvironment through Sialic Acid Binding of Vaccine Hemagglutinin. International Journal of Molecular Sciences. 2024; 25(1):225. https://doi.org/10.3390/ijms25010225
Chicago/Turabian StyleDaniels, Preston, Stefanie Cassoday, Kajal Gupta, Eileena Giurini, Malia E. Leifheit, Andrew Zloza, and Amanda L. Marzo. 2024. "Intratumoral Influenza Vaccine Administration Attenuates Breast Cancer Growth and Restructures the Tumor Microenvironment through Sialic Acid Binding of Vaccine Hemagglutinin" International Journal of Molecular Sciences 25, no. 1: 225. https://doi.org/10.3390/ijms25010225
APA StyleDaniels, P., Cassoday, S., Gupta, K., Giurini, E., Leifheit, M. E., Zloza, A., & Marzo, A. L. (2024). Intratumoral Influenza Vaccine Administration Attenuates Breast Cancer Growth and Restructures the Tumor Microenvironment through Sialic Acid Binding of Vaccine Hemagglutinin. International Journal of Molecular Sciences, 25(1), 225. https://doi.org/10.3390/ijms25010225






