The Ralstonia solanacearum Type III Effector RipAW Targets the Immune Receptor Complex to Suppress PAMP-Triggered Immunity
Abstract
:1. Introduction
2. Results
2.1. RipAW Impairs Plant Resistance to Different Pathogens in Arabidopsis thaliana
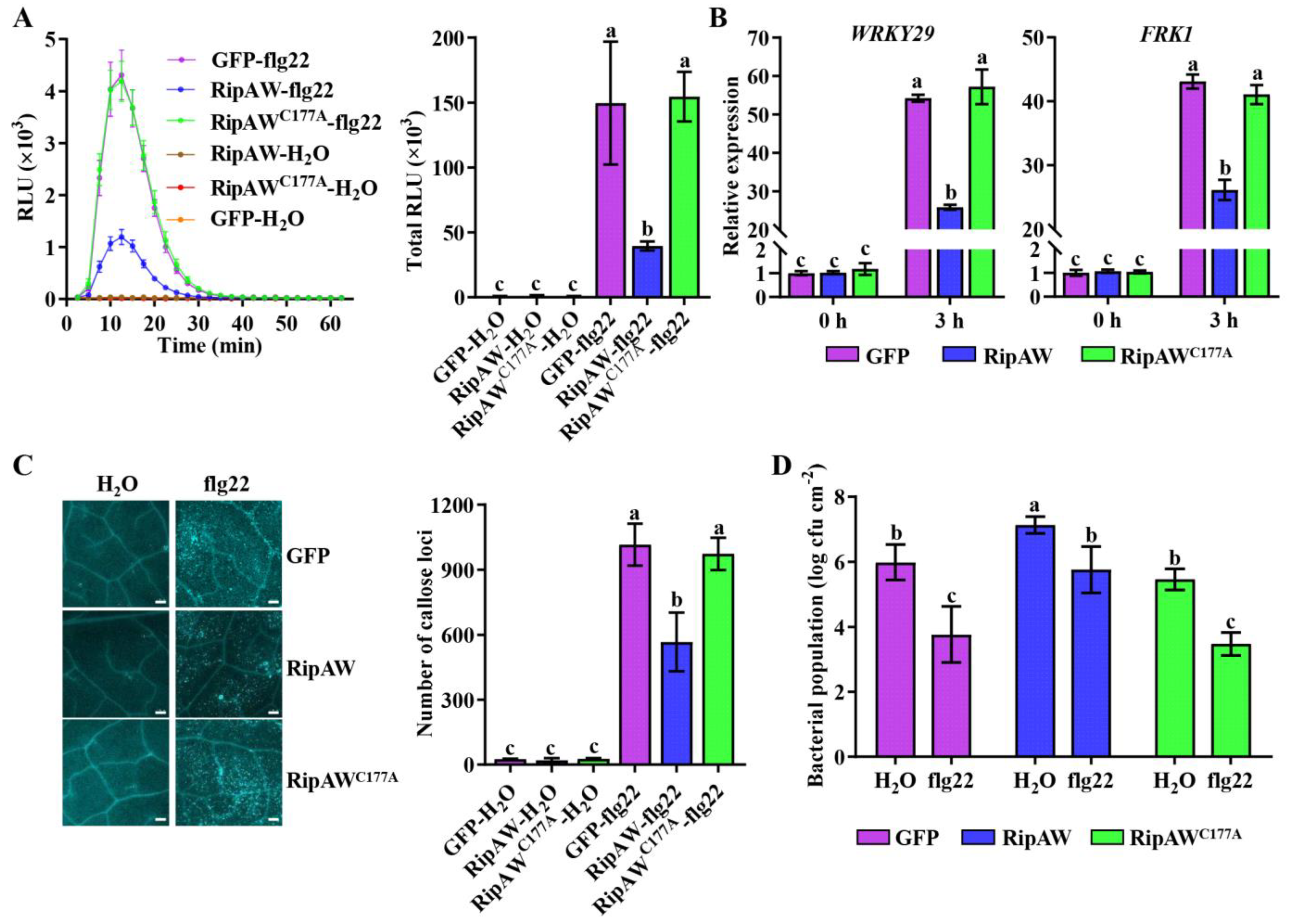
2.2. RipAW Suppresses PAMP-Triggered Immunity in A. thaliana
2.3. RipAW Associates with the Immune Receptor Complex
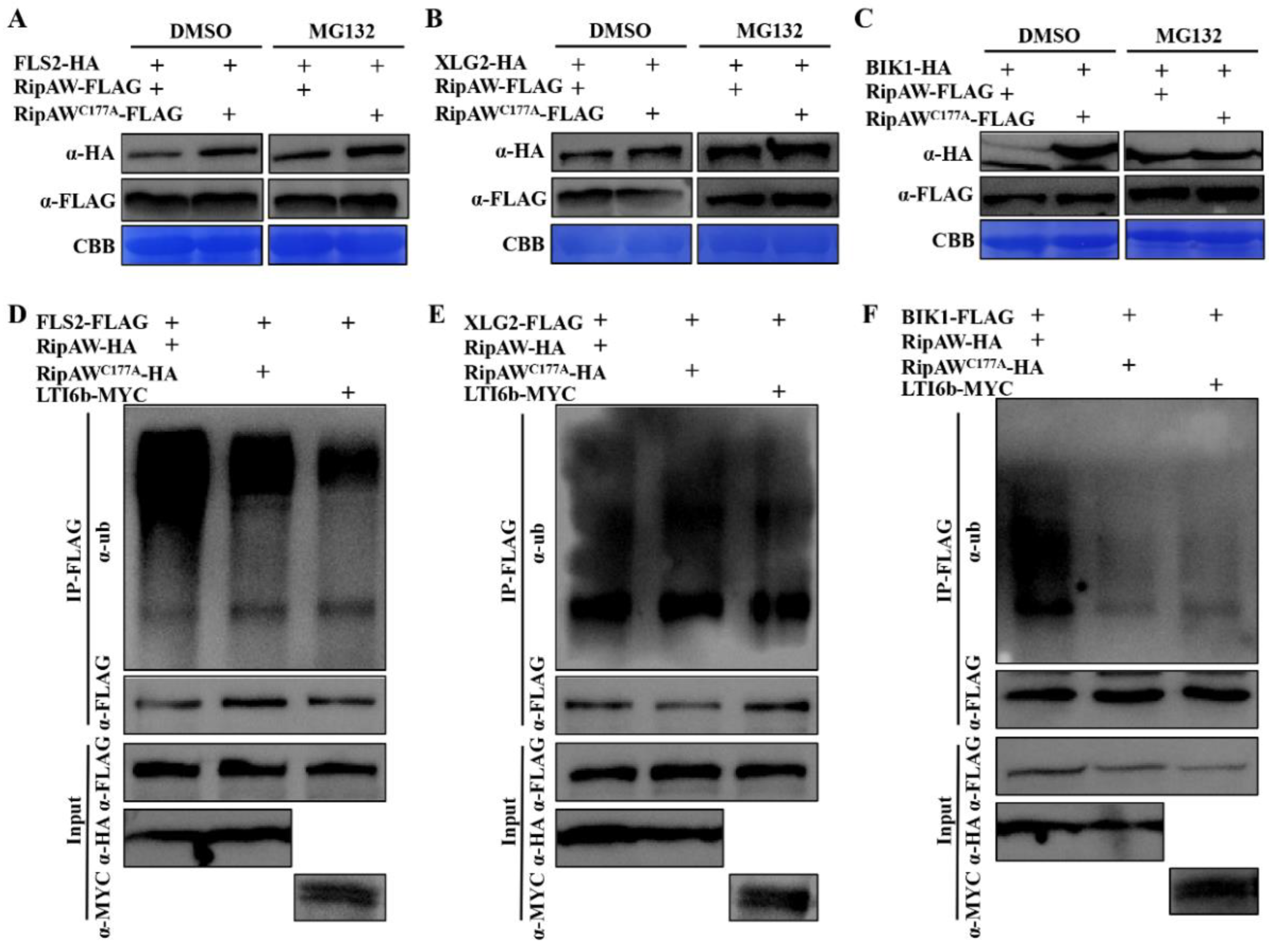
2.4. RipAW Ubiquitinates FLS2, XLG2, and BIK1 to Decrease Their Protein Accumulation through the 26S Proteasome
2.5. FLS2, XLG2, or BIK1 Can Partially Restore RipAW-Suppressed ROS Burst in N. benthamiana
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Pathogen Inoculation Assays
4.3. Agrobacterium-Mediated Transient Expression and Flg22-Induced ROS Burst
4.4. Co-Immunoprecipitation (co-IP)
4.5. Split-Luciferase Complementation (LCA) Assay
4.6. Bimolecular Fluorescence Complementation (BiFC) Assay
4.7. Quantitative Real-Time PCR
4.8. Callose Deposition Assay
4.9. In vivo Ubiquitination Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef]
- Ma, M.; Wang, W.; Fei, Y.; Cheng, H.-Y.; Song, B.; Zhou, Z.; Zhao, Y.; Zhang, X.; Li, L.; Chen, S. A surface-receptor-coupled G protein regulates plant immunity through nuclear protein kinases. Cell Host Microbe 2022, 30, 1602–1614.e5. [Google Scholar] [CrossRef]
- Ma, X.; Claus, L.A.; Leslie, M.E.; Tao, K.; Wu, Z.; Liu, J.; Yu, X.; Li, B.; Zhou, J.; Savatin, D.V. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature 2020, 581, 199–203. [Google Scholar] [CrossRef]
- Liang, X.; Ma, M.; Zhou, Z.; Wang, J.; Yang, X.; Rao, S.; Bi, G.; Li, L.; Zhang, X.; Chai, J. Ligand-triggered de-repression of Arabidopsis heterotrimeric G proteins coupled to immune receptor kinases. Cell Res. 2018, 28, 529–543. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Yu, L.; Zhou, Z.; Liang, X.; Liu, Z.; Cai, G.; Gao, L.; Zhang, X.; Wang, Y. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 2014, 15, 329–338. [Google Scholar] [CrossRef]
- Zhang, W.; He, S.Y.; Assmann, S.M. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 2008, 56, 984–996. [Google Scholar] [CrossRef]
- Liang, X.; Ding, P.; Lian, K.; Wang, J.; Ma, M.; Li, L.; Li, L.; Li, M.; Zhang, X.; Chen, S. Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. elife 2016, 5, e13568. [Google Scholar] [CrossRef]
- Lu, D.; Wu, S.; Gao, X.; Zhang, Y.; Shan, L.; He, P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 496–501. [Google Scholar] [CrossRef]
- Wang, J.; Grubb, L.E.; Wang, J.; Liang, X.; Li, L.; Gao, C.; Ma, M.; Feng, F.; Li, M.; Li, L. A regulatory module controlling homeostasis of a plant immune kinase. Mol. Cell 2018, 69, 493–504.e6. [Google Scholar] [CrossRef]
- Shan, L.; He, P.; Li, J.; Heese, A.; Peck, S.C.; Nürnberger, T.; Martin, G.B.; Sheen, J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 2008, 4, 17–27. [Google Scholar] [CrossRef]
- Xiang, T.; Zong, N.; Zou, Y.; Wu, Y.; Zhang, J.; Xing, W.; Li, Y.; Tang, X.; Zhu, L.; Chai, J. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr. Biol. 2008, 18, 74–80. [Google Scholar] [CrossRef]
- Rosebrock, T.R.; Zeng, L.; Brady, J.J.; Abramovitch, R.B.; Xiao, F.; Martin, G.B. A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 2007, 448, 370–374. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yamada, K.; Ishikawa, K.; Yoshimura, S.; Hayashi, N.; Uchihashi, K.; Ishihama, N.; Kishi-Kaboshi, M.; Takahashi, A.; Tsuge, S. A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity. Cell Host Microbe 2013, 13, 347–357. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Xiang, T.; Liu, Z.; Laluk, K.; Ding, X.; Zou, Y.; Gao, M.; Zhang, X.; Chen, S. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 2010, 7, 290–301. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Nakano, M.; Ichinose, Y.; Mukaihara, T. Ralstonia solanacearum type III effector RipAC targets SGT1 to suppress effector-triggered immunity. Plant Cell Physiol. 2020, 61, 2067–2076. [Google Scholar] [CrossRef]
- Qi, P.; Huang, M.; Hu, X.; Zhang, Y.; Wang, Y.; Li, P.; Chen, S.; Zhang, D.; Cao, S.; Zhu, W. A Ralstonia solanacearum effector targets TGA transcription factors to subvert salicylic acid signaling. Plant Cell 2022, 34, 1666–1683. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Deng, M.; Shen, D.; Dai, G.; Yao, N.; Lu, Y. The Ralstonia solanacearum effector RipAK suppresses plant hypersensitive response by inhibiting the activity of host catalases. Cell. Microbiol. 2017, 19, e12736. [Google Scholar] [CrossRef]
- Yu, G.; Xian, L.; Xue, H.; Yu, W.; Rufian, J.S.; Sang, Y.; Morcillo, R.J.; Wang, Y.; Macho, A.P. A bacterial effector protein prevents MAPK-mediated phosphorylation of SGT1 to suppress plant immunity. PLoS Pathog. 2020, 16, e1008933. [Google Scholar] [CrossRef]
- Nakano, M.; Oda, K.; Mukaihara, T. Ralstonia solanacearum novel E3 ubiquitin ligase (NEL) effectors RipAW and RipAR suppress pattern-triggered immunity in plants. Microbiology 2017, 163, 992–1002. [Google Scholar] [CrossRef]
- Niu, Y.; Fu, S.; Chen, G.; Wang, H.; Wang, Y.; Hu, J.; Jin, X.; Zhang, M.; Lu, M.; He, Y. Different epitopes of Ralstonia solanacearum effector RipAW are recognized by two Nicotiana species and trigger immune responses. Mol. Plant Pathol. 2022, 23, 188–203. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, J.; Sun, Z.; Wang, R.; Wu, X.; Li, B.; Song, C.; Liu, P.; Zhang, M. Ubiquitin E3 ligase activity of Ralstonia solanacearum effector RipAW is not essential for induction of plant defense in Nicotiana benthamiana. Front. Microbiol. 2023, 14, 1201444. [Google Scholar] [CrossRef]
- Peng, C.; Chen, J.-L.; Li, N.-N.; Zhang, S.-X.; Wang, R.-B.; Li, B.-J.; Liu, P.-Q.; An, Y.-Y.; Zhang, M.-X. Seedling Petri-dish inoculation method: A robust, easy-to-use and reliable assay for studying plant-Ralstonia solanacearum interactions. J. Integr. Agr. 2023, 22, 3709–3719. [Google Scholar] [CrossRef]
- Deng, W.-L.; Huang, H.-C. Cellular locations of Pseudomonas syringae pv. syringae HrcC and HrcJ proteins, required for harpin secretion via the type III pathway. J. Bacteriol. 1999, 181, 2298–2301. [Google Scholar] [CrossRef]
- Etchebar, C.; Trigalet-Demery, D.; van Gijsegem, F.; Vasse, J.; Trigalet, A. Xylem colonization by an HrcV mutant of Ralstonia solanacearum is a key factor for the efficient biological control of tomato bacterial wilt. Mol. Plant Microbe Interact. 1998, 11, 869–877. [Google Scholar] [CrossRef]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Shirasu, K.; Deng, X.W. The diverse roles of ubiquitin and the 26S proteasome in the life of plants. Nat. Rev. Genet. 2003, 4, 948–958. [Google Scholar] [CrossRef]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef]
- Sang, Y.; Yu, W.; Zhuang, H.; Wei, Y.; Derevnina, L.; Yu, G.; Luo, J.; Macho, A.P. Intra-strain elicitation and suppression of plant immunity by Ralstonia solanacearum type-III effectors in Nicotiana benthamiana. Plant Commun. 2020, 1, 100025. [Google Scholar] [CrossRef]
- Nakano, M.; Mukaihara, T. The type III effector RipB from Ralstonia solanacearum RS1000 acts as a major avirulence factor in Nicotiana benthamiana and other Nicotiana species. Mol. Plant Pathol. 2019, 20, 1237–1251. [Google Scholar] [CrossRef]
- Kim, B.; Kim, I.; Yu, W.; Li, M.; Kim, H.; Ahn, Y.J.; Sohn, K.H.; Macho, A.P.; Segonzac, C. The Ralstonia pseudosolanacearum effector RipE1 is recognized at the plasma membrane by NbPtr1, the Nicotiana benthamiana homologue of Pseudomonas tomato race 1. Mol. Plant Pathol. 2023, 24, 1312–1318. [Google Scholar] [CrossRef]
- Sang, Y.; Wang, Y.; Ni, H.; Cazalé, A.C.; She, Y.M.; Peeters, N.; Macho, A.P. The Ralstonia solanacearum type III effector RipAY targets plant redox regulators to suppress immune responses. Mol. Plant Pathol. 2018, 19, 129–142. [Google Scholar] [CrossRef]
- Cheng, D.; Zhou, D.; Wang, Y.; Wang, B.; He, Q.; Song, B.; Chen, H. Ralstonia solanacearum type III effector RipV2 encoding a novel E3 ubiquitin ligase (NEL) is required for full virulence by suppressing plant PAMP-triggered immunity. Biochem. Bioph. Res. Commun. 2021, 550, 120–126. [Google Scholar] [CrossRef]
- Tena, G.; Asai, T.; Chiu, W.-L.; Sheen, J. Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 2001, 4, 392–400. [Google Scholar] [CrossRef]
- Vailleau, F.; Sartorel, E.; Jardinaud, M.-F.; Chardon, F.; Genin, S.; Huguet, T.; Gentzbittel, L.; Petitprez, M. Characterization of the interaction between the bacterial wilt pathogen Ralstonia solanacearum and the model legume plant Medicago truncatula. Mol. Plant Microbe Interact. 2007, 20, 159–167. [Google Scholar] [CrossRef]
- Remigi, P.; Anisimova, M.; Guidot, A.; Genin, S.; Peeters, N. Functional diversification of the GALA type III effector family contributes to Ralstonia solanacearum adaptation on different plant hosts. New Phytol. 2011, 192, 976–987. [Google Scholar] [CrossRef]
- Wang, L.; Yu, G.; Macho, A.P.; Lozano-Durán, R. Split-luciferase complementation imaging assay to study protein-protein interactions in Nicotiana benthamiana. Bio-Protocol 2021, 11, e4237. [Google Scholar] [CrossRef]
- Cui, X.; Fan, B.; Scholz, J.; Chen, Z. Roles of Arabidopsis cyclin-dependent kinase C complexes in cauliflower mosaic virus infection, plant growth, and development. Plant Cell 2007, 19, 1388–1402. [Google Scholar] [CrossRef]
- Lajeunesse, G.; Roussin-Léveillée, C.; Boutin, S.; Fortin, É.; Laforest-Lapointe, I.; Moffett, P. Light prevents pathogen-induced aqueous microenvironments via potentiation of salicylic acid signaling. Nat. Commun. 2023, 14, 713. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Xie, Q. Plant Signalling Networks: Methods and Protocols, 1st ed.; Springer Link: Heidelberg, Germany, 2012; pp. 153–162. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.-M.; Zhang, Q.; Feng, Y.-X.; Zhang, S.-X.; Bai, B.-X.; Ouyang, X.; Xiao, Z.-L.; Meng, H.; Wang, X.-T.; He, J.-M.; et al. The Ralstonia solanacearum Type III Effector RipAW Targets the Immune Receptor Complex to Suppress PAMP-Triggered Immunity. Int. J. Mol. Sci. 2024, 25, 183. https://doi.org/10.3390/ijms25010183
Sun Z-M, Zhang Q, Feng Y-X, Zhang S-X, Bai B-X, Ouyang X, Xiao Z-L, Meng H, Wang X-T, He J-M, et al. The Ralstonia solanacearum Type III Effector RipAW Targets the Immune Receptor Complex to Suppress PAMP-Triggered Immunity. International Journal of Molecular Sciences. 2024; 25(1):183. https://doi.org/10.3390/ijms25010183
Chicago/Turabian StyleSun, Zhi-Mao, Qi Zhang, Yu-Xin Feng, Shuang-Xi Zhang, Bi-Xin Bai, Xue Ouyang, Zhi-Liang Xiao, He Meng, Xiao-Ting Wang, Jun-Min He, and et al. 2024. "The Ralstonia solanacearum Type III Effector RipAW Targets the Immune Receptor Complex to Suppress PAMP-Triggered Immunity" International Journal of Molecular Sciences 25, no. 1: 183. https://doi.org/10.3390/ijms25010183
APA StyleSun, Z.-M., Zhang, Q., Feng, Y.-X., Zhang, S.-X., Bai, B.-X., Ouyang, X., Xiao, Z.-L., Meng, H., Wang, X.-T., He, J.-M., An, Y.-Y., & Zhang, M.-X. (2024). The Ralstonia solanacearum Type III Effector RipAW Targets the Immune Receptor Complex to Suppress PAMP-Triggered Immunity. International Journal of Molecular Sciences, 25(1), 183. https://doi.org/10.3390/ijms25010183






