Effects of Argon Gas Plasma Treatment on Biocompatibility of Nanostructured Titanium
Abstract
:1. Introduction
2. Results
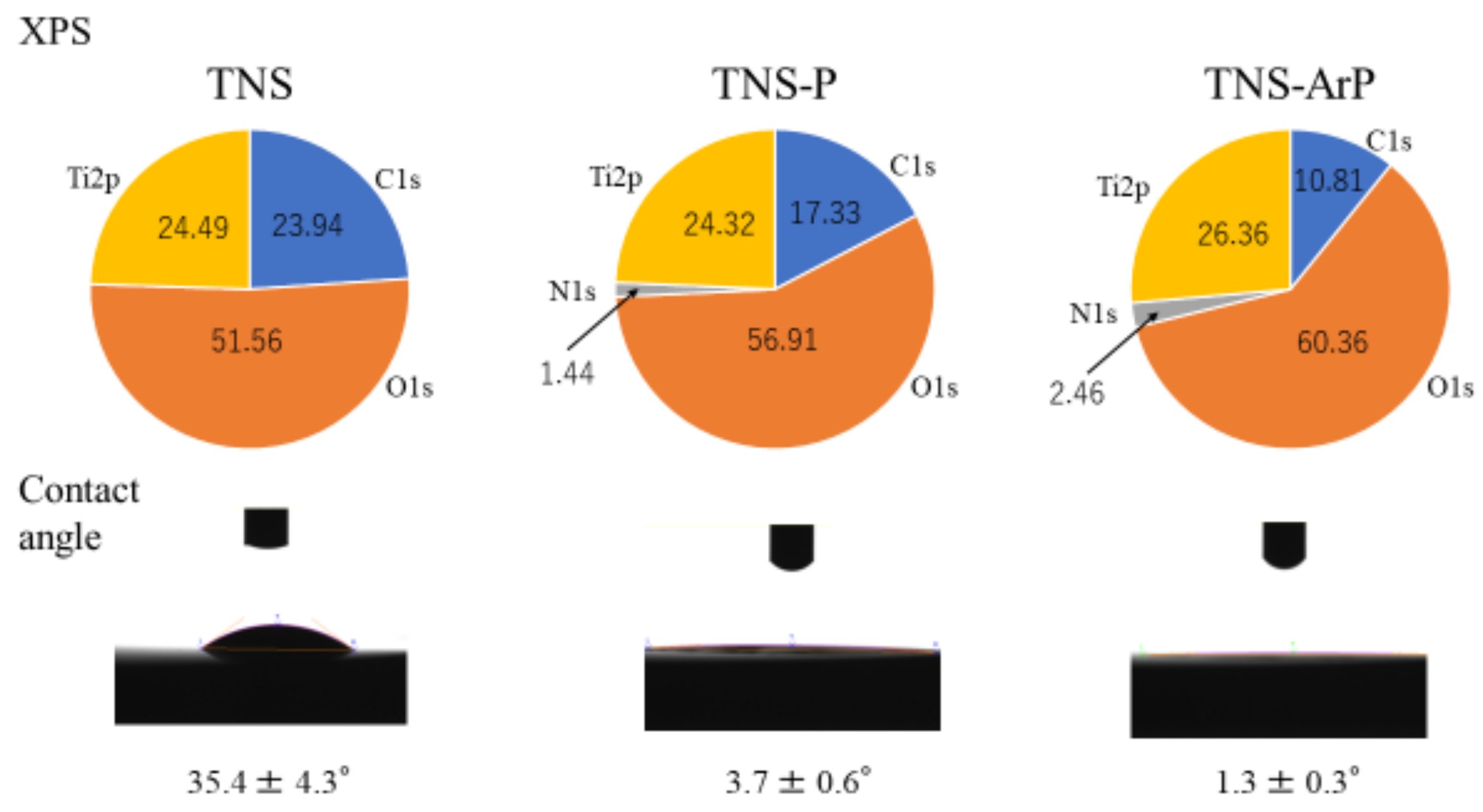
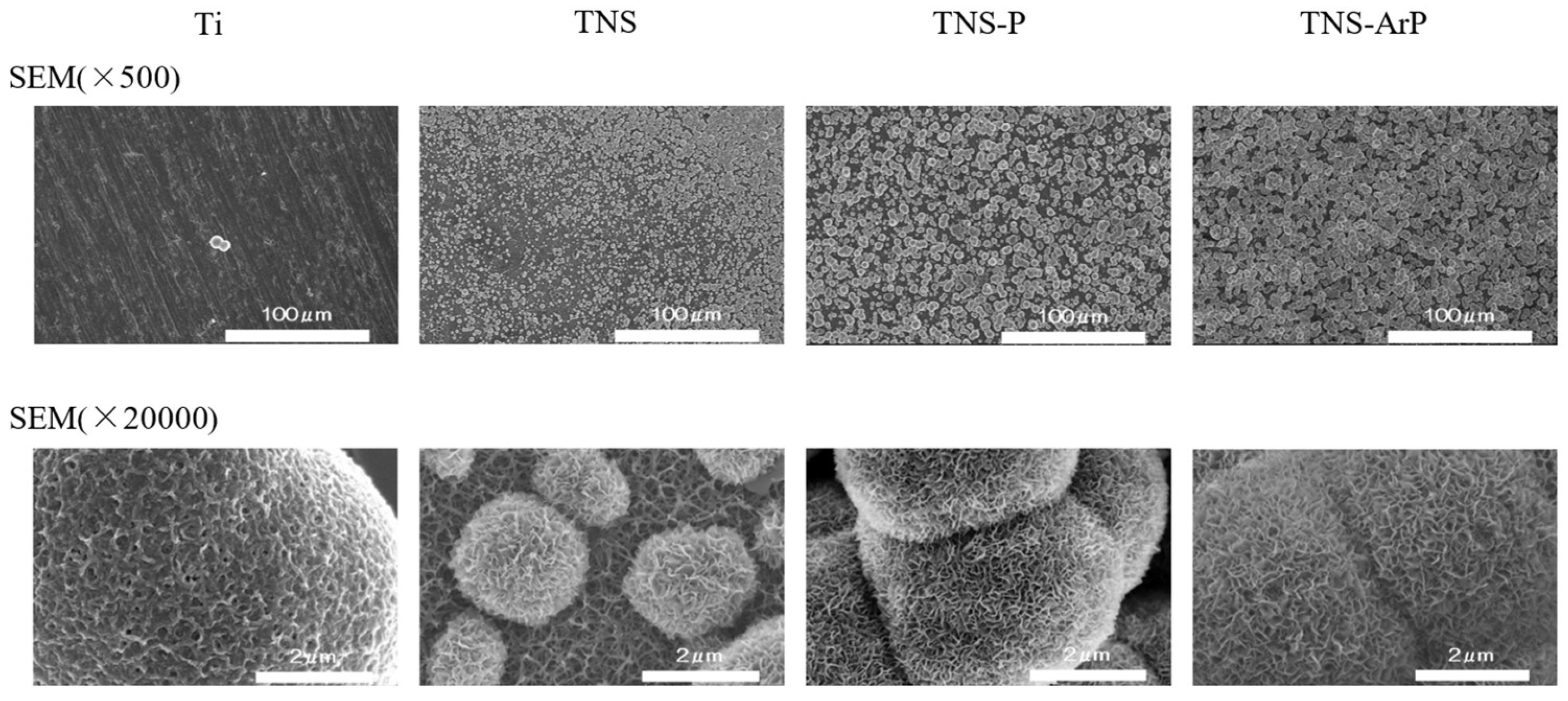
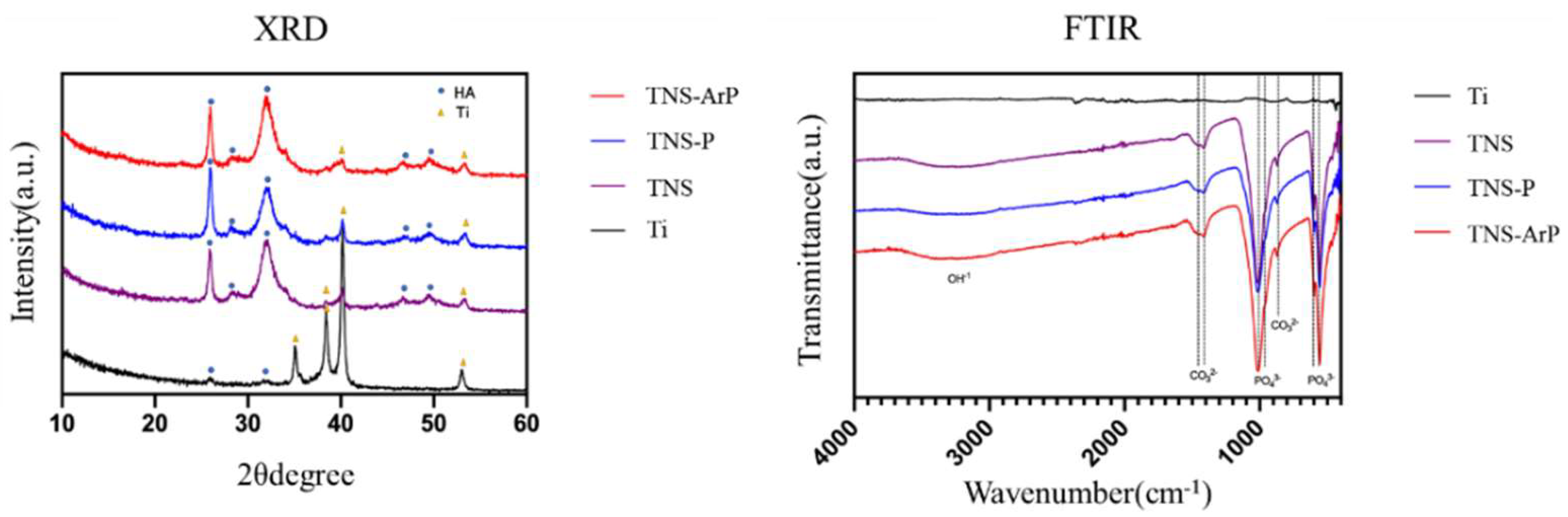
2.1. Surface Characterization
2.2. Evaluation of Protein Adsorption on the Plasma and Nano-Modified Titanium Surface
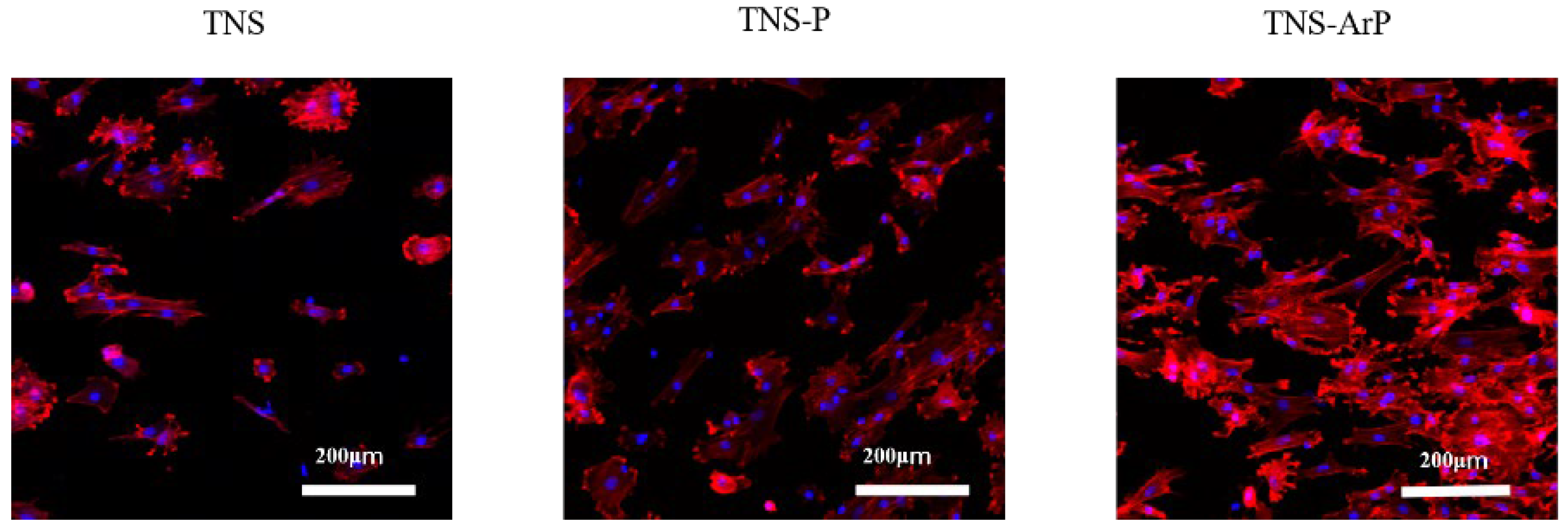
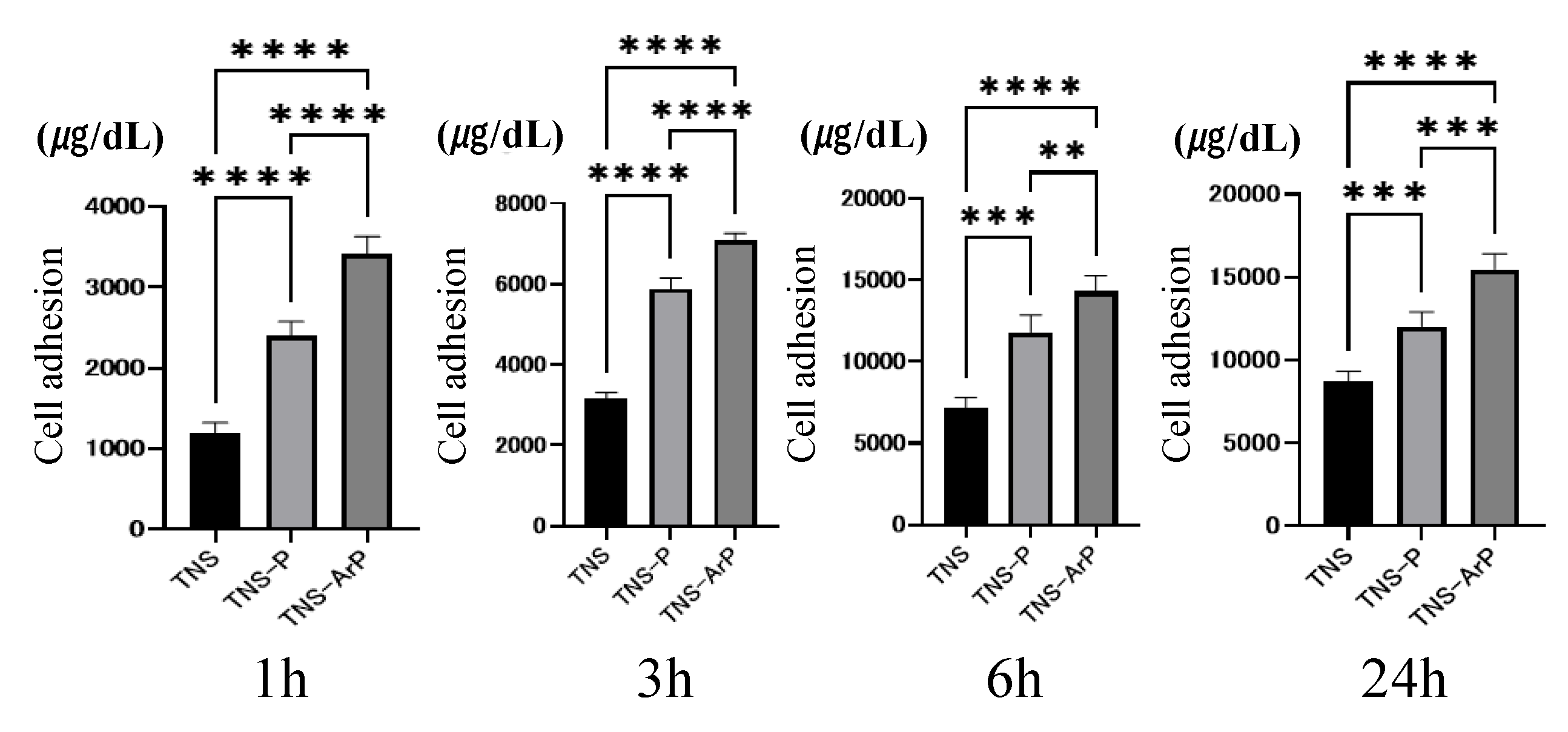
2.3. Effects of Plasma and Nano-Modified Titanium Surfaces on Cell Adhesion and Morphology in RBMCs
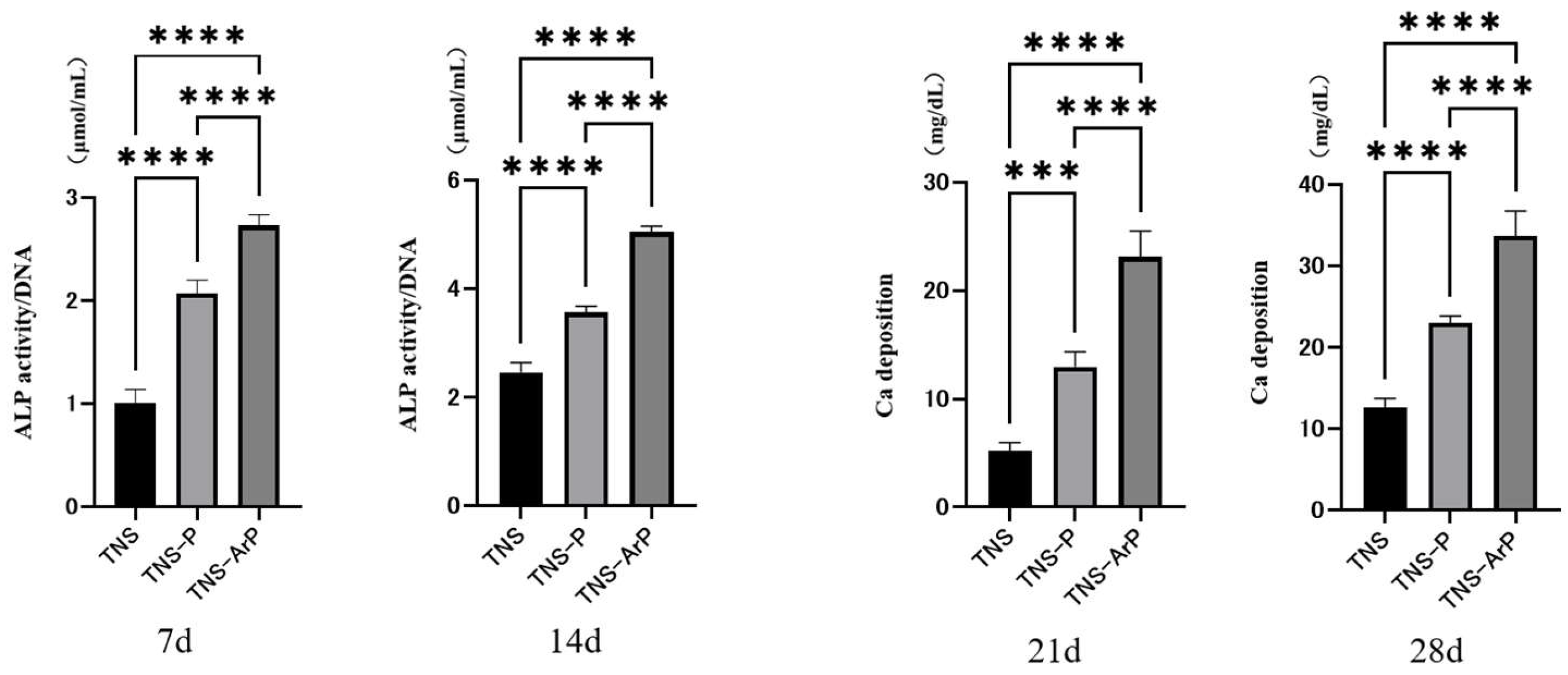
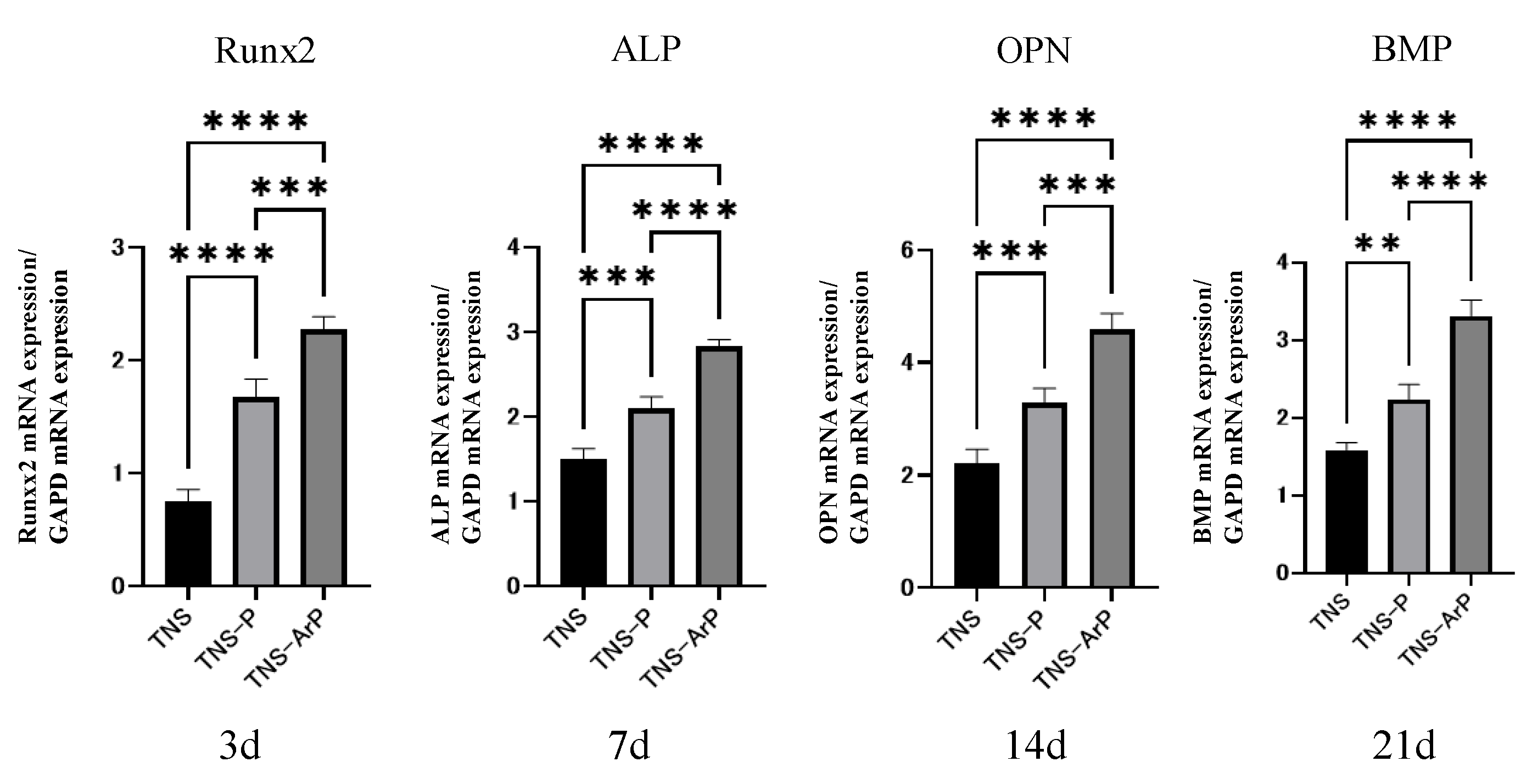
2.4. In Vitro Argon Plasma Treatment-Induced Bone Differentiation on the Nano-Modified Titanium Surface
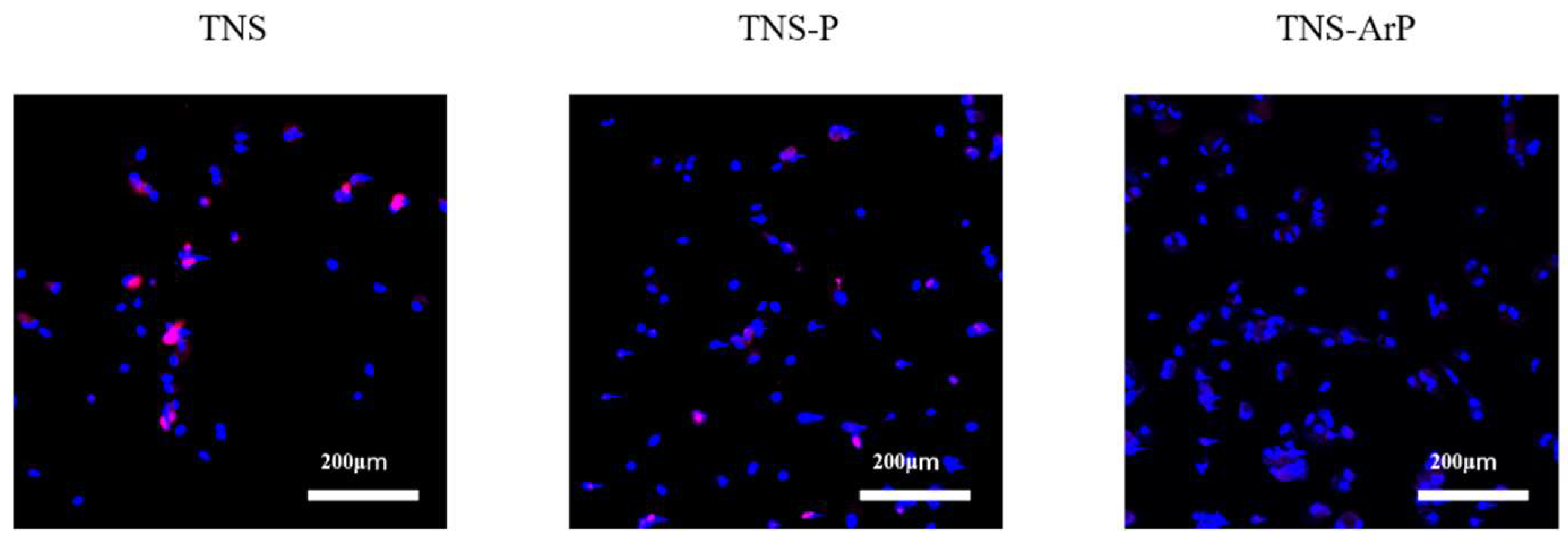
2.5. Cell Intracellular Reactive Oxygen Species (ROS) Level of RBMCs on Nano-Modified Titanium Surface
2.6. Simulated Body Fluid Immersion Experiment
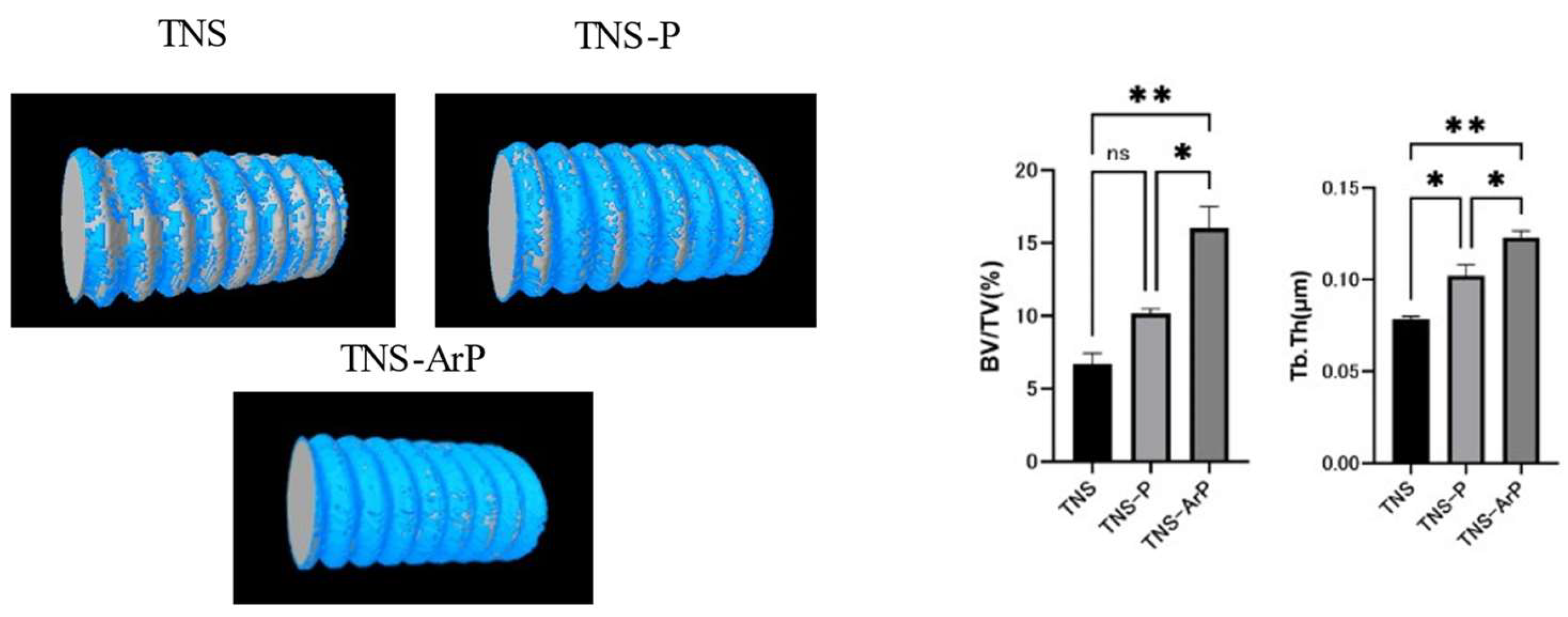
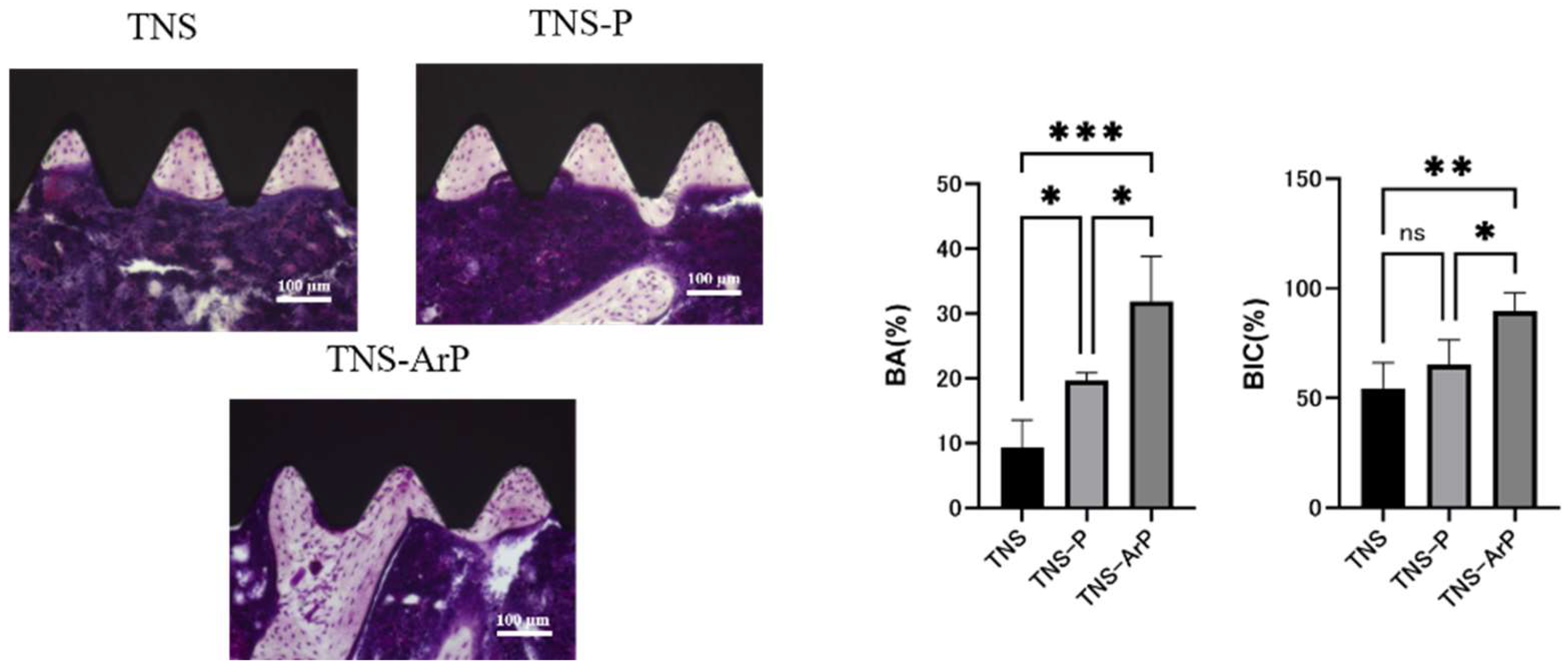
2.7. Evaluation of the Amount of New Bone Formation in the Tissue Surrounding the Nano-Modified Titanium Implant Placement In Vivo
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Surface Characterization
4.3. Protein Adsorption
4.4. Cell Culture
4.5. Cell Adhesion
4.6. Quantitative Reverse Transcription Polymerase Chain Reaction (Qrt-Pcr), Alp Activity, Dna Content, and Mineralization Determination
4.7. Intracellular ROS Levels in Rbmcs
4.8. Simulated Body Fluid (SBF) Test
4.9. Animal Model and Surgical Procedures
4.10. In Vivo Argon Plasma-Induced Bone Differentiation on the TNS-Modified Titanium Surface
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moradian-Oldak, J.; Wen, H.B.; Schneider, G.B.; Stanford, C.M. Tissue engineering strategies for the future generation of dental implants. Periodontology 2000 2006, 41, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Carlos, O.; Alejandro, D. Titanium as a Biomaterial for Implants. J. Arthroplast. 2012, 218, 149–162. [Google Scholar]
- John, W.N. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar]
- Romeo, E.; Storelli, S. Systematic review of the survival rate and the biological, technical, and aesthetic complications of fixed dental prostheses with cantilevers on implants reported in longitudinal studies with a mean of 5 years follow-up. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 39–49. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implant. 2003, 18, 200–210. [Google Scholar]
- Ogawa, T.; Ozawa, S.; Shih, J.H.; Ryu, K.H.; Sukotjo, C.; Yang, J.M.; Nishimura, I. Biomechanical evaluation of osseous implants having different surface topographies in rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef]
- Takeuchi, K.; Saruwatari, L.; Nakamura, H.K.; Yang, J.M.; Ogawa, T. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J. Biomed. Mater. Res. A 2005, 72, 296–305. [Google Scholar] [CrossRef]
- Xuanyong, L.; Paul, K.C.; Chuanxian, D. Surface modification of titanium, titanium alloys, and related materials for bio-medical applications. Mater. Sci. Eng. R. Rep. 2004, 47, 49–121. [Google Scholar]
- Ulrike, D. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar]
- Vandrovcová, M.; Bačáková, L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Komasa, S.; Taguchi, Y.; Nishida, H.; Tanaka, M.; Kawazoe, T. Bioactivity of nanostructure on titanium surface modified by chemical processing at room temperature. J. Prosthodont. Res. 2012, 56, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Taguchi, Y.; Komasa, S.; Sekino, T.; Tanaka, M. Cell differentiation on nanoscale features of a titanium surface: Effects of deposition time in NaOH solution. J. Hard Tissue Biol. 2014, 23, 63–70. [Google Scholar] [CrossRef]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K.K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Oliveira, M.; Radi, P.; Reis, D.; Reis, A. Titanium Bioactive Surface Formation Via Alkali and Heat Treatments for Rapid Osseointegration. J. Mater. Res. 2021, 24, 20200514. [Google Scholar] [CrossRef]
- Mohamed, M.; Samy, E.; Eman, E. Effect of alkaline treatment with sodium hydroxide on wettability and bioactivity of some commercial dental implants: An in-vitro study. Tanta. Dent. J. 2022, 19, 140–145. [Google Scholar]
- Umehara, H.; Doi, K.; Oki, Y.; Kobatake, R.; Makihara, Y.; Kubo, T.; Tsuga, K. Development of a novel bioactive titanium membrane with alkali treatment for bone regeneration. Dent. Mater. J. 2020, 39, 877–882. [Google Scholar] [CrossRef]
- Seyda, V.; Niko, K.; Claus, E. Investigation of aging processes of Ti-6Al-4 V powder material in laser melting. Phys. Procedia 2012, 39, 425–431. [Google Scholar] [CrossRef]
- Wang, K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. A 1996, 213, 134–137. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and surface free energy of graphene films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef] [PubMed]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Ohko, Y.; Hashimoto, K.; Fujishima, A. Kinetics of photocatalytic reactions under extremely low-intensity UV illumination on titanium dioxide thin films. J. Phys. Chem. A 1997, 101, 8057–8062. [Google Scholar] [CrossRef]
- Park, J.H.; Schwartz, Z.; Olivares-Navarrete, R.; Boyan, B.D.; Tannenbaum, R. Enhancement of surface wettability via the modification of microtextured titanium implant surfaces with polyelectrolytes. Langmuir 2011, 27, 5976–5985. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef]
- Hatoko, M.; Komasa, S.; Zhang, H.; Sekino, T.; Okazaki, J. UV treatment improves the biocompatibility and antibacterial properties of crystallized nanostructured titanium surface. Int. J. Mol. Sci. 2019, 20, 5991. [Google Scholar] [CrossRef]
- Ujino, D.; Nishizaki, H.; Higuchi, S.; Komasa, S.; Okazaki, J. Effect of plasma treatment of titanium surface on biocompatibility. Appl. Sci. 2019, 9, 2257. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tashiro, Y.; Komasa, S.; Miyake, A.; Komasa, Y.; Okazaki, J. Effects of surface modification on adsorption behavior of cell and protein on titanium surface by using quartz crystal microbalance system. Materials 2021, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Takao, S.; Komasa, S.; Agariguchi, A.; Kusumoto, T.; Pezzotti, G.; Okazaki, J. Effects of plasma treatment on the bioactivity of alkali-treated ceria-stabilised Zirconia/Alumina nanocomposite (NANOZR). Int. J. Mol. Sci. 2020, 21, 7476. [Google Scholar] [CrossRef] [PubMed]
- Komasa, S.; Takao, S.; Yang, Y.; Zeng, Y.; Li, M.; Yan, S.; Zhang, H.; Komasa, C.; Kobayashi, Y.; Nishizaki, H. Effects of UV treatment on ceria-stabilized zirconia/alumina nanocomposite (NANOZR). Materials 2020, 13, 2772. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Barreto, A.; Seixas, R.; Paes, P.; Lunz, J.; Thire, R.; Jardim, P. Novel Strategy for Surface Modification of Titanium Implants towards the Improvement of Osseointegration Property and Antibiotic Local Delivery. Materials 2023, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of argon affects the earliest biological response of different implant surfaces: An in vitro comparative study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Zhao, H.; Liu, Y.; Liu, J.; Bai, N. Bioactive Effects of Low-Temperature Argon–Oxygen Plasma on a Titanium Implant Surface. ACS Omega 2020, 5, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Wang, H.L.; Carossa, S.; Mussano, F. Plasma of argon increases cell attachment and bacterial decontamination on different implant surfaces. Int. J. Oral Maxillofac. Implant. 2017, 32, 1315–1323. [Google Scholar] [CrossRef]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. A 2012, 100, 1901–1906. [Google Scholar] [CrossRef]
- Carossa, M.; Cavagnetto, D.; Mancini, F.; Balma, A.; Mussano, F. Plasma of Argon Treatment of the Implant Surface, Systematic Review of In Vitro Studies. Biomolecules 2022, 12, 1219. [Google Scholar] [CrossRef]
- Canullo, L.; Rakic, M.; Corvino, E.; Burton, M.; Krumbeck, J.; Prem, A.; Ravida, A.; Ignjatovic, N.; Sculean, A.; Menini, M.; et al. Effect of argon plasma pre-treatment of healing abutments on peri-implant microbiome and soft tissue integration: A proof-of-concept randomized study. BMC Oral Health 2023, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Donato, A.; Savadori, P.; Radovanovic, S.; Lacono, R.; Rakic, M. Effect of argon plasma abutment activation on soft tissue healing: RCT with histological assessment. Clin. Implant. Dent. Relat. Res. 2023, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.J.; Kim, H.J.; Kim, A.J.; Bae, Y.S.; Bae, S.S.; Yun, J. UV light induces premature senescence in Akt1-null mouse em-bryonic fibroblasts by increasing intracellular levels of ROS. Biochem. Biophys. Res. Commun. 2009, 383, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Scharffetter-Kochanek, K.; Wlaschek, M.; Brenneisen, P.; Schauen, M.; Blaudschun, R.; Wenk, J. UV-induced reactive ox-ygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997, 378, 1247–1257. [Google Scholar] [PubMed]
- Tang, K.; Zhan, J.C.; Yang, H.R.; Huang, W.D. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Amir, Z. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar]
- Bharati, S.; Mithiles, K.S.; Basu, D. Hydroxyapatite coating by biomimetic method on titanium alloy using concentrated SBF. Bulletin. Mater. Sci. 2005, 28, 617–621. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayed hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Liang, F.; Zhou, L.; Wang, K. Apatite formation on porous titanium by alkali and heat-treatment. Surf. Coat. Tech. 2003, 165, 133–139. [Google Scholar] [CrossRef]
- Jonášová, L.; Muller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Hydroxyapatite formation on alkali-treated titanium with different content of Na+ in the surface layer. Biomaterials 2002, 23, 3095–3101. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Li, Y.C.; Hodgson, P.D.; Wen, C. The importance of particle size in porous titanium and nonporous counter-parts for surface energy and its impact on apatite formation. Acta Biomater. 2009, 5, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Hieda, J.; Sakaguchi, A.; Nakano, M.; Akasaka, H.; Ohtake, N. Relationships between surface energy and charge of sur-face-modified titanium and HAp formation. Appl. Surf. Sci. 2019, 465, 509–516. [Google Scholar] [CrossRef]
- Hayakawa, S.; Matsumoto, Y.; Uetsuki, K.; Shirosaki, Y.; Osaka, A. In vitro apatite formation on nano-crystalline titania layer aligned parallel to Ti6Al4V alloy substrates with sub-millimeter gap. J. Mater. Sci. Mater. Med. 2015, 26, 190. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Okamoto, K.; Yoshioka, T. Accelerated induction of in vitro apatite formation by parallel alignment of hydrothermally oxidized titanium substrates separated by sub-millimeter gaps. J. Asian Ceram. Soc. 2019, 7, 90–100. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Nishimura, D.; Doi, H.; Nomura, N.; Hanawa, T. Difference in surface reactions between titanium and zirconium in Hanks’ solution to elucidate mechanism of calcium phosphate formation on titanium using XPS and cathodic polarization. Mater. Sci. Eng. C 2009, 29, 1702–1708. [Google Scholar] [CrossRef]
- Wei, Y.; Jing, Q.; Ling, X.; Fu, Z. Corrosion behaviors of TiO2 nanotube layers on titanium in Hank’s solution. Biomed. Mater. 2009, 4, 065012. [Google Scholar]
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M.; et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration–communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Zhong, J.; Li, X.; Yao, Y.; Zhou, J.; Cao, S.; Zhang, X.; Jian, Y.; Zhao, K. Effect of acid-alkali treatment on serum protein adsorption and bacterial adhesion to porous titanium. J. Mater. Sci. Mater. Med. 2022, 33, 20. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, R.; Takao, S.; Komasa, S.; Sekino, T.; Kusumoto, T.; Maekawa, K. Effects of Argon Gas Plasma Treatment on Biocompatibility of Nanostructured Titanium. Int. J. Mol. Sci. 2024, 25, 149. https://doi.org/10.3390/ijms25010149
Hayashi R, Takao S, Komasa S, Sekino T, Kusumoto T, Maekawa K. Effects of Argon Gas Plasma Treatment on Biocompatibility of Nanostructured Titanium. International Journal of Molecular Sciences. 2024; 25(1):149. https://doi.org/10.3390/ijms25010149
Chicago/Turabian StyleHayashi, Rina, Seiji Takao, Satoshi Komasa, Tohru Sekino, Tetsuji Kusumoto, and Kenji Maekawa. 2024. "Effects of Argon Gas Plasma Treatment on Biocompatibility of Nanostructured Titanium" International Journal of Molecular Sciences 25, no. 1: 149. https://doi.org/10.3390/ijms25010149
APA StyleHayashi, R., Takao, S., Komasa, S., Sekino, T., Kusumoto, T., & Maekawa, K. (2024). Effects of Argon Gas Plasma Treatment on Biocompatibility of Nanostructured Titanium. International Journal of Molecular Sciences, 25(1), 149. https://doi.org/10.3390/ijms25010149





