In Vivo Functional Assay in Fish Gills: Exploring Branchial Acid-Excreting Mechanisms in Zebrafish
Abstract
1. Introduction
2. Results
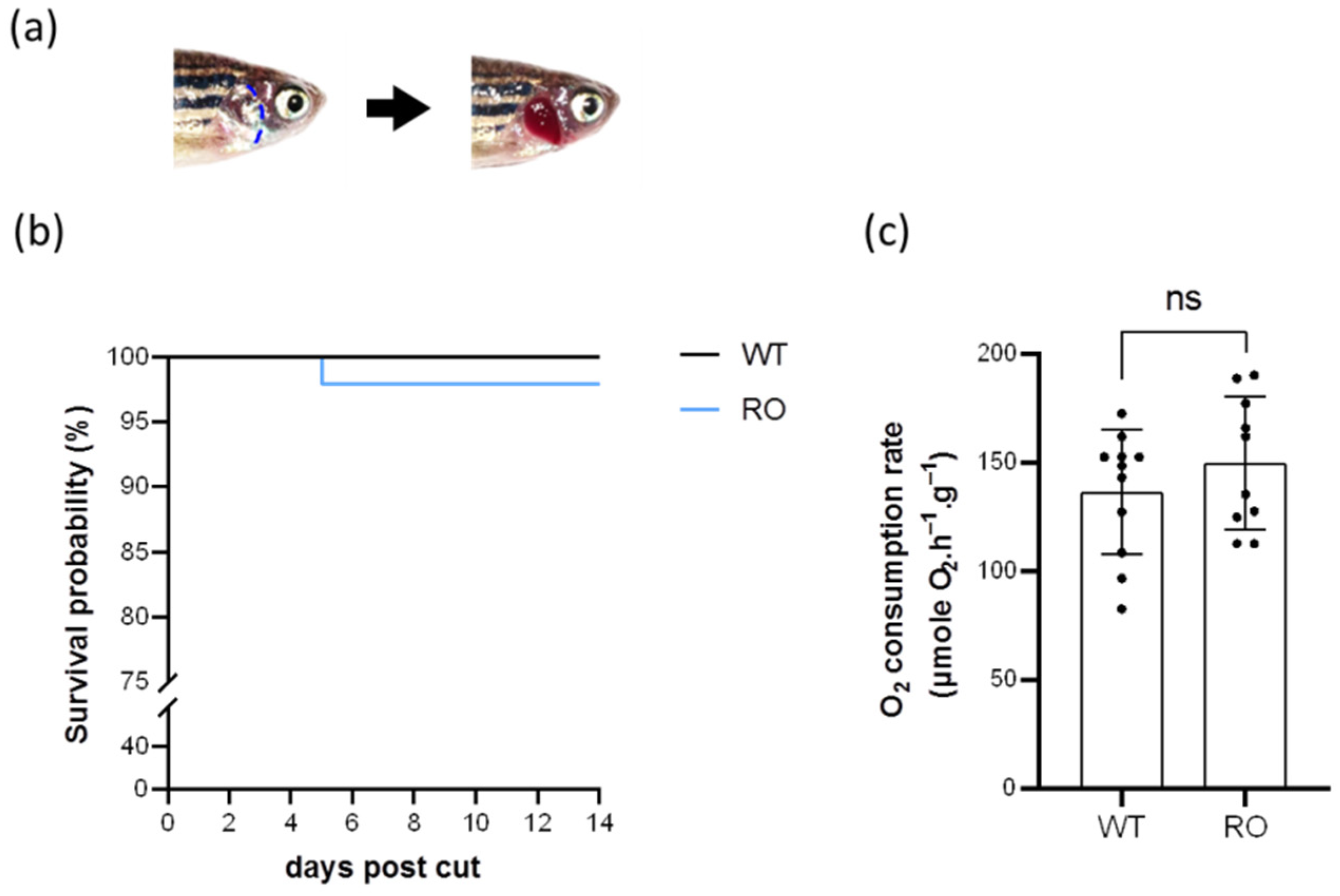
2.1. Removal of the Opercula Did Not Contribute to High Mortality or Abnormal Oxygen Consumption
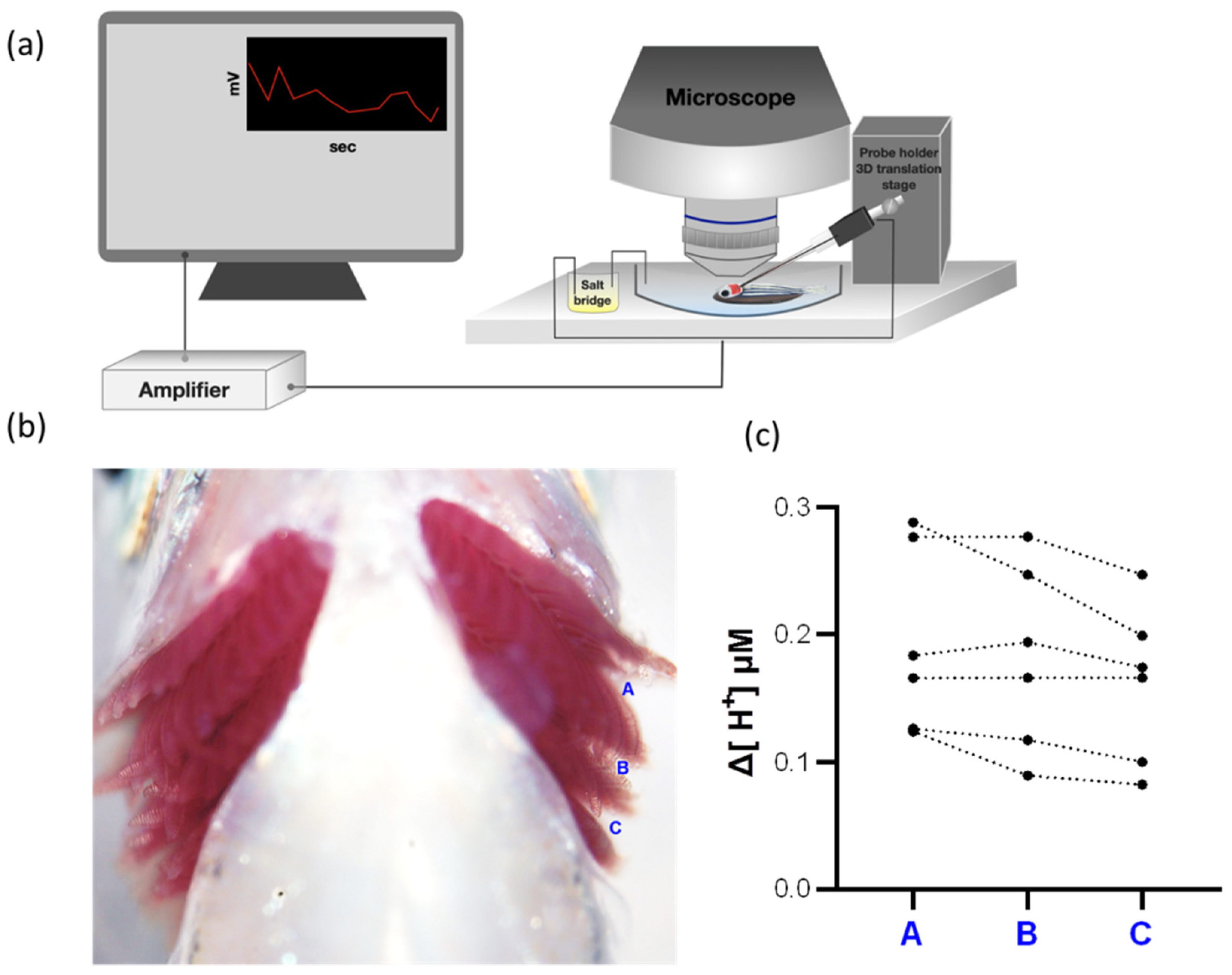
2.2. H+ Activities Could Be Detected at Different Positions in the Gills
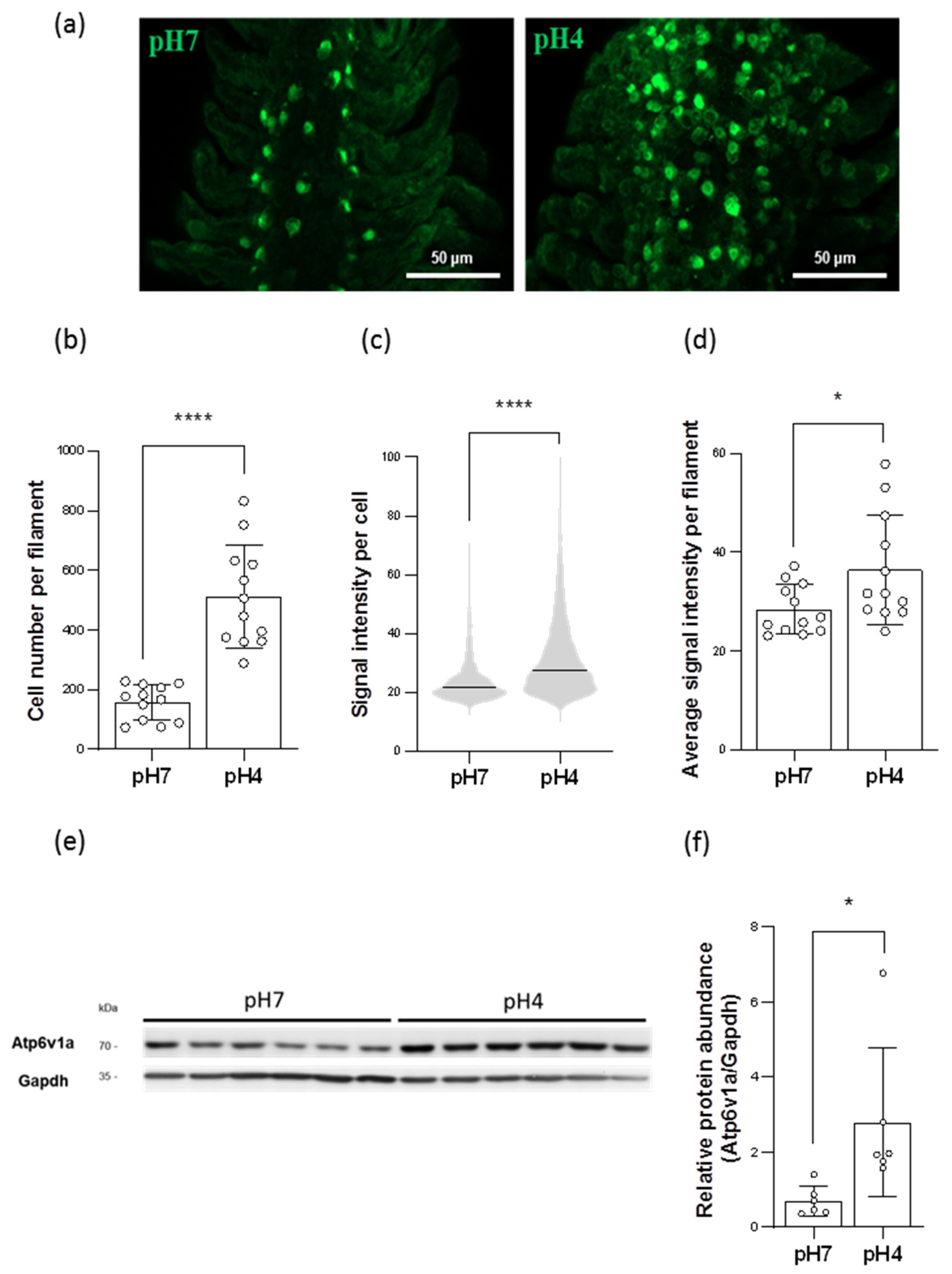
2.3. Acidic Environments Increased the Number of HR Ionocytes
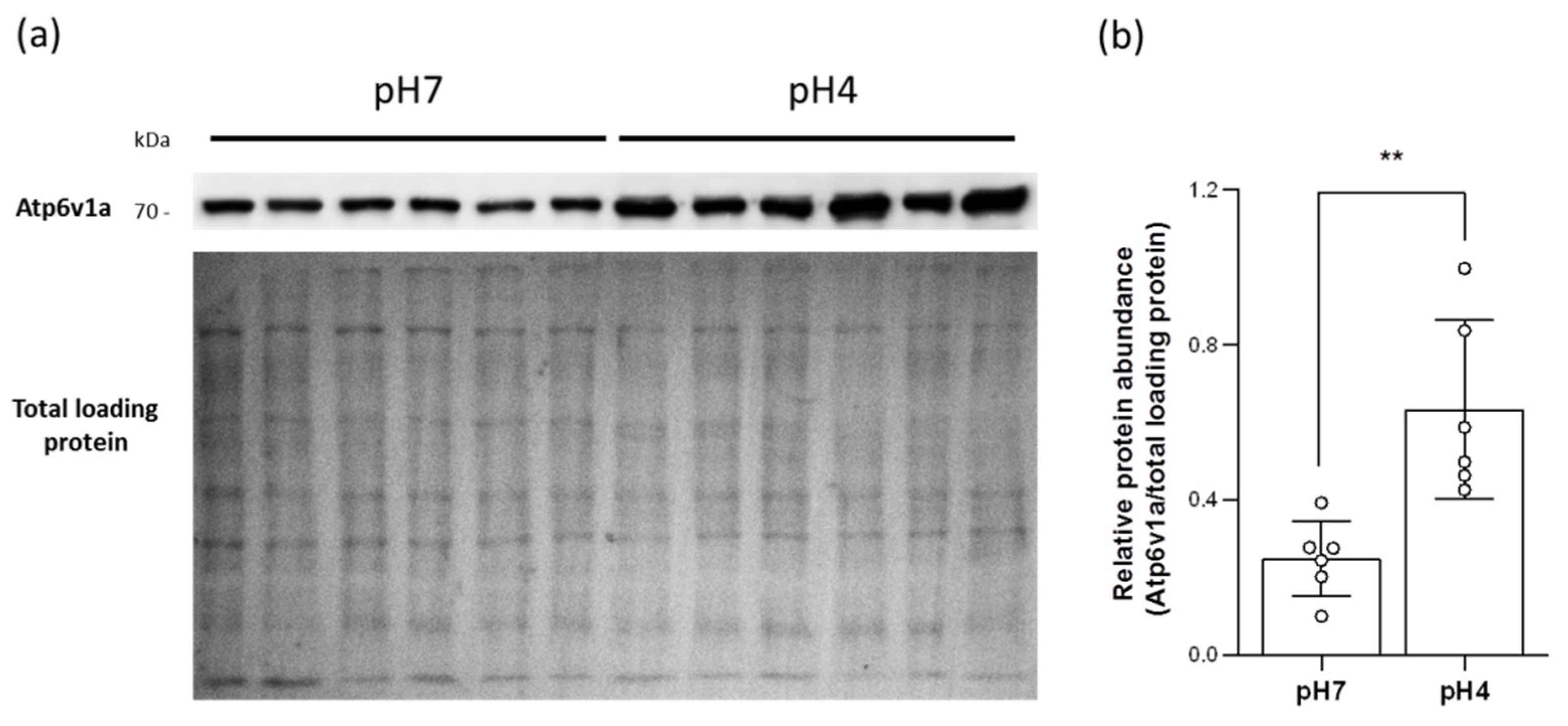
2.4. Acidic Environments Increased the Expression of HA and Other H+ Excretion-Related Transporters/Enzymes
2.5. Acidic Environments Promote Plasma Membrane HA Accumulation
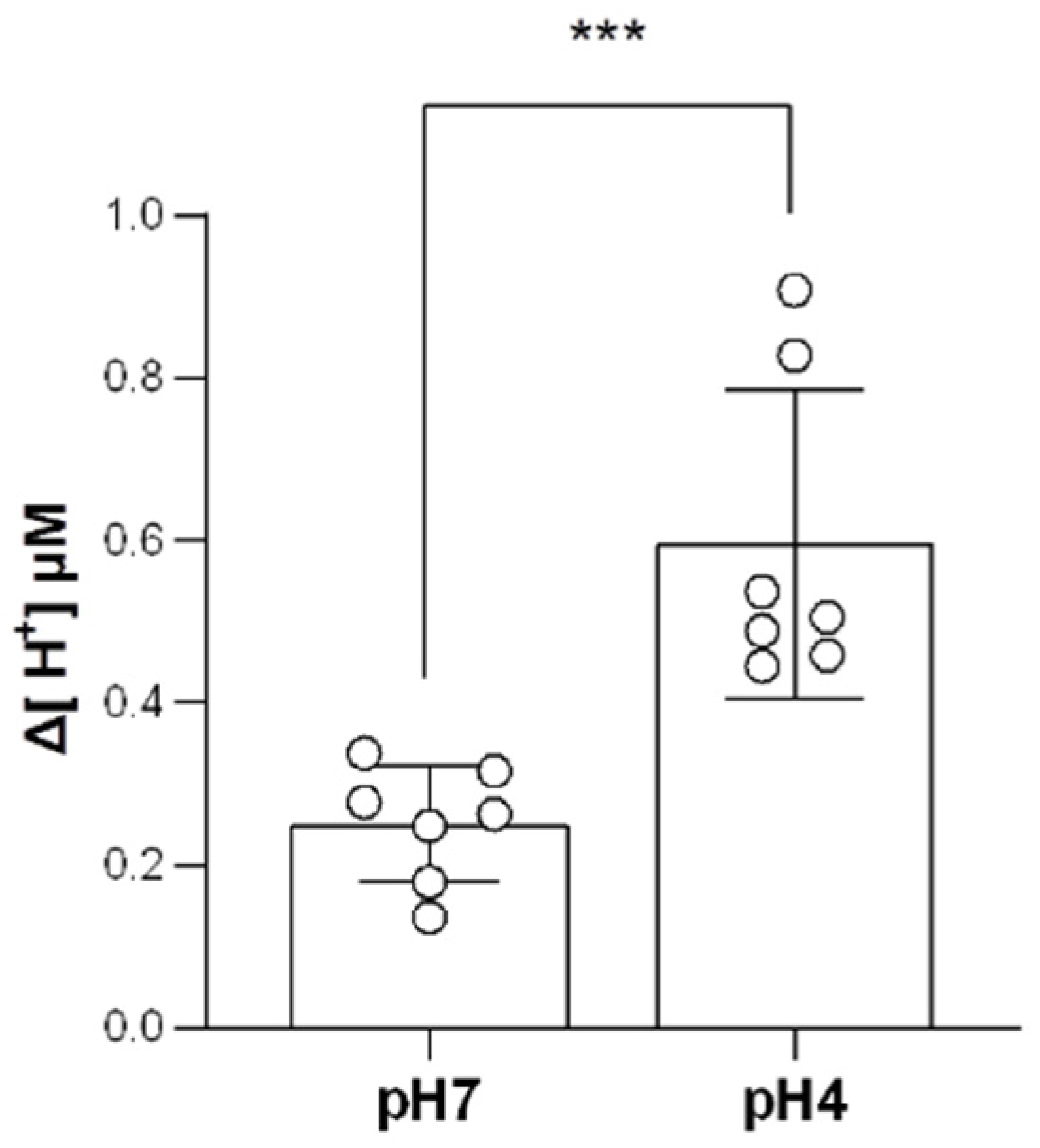
2.6. Branchial H+-Excreting Capacity Was Elevated after Acid Acclimation
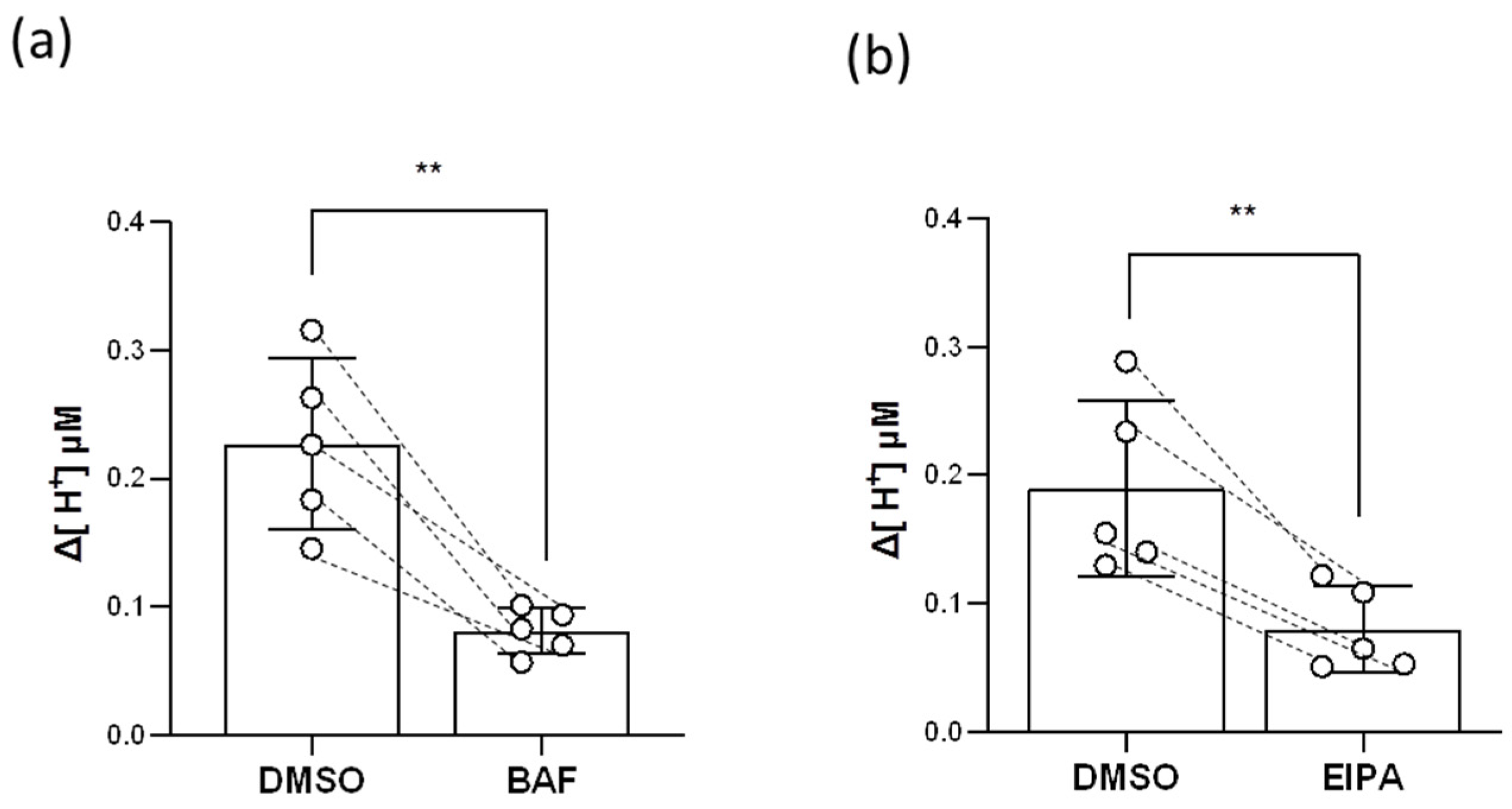
2.7. HR Ionocytes Excreted H+ through HA and Nhe
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Measurements of the Oxygen Consumption Rate
4.3. Acid Acclimation Experiment
4.4. Complementary DNA (cDNA) Preparation
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Immunofluorescence (IF)
4.7. Western Blot
4.8. Scanning Ion-Selective Electrode Technique (SIET)
4.9. Pharmacological Treatments
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ae1b | Anion exchanger 1b |
| BAF | Bafilomycin A1 |
| Ca2 | Carbonic anhydrase 2 |
| EIPA | 5-ethylisopropyl amiloride |
| FW | Freshwater |
| HA | H+-ATPase |
| HR | H+-ATPase-rich |
| IF | Immunofluorescence |
| Nhe | Na+/H+ exchanger |
| Rhcg2 | Rhesus C glycoprotein 2 |
| RO | Removal of the opercula |
| SIET | Scanning ion-selective electrode technique |
| SW | Seawater |
References
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 28–47. [Google Scholar] [CrossRef]
- Takei, Y.; Hiroi, J.; Takahashi, H.; Sakamoto, T. Diverse mechanisms for body fluid regulation in teleost fishes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Lee, T.H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D.; Bradshaw, D. Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 2006, 147, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, J.B.; Edwards, S.L.; Morrison-Shetlar, A.I. Acid-base regulation in fishes: Cellular and molecular mechanisms. J. Exp. Zool. 2002, 293, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.C.; Yan, J.J.; Furukawa, F.; Hwang, P.P. Did acidic stress resistance in vertebrates evolve as Na+/H+ exchanger-mediated ammonia excretion in fish? BioEssays 2020, 42, 1900161. [Google Scholar] [CrossRef]
- Yan, J.J.; Chou, M.Y.; Kaneko, T.; Hwang, P.P. Gene expression of Na+/H+ exchanger in zebrafish H+-ATPase-rich cells during acclimation to low-Na+ and acidic environments. Am. J. Physiol. Cell Physiol. 2007, 293, 1814–1823. [Google Scholar] [CrossRef]
- Lin, L.Y.; Horng, J.L.; Kunkel, J.G.; Hwang, P.P. Proton pump-rich cell secretes acid in skin of zebrafish larvae. Am. J. Physiol. Cell Physiol. 2006, 290, 371–378. [Google Scholar] [CrossRef]
- Guh, Y.J.; Tseng, Y.C.; Yang, C.Y.; Hwang, P.P. Endothelin-1 regulates H+-ATPase-dependent transepithelial H+ secretion in zebrafish. Endocrinology 2014, 155, 1728–1737. [Google Scholar] [CrossRef][Green Version]
- Chang, W.J.; Wang, Y.F.; Hu, H.J.; Wang, J.H.; Lee, T.H.; Hwang, P.P. Compensatory regulation of Na+ absorption by Na+/H+ exchanger and Na+-Cl− cotransporter in zebrafish (Danio rerio). Front Zool. 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Yan, J.J.; Cruz, S.A.; Horng, J.L.; Hwang, P.P. Anion exchanger 1b, but not sodium-bicarbonate cotransporter 1b, plays a role in transport functions of zebrafish H+-ATPase-rich cells. Am. J. Physiol. Cell Physiol. 2011, 300, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Hwang, P.P. Novel discoveries in acid-base regulation and osmoregulation: A review of selected hormonal actions in zebrafish and medaka. Gen. Comp. Endocrinol. 2019, 277, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Kwong, R.W. Zebrafish as a model system for investigating the compensatory regulation of ionic balance during metabolic acidosis. Int. J. Mol. Sci. 2018, 19, 1087. [Google Scholar] [CrossRef]
- Hwang, P.P.; Chou, M.Y. Zebrafish as an animal model to study ion homeostasis. Pflugers. Arch. 2013, 465, 1233–1247. [Google Scholar] [CrossRef]
- Horng, J.L.; Lin, L.Y.; Hwang, P.P. Functional regulation of H+-ATPase-rich cells in zebrafish embryos acclimated to an acidic environment. Am. J. Physiol. Cell Physiol. 2009, 296, 682–692. [Google Scholar] [CrossRef]
- Horng, J.L.; Lin, L.Y.; Huang, C.J.; Katoh, F.; Kaneko, T.; Hwang, P.P. Knockdown of V-ATPase subunit A (atp6v1a) impairs acid secretion and ion balance in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, 2068–2076. [Google Scholar] [CrossRef]
- Avella, M.; Berhaut, J.; Payan, P. Primary culture of gill epithelial cells from the sea bass Dicentrarchus Labrax. In Vitro Cell. Dev. Biol. Anim. 1994, 30, 41–49. [Google Scholar] [CrossRef]
- Schnell, S.; Stott, L.C.; Hogstrand, C.; Wood, C.M.; Kelly, S.P.; Pärt, P.; Owen, S.F.; Bury, N.R. Procedures for the reconstruction, primary culture and experimental use of rainbow trout gill epithelia. Nat. Protoc. 2016, 11, 490–498. [Google Scholar] [CrossRef]
- Bury, N.R.; Jie, L.; Flik, G.; Lock, R.A.C.; Bonga, S.E.W. Cortisol protects against copper induced necrosis and promotes apoptosis in fish gill chloride cells in vitro. Aquat. Toxicol. 1998, 40, 193–202. [Google Scholar] [CrossRef]
- Lin, C.H.; Tsai, I.L.; Su, C.H.; Tseng, D.Y.; Hwang, P.P. Reverse effect of mammalian hypocalcemic cortisol in fish: Cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLoS ONE 2011, 6, 23689. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Horng, J.L.; Yan, J.J.; Hsiao, C.D.; Hwang, P.P. The transcription factor, glial cell missing 2, is involved in differentiation and functional regulation of H+-ATPase-rich cells in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Karnaky, K.G., Jr.; Kinter, W.B. Killifish opercular skin: A flat epithelium with a high density of chloride cells. J. Exp. Zool. 1977, 199, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Kaneko, T.; Miyazaki, H.; Hasegawa, S.; Hirano, T. Excellent salinity tolerance of Mozambique tilapia (Oreochromis mossambicus): Elevated chloride cell activity in the branchial and opercular epithelia of the fish adapted to concentrated seawater. Zool. Sci. 2000, 17, 149–160. [Google Scholar] [CrossRef]
- Kang, C.K.; Yang, S.Y.; Lin, S.T.; Lee, T.H. The inner opercular membrane of the euryhaline teleost: A useful surrogate model for comparisons of different characteristics of ionocytes between seawater-and freshwater-acclimated medaka. Histochem. Cell Biol. 2015, 143, 69–81. [Google Scholar] [CrossRef]
- Marshall, W.S.; Bryson, S.E.; Darling, P.; Whitten, C.; Patrick, M.; Wilkie, M.; Wood, C.M.; Buckland-Nicks, J. NaCl transport and ultrastructure of opercular epithelium from a freshwater-adapted euryhaline teleost, Fundulus heteroclitus. J. Exp. Zool. 1997, 277, 23–37. [Google Scholar] [CrossRef]
- Karnaky, K.G., Jr.; Degnan, K.J.; Zadunaisky, J.A. Chloride transport across isolated opercular epithelium of killifish: A membrane rich in chloride cells. Science 1977, 195, 203–205. [Google Scholar] [CrossRef]
- Marshall, W.S. Rapid regulation of NaCl secretion by estuarine teleost fish: Coping strategies for short-duration freshwater exposures. Biochim. Biophys. Acta 2003, 1618, 95–105. [Google Scholar] [CrossRef]
- Foskett, J.K.; Machen, T.E.; Bern, H.A. Chloride secretion and conductance of teleost opercular membrane: Effects of prolactin. Am. J. Physiol. 1982, 242, 380–389. [Google Scholar] [CrossRef]
- McCormick, S.; Hasegawa, S.; Hirano, T. Calcium uptake in the skin of a freshwater teleost. Proc. Natl. Acad. Sci. USA 1992, 89, 3635–3638. [Google Scholar] [CrossRef]
- Verbost, P.M.; Bryson, S.E.; Bonga, S.E.; Marshall, W.S. Na+-dependent Ca2+ uptake in isolated opercular epithelium of Fundulus heteroclitus. J. Comp. Physiol. B 1997, 167, 205–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silver, R.B.; Breton, S.; Brown, D. Potassium depletion increases proton pump (H+-ATPase) activity in intercalated cells of cortical collecting duct. Am. J. Physiol. Renal Physiol. 2000, 279, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.H.; Horng, J.L.; Liu, S.T.; Hwang, P.P.; Lin, L.Y. Rhcg1 and NHE3b are involved in ammonium-dependent sodium uptake by zebrafish larvae acclimated to low-sodium water. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Shih, T.H.; Liu, S.T.; Hsu, H.H.; Hwang, P.P. Cortisol regulates acid secretion of H+-ATPase-rich ionocytes in zebrafish (Danio rerio) embryos. Front. Physiol. 2015, 6, 328. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.H.; Horng, J.L.; Hwang, P.P.; Lin, L.Y. Ammonia excretion by the skin of zebrafish (Danio rerio) larvae. Am. J. Physiol. Cell Physiol. 2008, 295, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Al-bataineh, M.M.; Pastor-Soler, N.M. Collecting duct intercalated cell function and regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 305–324. [Google Scholar] [CrossRef]
- Sabolić, I.; Brown, D.; Gluck, S.L.; Alper, S.L. Regulation of AE1 anion exchanger and H+-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int. 1997, 51, 125–137. [Google Scholar] [CrossRef]
- Purkerson, J.M.; Schwaderer, A.L.; Nakamori, A.; Schwartz, G.J. Distinct α-intercalated cell morphology and its modification by acidosis define regions of the collecting duct. Am. J. Physiol. Renal Physiol. 2015, 309, 464–473. [Google Scholar] [CrossRef][Green Version]
- Tsuruoka, S.; Schwartz, G.J. HCO3− absorption in rabbit outer medullary collecting duct: Role of luminal carbonic anhydrase. Am. J. Physiol. 1998, 274, 139–147. [Google Scholar]
- Tsuruoka, S.; Schwartz, G.J. Adaptation of the outer medullary collecting duct to metabolic acidosis in vitro. Am. J. Physiol. 1998, 275, 982–990. [Google Scholar] [CrossRef]
- Liu, S.T.; Horng, J.L.; Chen, P.Y.; Hwang, P.P.; Lin, L.Y. Salt secretion is linked to acid-base regulation of ionocytes in seawater-acclimated medaka: New insights into the salt-secreting mechanism. Sci. Rep. 2016, 6, 31433. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Lee, T.H. Ion-deficient environment induces the expression of basolateral chloride channel, ClC-3-like protein, in gill mitochondrion-rich cells for chloride uptake of the tilapia Oreochromis mossambicus. Physiol. Biochem. Zool. 2011, 84, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.W.; Yan, J.J.; Wang, Y.H.; Tsou, Y.L.; Chiu, L.; Tseng, Y.C.; Chou, M.Y.; Hwang, P.P. Estrogen-related receptor γ2 controls NaCl uptake to maintain ionic homeostasis. J. Endocrinol. 2021, 251, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.; Tsung, L.; Horng, J.L.; Lin, L.Y. Proton-facilitated ammonia excretion by ionocytes of medaka (Oryzias latipes) acclimated to seawater. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 242–251. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, S.-W.; Yan, J.-J.; Tsou, Y.-L.; Lu, S.-W.; Wang, M.-C.; Chou, M.-Y.; Hwang, P.-P. In Vivo Functional Assay in Fish Gills: Exploring Branchial Acid-Excreting Mechanisms in Zebrafish. Int. J. Mol. Sci. 2022, 23, 4419. https://doi.org/10.3390/ijms23084419
Shih S-W, Yan J-J, Tsou Y-L, Lu S-W, Wang M-C, Chou M-Y, Hwang P-P. In Vivo Functional Assay in Fish Gills: Exploring Branchial Acid-Excreting Mechanisms in Zebrafish. International Journal of Molecular Sciences. 2022; 23(8):4419. https://doi.org/10.3390/ijms23084419
Chicago/Turabian StyleShih, Shang-Wu, Jia-Jiun Yan, Yi-Ling Tsou, Shao-Wei Lu, Min-Chen Wang, Ming-Yi Chou, and Pung-Pung Hwang. 2022. "In Vivo Functional Assay in Fish Gills: Exploring Branchial Acid-Excreting Mechanisms in Zebrafish" International Journal of Molecular Sciences 23, no. 8: 4419. https://doi.org/10.3390/ijms23084419
APA StyleShih, S.-W., Yan, J.-J., Tsou, Y.-L., Lu, S.-W., Wang, M.-C., Chou, M.-Y., & Hwang, P.-P. (2022). In Vivo Functional Assay in Fish Gills: Exploring Branchial Acid-Excreting Mechanisms in Zebrafish. International Journal of Molecular Sciences, 23(8), 4419. https://doi.org/10.3390/ijms23084419






