Chaenomeles Fructus (CF), the Fruit of Chaenomeles sinensis Alleviates IL-1β Induced Cartilage Degradation in Rat Articular Chondrocytes
Abstract
1. Introduction
2. Results
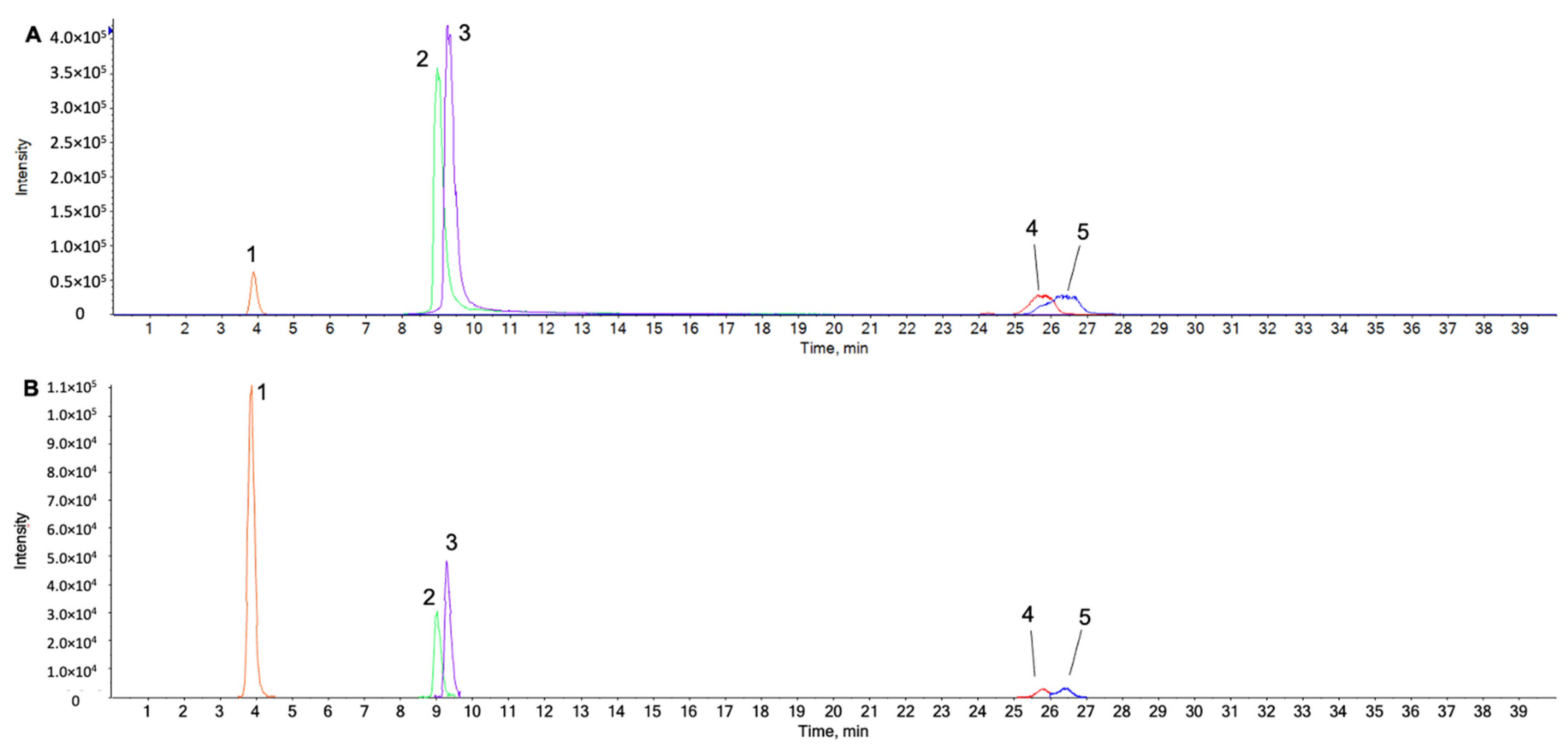
2.1. UPLC-ESI-QTOF MS/MS Analysis for Identification of Chemical Components in the Water Extract from Chaenomeles Fructus
2.2. CF was Not Cytotoxic to Rat Primary Chondrocytes
2.3. CF Suppressed IL-1β-Induced Cartilage Gene Expression and the Protein Level of MMP3/13 and Adamts5 in Rat Chondrocytes
2.4. CF Prevented IL-1β-Induced Degradation of aggrecan and Col2a1 and Reversed IL-1β-Induced Alcian Blue Staining Loss
2.5. CF Inhibited IL-1β-Induced NF-κB Activation in Rat Chondrocytes
2.6. CF Repressed IL-1β-Induced ERK2 Activation in Rat Chondrocytes
2.7. CF Reduced IL-1β-Induced Reactive Oxygen Species (ROS) Production in Rat Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Preparation of Extracts from CF Fruit
4.2. Primary Rat Chondrocyte Isolation and Culture
4.3. Cell Viability
4.4. Nuclear and Cytoplasmic Extraction
4.5. Western Blot Analysis
4.6. Immunofluorescence Assay
4.7. Quantitative Real-Time PCR
4.8. Alcian Blue Stain
4.9. Flow Cytometry
4.10. Liquid Chromatography–Mass Spectrometry Based Analysis of the Water Extract from Chaenomeles Sinensis Fruit
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OA | osteoarthritis |
| CF | Chaenomeles Fructus |
| ECM | extracellular matrix |
| IL-1β | interleukin 1 beta |
| MMP | matrix metallopeptidase |
| ADAMTs | a disintegrin and metalloproteinase with thrombospondin motifs |
| ROS | reactive oxygen species |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinase |
| JNK | c-Jun NH2-terminal kinase |
| NF-κb | nuclear factor kappa B |
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, L.; Billinghurst, R.C.; Manner, P.; Nelson, F.; Webb, G.; Ionescu, M.; Reiner, A.; Tanzer, M.; Zukor, D.; Chen, J.; et al. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1). Arthritis Rheum. 2000, 43, 673–682. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Wang, X.; Simpson, E.R.; Brown, K.A. Correction: p53: Protection against Tumor Growth beyond Effects on Cell Cycle and Apoptosis. Cancer Res. 2016, 76, 1668. [Google Scholar] [CrossRef][Green Version]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Song, J.S.; Hong, K.T.; Kim, N.M.; Jung, J.Y.; Park, H.S.; Lee, S.H.; Cho, Y.J.; Kim, S.J. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regen. Ther. 2020, 14, 32–39. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018, 40, 74–80. [Google Scholar] [CrossRef]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Ding, S.L.; Pang, Z.Y.; Chen, X.M.; Li, Z.; Liu, X.X.; Zhai, Q.L.; Huang, J.M.; Ruan, Z.Y. Urolithin a attenuates IL-1beta-induced inflammatory responses and cartilage degradation via inhibiting the MAPK/NF-kappaB signaling pathways in rat articular chondrocytes. J. Inflamm. 2020, 17, 13. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.S.; Kim, J.H.; Kim, E.Y.; Lee, B.; Lee, S.Y.; Jun, J.Y.; Kim, M.B.; Sohn, Y.; Jung, H.S. Chaenomelis fructus inhibits osteoclast differentiation by suppressing NFATc1 expression and prevents ovariectomy-induced osteoporosis. BMC Complement. Med. Ther. 2020, 20, 35. [Google Scholar] [CrossRef]
- Jang, G.; Yun-Kyoung, S.; Hyung-Ho, L. Effect of aqueous extract of sukjiyanggeun-tang (shudiyangjin-tang) on functional recovery and expressions of inflammatory mediators after sciatic nerve crushed injury in rat. J. Korean Med. Rehabil. 2013, 23, 16. [Google Scholar]
- Zhu, L.; Fang, L.; Li, Z.; Xie, X.; Zhang, L. A HPLC fingerprint study on chaenomelis fructus. BMC Chem. 2019, 13, 7. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Li, H.; Zhong, P.X.; Xu, Y.J.; Li, C.H.; Ji, K.X.; Wang, L.S. Polyphenols and triterpenes from chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, B.; Chen, X.; Chen, D.; Yang, H. Protocatechuic acid attenuates anterior cruciate ligament transection-induced osteoarthritis by suppressing osteoclastogenesis. Exp. Ther. Med. 2020, 19, 232–240. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1beta-induced in fl ammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharm. 2019, 109, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Salman, I.; Fakhoury, M.; Fouani, M.; Lawand, N. Peripheral anti-nociceptive and anti-inflammatory effect of oleanolic acid in a rat model of osteoarthritis. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2021, 20, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, Y.; Zhang, Z.; Chen, C.; Chi, Q.; Xu, K.; Yang, L. Ursolic acid protects chondrocytes, exhibits anti-inflammatory properties via regulation of the NF-kappaB/NLRP3 inflammasome pathway and ameliorates osteoarthritis. Biomed. Pharm. 2020, 130, 110568. [Google Scholar] [CrossRef]
- Tabeian, H.; Betti, B.F.; Dos Santos Cirqueira, C.; de Vries, T.J.; Lobbezoo, F.; Ter Linde, A.V.; Zandieh-Doulabi, B.; Koenders, M.I.; Everts, V.; Bakker, A.D. IL-1beta damages fibrocartilage and upregulates MMP-13 expression in fibrochondrocytes in the condyle of the temporomandibular joint. Int. J. Mol. Sci. 2019, 20, 2260. [Google Scholar] [CrossRef]
- Tu, C.; Ma, Y.; Song, M.; Yan, J.; Xiao, Y.; Wu, H. Liquiritigenin inhibits IL-1beta-induced inflammation and cartilage matrix degradation in rat chondrocytes. Eur. J. Pharmacol. 2019, 858, 172445. [Google Scholar] [CrossRef]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Onnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef]
- Ji, B.; Ma, Y.; Wang, H.; Fang, X.; Shi, P. Activation of the P38/CREB/MMP13 axis is associated with osteoarthritis. Drug Des. Dev. Ther. 2019, 13, 2195–2204. [Google Scholar] [CrossRef]
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, R.C.; Zhang, J. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-kappaB pathway. Aging 2019, 11, 11391–11415. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- Guo, H.; Yin, W.; Zou, Z.; Zhang, C.; Sun, M.; Min, L.; Yang, L.; Kong, L. Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study. J. Adv. Res. 2021, 28, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Ma, C.; Xu, K.; Xu, L.; He, Y.; Moqbel, S.A.A.; Hu, P.; Jiang, L.; Chen, W.; Bao, J.; et al. Schisandrin B ameliorated chondrocytes inflammation and osteoarthritis via suppression of NF-kappaB and MAPK signal pathways. Drug Des. Devel. Ther. 2018, 12, 1195–1204. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Kongtharvonskul, J.; Anothaisintawee, T.; McEvoy, M.; Attia, J.; Woratanarat, P.; Thakkinstian, A. Efficacy and safety of glucosamine, diacerein, and NSAIDs in osteoarthritis knee: A systematic review and network meta-analysis. Eur. J. Med. Res. 2015, 20, 24. [Google Scholar] [CrossRef]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Tang, Y.; Lu, H.; Qi, Y.; Li, G.; He, H.; Lu, F.; Yang, Y.; Sun, H. Quercetin alleviates osteoarthritis progression in rats by suppressing inflammation and apoptosis via inhibition of IRAK1/NLRP3 signaling. J. Inflamm. Res. 2021, 14, 3393–3403. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Tang, L.; Ji, Y.; Yan, C.; Zhang, X. Protective effects of quercetin against inflammation and oxidative stress in a rabbit model of knee osteoarthritis. Drug Dev. Res. 2019, 80, 360–367. [Google Scholar] [CrossRef]
- Huang, K.; Wu, L.D. Aggrecanase and aggrecan degradation in osteoarthritis: A review. J. Int. Med. Res. 2008, 36, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Miranda-Hernandez, S.; Alim, M.A.; Hayes, L.; Bird, G.; Ketheesan, N. Therapeutic effect of quercetin in collagen-induced arthritis. Biomed. Pharm. 2017, 90, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Heydari Nasrabadi, M.; Parsivand, M.; Mohammadi, N.; Asghari Moghaddam, N. Comparison of Elaeagnus angustifolia L. extract and quercetin on mouse model of knee osteoarthritis. J. Ayurveda Integr. Med. 2021, 13, 100529. [Google Scholar] [CrossRef]






| Antibody | Company | Dilution | Product No. |
|---|---|---|---|
| β-Actin | Santa cruz | 1:1000 | sc-47778 |
| ADAMTS5 | Abcam | 1:500 | ab41037 |
| MMP-3 | Abcam | 1:1000 | ab53015 |
| MMP-13 | Abcam | 1:1000 | ab39012 |
| COL2A1 | Santa Cruz | 1:1000 | sc-52658 |
| Aggrecan | Abcam | 1:1000 | ab36861 |
| NF-κB p65 | Cell signaling | 1:1000 | 8242 s |
| p-NF-κB p65 | Cell signaling | 1:1000 | #3033 |
| ERK | Santa cruz | 1:1000 | sc-81457 |
| p-ERK | Cell signaling | 1:2000 | #4370 |
| JNK | Cell signaling | 1:1000 | #9252 |
| P-JNK | Cell signaling | 1:2000 | #9255 |
| P38 | Cell signaling | 1:1000 | #8690 |
| p-P38 | Cell signaling | 1:1000 | #4511 |
| Histione H3 | Cell signaling | 1:1000 | #9715 |
| IkB-alpha | Cell signaling | 1:1000 | #9242 |
| Primer | Forward | Reverse |
|---|---|---|
| MMP3 | ATGATGAACGATGGACAGATGA | CATTGGCTGAGTGAAAGAGACC |
| MMP13 | TGCTGCATACGAGCATCCAT | TGTCCTCAAAGTGAACCGCA |
| COL2A1 | TCAACAATGGGAAGGCGTGAG | GTTCACGTACACTGCCCTGAAG |
| Aggrecan | GCCTCTCAAGCCCTTGTCTG | GATCTCACACAGGTCCCCTC |
| Adamts5 | CAAGTGTGGAGTGTGTGGAG | GTCTTTGGCTTTGAACTGTCG |
| β-Actin | GCTACAGCTTCACCACCACA | GCCATCTCTTGCTCGAAGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, C.; Ahn, C.R.; Kim, J.-E.; Kim, Y.W.; Park, J.; Ahn, K.S.; Ha, I.J.; Lee, Y.J.; Baek, S.H.; Ha, I.-H. Chaenomeles Fructus (CF), the Fruit of Chaenomeles sinensis Alleviates IL-1β Induced Cartilage Degradation in Rat Articular Chondrocytes. Int. J. Mol. Sci. 2022, 23, 4360. https://doi.org/10.3390/ijms23084360
Yeo C, Ahn CR, Kim J-E, Kim YW, Park J, Ahn KS, Ha IJ, Lee YJ, Baek SH, Ha I-H. Chaenomeles Fructus (CF), the Fruit of Chaenomeles sinensis Alleviates IL-1β Induced Cartilage Degradation in Rat Articular Chondrocytes. International Journal of Molecular Sciences. 2022; 23(8):4360. https://doi.org/10.3390/ijms23084360
Chicago/Turabian StyleYeo, Changhwan, Chae Ryeong Ahn, Jai-Eun Kim, Young Woo Kim, Jinbong Park, Kwang Seok Ahn, In Jin Ha, Yoon Jae Lee, Seung Ho Baek, and In-Hyuk Ha. 2022. "Chaenomeles Fructus (CF), the Fruit of Chaenomeles sinensis Alleviates IL-1β Induced Cartilage Degradation in Rat Articular Chondrocytes" International Journal of Molecular Sciences 23, no. 8: 4360. https://doi.org/10.3390/ijms23084360
APA StyleYeo, C., Ahn, C. R., Kim, J.-E., Kim, Y. W., Park, J., Ahn, K. S., Ha, I. J., Lee, Y. J., Baek, S. H., & Ha, I.-H. (2022). Chaenomeles Fructus (CF), the Fruit of Chaenomeles sinensis Alleviates IL-1β Induced Cartilage Degradation in Rat Articular Chondrocytes. International Journal of Molecular Sciences, 23(8), 4360. https://doi.org/10.3390/ijms23084360









