Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration
Abstract
1. Introduction
2. RGC Classification and Injury Sensitivity
| Main Feature | ON/OFF Feature | Subtypes |
|---|---|---|
| Sustained | ON | ON alpha, Pix ON, M2, M1 |
| OFF | OFF s alpha, OFF s med, OFF s EW1 no, OFF s EW3o, OFFhOS, OFFvOS | |
| Transient | ON | M6, ON tr MeRF, ON tr SmRF, ON tr EW6t |
| OFF | OFF tr alpha, OFF tr MeRF, OFF tr SmRF | |
| Orientation selective | ON | ONhOS SmRF, ONvOS SmRF, ONhOS LgRF, ONvOS LgRF |
| Direction selective | ON | ONDS s (3 subtypes), ONDS tr (1 subtypes) |
| ON-OFF | OODS (4 subtypes) | |
| ON-OFF Small RF | ON-OFF | HD1, HD2, UHD, LED, F-mini ON, F-mini OFF |
| SbC/Others | ON delayed, ON bursty, bSbC, sSbC EW27, sSbC EW28, ON Sm OFF Lg, Motion sensor |
3. RGC Labeling
3.1. Dyes for RGC Labeling
3.2. Immunostaining: Specific Antibodies against RGC Subtypes
3.3. Transgenic Models
4. Neurodegeneration and Death of Retinal Ganglion Cells
4.1. Retinal Images Techniques
4.2. Apoptosis Detection Techniques
4.3. Caspase Activation Detection
4.4. Detection of Changes in the Apoptotic Membrane
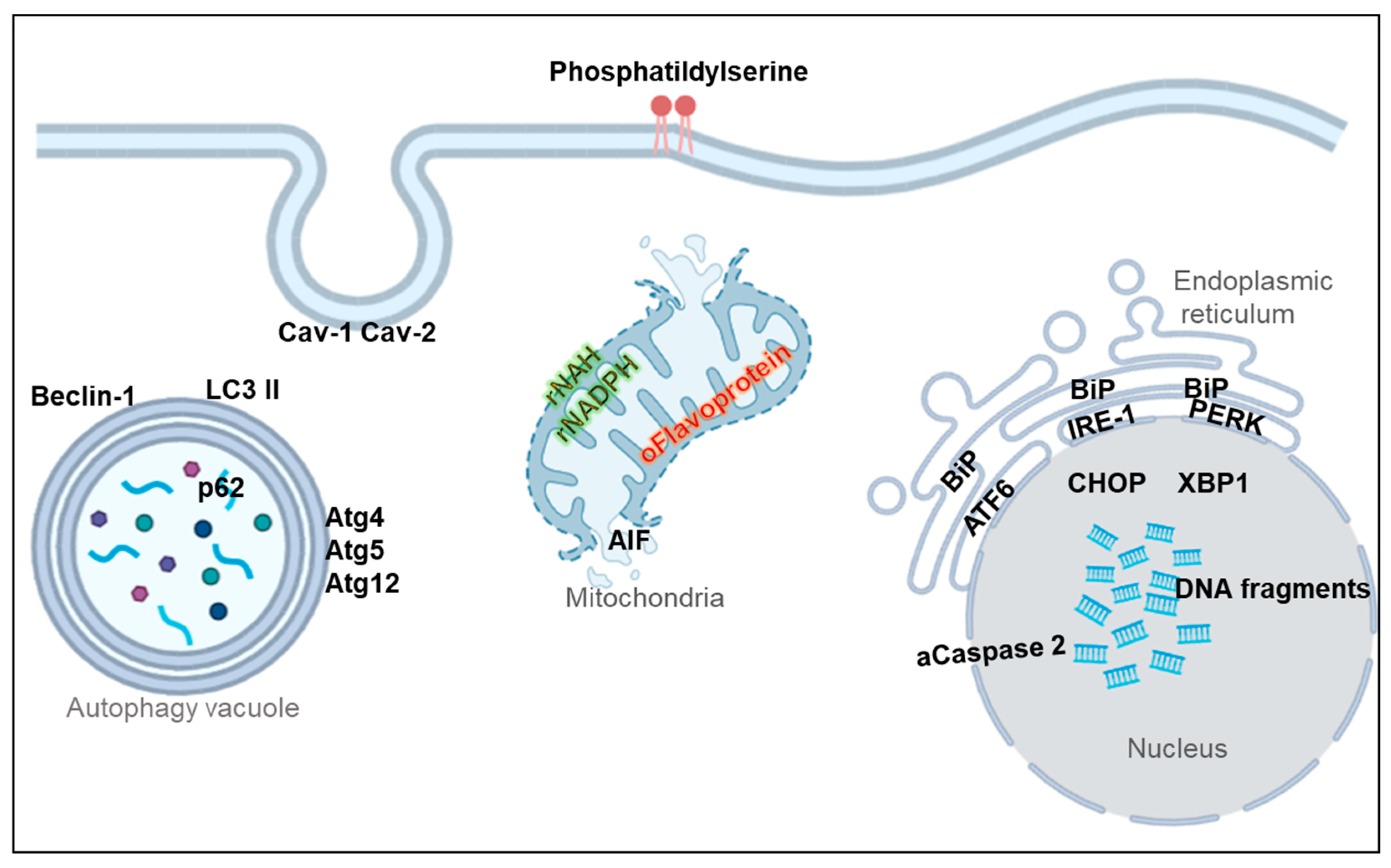
5. Non-Apoptotic Biomarkers
5.1. Membrane Markers
5.2. Oxidative Stress Markers
5.3. Mitochondrial Dysfunction Markers
5.4. Endoplasmic Reticulum (ER) Stress Markers
5.5. Autophagy Markers
5.6. Necroptosis Markers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacCormick, I.J.C.; Czanner, G.; Faragher, B. Developing Retinal Biomarkers of Neurological Disease: An Analytical Perspective. Biomark. Med. 2015, 9, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Asanad, S.; Felix, C.M.; Fantini, M.; Harrington, M.G.; Sadun, A.A.; Karanjia, R. Retinal Ganglion Cell Dysfunction in Preclinical Alzheimer’s Disease: An Electrophysiologic Biomarker Signature. Sci. Rep. 2021, 11, 6344. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.C. Chapter 4—Functional Anatomy of the Mammalian Retina. In Retina (Fourth Edition); Ryan, S.J., Hinton, D.R., Schachat, A.P., Wilkinson, C.P., Eds.; Mosby: Edinburgh, UK, 2006; pp. 43–82. ISBN 978-0-323-02598-0. [Google Scholar]
- Feng, G.; Mellor, R.H.; Bernstein, M.; Keller-Peck, C.; Nguyen, Q.T.; Wallace, M.; Nerbonne, J.M.; Lichtman, J.W.; Sanes, J.R. Imaging Neuronal Subsets in Transgenic Mice Expressing Multiple Spectral Variants of GFP. Neuron 2000, 28, 41–51. [Google Scholar] [CrossRef]
- Cordeiro, M.F.; Guo, L.; Luong, V.; Harding, G.; Wang, W.; Jones, H.E.; Moss, S.E.; Sillito, A.M.; Fitzke, F.W. Real-Time Imaging of Single Nerve Cell Apoptosis in Retinal Neurodegeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 13352–13356. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.C.; Merigan, W.; Wolfing, J.I.; Gee, B.P.; Porter, J.; Dubra, A.; Twietmeyer, T.H.; Ahamd, K.; Tumbar, R.; Reinholz, F.; et al. In Vivo Fluorescence Imaging of Primate Retinal Ganglion Cells and Retinal Pigment Epithelial Cells. Opt. Express 2006, 14, 7144–7158. [Google Scholar] [CrossRef]
- Kerrison, J.B.; Duh, E.J.; Yu, Y.; Otteson, D.C.; Zack, D.J. A System for Inducible Gene Expression in Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Gollisch, T.; Meister, M. Eye Smarter than Scientists Believed: Neural Computations in Circuits of the Retina. Neuron 2010, 65, 150–164. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.; Xing, Y.; Yang, N. Differential Susceptibility of Retinal Ganglion Cell Subtypes against Neurodegenerative Diseases. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2022, 260, 1–15. [Google Scholar] [CrossRef]
- Boycott, B.B.; Wässle, H. The Morphological Types of Ganglion Cells of the Domestic Cat’s Retina. J. Physiol. 1974, 240, 397–419. [Google Scholar] [CrossRef]
- Briggman, K.L.; Helmstaedter, M.; Denk, W. Wiring Specificity in the Direction-Selectivity Circuit of the Retina. Nature 2011, 471, 183–188. [Google Scholar] [CrossRef]
- Morin, L.P.; Studholme, K.M. Retinofugal Projections in the Mouse. J. Comp. Neurol. 2014, 522, 3733–3753. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.R.; Masland, R.H. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.A.; Mu, S.; Kim, J.S.; Turner, N.L.; Tartavull, I.; Kemnitz, N.; Jordan, C.S.; Norton, A.D.; Silversmith, W.M.; Prentki, R.; et al. Digital Museum of Retinal Ganglion Cells with Dense Anatomy and Physiology. Cell 2018, 173, 1293–1306.e19. [Google Scholar] [CrossRef] [PubMed]
- Laboissonniere, L.A.; Goetz, J.J.; Martin, G.M.; Bi, R.; Lund, T.J.S.; Ellson, L.; Lynch, M.R.; Mooney, B.; Wickham, H.; Liu, P.; et al. Molecular Signatures of Retinal Ganglion Cells Revealed through Single Cell Profiling. Sci. Rep. 2019, 9, 15778. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A Novel Human Opsin in the Inner Retina. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef]
- Mayer, C.; Bruehl, C.; Salt, E.L.; Diem, R.; Draguhn, A.; Fairless, R. Selective Vulnerability of AOFF Retinal Ganglion Cells during Onset of Autoimmune Optic Neuritis. Neuroscience 2018, 393, 258–272. [Google Scholar] [CrossRef]
- VanderWall, K.B.; Lu, B.; Alfaro, J.S.; Allsop, A.R.; Carr, A.S.; Wang, S.; Meyer, J.S. Differential Susceptibility of Retinal Ganglion Cell Subtypes in Acute and Chronic Models of Injury and Disease. Sci. Rep. 2020, 10, 17359. [Google Scholar] [CrossRef]
- DeParis, S.; Caprara, C.; Grimm, C. Intrinsically Photosensitive Retinal Ganglion Cells Are Resistant to N-Methyl-D-Aspartic Acid Excitotoxicity. Mol. Vis. 2012, 18, 2814–2827. [Google Scholar]
- Pérez de Sevilla Müller, L.; Sargoy, A.; Rodriguez, A.R.; Brecha, N.C. Melanopsin Ganglion Cells Are the Most Resistant Retinal Ganglion Cell Type to Axonal Injury in the Rat Retina. PLoS ONE 2014, 9, e93274. [Google Scholar] [CrossRef]
- Honda, S.; Namekata, K.; Kimura, A.; Guo, X.; Harada, C.; Murakami, A.; Matsuda, A.; Harada, T. Survival of Alpha and Intrinsically Photosensitive Retinal Ganglion Cells in NMDA-Induced Neurotoxicity and a Mouse Model of Normal Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3696–3707. [Google Scholar] [CrossRef]
- Mey, J.; Thanos, S. Intravitreal Injections of Neurotrophic Factors Support the Survival of Axotomized Retinal Ganglion Cells in Adult Rats in Vivo. Brain Res. 1993, 602, 304–317. [Google Scholar] [CrossRef]
- Estevez, M.E.; Fogerson, P.M.; Ilardi, M.C.; Borghuis, B.G.; Chan, E.; Weng, S.; Auferkorte, O.N.; Demb, J.B.; Berson, D.M. Form and Function of the M4 Cell, an Intrinsically Photosensitive Retinal Ganglion Cell Type Contributing to Geniculocortical Vision. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 13608–13620. [Google Scholar] [CrossRef] [PubMed]
- Vaarmann, A.; Kovac, S.; Holmström, K.M.; Gandhi, S.; Abramov, A.Y. Dopamine Protects Neurons against Glutamate-Induced Excitotoxicity. Cell Death Dis. 2013, 4, e455. [Google Scholar] [CrossRef] [PubMed]
- Vugler, A.A.; Redgrave, P.; Semo, M.; Lawrence, J.; Greenwood, J.; Coffey, P.J. Dopamine Neurones Form a Discrete Plexus with Melanopsin Cells in Normal and Degenerating Retina. Exp. Neurol. 2007, 205, 26–35. [Google Scholar] [CrossRef]
- Joo, H.R.; Peterson, B.B.; Dacey, D.M.; Hattar, S.; Chen, S.-K. Recurrent Axon Collaterals of Intrinsically Photosensitive Retinal Ganglion Cells. Vis. Neurosci. 2013, 30, 175–182. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. MTOR Is a Key Modulator of Ageing and Age-Related Disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Rane, S.G.; Reddy, E.P. Janus Kinases: Components of Multiple Signaling Pathways. Oncogene 2000, 19, 5662–5679. [Google Scholar] [CrossRef]
- Reglodi, D.; Kiss, P.; Szabadfi, K.; Atlasz, T.; Gabriel, R.; Horvath, G.; Szakaly, P.; Sandor, B.; Lubics, A.; Laszlo, E.; et al. PACAP Is an Endogenous Protective Factor-Insights from PACAP-Deficient Mice. J. Mol. Neurosci. MN 2012, 48, 482–492. [Google Scholar] [CrossRef]
- Hannibal, J.; Hindersson, P.; Ostergaard, J.; Georg, B.; Heegaard, S.; Larsen, P.J.; Fahrenkrug, J. Melanopsin Is Expressed in PACAP-Containing Retinal Ganglion Cells of the Human Retinohypothalamic Tract. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4202–4209. [Google Scholar] [CrossRef]
- Kreuzberg, M.M.; Schrickel, J.W.; Ghanem, A.; Kim, J.-S.; Degen, J.; Janssen-Bienhold, U.; Lewalter, T.; Tiemann, K.; Willecke, K. Connexin30.2 Containing Gap Junction Channels Decelerate Impulse Propagation through the Atrioventricular Node. Proc. Natl. Acad. Sci. USA 2006, 103, 5959–5964. [Google Scholar] [CrossRef]
- Johnson, K.P.; Zhao, L.; Kerschensteiner, D. A Pixel-Encoder Retinal Ganglion Cell with Spatially Offset Excitatory and Inhibitory Receptive Fields. Cell Rep. 2018, 22, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- RGC Types. Available online: http://rgctypes.org/ (accessed on 12 April 2022).
- Balendra, S.I.; Normando, E.M.; Bloom, P.A.; Cordeiro, M.F. Advances in Retinal Ganglion Cell Imaging. Eye Lond. Engl. 2015, 29, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.; Chang, Q.; Zhang, X.; Barnett, E.M.; Piwnica-Worms, D. An Improved Cell-Penetrating, Caspase-Activatable, near-Infrared Fluorescent Peptide for Apoptosis Imaging. Bioconjug. Chem. 2009, 20, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Johnson, J.R.; Wilson, B.S.; Gammon, S.T.; Piwnica-Worms, D.; Barnett, E.M. Single-Cell Resolution Imaging of Retinal Ganglion Cell Apoptosis in Vivo Using a Cell-Penetrating Caspase-Activatable Peptide Probe. PLoS ONE 2014, 9, e88855. [Google Scholar] [CrossRef][Green Version]
- Nickerson, J.M.; Getz, S.E.; Sellers, J.T.; Chrenek, M.A.; Goodman, P.; Bernal, C.J.; Boatright, J.H. DNA Delivery in Adult Mouse Eyes: An Update with Corneal Outcomes. Methods Mol. Biol. Clifton NJ 2014, 1121, 165–177. [Google Scholar] [CrossRef]
- Dezawa, M.; Takano, M.; Negishi, H.; Mo, X.; Oshitari, T.; Sawada, H. Gene Transfer into Retinal Ganglion Cells by in Vivo Electroporation: A New Approach. Micron Oxf. Engl. 1993 2002, 33, 1–6. [Google Scholar] [CrossRef]
- Matsuda, T.; Cepko, C.L. Electroporation and RNA Interference in the Rodent Retina in Vivo and in Vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 16–22. [Google Scholar] [CrossRef]
- Köbbert, C.; Apps, R.; Bechmann, I.; Lanciego, J.L.; Mey, J.; Thanos, S. Current Concepts in Neuroanatomical Tracing. Prog. Neurobiol. 2000, 62, 327–351. [Google Scholar] [CrossRef]
- Lafuente, M.P.; Villegas-Pérez, M.P.; Sellés-Navarro, I.; Mayor-Torroglosa, S.; Miralles de Imperial, J.; Vidal-Sanz, M. Retinal Ganglion Cell Death after Acute Retinal Ischemia Is an Ongoing Process Whose Severity and Duration Depends on the Duration of the Insult. Neuroscience 2002, 109, 157–168. [Google Scholar] [CrossRef]
- Villegas-Pérez, M.P.; Vidal-Sanz, M.; Rasminsky, M.; Bray, G.M.; Aguayo, A.J. Rapid and Protracted Phases of Retinal Ganglion Cell Loss Follow Axotomy in the Optic Nerve of Adult Rats. J. Neurobiol. 1993, 24, 23–36. [Google Scholar] [CrossRef]
- Grünert, U.; Lee, S.C.S.; Kwan, W.C.; Mundinano, I.-C.; Bourne, J.A.; Martin, P.R. Retinal Ganglion Cells Projecting to Superior Colliculus and Pulvinar in Marmoset. Brain Struct. Funct. 2021, 226, 2745–2762. [Google Scholar] [CrossRef] [PubMed]
- Germain, F.; Istillarte, M.; Gómez-Vicente, V.; Pérez-Rico, C.; de la Villa, P. Electroretinographical and Histological Study of Mouse Retina after Optic Nerve Section: A Comparison between Wild-Type and Retinal Degeneration 1 Mice. Clin. Exp. Ophthalmol. 2013, 41, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sanz, M.; Valiente-Soriano, F.J.; Ortín-Martínez, A.; Nadal-Nicolás, F.M.; Jiménez-López, M.; Salinas-Navarro, M.; Alarcón-Martínez, L.; García-Ayuso, D.; Avilés-Trigueros, M.; Agudo-Barriuso, M.; et al. Retinal Neurodegeneration in Experimental Glaucoma. Prog. Brain Res. 2015, 220, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Thanos, S.; Fischer, D.; Pavlidis, M.; Heiduschka, P.; Bodeutsch, N. Glioanatomy Assessed by Cell-Cell Interactions and Phagocytotic Labelling. J. Neurosci. Methods 2000, 103, 39–50. [Google Scholar] [CrossRef]
- Bodeutsch, N.; Thanos, S. Migration of Phagocytotic Cells and Development of the Murine Intraretinal Microglial Network: An in Vivo Study Using Fluorescent Dyes. Glia 2000, 32, 91–101. [Google Scholar] [CrossRef]
- Nuschke, A.C.; Farrell, S.R.; Levesque, J.M.; Chauhan, B.C. Assessment of Retinal Ganglion Cell Damage in Glaucomatous Optic Neuropathy: Axon Transport, Injury and Soma Loss. Exp. Eye Res. 2015, 141, 111–124. [Google Scholar] [CrossRef]
- Salinas-Navarro, M.; Jiménez-López, M.; Valiente-Soriano, F.J.; Alarcón-Martínez, L.; Avilés-Trigueros, M.; Mayor, S.; Holmes, T.; Lund, R.D.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Retinal Ganglion Cell Population in Adult Albino and Pigmented Mice: A Computerized Analysis of the Entire Population and Its Spatial Distribution. Vision Res. 2009, 49, 637–647. [Google Scholar] [CrossRef]
- Wang, X.; Archibald, M.L.; Stevens, K.; Baldridge, W.H.; Chauhan, B.C. Cyan Fluorescent Protein (CFP) Expressing Cells in the Retina of Thy1-CFP Transgenic Mice before and after Optic Nerve Injury. Neurosci. Lett. 2010, 468, 110–114. [Google Scholar] [CrossRef]
- Sanchez, J.; Holmgren, J. Cholera Toxin—A Foe & a Friend. Indian J. Med. Res. 2011, 133, 153–163. [Google Scholar]
- Smith, C.A.; Chauhan, B.C. Imaging Retinal Ganglion Cells: Enabling Experimental Technology for Clinical Application. Prog. Retin. Eye Res. 2015, 44, 1–14. [Google Scholar] [CrossRef]
- Calvo, E.; Milla-Navarro, S.; Ortuño-Lizarán, I.; Gómez-Vicente, V.; Cuenca, N.; De la Villa, P.; Germain, F. Deleterious Effect of NMDA Plus Kainate on the Inner Retinal Cells and Ganglion Cell Projection of the Mouse. Int. J. Mol. Sci. 2020, 21, 1570. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Salinas-Navarro, M.; Sobrado-Calvo, P.; Alburquerque-Béjar, J.J.; Vidal-Sanz, M.; Agudo-Barriuso, M. Whole Number, Distribution and Co-Expression of Brn3 Transcription Factors in Retinal Ganglion Cells of Adult Albino and Pigmented Rats. PLoS ONE 2012, 7, e49830. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; de Sevilla Müller, L.P.; Brecha, N.C. The RNA Binding Protein RBPMS Is a Selective Marker of Ganglion Cells in the Mammalian Retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef] [PubMed]
- Surgucheva, I.; Weisman, A.D.; Goldberg, J.L.; Shnyra, A.; Surguchov, A. Gamma-Synuclein as a Marker of Retinal Ganglion Cells. Mol. Vis. 2008, 14, 1540–1548. [Google Scholar] [PubMed]
- Chidlow, G.; Osborne, N.N. Rat Retinal Ganglion Cell Loss Caused by Kainate, NMDA and Ischemia Correlates with a Reduction in MRNA and Protein of Thy-1 and Neurofilament Light. Brain Res. 2003, 963, 298–306. [Google Scholar] [CrossRef]
- Higashide, T.; Kawaguchi, I.; Ohkubo, S.; Takeda, H.; Sugiyama, K. In Vivo Imaging and Counting of Rat Retinal Ganglion Cells Using a Scanning Laser Ophthalmoscope. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2943–2950. [Google Scholar] [CrossRef][Green Version]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a Neuronal Specific Nuclear Protein in Vertebrates. Dev. Camb. Engl. 1992, 116, 201–211. [Google Scholar] [CrossRef]
- Buckingham, B.P.; Inman, D.M.; Lambert, W.; Oglesby, E.; Calkins, D.J.; Steele, M.R.; Vetter, M.L.; Marsh-Armstrong, N.; Horner, P.J. Progressive Ganglion Cell Degeneration Precedes Neuronal Loss in a Mouse Model of Glaucoma. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 2735–2744. [Google Scholar] [CrossRef]
- Raymond, I.D.; Vila, A.; Huynh, U.-C.N.; Brecha, N.C. Cyan Fluorescent Protein Expression in Ganglion and Amacrine Cells in a Thy1-CFP Transgenic Mouse Retina. Mol. Vis. 2008, 14, 1559–1574. [Google Scholar]
- Johansson, U.E.; Eftekhari, S.; Warfvinge, K. A Battery of Cell- and Structure-Specific Markers for the Adult Porcine Retina. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2010, 58, 377–389. [Google Scholar] [CrossRef]
- Kay, J.N.; De la Huerta, I.; Kim, I.-J.; Zhang, Y.; Yamagata, M.; Chu, M.W.; Meister, M.; Sanes, J.R. Retinal Ganglion Cells with Distinct Directional Preferences Differ in Molecular Identity, Structure, and Central Projections. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 7753–7762. [Google Scholar] [CrossRef] [PubMed]
- Dhande, O.S.; Estevez, M.E.; Quattrochi, L.E.; El-Danaf, R.N.; Nguyen, P.L.; Berson, D.M.; Huberman, A.D. Genetic Dissection of Retinal Inputs to Brainstem Nuclei Controlling Image Stabilization. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 17797–17813. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Lee, P.; Pan, Z.-H. Characterization of Multiple Bistratified Retinal Ganglion Cells in a Purkinje Cell Protein 2-Cre Transgenic Mouse Line. J. Comp. Neurol. 2013, 521, 2165–2180. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, S.; Berg, D.; Papendorp, C.; Briggman, K.L.; Berson, D.M. A Cre Mouse Line for Probing Irradiance- and Direction-Encoding Retinal Networks. eNeuro 2017, 4, ENEURO.0065-17.2017. [Google Scholar] [CrossRef]
- Krieger, B.; Qiao, M.; Rousso, D.L.; Sanes, J.R.; Meister, M. Four Alpha Ganglion Cell Types in Mouse Retina: Function, Structure, and Molecular Signatures. PLoS ONE 2017, 12, e0180091. [Google Scholar] [CrossRef]
- Rousso, D.L.; Qiao, M.; Kagan, R.D.; Yamagata, M.; Palmiter, R.D.; Sanes, J.R. Two Pairs of ON and OFF Retinal Ganglion Cells Are Defined by Intersectional Patterns of Transcription Factor Expression. Cell Rep. 2016, 15, 1930–1944. [Google Scholar] [CrossRef]
- Quattrochi, L.E.; Stabio, M.E.; Kim, I.; Ilardi, M.C.; Michelle Fogerson, P.; Leyrer, M.L.; Berson, D.M. The M6 Cell: A Small-Field Bistratified Photosensitive Retinal Ganglion Cell. J. Comp. Neurol. 2019, 527, 297–311. [Google Scholar] [CrossRef]
- Stabio, M.E.; Sabbah, S.; Quattrochi, L.E.; Ilardi, M.C.; Fogerson, P.M.; Leyrer, M.L.; Kim, M.T.; Kim, I.; Schiel, M.; Renna, J.M.; et al. The M5 Cell: A Color-Opponent Intrinsically Photosensitive Retinal Ganglion Cell. Neuron 2018, 97, 150–163.e4. [Google Scholar] [CrossRef]
- Berson, D.M.; Castrucci, A.M.; Provencio, I. Morphology and Mosaics of Melanopsin-Expressing Retinal Ganglion Cell Types in Mice. J. Comp. Neurol. 2010, 518, 2405–2422. [Google Scholar] [CrossRef]
- Quina, L.A.; Pak, W.; Lanier, J.; Banwait, P.; Gratwick, K.; Liu, Y.; Velasquez, T.; O’Leary, D.D.M.; Goulding, M.; Turner, E.E. Brn3a-Expressing Retinal Ganglion Cells Project Specifically to Thalamocortical and Collicular Visual Pathways. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 11595–11604. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Avilés-Trigueros, M.; Jiménez-López, M.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolás, F.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axotomy-Induced Retinal Ganglion Cell Death in Adult Mice: Quantitative and Topographic Time Course Analyses. Exp. Eye Res. 2011, 92, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Stevens, K.T.; Levesque, J.M.; Nuschke, A.C.; Sharpe, G.P.; O’Leary, N.; Archibald, M.L.; Wang, X. Longitudinal in Vivo Imaging of Retinal Ganglion Cells and Retinal Thickness Changes Following Optic Nerve Injury in Mice. PLoS ONE 2012, 7, e40352. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.; Lindsey, J.D.; Crowston, J.G.; Lijia, C.; Chiang, S.; Weinreb, R.N. Longitudinal Profile of Retinal Ganglion Cell Damage after Optic Nerve Crush with Blue-Light Confocal Scanning Laser Ophthalmoscopy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harwerth, R.S.; Carter-Dawson, L.; Shen, F.; Smith, E.L.; Crawford, M.L. Ganglion Cell Losses Underlying Visual Field Defects from Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2242–2250. [Google Scholar]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Liebmann, J.M.; Girkin, C.; Zangwill, L.M. Retinal Ganglion Cell Count Estimates Associated with Early Development of Visual Field Defects in Glaucoma. Ophthalmology 2013, 120, 736–744. [Google Scholar] [CrossRef]
- Birt, C.; Shin, D.; Samudrala, V.; Hughes, B.; Kim, C.; Lee, D. Analysis of Reliability Indices from Humphrey Visual Field Tests in an Urban Glaucoma Population. Ophthalmology 1997, 104, 1126–1130. [Google Scholar] [CrossRef]
- Gardiner, S.K.; Demirel, S.; Johnson, C.A. Is There Evidence for Continued Learning over Multiple Years in Perimetry? Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2008, 85, 1043–1048. [Google Scholar] [CrossRef]
- Humayun, M.S.; Prince, M.; de Juan, E.; Barron, Y.; Moskowitz, M.; Klock, I.B.; Milam, A.H. Morphometric Analysis of the Extramacular Retina from Postmortem Eyes with Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 1999, 40, 143–148. [Google Scholar]
- Medeiros, N.E.; Curcio, C.A. Preservation of Ganglion Cell Layer Neurons in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 795–803. [Google Scholar]
- Fischer, U.; Schulze-Osthoff, K. New Approaches and Therapeutics Targeting Apoptosis in Disease. Pharmacol. Rev. 2005, 57, 187–215. [Google Scholar] [CrossRef]
- Zollet, P.; Yap, T.E.; Cordeiro, M.F. Detecting Apoptosis as a Clinical Endpoint for Proof of a Clinical Principle. Ophthalmol. J. Int. Ophtalmol. Int. J. Ophthalmol. Z. Augenheilkd. 2021, 244, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Guo, L.; Coxon, K.M.; Duggan, J.; Nizari, S.; Normando, E.M.; Sensi, S.L.; Sillito, A.M.; Fitzke, F.W.; Salt, T.E.; et al. Imaging Multiple Phases of Neurodegeneration: A Novel Approach to Assessing Cell Death in Vivo. Cell Death Dis. 2010, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- Van Velthoven, M.E.J.; Verbraak, F.D.; Yannuzzi, L.A.; Rosen, R.B.; Podoleanu, A.G.H.; de Smet, M.D. Imaging the Retina by En Face Optical Coherence Tomography. Retina Phila. Pa 2006, 26, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Skaf, M.; Melo, L.A.S.; Calucci, D.; Cardillo, J.A.; Castro, J.C.; Huang, D.; Wojtkowski, M. Retinal Assessment Using Optical Coherence Tomography. Prog. Retin. Eye Res. 2006, 25, 325–353. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.S. Spectral Domain Optical Coherence Tomography for Glaucoma (an AOS Thesis). Trans. Am. Ophthalmol. Soc. 2008, 106, 426–458. [Google Scholar]
- Petzold, A.; de Boer, J.F.; Schippling, S.; Vermersch, P.; Kardon, R.; Green, A.; Calabresi, P.A.; Polman, C. Optical Coherence Tomography in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Lancet Neurol. 2010, 9, 921–932. [Google Scholar] [CrossRef]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for Glaucoma Diagnosis, Screening and Detection of Glaucoma Progression. Br. J. Ophthalmol. 2014, 98 (Suppl. S2), ii15–ii19. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Dragostinoff, N.; Pircher, M.; Götzinger, E.; Hitzenberger, C.K.; Leitgeb, R.A.; Schmetterer, L. Bidirectional Doppler Fourier-Domain Optical Coherence Tomography for Measurement of Absolute Flow Velocities in Human Retinal Vessels. Opt. Lett. 2008, 33, 2967–2969. [Google Scholar] [CrossRef]
- Pircher, M.; Hitzenberger, C.K.; Schmidt-Erfurth, U. Polarization Sensitive Optical Coherence Tomography in the Human Eye. Prog. Retin. Eye Res. 2011, 30, 431–451. [Google Scholar] [CrossRef]
- Chauhan, B.C. Confocal Scanning Laser Tomography. Can. J. Ophthalmol. 1996, 31, 152–156. [Google Scholar]
- Keane, P.A.; Ruiz-Garcia, H.; Sadda, S.R. Clinical Applications of Long-Wavelength (1000-Nm) Optical Coherence Tomography. Ophthalmic Surg. Lasers Imaging Off. J. Int. Soc. Imaging Eye 2011, 42 (Suppl. S4), S67–S74. [Google Scholar] [CrossRef]
- Webb, R.H.; Hughes, G.W.; Delori, F.C. Confocal Scanning Laser Ophthalmoscope. Appl. Opt. 1987, 26, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Kocaoglu, O.P.; Wang, Q.; Lee, S. Adaptive Optics and the Eye (Super Resolution OCT). Eye Lond. Engl. 2011, 25, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Grimm, B.; Goelz, S.; Bille, J.F. Objective Measurement of Wave Aberrations of the Human Eye with the Use of a Hartmann-Shack Wave-Front Sensor. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1994, 11, 1949–1957. [Google Scholar] [CrossRef]
- Chen, T.C.; Cense, B.; Pierce, M.C.; Nassif, N.; Park, B.H.; Yun, S.H.; White, B.R.; Bouma, B.E.; Tearney, G.J.; de Boer, J.F. Spectral Domain Optical Coherence Tomography: Ultra-High Speed, Ultra-High Resolution Ophthalmic Imaging. Arch. Ophthalmol. Chic. Ill 1960 2005, 123, 1715–1720. [Google Scholar] [CrossRef]
- Truong, S.N.; Alam, S.; Zawadzki, R.J.; Choi, S.S.; Telander, D.G.; Park, S.S.; Werner, J.S.; Morse, L.S. High Resolution Fourier-Domain Optical Coherence Tomography of Retinal Angiomatous Proliferation. Retina Phila. Pa 2007, 27, 915–925. [Google Scholar] [CrossRef]
- Biss, D.P.; Sumorok, D.; Burns, S.A.; Webb, R.H.; Zhou, Y.; Bifano, T.G.; Côté, D.; Veilleux, I.; Zamiri, P.; Lin, C.P. In Vivo Fluorescent Imaging of the Mouse Retina Using Adaptive Optics. Opt. Lett. 2007, 32, 659–661. [Google Scholar] [CrossRef]
- Williams, D.R. Imaging Single Cells in the Living Retina. Vision Res. 2011, 51, 1379–1396. [Google Scholar] [CrossRef]
- Chen, M.F.; Chui, T.Y.P.; Alhadeff, P.; Rosen, R.B.; Ritch, R.; Dubra, A.; Hood, D.C. Adaptive Optics Imaging of Healthy and Abnormal Regions of Retinal Nerve Fiber Bundles of Patients with Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 674–681. [Google Scholar] [CrossRef]
- Cilkova, M.; Matlach, J.; Chopra, R.; Rider, A.; Shah, N.; Mulholland, P.; Dakin, S.C.; Tufail, A.; Anderson, R.S. Repeatability and Inter-Observer Variability of in Vivo Retinal Cone Imaging Using a Modified Heidelberg Retinal Angiography (HRA2) in Normal Subjects. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4921. [Google Scholar]
- Meers, P.; Mealy, T. Calcium-Dependent Annexin V Binding to Phospholipids: Stoichiometry, Specificity, and the Role of Negative Charge. Biochemistry 1993, 32, 11711–11721. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.E.; Davis, B.M.; Guo, L.; Normando, E.M.; Cordeiro, M.F. Annexins in Glaucoma. Int. J. Mol. Sci. 2018, 19, 1218. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of Phosphatidylserine on the Surface of Apoptotic Lymphocytes Triggers Specific Recognition and Removal by Macrophages. J. Immunol. Baltim. Md 1950 1992, 148, 2207–2216. [Google Scholar]
- Adanja, I.; Debeir, O.; Mégalizzi, V.; Kiss, R.; Warzée, N.; Decaestecker, C. Automated Tracking of Unmarked Cells Migrating in Three-Dimensional Matrices Applied to Anti-Cancer Drug Screening. Exp. Cell Res. 2010, 316, 181–193. [Google Scholar] [CrossRef] [PubMed]
- D’Arceuil, H.; Rhine, W.; de Crespigny, A.; Yenari, M.; Tait, J.F.; Strauss, W.H.; Engelhorn, T.; Kastrup, A.; Moseley, M.; Blankenberg, F.G. 99mTc Annexin V Imaging of Neonatal Hypoxic Brain Injury. Stroke 2000, 31, 2692–2700. [Google Scholar] [CrossRef]
- Guo, L.; Moss, S.E.; Alexander, R.A.; Ali, R.R.; Fitzke, F.W.; Cordeiro, M.F. Retinal Ganglion Cell Apoptosis in Glaucoma Is Related to Intraocular Pressure and IOP-Induced Effects on Extracellular Matrix. Investig. Ophthalmol. Vis. Sci. 2005, 46, 175–182. [Google Scholar] [CrossRef]
- Guo, L.; Duggan, J.; Cordeiro, M.F. Alzheimer’s Disease and Retinal Neurodegeneration. Curr. Alzheimer Res. 2010, 7, 3–14. [Google Scholar] [CrossRef]
- Baltmr, A.; Duggan, J.; Nizari, S.; Salt, T.; Cordeiro, M. Neuroprotection in Glaucoma—Is There a Future Role? Exp. Eye Res. 2010, 91, 554–566. [Google Scholar] [CrossRef]
- Davis, B.M.; Tian, K.; Pahlitzsch, M.; Brenton, J.; Ravindran, N.; Butt, G.; Malaguarnera, G.; Normando, E.M.; Guo, L.; Cordeiro, M.F. Topical Coenzyme Q10 Demonstrates Mitochondrial-Mediated Neuroprotection in a Rodent Model of Ocular Hypertension. Mitochondrion 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Borrie, S.C.; Cheung, W.; Guo, L.; Barber, A.J.; Singh, R.S.J.; Gardner, T.W.; Cordeiro, M.F. Diabetic Retinal Neurodegeneration: In Vivo Imaging of Retinal Ganglion Cell Apoptosis in the Ins2Akita/J Mouse. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4924. [Google Scholar]
- Schmitz-Valckenberg, S.; Guo, L.; Cheung, W.; Moss, S.E.; Fitzke, F.W.; Cordeiro, M.F. [In vivo imaging of retinal cell apoptosis following acute light exposure]. Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2010, 107, 22–29. [Google Scholar] [CrossRef]
- Nizari, S.; Guo, L.; Davis, B.M.; Normando, E.M.; Galvao, J.; Turner, L.A.; Bizrah, M.; Dehabadi, M.; Tian, K.; Cordeiro, M.F. Non-Amyloidogenic Effects of A2 Adrenergic Agonists: Implications for Brimonidine-Mediated Neuroprotection. Cell Death Dis. 2016, 7, e2514. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Salt, T.E.; Maass, A.; Luong, V.; Moss, S.E.; Fitzke, F.W.; Cordeiro, M.F. Assessment of Neuroprotective Effects of Glutamate Modulation on Glaucoma-Related Retinal Ganglion Cell Apoptosis in Vivo. Investig. Ophthalmol. Vis. Sci. 2006, 47, 626–633. [Google Scholar] [CrossRef]
- Galvao, J.; Elvas, F.; Martins, T.; Cordeiro, M.F.; Ambrósio, A.F.; Santiago, A.R. Adenosine A3 Receptor Activation Is Neuroprotective against Retinal Neurodegeneration. Exp. Eye Res. 2015, 140, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Davis, B.; Nizari, S.; Normando, E.M.; Shi, H.; Galvao, J.; Turner, L.; Shi, J.; Clements, M.; Parrinello, S.; et al. Direct Optic Nerve Sheath (DONS) Application of Schwann Cells Prolongs Retinal Ganglion Cell Survival in Vivo. Cell Death Dis. 2014, 5, e1460. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Davis, B.M.; Ravindran, N.; Galvao, J.; Kapoor, N.; Haamedi, N.; Shamsher, E.; Luong, V.; Fico, E.; Cordeiro, M.F. Topical Recombinant Human Nerve Growth Factor (Rh-NGF) Is Neuroprotective to Retinal Ganglion Cells by Targeting Secondary Degeneration. Sci. Rep. 2020, 10, 3375. [Google Scholar] [CrossRef]
- Guo, L.; Salt, T.E.; Luong, V.; Wood, N.; Cheung, W.; Maass, A.; Ferrari, G.; Russo-Marie, F.; Sillito, A.M.; Cheetham, M.E.; et al. Targeting Amyloid-Beta in Glaucoma Treatment. Proc. Natl. Acad. Sci. USA 2007, 104, 13444–13449. [Google Scholar] [CrossRef]
- Salt, T.E.; Nizari, S.; Cordeiro, M.F.; Russ, H.; Danysz, W. Effect of the Aβ Aggregation Modulator MRZ-99030 on Retinal Damage in an Animal Model of Glaucoma. Neurotox. Res. 2014, 26, 440–446. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small Weinh. Bergstr. Ger. 2018, 14, 1701808. [Google Scholar] [CrossRef]
- Normando, E.M.; Davis, B.M.; De Groef, L.; Nizari, S.; Turner, L.A.; Ravindran, N.; Pahlitzsch, M.; Brenton, J.; Malaguarnera, G.; Guo, L.; et al. The Retina as an Early Biomarker of Neurodegeneration in a Rotenone-Induced Model of Parkinson’s Disease: Evidence for a Neuroprotective Effect of Rosiglitazone in the Eye and Brain. Acta Neuropathol. Commun. 2016, 4, 86. [Google Scholar] [CrossRef]
- Yoshimura, N. [Retinal neuronal cell death: Molecular mechanism and neuroprotection]. Nippon Ganka Gakkai Zasshi 2001, 105, 884–902. [Google Scholar] [CrossRef]
- Griffin, R.J.; Williams, B.W.; Bischof, J.C.; Olin, M.; Johnson, G.L.; Lee, B.W. Use of a Fluorescently Labeled Poly-Caspase Inhibitor for in Vivo Detection of Apoptosis Related to Vascular-Targeting Agent Arsenic Trioxide for Cancer Therapy. Technol. Cancer Res. Treat. 2007, 6, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Devi, T.S.; Hosoya, K.-I.; Terasaki, T.; Singh, L.P. Inhibition of TXNIP Expression in Vivo Blocks Early Pathologies of Diabetic Retinopathy. Cell Death Dis. 2010, 1, e65. [Google Scholar] [CrossRef]
- Bosco, A.; Crish, S.D.; Steele, M.R.; Romero, C.O.; Inman, D.M.; Horner, P.J.; Calkins, D.J.; Vetter, M.L. Early Reduction of Microglia Activation by Irradiation in a Model of Chronic Glaucoma. PLoS ONE 2012, 7, e43602. [Google Scholar] [CrossRef] [PubMed]
- Subramani, M.; Murugeswari, P.; Dhamodaran, K.; Chevour, P.; Gunasekaran, S.; Kumar, R.S.; Jayadev, C.; Shetty, R.; Begum, N.; Das, D. Short Pulse of Clinical Concentration of Bevacizumab Modulates Human Retinal Pigment Epithelial Functionality. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Dib, B.; Lin, H.; Maidana, D.E.; Tian, B.; Miller, J.B.; Bouzika, P.; Miller, J.W.; Vavvas, D.G. Mitochondrial DNA Has a Pro-Inflammatory Role in AMD. Biochim. Biophys. Acta 2015, 1853, 2897–2906. [Google Scholar] [CrossRef]
- O’Brien, M.A.; Daily, W.J.; Hesselberth, P.E.; Moravec, R.A.; Scurria, M.A.; Klaubert, D.H.; Bulleit, R.F.; Wood, K.V. Homogeneous, Bioluminescent Protease Assays: Caspase-3 as a Model. J. Biomol. Screen. 2005, 10, 137–148. [Google Scholar] [CrossRef]
- Scabini, M.; Stellari, F.; Cappella, P.; Rizzitano, S.; Texido, G.; Pesenti, E. In Vivo Imaging of Early Stage Apoptosis by Measuring Real-Time Caspase-3/7 Activation. Apoptosis Int. J. Program. Cell Death 2011, 16, 198–207. [Google Scholar] [CrossRef]
- Grimberg, H.; Levin, G.; Shirvan, A.; Cohen, A.; Yogev-Falach, M.; Reshef, A.; Ziv, I. Monitoring of Tumor Response to Chemotherapy in Vivo by a Novel Small-Molecule Detector of Apoptosis. Apoptosis Int. J. Program. Cell Death 2009, 14, 257–267. [Google Scholar] [CrossRef]
- Damianovich, M.; Ziv, I.; Heyman, S.N.; Rosen, S.; Shina, A.; Kidron, D.; Aloya, T.; Grimberg, H.; Levin, G.; Reshef, A.; et al. ApoSense: A Novel Technology for Functional Molecular Imaging of Cell Death in Models of Acute Renal Tubular Necrosis. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 281–291. [Google Scholar] [CrossRef]
- Shirvan, A.; Reshef, A.; Yogev-Falach, M.; Ziv, I. Molecular Imaging of Neurodegeneration by a Novel Cross-Disease Biomarker. Exp. Neurol. 2009, 219, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Ziv, I.; Aloya, T.; Levin, G.; Kidron, D.; Grimberg, H.; Reshef, A.; Shirvan, A. Monitoring of Chemotherapy-Induced Cell Death in Melanoma Tumors by N,N′-Didansyl-L-Cystine. Technol. Cancer Res. Treat. 2007, 6, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Shirvan, A.; Grimberg, H.; Reshef, A.; Yogev-Falach, M.; Cohen, A.; Ziv, I. Novel Fluorescence Molecular Imaging of Chemotherapy-Induced Intestinal Apoptosis. J. Biomed. Opt. 2009, 14, 054019. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Shirvan, A.; Antoni, G.; Gustavsson, S.-Å.; Långström, B.; Ringheim, A.; Sörensen, J.; Ben-Ami, M.; Ziv, I. 18F-ML-10, a PET Tracer for Apoptosis: First Human Study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2011, 52, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Heaton, G.R.; Davis, B.M.; Turner, L.A.; Cordeiro, M.F. Ocular Biomarkers of Alzheimer’s Disease. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 117–125. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Lee, R.; Grus, F. Molecular Biomarkers in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 121–131. [Google Scholar] [CrossRef]
- Kim, H.; Lee, T.; Lee, J.; Ahn, M.; Moon, C.; Wie, M.; Shin, T. Immunohistochemical Study of Caveolin-1 and -2 in the Rat Retina. J. Vet. Sci. 2006, 7, 101–104. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.; Hewitt, A.; Masson, G.; Helgason, A.; DeWan, A.; Sigurdsson, A.; Jonasdottir, A.; Gudjonsson, S.; Magnusson, K.; et al. Common Variants near CAV1 and CAV2 Are Associated with Primary Open-Angle Glaucoma. Nat. Genet. 2010, 42, 906–909. [Google Scholar] [CrossRef]
- Loo, J.; Lee, Y.; Woon, C.; Yong, V.; Tan, B.; Schmetterer, L.; Chong, R. Loss of Caveolin-1 Impairs Light Flicker-Induced Neurovascular Coupling at the Optic Nerve Head. Front. Neurosci. 2021, 15, 764898. [Google Scholar] [CrossRef]
- Ray, P.; Huang, B.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Kumar, D.; Agarwal, N. Oxidative Stress in Glaucoma: A Burden of Evidence. J. Glaucoma 2007, 16, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Hirooka, K.; Mizote, M.; Nakamura, T.; Itano, T.; Shiraga, F. Neuroprotection against Retinal Ischemia-Reperfusion Injury by Blocking the Angiotensin II Type 1 Receptor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Chitranshi, N.; Dheer, Y.; Abbasi, M.; You, Y.; Graham, S.; Gupta, V. Glaucoma Pathogenesis and Neurotrophins: Focus on the Molecular and Genetic Basis for Therapeutic Prospects. Curr. Neuropharmacol. 2018, 16, 1018–1035. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.-P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lewin, A.; Hauswirth, W.; Guy, J. Optic Neuropathy Induced by Reductions in Mitochondrial Superoxide Dismutase. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1088–1096. [Google Scholar] [CrossRef]
- Himori, N.; Yamamoto, K.; Maruyama, K.; Ryu, M.; Taguchi, K.; Yamamoto, M.; Nakazawa, T. Critical Role of Nrf2 in Oxidative Stress-Induced Retinal Ganglion Cell Death. J. Neurochem. 2013, 127, 669–680. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, D.; Xie, T.; Hao, J.; Malik, T.; Lu, C.; Qi, J.; Pant, O.; Lu, C. The Nrf2 Signaling in Retinal Ganglion Cells under Oxidative Stress in Ocular Neurodegenerative Diseases. Int. J. Biol. Sci. 2018, 14, 1090–1098. [Google Scholar] [CrossRef]
- Xu, Z.; Cho, H.; Hartsock, M.; Mitchell, K.; Gong, J.; Wu, L.; Wei, Y.; Wang, S.; Thimmulappa, R.; Sporn, M.; et al. Neuroprotective Role of Nrf2 for Retinal Ganglion Cells in Ischemia-Reperfusion. J. Neurochem. 2015, 133, 233–241. [Google Scholar] [CrossRef]
- Picard, E.; Daruich, A.; Youale, J.; Courtois, Y.; Behar-Cohen, F. From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology. Cells 2020, 9, 705. [Google Scholar] [CrossRef]
- Farkas, R.H.; Chowers, I.; Hackam, A.S.; Kageyama, M.; Nickells, R.W.; Otteson, D.C.; Duh, E.J.; Wang, C.; Valenta, D.F.; Gunatilaka, T.L.; et al. Increased Expression of Iron-Regulating Genes in Monkey and Human Glaucoma. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Gokulgandhi, M.; Mitra, A. Mitochondrial Dysfunction in Retinal Diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Lenaers, G.; Neutzner, A.; Le Dantec, Y.; Jüschke, C.; Xiao, T.; Decembrini, S.; Swirski, S.; Kieninger, S.; Agca, C.; Kim, U.; et al. Dominant Optic Atrophy: Culprit Mitochondria in the Optic Nerve. Prog. Retin. Eye Res. 2021, 83, 100935. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, Y.; Kitaoka, Y.; Kuribayashi, J.; Ueno, S. Modulation of Mitochondria in the Axon and Soma of Retinal Ganglion Cells in a Rat Glaucoma Model. J. Neurochem. 2010, 115, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Conti, T.; Hom, G.; Greenlee, T.; Raimondi, R.; Briskin, I.; Rich, C.; Kampani, R.; Engel, R.; Sharma, S.; et al. Functional Imaging of Mitochondria in Retinal Diseases Using Flavoprotein Fluorescence. Eye Lond. Engl. 2021, 35, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Mauro-Lizcano, M.; Esteban-Martínez, L.; Seco, E.; Serrano-Puebla, A.; Garcia-Ledo, L.; Figueiredo-Pereira, C.; Vieira, H.; Boya, P. New Method to Assess Mitophagy Flux by Flow Cytometry. Autophagy 2015, 11, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Tewari, S.; Goldberg, A.; Kowluru, R. Mitochondrial Biogenesis and the Development of Diabetic Retinopathy. Free Radic. Biol. Med. 2011, 51, 1849–1860. [Google Scholar] [CrossRef]
- Daugas, E.; Susin, S.; Zamzami, N.; Ferri, K.; Irinopoulou, T.; Larochette, N.; Prévost, M.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-Nuclear Translocation of AIF in Apoptosis and Necrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 729–739. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X. Caspase-Independent Component of Retinal Ganglion Cell Death, in Vitro. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4049–4059. [Google Scholar] [CrossRef]
- Zhang, S.; Sanders, E.; Fliesler, S.; Wang, J. Endoplasmic Reticulum Stress and the Unfolded Protein Responses in Retinal Degeneration. Exp. Eye Res. 2014, 125, 30–40. [Google Scholar] [CrossRef]
- Kasetti, R.; Patel, P.; Maddineni, P.; Patil, S.; Kiehlbauch, C.; Millar, J.C.; Searby, C.C.; Raghunathan, V.; Sheffield, V.C.; Zode, G.S. ATF4 Leads to Glaucoma by Promoting Protein Synthesis and ER Client Protein Load. Nat. Commun. 2020, 11, 5594. [Google Scholar] [CrossRef] [PubMed]
- Doh, S.; Kim, J.; Lee, K.; Park, H.; Park, C. Retinal Ganglion Cell Death Induced by Endoplasmic Reticulum Stress in a Chronic Glaucoma Model. Brain Res. 2010, 1308, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, K.; Reddy, S.S.; Reddy, G.B. Ubiquitin-Proteasome System and ER Stress in the Retina of Diabetic Rats. Arch. Biochem. Biophys. 2017, 627, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S.; Liu, Y.; Kim, B.; Clark, A.; Pang, I. Role of C/EBP Homologous Protein in Retinal Ganglion Cell Death after Ischemia/Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 2014, 56, 221–231. [Google Scholar] [CrossRef]
- Boya, P.; Esteban-Martínez, L.; Serrano-Puebla, A.; Gómez-Sintes, R.; Villarejo-Zori, B. Autophagy in the Eye: Development, Degeneration, and Aging. Prog. Retin. Eye Res. 2016, 55, 206–245. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Muela, N.; Germain, F.; Mariño, G.; Fitze, P.S.; Boya, P. Autophagy Promotes Survival of Retinal Ganglion Cells after Optic Nerve Axotomy in Mice. Cell Death Differ. 2012, 19, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Munemasa, Y.; Kwong, J.M.K.; Ahn, J.H.; Mareninov, S.; Gordon, L.K.; Caprioli, J.; Piri, N. Activation of autophagy in retinal ganglion cells. J. Neurosci. Res. 2008, 86, 2943–2951. [Google Scholar] [CrossRef]
- Park, H.-Y.L.; Kim, J.H.; Park, C.K. Activation of Autophagy Induces Retinal Ganglion Cell Death in a Chronic Hypertensive Glaucoma Model. Cell Death Dis. 2012, 3, e290. [Google Scholar] [CrossRef]
- Lee, S.; Shim, K.; Kim, C.; Park, T. Characterization of the Role of Autophagy in Retinal Ganglion Cell Survival over Time Using a Rat Model of Chronic Ocular Hypertension. Sci. Rep. 2021, 11, 5767. [Google Scholar] [CrossRef]
- Runwal, G.; Stamatakou, E.; Siddiqi, F.H.; Puri, C.; Zhu, Y.; Rubinsztein, D.C. LC3-Positive Structures Are Prominent in Autophagy-Deficient Cells. Sci. Rep. 2019, 9, 10147. [Google Scholar] [CrossRef]
- Fan, X.; Huang, T.; Tong, Y.; Fan, Z.; Yang, Z.; Yang, D.; Mao, X.; Yang, M. P62 Works as a Hub Modulation in the Ageing Process. Ageing Res. Rev. 2022, 73, 101538. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Munemasa, Y.; Kojima, K.; Hirano, A.; Ueno, S.; Takagi, H. Axonal Protection by Nmnat3 Overexpression with IPeng, J.Autophagy in Optic Nerve Degeneration. Cell Death Dis. 2013, 4, e860. [Google Scholar] [CrossRef] [PubMed]
- Adornetto, A.; Parisi, V.; Morrone, L.; Corasaniti, M.; Bagetta, G.; Tonin, P.; Russo, R. The Role of Autophagy in Glaucomatous Optic Neuropathy. Front. Cell Dev. Biol. 2020, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shosha, E.; Yokota, H.; Saul, A.; Rojas, M.; Caldwell, R.; Caldwell, R.; Narayanan, S. Arginase 2 Promotes Neurovascular Degeneration during Ischemia/Reperfusion Injury. Cell Death Dis. 2016, 7, e2483. [Google Scholar] [CrossRef]
- Peng, J.; Song, W.; Yao, F.; Zhang, X.; Peng, J.; Luo, X.; Xia, X. Involvement of Regulated Necrosis in Blinding Diseases: Focus on Necroptosis and Ferroptosis. Exp. Eye Res. 2020, 191, 107922. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Cell Type | Reference |
|---|---|---|
| Anti-NeuN | RGC and amacrine nucleus | [60,61,62,63] |
| Anti- cocaine- and amphetamine-regulated transcript (CART) | Superior Inferior and posterior ooDSGC subtypes, does not stain A/T-ooDSGC. Purkinje+ RGC R-RGC | [64,65,66,67] |
| Anti-matrix metalloprotease 17 (Mmp17) | ≤5% of all RGCs, some SACs 70% of MMP17 + RGCs are DRD4 + (subtype of N-ooDSGC) >90% of DRD4 + are Mmp17 + | [64] |
| Anti-cadherin 6 (Cdh6) | Marks both I-ooDSGC and S-ooDSGC in similar proportion. Marks SACs | [64] |
| Anti-collagen 25a1 (Col25a1) | Marks both I-ooDSGC and S-ooDSGC. Marks SACs | [64] |
| Anti-SMI 32 (neurofilaments) | Marks Alpha RGCs | [68] |
| Anti- osteopontin (SPP1) | Marks Alpha RGCs | [68] |
| Anti-Brn3b/Po4f2 | Marks Alpha RGCs (67+/5%). Functions as a co-marker of F-mini RGC | [68,69] |
| Anti-Paralbumin (PV) | Marks Alpha RGCs (73+/−4%) | [68] |
| Anti-Melanopsin | May works as co-marker of ON-sustained Alpha RGCs (M4) M1: soma, dendrites and axons up to the optic nerve M2: all cellular structures are marked M3: all cellular structures are marked M4: Soma and dendrites, only with tyramide signal amplification [41] M5: Weak perisomatic staining, greater percentage with tyramide signal amplification. M6: Weak soma staining, only with tyramide signal amplification. | [24,68,70,71,72] |
| Anti-Brn3a/Pou4f1 | May works as co-marker of ON Alpha RGCs. May be excluded from the ipsilateral pathway Marks >95% BD, DRD4, Cdh6-RGCs, and CART+ RGC | [64,68,73,74] |
| Anti-Brn3c/Pou4f1 | May work as co-marker of OFF-Transient Alpha RGCs | [68] |
| Anti-Calbindin | May work as co-marker of ON-Sustained Alpha RGCs | [68] |
| Anti-Foxp1 | Marks all ON F-RGC | [69] |
| Anti-Foxp2 | Marks all F-RGC | [69] |
| Anti-Calretinine | Marks S-BGC, M-BGC and B-BGC | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corral-Domenge, C.; de la Villa, P.; Mansilla, A.; Germain, F. Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration. Int. J. Mol. Sci. 2022, 23, 4287. https://doi.org/10.3390/ijms23084287
Corral-Domenge C, de la Villa P, Mansilla A, Germain F. Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration. International Journal of Molecular Sciences. 2022; 23(8):4287. https://doi.org/10.3390/ijms23084287
Chicago/Turabian StyleCorral-Domenge, Ciriaco, Pedro de la Villa, Alicia Mansilla, and Francisco Germain. 2022. "Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration" International Journal of Molecular Sciences 23, no. 8: 4287. https://doi.org/10.3390/ijms23084287
APA StyleCorral-Domenge, C., de la Villa, P., Mansilla, A., & Germain, F. (2022). Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration. International Journal of Molecular Sciences, 23(8), 4287. https://doi.org/10.3390/ijms23084287







