Cellular Computational Logic Using Toehold Switches
Abstract
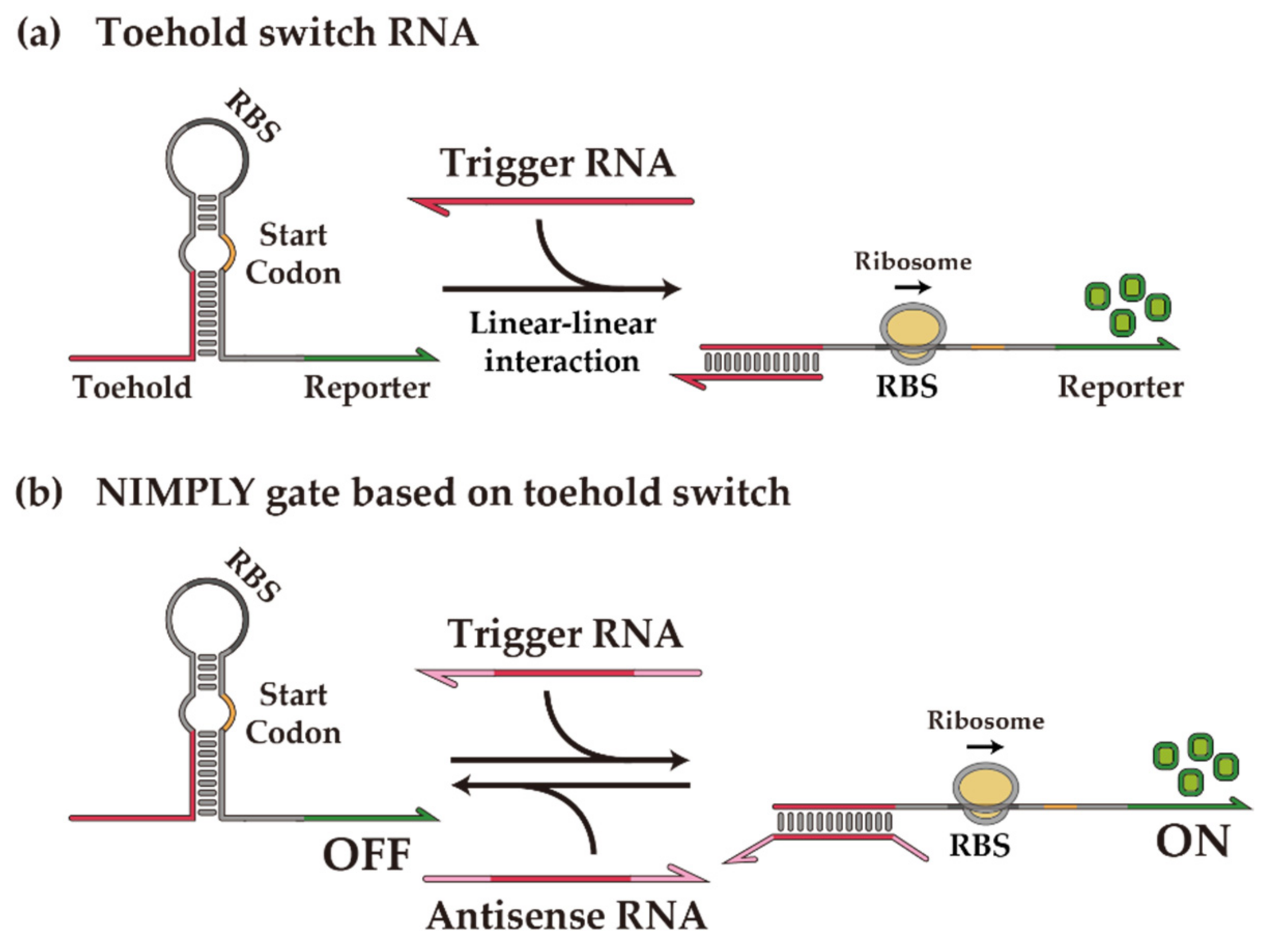
1. Introduction
2. Results
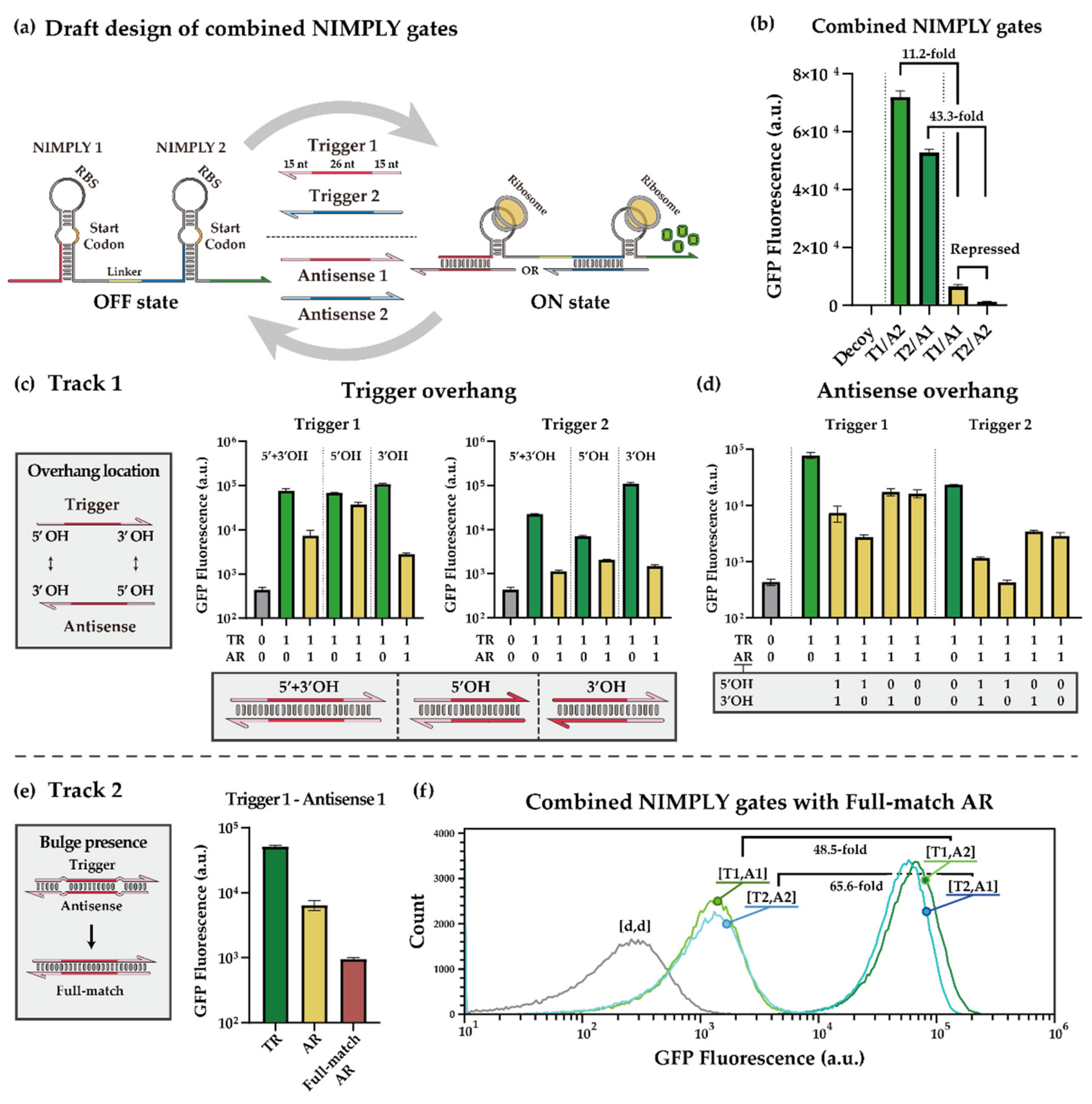
2.1. XOR Gate
2.1.1. Design of XOR Gate with Toehold Switches
2.1.2. Optimization Strategies for Toehold-Switch-Based XOR Gate
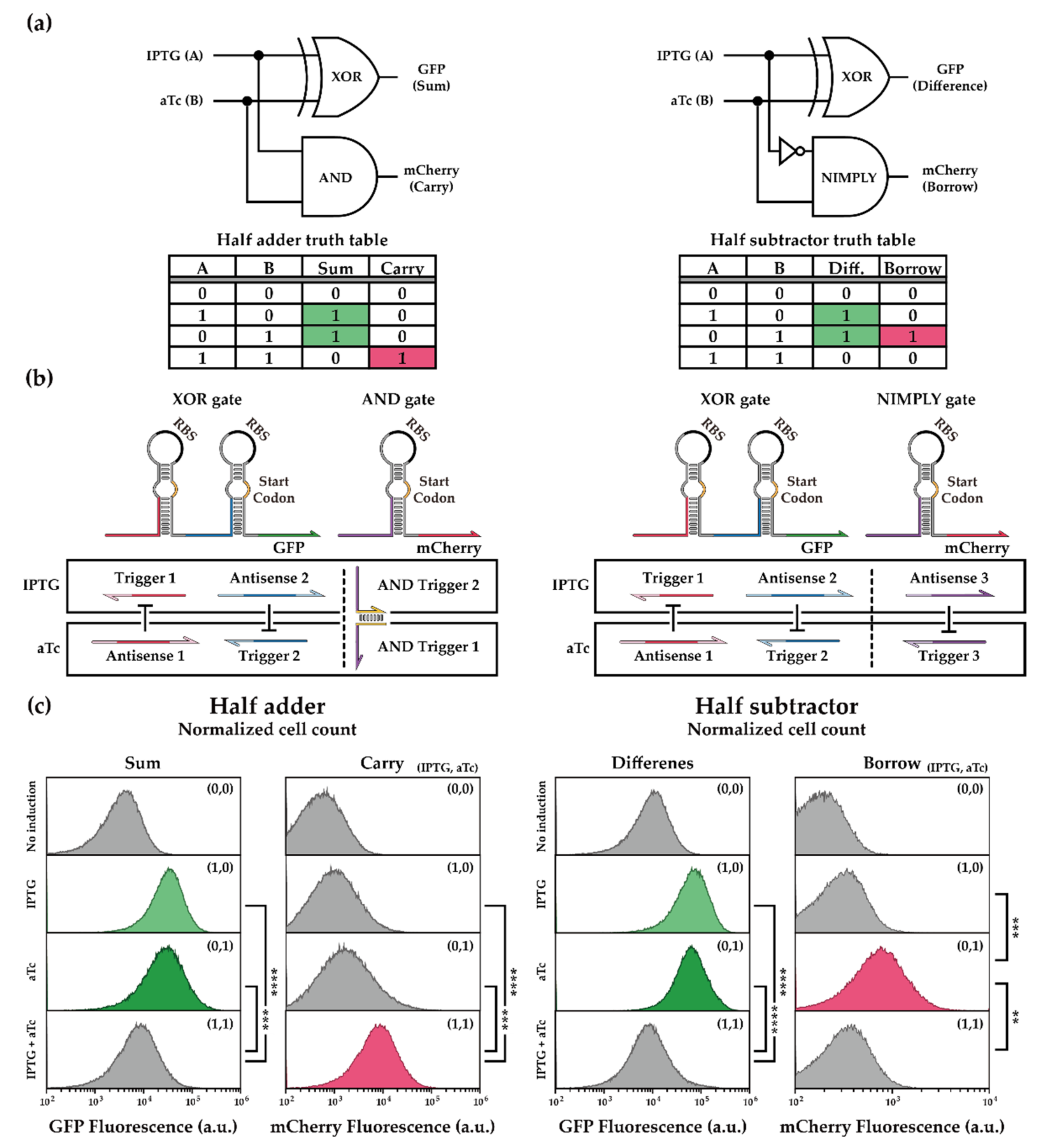
2.2. In Vivo Characterization of XOR Gate
2.2.1. Cellular Arithmetic Operation of a Half Adder and a Half Subtractor
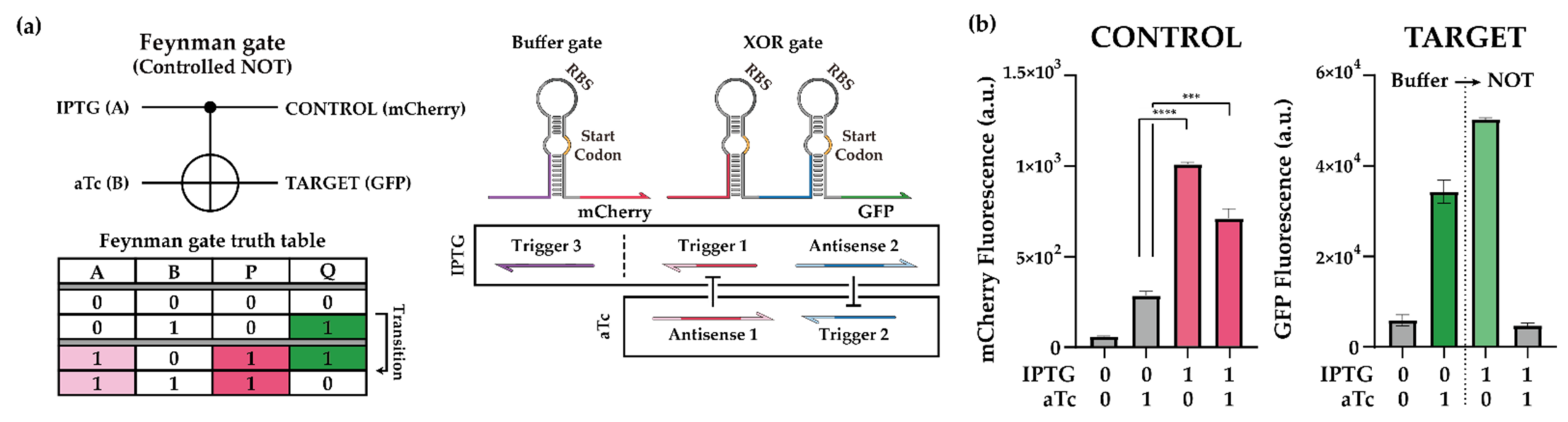
2.2.2. Cellular Reversible Logic Operation of Feynman Gate
3. Discussion
4. Materials and Methods
4.1. E. coli Strains and Plasmid Construction
4.2. Cell Culture and Induction Condition
4.3. Microplate Reader Analysis
4.4. Fluorescence Measurements Using Flow Cytometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cameron, D.E.; Bashor, C.J.; Collins, J.J. A brief history of synthetic biology. Nat. Rev. Microbiol 2014, 12, 381–390. [Google Scholar] [CrossRef]
- Gardner, T.S.; Cantor, C.R.; Collins, J.J. Construction of a genetic toggle switch in Escherichia coli. Nature 2000, 403, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Elowitz, M.B.; Leibler, S.A. synthetic oscillatory network of transcriptional regulators. Nature 2000, 403, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Weitz, M.; Kim, J.; Kapsner, K.; Winfree, E.; Franco, E.; Simmel, F.C. Diversity in the dynamical behaviour of a compartmentalized programmable biochemical oscillator. Nat. Chem. 2014, 6, 295–302. [Google Scholar] [CrossRef]
- Weinberg, B.H.; Pham, N.T.H.; Caraballo, L.D.; Lozanoski, T.; Engel, A.; Bhatia, S.; Wong, W.W. Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat. Biotechnol. 2017, 35, 453–462. [Google Scholar] [CrossRef]
- Auslander, D.; Auslander, S.; Pierrat, X.; Hellmann, L.; Rachid, L.; Fussenegger, M. Programmable full-adder computations in communicating three-dimensional cell cultures. Nat. Methods 2018, 15, 57–60. [Google Scholar] [CrossRef]
- Sexton, J.T.; Tabor, J.J. Multiplexing cell-cell communication. Mol. Syst. Biol. 2020, 16, e9618. [Google Scholar] [CrossRef]
- Munck, C.; Sheth, R.U.; Freedberg, D.E.; Wang, H.H. Recording mobile DNA in the gut microbiota using an Escherichia coli CRISPR-Cas spacer acquisition platform. Nat. Commun. 2020, 11, 95. [Google Scholar] [CrossRef]
- Shipman, S.L.; Nivala, J.; Macklis, J.D.; Church, G.M. CRISPR-Cas encoding of a digital movie into the genomes of a population of living bacteria. Nature 2017, 547, 345–349. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Chen, J.; Chirasani, V.R.; Tabdanov, E.D.; Dokholyan, N.V. Two-input protein logic gate for computation in living cells. Nat. Commun. 2021, 12, 6615. [Google Scholar] [CrossRef]
- Gao, X.J.; Chong, L.S.; Kim, M.S.; Elowitz, M.B. Programmable protein circuits in living cells. Science 2018, 361, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kibler, R.D.; Hunt, A.; Busch, F.; Pearl, J.; Jia, M.; VanAernum, Z.L.; Wicky, B.I.M.; Dods, G.; Liao, H.; et al. De novo design of protein logic gates. Science 2020, 368, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, J.; Yin, P.; Voigt, C.A. Genetic encoding of DNA nanostructures and their self-assembly in living bacteria. Nat. Commun. 2016, 7, 11179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Elowitz, M.B. Programmable protein circuit design. Cell 2021, 184, 2284–2301. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.C.; Nielsen, A.A.; Tamsir, A.; Clancy, K.; Peterson, T.; Voigt, C.A. Genomic mining of prokaryotic repressors for orthogonal logic gates. Nat. Chem. Biol. 2014, 10, 99–105. [Google Scholar] [CrossRef]
- Bolognesi, B.; Lehner, B. Reaching the limit. Elife 2018, 7, e39804. [Google Scholar] [CrossRef]
- Ceroni, F.; Boo, A.; Furini, S.; Gorochowski, T.E.; Borkowski, O.; Ladak, Y.N.; Awan, A.R.; Gilbert, C.; Stan, G.B.; Ellis, T. Burden-driven feedback control of gene expression. Nat. Methods 2018, 15, 387–393. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, Y.; Zhang, Y.; Li, Y. Engineering the robustness of industrial microbes through synthetic biology. Trends Microbiol. 2012, 20, 94–101. [Google Scholar] [CrossRef]
- Purnick, P.E.; Weiss, R. The second wave of synthetic biology: From modules to systems. Nat. Rev. Mol. Cell. Biol. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Kim, J.; White, K.S.; Winfree, E. Construction of an in vitro bistable circuit from synthetic transcriptional switches. Mol. Syst. Biol. 2006, 2, 68. [Google Scholar] [CrossRef]
- Qian, L.; Winfree, E. Scaling up digital circuit computation with DNA strand displacement cascades. Science 2011, 332, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Winfree, E.; Bruck, J. Neural network computation with DNA strand displacement cascades. Nature 2011, 475, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Cherry, K.M.; Qian, L. Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. Nature 2018, 559, 370–376. [Google Scholar] [CrossRef]
- Su, H.; Xu, J.; Wang, Q.; Wang, F.; Zhou, X. High-efficiency and integrable DNA arithmetic and logic system based on strand displacement synthesis. Nat. Commun. 2019, 10, 5390. [Google Scholar] [CrossRef]
- Yordanov, B.; Kim, J.; Petersen, R.L.; Shudy, A.; Kulkarni, V.V.; Phillips, A. Computational Design of Nucleic Acid Feedback Control Circuits. ACS Synth. Biol. 2014, 3, 600–616. [Google Scholar] [CrossRef]
- Lakin, M.R.; Youssef, S.; Polo, F.; Emmott, S.; Phillips, A. Visual DSD: A design and analysis tool for DNA strand displacement systems. Bioinformatics 2011, 27, 3211–3213. [Google Scholar] [CrossRef]
- Groves, B.; Chen, Y.J.; Zurla, C.; Pochekailov, S.; Kirschman, J.L.; Santangelo, P.J.; Seelig, G. Computing in mammalian cells with nucleic acid strand exchange. Nat. Nanotechnol. 2016, 11, 287–294. [Google Scholar] [CrossRef]
- Choi, H.M.; Chang, J.Y.; Trinh le, A.; Padilla, J.E.; Fraser, S.E.; Pierce, N.A. Programmable in situ amplification for multiplexed imaging of mRNA expression. Nat. Biotechnol. 2010, 28, 1208–1212. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 2011, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Pan, T.; Sosnick, T. RNA folding during transcription. Annu Rev. Biophys. Biomol. Struct. 2006, 35, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Watters, K.E.; Strobel, E.J.; Yu, A.M.; Lis, J.T.; Lucks, J.B. Cotranscriptional folding of a riboswitch at nucleotide resolution. Nat. Struct. Mol. Biol. 2016, 23, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Rammohan, J.; Anderson, D.A.; Alperovich, N.; Ross, D.; Voigt, C.A. Single-cell measurement of plasmid copy number and promoter activity. Nat. Commun 2021, 12, 1475. [Google Scholar] [CrossRef] [PubMed]
- Itzkovitz, S.; van Oudenaarden, A. Validating transcripts with probes and imaging technology. Nat. Methods 2011, 8, S12–S19. [Google Scholar] [CrossRef]
- Coller, J.; Wickens, M. Tethered function assays: An adaptable approach to study RNA regulatory proteins. Methods Enzymol. 2007, 429, 299–321. [Google Scholar]
- Woo, C.H.; Jang, S.; Shin, G.; Jung, G.Y.; Lee, J.W. Sensitive fluorescence detection of SARS-CoV-2 RNA in clinical samples via one-pot isothermal ligation and transcription. Nat. Biomed. Eng. 2020, 4, 1168–1179. [Google Scholar] [CrossRef]
- Santiago-Frangos, A.; Hall, L.N.; Nemudraia, A.; Nemudryi, A.; Krishna, P.; Wiegand, T.; Wilkinson, R.A.; Snyder, D.T.; Hedges, J.F.; Cicha, C.; et al. Intrinsic signal amplification by type III CRISPR-Cas systems provides a sequence-specific SARS-CoV-2 diagnostic. Cell Rep. Med. 2021, 2, 100319. [Google Scholar] [CrossRef]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Isaacs, F.J.; Dwyer, D.J.; Ding, C.; Pervouchine, D.D.; Cantor, C.R.; Collins, J.J. Engineered riboregulators enable post-transcriptional control of gene expression. Nat. Biotechnol. 2004, 22, 841–847. [Google Scholar] [CrossRef]
- Green, A.A.; Silver, P.A.; Collins, J.J.; Yin, P. Toehold switches: De-novo-designed regulators of gene expression. Cell 2014, 159, 925–939. [Google Scholar] [CrossRef]
- Chappell, J.; Takahashi, M.K.; Lucks, J.B. Creating small transcription activating RNAs. Nat. Chem. Biol. 2015, 11, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.; Westbrook, A.; Verosloff, M.; Lucks, J.B. Computational design of small transcription activating RNAs for versatile and dynamic gene regulation. Nat. Commun. 2017, 8, 1051. [Google Scholar] [CrossRef]
- Jang, S.; Jang, S.; Xiu, Y.; Kang, T.J.; Lee, S.-H.; Koffas, M.A.A.G.; Jung, G.Y. Development of Artificial Riboswitches for Monitoring of Naringenin In Vivo. ACS Synth. Biol. 2017, 6, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Hanewich-Hollatz, M.H.; Chen, Z.; Hochrein, L.M.; Huang, J.; Pierce, N.A. Conditional Guide RNAs: Programmable Conditional Regulation of CRISPR/Cas Function in Bacterial and Mammalian Cells via Dynamic RNA Nanotechnology. ACS Cent. Sci. 2019, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Serganov, A.; Nudler, E. A Decade of Riboswitches. Cell 2013, 152, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Kim, J.; Ma, D.; Silver, P.A.; Collins, J.J.; Yin, P. Complex cellular logic computation using ribocomputing devices. Nature 2017, 548, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhou, Y.; Carlson, P.D.; Teichmann, M.; Chaudhary, S.; Simmel, F.C.; Silver, P.A.; Collins, J.J.; Lucks, J.B.; Yin, P.; et al. De novo-designed translation-repressing riboregulators for multi-input cellular logic. Nat. Chem. Biol. 2019, 15, 1173–1182. [Google Scholar] [CrossRef]
- Hong, S.; Jeong, D.; Ryan, J.; Foo, M.; Tang, X.; Kim, J. Design and evaluation of synthetic RNA-based incoherent feed-forward loop circuits. Biomolecules 2021, 11, 1182. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.; Kim, J. Multilevel Gene Regulation Using Switchable Transcription Terminator and Toehold Switch in Escherichia coli. Appl. Sci. 2021, 11, 4532. [Google Scholar] [CrossRef]
- Yang, J.; Han, Y.H.; Im, J.; Seo, S.W. Synthetic protein quality control to enhance full-length translation in bacteria. Nat. Chem. Biol. 2021, 17, 421–427. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, S.G.; Jang, S.; Kim, J.; Jung, G.Y. Signal amplification and optimization of riboswitch-based hybrid inputs by modular and titratable toehold switches. J. Biol. Eng. 2021, 15, 11. [Google Scholar] [CrossRef]
- Zhao, E.M.; Mao, A.S.; de Puig, H.; Zhang, K.; Tippens, N.D.; Tan, X.; Ran, F.A.; Han, I.; Nguyen, P.Q.; Chory, E.J.; et al. RNA-responsive elements for eukaryotic translational control. Nat. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Huang, A.; Nguyen, P.Q.; Stark, J.C.; Takahashi, M.K.; Donghia, N.; Ferrante, T.; Dy, A.J.; Hsu, K.J.; Dubner, R.S.; Pardee, K.; et al. BioBits™ Explorer: A modular synthetic biology education kit. Sci. Adv. 2018, 4, eaat5105. [Google Scholar] [CrossRef] [PubMed]
- McNerney, M.P.; Zhang, Y.; Steppe, P.; Silverman, A.D.; Jewett, M.C.; Styczynski, M.P. Point-of-care biomarker quantification enabled by sample-specific calibration. Sci. Adv. 2019, 5, eaax4473. [Google Scholar] [CrossRef] [PubMed]
- Sadat Mousavi, P.; Smith, S.J.; Chen, J.B.; Karlikow, M.; Tinafar, A.; Robinson, C.; Liu, W.; Ma, D.; Green, A.A.; Kelley, S.O.; et al. A multiplexed, electrochemical interface for gene-circuit-based sensors. Nat. Chem. 2020, 12, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.; Soenksen, L.R.; Donghia, N.M.; Angenent-Mari, N.M.; de Puig, H.; Huang, A.; Lee, R.; Slomovic, S.; Galbersanini, T.; Lansberry, G.; et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 2021, 39, 1366–1374. [Google Scholar] [CrossRef]
- Amalfitano, E.; Karlikow, M.; Norouzi, M.; Jaenes, K.; Cicek, S.; Masum, F.; Sadat Mousavi, P.; Guo, Y.; Tang, L.; Sydor, A.; et al. A glucose meter interface for point-of-care gene circuit-based diagnostics. Nat. Commun. 2021, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.K.; Tan, X.; Dy, A.J.; Braff, D.; Akana, R.T.; Furuta, Y.; Donghia, N.; Ananthakrishnan, A.; Collins, J.J. A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers. Nat. Commun. 2018, 9, 3347. [Google Scholar] [CrossRef]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef]
- Pardee, K.; Green, A.A.; Ferrante, T.; Cameron, D.E.; DaleyKeyser, A.; Yin, P.; Collins, J.J. Paper-Based Synthetic Gene Networks. Cell 2014, 159, 940–954. [Google Scholar] [CrossRef]
- Wong, A.; Wang, H.; Poh, C.L.; Kitney, R.I. Layering genetic circuits to build a single cell, bacterial half adder. BMC Biol. 2015, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Rosado, A.; Cordero, T.; Rodrigo, G. Binary addition in a living cell based on riboregulation. PLoS Genet. 2018, 14, e1007548. [Google Scholar] [CrossRef] [PubMed]
- Goldsworthy, V.; LaForce, G.; Abels, S.; Khisamutdinov, E.F. Fluorogenic RNA Aptamers: A Nano-platform for Fabrication of Simple and Combinatorial Logic Gates. Nanomaterials 2018, 8, 984. [Google Scholar] [CrossRef]
- Buchler, N.E.; Gerland, U.; Hwa, T. On schemes of combinatorial transcription logic. Proc. Natl. Acad. Sci. USA 2003, 100, 5136–5141. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.; Yin, P.; Ortiz, M.E.; Subsoontorn, P.; Endy, D. Amplifying genetic logic gates. Science 2013, 340, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Auslander, S.; Auslander, D.; Muller, M.; Wieland, M.; Fussenegger, M. Programmable single-cell mammalian biocomputers. Nature 2012, 487, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Ono, H.; Kawasaki, S.; Kuang, Y.; Fujita, Y.; Saito, H. Synthetic RNA-based logic computation in mammalian cells. Nat. Commun. 2018, 9, 4847. [Google Scholar] [CrossRef]
- Kim, H.; Bojar, D.; Fussenegger, M. A CRISPR/Cas9-based central processing unit to program complex logic computation in human cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7214–7219. [Google Scholar] [CrossRef]
- Fratto, B.E.; Katz, E. Reversible Logic Gates Based on Enzyme-Biocatalyzed Reactions and Realized in Flow Cells: A Modular Approach. ChemPhysChem 2015, 16, 1405–1415. [Google Scholar] [CrossRef]
- Tan, S.-I.; Ng, I.S. CRISPRi-Mediated NIMPLY Logic Gate for Fine-Tuning the Whole-Cell Sensing toward Simple Urine Glucose Detection. ACS Synth. Biol. 2021, 10, 412–421. [Google Scholar] [CrossRef]
- Fornace, M.E.; Porubsky, N.J.; Pierce, N.A. A Unified Dynamic Programming Framework for the Analysis of Interacting Nucleic Acid Strands: Enhanced Models, Scalability, and Speed. ACS Synth. Biol. 2020, 9, 2665–2678. [Google Scholar] [CrossRef] [PubMed]
- Dirks, R.M.; Bois, J.S.; Schaeffer, J.M.; Winfree, E.; Pierce, N.A. Thermodynamic Analysis of Interacting Nucleic Acid Strands. SIAM Rev. 2007, 49, 65–88. [Google Scholar] [CrossRef]
- Dirks, R.M.; Pierce, N.A. An algorithm for computing nucleic acid base-pairing probabilities including pseudoknots. J. Comput. Chem. 2004, 25, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Dirks, R.M.; Pierce, N.A. A partition function algorithm for nucleic acid secondary structure including pseudoknots. J. Comput. Chem. 2003, 24, 1664–1677. [Google Scholar] [CrossRef]
- Wolfe, B.R.; Pierce, N.A. Sequence Design for a Test Tube of Interacting Nucleic Acid Strands. ACS Synth. Biol. 2015, 4, 1086–1100. [Google Scholar] [CrossRef]
- Nicholson, A.W. Ribonuclease III mechanisms of double-stranded RNA cleavage. Wiley Interdiscip. Rev. RNA 2014, 5, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Court, D.L.; Gan, J.; Liang, Y.H.; Shaw, G.X.; Tropea, J.E.; Costantino, N.; Waugh, D.S.; Ji, X. RNase III: Genetics and function; structure and mechanism. Annu Rev. Genet. 2013, 47, 405–431. [Google Scholar] [CrossRef]
- Zadeh, J.N.; Wolfe, B.R.; Pierce, N.A. Nucleic acid sequence design via efficient ensemble defect optimization. J. Comput. Chem. 2011, 32, 439–452. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Winfree, E. Control of DNA Strand Displ.lacement Kinetics Using Toehold Exchange. J. Am. Chem. Soc. 2009, 131, 17303–17314. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Chen, S.X.; Yin, P. Optimizing the specificity of nucleic acid hybridization. Nat. Chem. 2012, 4, 208–214. [Google Scholar] [CrossRef]
- Mancuso, C.P.; Kiriakov, S.; Khalil, A.S. Cellular Advantages to Signaling in a Digital World. Cell Syst. 2016, 3, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Rubens, J.R.; Selvaggio, G.; Lu, T.K. Synthetic mixed-signal computation in living cells. Nat. Commun. 2016, 7, 11658. [Google Scholar] [CrossRef]
- Balazsi, G.; van Oudenaarden, A.; Collins, J.J. Cellular decision making and biological noise: From microbes to mammals. Cell 2011, 144, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.M.; Schleif, R.F. A Regulatory Cascade in the Induction of rhaBAD. J. Mol. Biol. 1993, 234, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef]
- Ruegg, T.L.; Pereira, J.H.; Chen, J.C.; DeGiovanni, A.; Novichkov, P.; Mutalik, V.K.; Tomaleri, G.P.; Singer, S.W.; Hillson, N.J.; Simmons, B.A.; et al. Jungle Express is a versatile repressor system for tight transcriptional control. Nat. Commun. 2018, 9, 3617. [Google Scholar] [CrossRef]
- Tamsir, A.; Tabor, J.J.; Voigt, C.A. Robust multicellular computing using genetically encoded NOR gates and chemical ‘wires’. Nature 2011, 469, 212–215. [Google Scholar] [CrossRef]
- Regot, S.; Macia, J.; Conde, N.; Furukawa, K.; Kjellen, J.; Peeters, T.; Hohmann, S.; de Nadal, E.; Posas, F.; Sole, R. Distributed biological computation with multicellular engineered networks. Nature 2011, 469, 207–211. [Google Scholar] [CrossRef]
- Osmekhina, E.; Jonkergouw, C.; Schmidt, G.; Jahangiri, F.; Jokinen, V.; Franssila, S.; Linder, M.B. Controlled communication between physically separated bacterial populations in a microfluidic device. Commun. Biol. 2018, 1, 97. [Google Scholar] [CrossRef]
- Callura, J.M.; Dwyer, D.J.; Isaacs, F.J.; Cantor, C.R.; Collins, J.J. Tracking, tuning, and terminating microbial physiology using synthetic riboregulators. Proc. Natl. Acad. Sci. USA 2010, 107, 15898. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, F.; Algar, R.; Stan, G.-B.; Ellis, T. Quantifying cellular capacity identifies gene expression designs with reduced burden. Nat. Methods 2015, 12, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, D.; Wu, F.; Standage-Beier, K.; Chen, X.; Wu, K.; Green, A.A.; Wang, X. Predictable control of RNA lifetime using engineered degradation-tuning RNAs. Nat. Chem. Biol. 2021, 17, 828–836. [Google Scholar] [CrossRef]
- Bhat, G.J.; Lodes, M.J.; Myler, P.J.; Stuart, K.D. A simple method for cloning blunt ended DNA fragments. Nucleic Acids. Res. 1991, 19, 398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., III; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Tian, J. Circular polymerase extension cloning for high-throughput cloning of complex and combinatorial DNA libraries. Nat. Protoc. 2011, 6, 242–251. [Google Scholar] [CrossRef]
- Liu, H.; Naismith, J.H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol 2008, 8, 91. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. The Inoue Method for Preparation and Transformation of Competent Escherichia coli: “Ultracompetent” Cells. Cold Spring Harb. Protoc. 2020, 2020, 101196. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Lee, G.; Kim, J. Cellular Computational Logic Using Toehold Switches. Int. J. Mol. Sci. 2022, 23, 4265. https://doi.org/10.3390/ijms23084265
Choi S, Lee G, Kim J. Cellular Computational Logic Using Toehold Switches. International Journal of Molecular Sciences. 2022; 23(8):4265. https://doi.org/10.3390/ijms23084265
Chicago/Turabian StyleChoi, Seungdo, Geonhu Lee, and Jongmin Kim. 2022. "Cellular Computational Logic Using Toehold Switches" International Journal of Molecular Sciences 23, no. 8: 4265. https://doi.org/10.3390/ijms23084265
APA StyleChoi, S., Lee, G., & Kim, J. (2022). Cellular Computational Logic Using Toehold Switches. International Journal of Molecular Sciences, 23(8), 4265. https://doi.org/10.3390/ijms23084265





