Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials
Abstract
:1. Introduction
2. Nanoliposomes
3. Application of Nanoliposomes in Cancer Therapy
4. Clinical Trials with Nanoliposomes in Cancer Therapy
- The efficacy and safety of treatment with Paclitaxel liposome and S-1 as first-line therapy in advanced pancreatic cancer patients aged between 18 and 75 years [78] is under investigation. This study is still in the preparation phase, and patient recruitment has not yet been completed.
- At the same stage is another study in adult women with epithelial ovarian cancer, to compare the efficacy of the combination of pegylated liposomal doxorubicin with carboplatin (experimental study group) versus the combination of paclitaxel with carboplatin (active comparator) [79].
- Rhenium Nanoliposomes in recurrent glioma (ReSPECT) [80]. Rhenium 186 emits beta and gamma particles and has a half-life of 3.8 days. It can complex with hydroxyethylene diphosphonic acid to target it to bone. It has been studied in patients with metastatic cancers. It has shown both analgesic and therapeutic effects. In this clinical dual-phase 1/2 trial, its maximum tolerated dose, safety, and efficacy have been studied. Its completion is planned for 2025.
- C6 Ceramide NanoLiposome (CNL) in patients with relapsed/refractory acute myeloid leukemia [81] is an interesting phase 1 study to assess if combinations with this type of liposomes improve the clinical therapeutic index of already known antineoplastics in myeloid leukemia [82]. Completion is scheduled for June 2022.
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
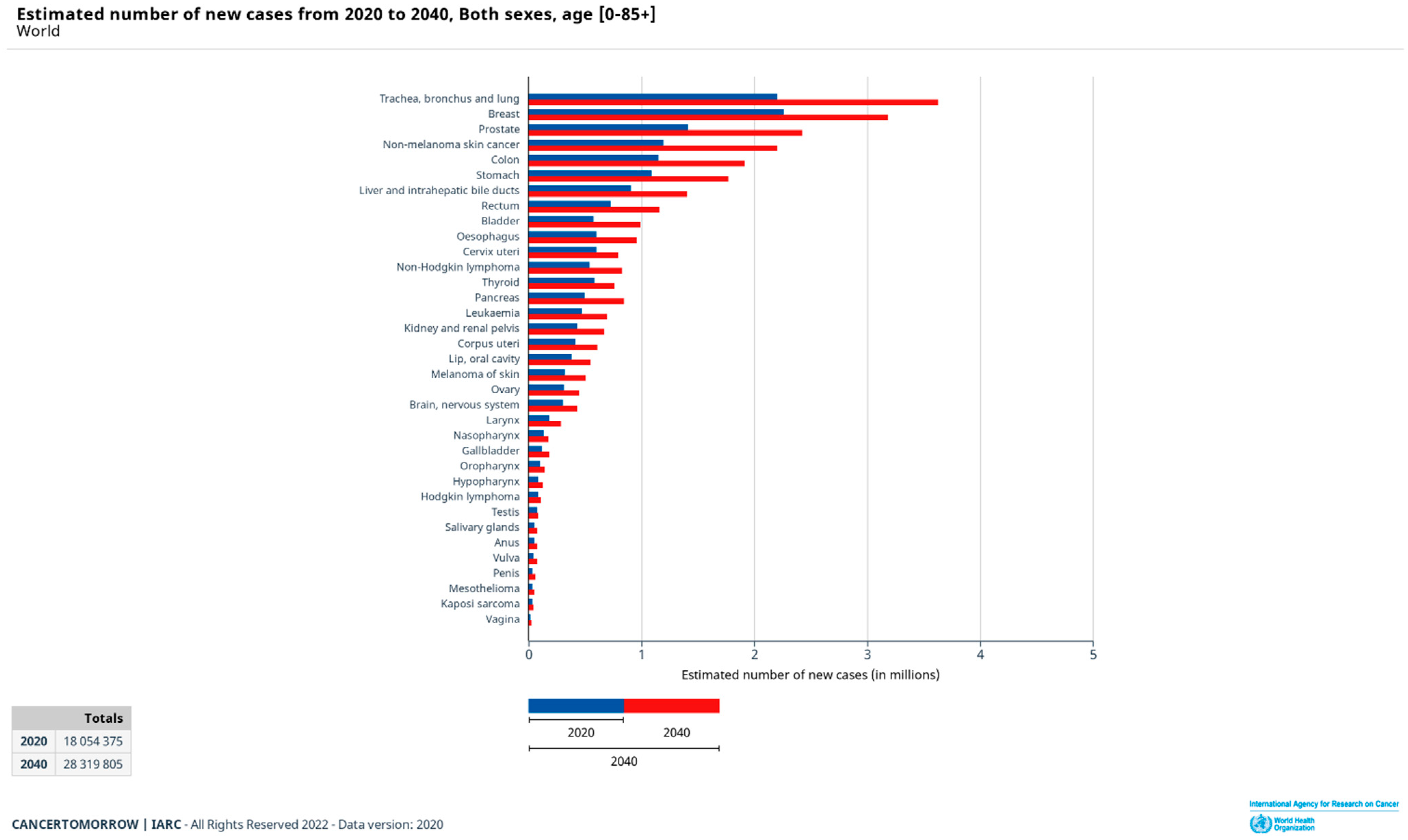
- International Agency for Research on Cancer. Elaborated Online on the Website Global Cancer Observatory. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/bars?types=0&sexes=0&mode=cancer&group_populations=0&multiple_populations=0&multiple_cancers=1&cancers=10_9_1_3_5_7_12_16_36_2_4_6_8_13_11_14_15_17_18_19_20_21_23_22_24_25_26_27_28_29_30_31_32_33_34&populations=900&bar_mode=stacked&group_cancers=0&key=total&show_bar_mode_prop=1 (accessed on 17 February 2022).
- Abd Elkodous, M.; El-Sayyad, G.S.; Abdelrahman, I.Y.; El-Bastawisy, H.S.; Mohamed, A.E.; Mosallam, F.M.; Nasser, H.A.; Gobara, M.; Baraka, A.; Elsayed, M.A.; et al. Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. B Biointerfaces 2019, 180, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bargel, H.; Scheibel, T. Recombinant Spider Silk–Silica Hybrid Scaffolds with Drug-Releasing Properties for Tissue Engineering Applications. Macromol. Rapid Commun. 2019, 41, 1900426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. Nanotechnology. Available online: https://www.ema.europa.eu/en/glossary/nanotechnology (accessed on 20 February 2022).
- Boey, A.; Ho, H.K. All Roads Lead to the Liver: Metal Nanoparticles and Their Implications for Liver Health. Small 2020, 16, 2000153. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Carreño, F.; Paese, K.; Silva, C.M.; Guterres, S.S.; Dalla Costa, T. Pre-Clinical Investigation of the Modulation of Quetiapine Plasma Pharmacokinetics and Tissues Biodistribution by Lipid-Core Nanocapsules. J. Pharm. Biomed. Anal. 2016, 119, 152–158. [Google Scholar] [CrossRef]
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyás, B. Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Adv. Sci. 2016, 4, 1600279. [Google Scholar] [CrossRef]
- Fontana, M.C.; Laureano, J.V.; Forgearini, B.; dos Santos, J.; Pohlmann, A.R.; Guterres, S.S.; de Araujo, B.V.; Beck, R.C. Spray-Dried Raloxifene Submicron Particles for Pulmonary Delivery: Development and in Vivo Pharmacokinetic Evaluation in Rats. Int. J. Pharm. 2020, 585, 119429. [Google Scholar] [CrossRef]
- Teleanu, D.; Chircov, C.; Grumezescu, A.; Teleanu, R. Neurotoxicity of Nanomaterials: An up-to-Date Overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.R.; Guterres, S.S. Poly(ϵ-Caprolactone) Microcapsules and Nanocapsules in Drug Delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An Update on Anticancer Molecular Action, Toxicity and Novel Drug Delivery Systems. J. Pharm. Pharmacol. 2012, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Ilić-Stojanović, S.; Petrović, S.; Tačić, A.; Nikolić, L. Administration Routes for Nano Drugs and Characterization of Nano Drug Loading. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 587–625. [Google Scholar]
- Nabi, B.; Rehman, S.; Baboota, S.; Ali, J. Insights on Oral Drug Delivery of Lipid Nanocarriers: A Win-Win Solution for Augmenting Bioavailability of Antiretroviral Drugs. AAPS PharmSciTech 2019, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Allaw, M.; Pleguezuelos-Villa, M.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Nacher, A.; Diez-Sales, O.; Saurí, A.R.; Ferrer, E.E.; Fadda, A.M.; et al. Innovative Strategies to Treat Skin Wounds with Mangiferin: Fabrication of Transfersomes Modified with Glycols and Mucin. Nanomedicine 2020, 15, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.A.; Maldonado, M.; Chen, J.; Zhong, Y.; Gu, J. Development and Evaluation of Curcumin Encapsulated Self-Assembled Nanoparticles as Potential Remedial Treatment for PCOS in a Female Rat Model. Int. J. Nanomed. 2021, 16, 6231–6247. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Fathi, M.; Aboutorabzade, S.M.; Sabbagh, F. Development of Curcumin-Loaded Prunus Armeniaca Gum Nanoparticles: Synthesis, Characterization, Control Release Behavior, and Evaluation of Anticancer and Antimicrobial Properties. Food Sci. Nutr. 2021, 9, 6109–6119. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, L.; Singh, G.; Dhawan, R.K.; Mahajan, A. Nanotechnology Based Herbal Formulations: A Survey of Recent Patents, Advancements and Transformative Headways. Recent Pat. Nanotechnol. 2022, 16, 295–307. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, Stimuli-Sensitive Nanoparticulate Systems for Drug Delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Bangham, A.D. Lipid bilayers and biomembranes. Annu. Rev. Biochem. 1972, 41, 753–776. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Khosravi-Darani, K.; Borazan, G.G.; Cui, J.; Pardakhty, A.; Yurdugul, S. Encapsulation of Food Ingredients Using Nanoliposome Technology. Int. J. Food Prop. 2008, 11, 833–844. [Google Scholar] [CrossRef]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery. Appl. Nanotechnol. Drug Deliv. 2014, 1, 1–50. [Google Scholar]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Singh, J.; Garg, T.; Rath, G.; Goyal, A.K. Advances in Nanotechnology-Based Carrier Systems for Targeted Delivery of Bioactive Drug Molecules with Special Emphasis on Immunotherapy in Drug Resistant Tuberculosis—A Critical Review. Drug Deliv. 2015, 23, 1676–1698. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D. Liposomes: The babraham connection. Chem. Phys. Lipids 1993, 64, 275–285. [Google Scholar] [CrossRef]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 5005. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Aguirre, Y.; Aguado-Castrejón, K.; González-Méndez, I. La Nanomedicina y Los Sistemas De Liberación De Fármacos: ¿La (R)Evolución De La Terapia Contra El Cáncer? Educ. Química 2016, 27, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, Y.; Deng, Y.; Wang, Y.; Ke, X.; Ci, T. Effects of Surface Charge of Low Molecular Weight Heparin-Modified Cationic Liposomes on Drug Efficacy and Toxicity. Drug Dev. Ind. Pharm. 2017, 43, 1163–1172. [Google Scholar] [CrossRef]
- Tereshkina, Y.A.; Torkhovskaya, T.I.; Tikhonova, E.G.; Kostryukova, L.V.; Sanzhakov, M.A.; Korotkevich, E.I.; Khudoklinova, Y.Y.; Orlova, N.A.; Kolesanova, E.F. Nanoliposomes as Drug Delivery Systems: Safety Concerns. J. Drug Target. 2021, 30, 313–325. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Qin, L.; Song, R.; Su, J.; Yuan, Y.; Zhang, X.; Mao, S. Elucidating Inhaled Liposome Surface Charge on Its Interaction with Biological Barriers in the Lung. Eur. J. Pharm. Biopharm. 2022, 172, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, H.; Vertut-Doi, A.; Hirose, T.; Miyajima, K. Physical-Chemistry Characteristics and Biodistribution of Poly(ethylene glycol)-Coated Liposomes Using Poly(oxyethylene) Cholesteryl Ether. Chem. Pharm. Bull. 1995, 43, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Ferreira, H.; Cavaco-Paulo, A. Sonoproduction of Liposomes and Protein Particles as Templates for Delivery Purposes. Biomacromolecules 2011, 12, 3353–3368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, N. Gadolinium Loaded Nanoparticles in Theranostic Magnetic Resonance Imaging. Biomaterials 2012, 33, 5363–5375. [Google Scholar] [CrossRef]
- Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2021, 11, e2100639. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [Green Version]
- Farooque, F.; Wasi, M.; Mughees, M.M. Liposomes as Drug Delivery System: An Updated Review. J. Drug Deliv. Ther. 2021, 11 (Suppl. S5), 149–158. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheibi Hayat, S.M.; Jaafari, M.R.; Hatamipour, M.; Jamialahmadi, T.; Sahebkar, A. Harnessing CD47 Mimicry to Inhibit Phagocytic Clearance and Enhance Anti-Tumor Efficacy of Nanoliposomal Doxorubicin. Expert Opin. Drug Deliv. 2020, 17, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Manjila, S.B.; Baby, J.N.; Bijin, E.N.; Constantine, I.; Pramod, K.; Valsalakumari, J. Novel gene delivery systems. Int. J. Pharm. Investig. 2013, 3, 1–7. [Google Scholar]
- Giulimondi, F.; Vulpis, E.; Digiacomo, L.; Giuli, M.V.; Mancusi, A.; Capriotti, A.L.; Laganà, A.; Cerrato, A.; Zenezini Chiozzi, R.; Nicoletti, C.; et al. Opsonin-Deficient Nucleoproteic Corona Endows Unpegylated Liposomes with Stealth Properties in Vivo. ACS Nano 2022, 16, 2088–2100. [Google Scholar] [CrossRef]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” Nanomedicine: Co-Delivery of Multi-Modal Therapeutics for Efficient, Targeted, and Safe Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Huang, Y. Combination Therapy Based on Nano Codelivery for Overcoming Cancer Drug Resistance. Med. Drug Discov. 2020, 6, 100024. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer Nanotechnology: Application of Nanotechnology in Cancer Therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Auguste, D.T. Cancer Targeted Therapeutics: From Molecules to Drug Delivery Vehicles. J. Control. Release 2015, 219, 632–643. [Google Scholar] [CrossRef] [Green Version]
- Gilabert-Oriol, R.; Ryan, G.; Leung, A.; Firmino, N.; Bennewith, K.; Bally, M. Liposomal Formulations to Modulate the Tumour Microenvironment and Antitumour Immune Response. Int. J. Mol. Sci. 2018, 19, 2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbato, L.; Bocchetti, M.; Di Biase, A.; Regad, T. Cancer stem cells and targeting strategies. Cells 2019, 8, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Huang, G.; Chen, Z.; Zhang, Y. Nanomaterials in targeting cancer stem cells for cancer therapy. Front. Pharmacol. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Misra, S.K.; Kumar, K.; Kondaiah, P.; Tran, P.; Duan, W.; Bhattacharya, S. Cancer stem cell-targeted gene delivery mediated by aptamer-decorated ph-sensitive Nanoliposomes. ACS Biomater. Sci. Eng. 2021, 7, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Prabhakaran, M.P.; Rodríguez, A.P.; Ramakrishna, S. Drug Delivery Vehicles on a Nano-Engineering Perspective. Mater. Sci. Eng. C 2014, 41, 178–195. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (Vincristine Sulfate Liposome Injection) Improves the Pharmacokinetics and Pharmacodynamics of Vincristine. Cancer Chemother. Pharmacol. 2012, 71, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Barenholz, Y.C. Doxil®—the First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Current Nanotechnology Treatments. Available online: https://www.cancer.gov/nano/cancer-nanotechnology/current-treatments (accessed on 28 February 2022).
- Escudero-Ortiz, V.; Ramón-López, A.; Duart, M.J.; Pérez-Ruixo, J.J.; Valenzuela, B. Farmacocinética Poblacional De Doxorubicina Aplicada a La Personalización De Su Dosificación En Pacientes Oncológicos. Farm. Hosp. 2012, 36, 282–291. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Patil, Y.; La-Beck, N.M. New Insights and Evolving Role of Pegylated Liposomal Doxorubicin in Cancer Therapy. Drug Resist. Updates 2016, 29, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Rivankar, S. An Overview of Doxorubicin Formulations in Cancer Therapy. J. Cancer Res. Ther. 2014, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N.; Scott, L.J. Liposomal Irinotecan: A Review in Metastatic Pancreatic Adenocarcinoma. Drugs 2017, 77, 785–792. [Google Scholar] [CrossRef] [PubMed]
- The European Medicines Agency—Caelyx. Available online: https://www.ema.europa.eu/en/documents/product-information/caelyx-epar-product-information_es.pdf (accessed on 6 April 2022).
- Ministerio de Sanidad Informe de Posicionamiento Terapéutico de Irinotecan Liposomal Páncreas Metastásico. Available online: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-irinotecan-liposomal-pegilado-Onivyde-cancer-pancreas.pdf?x17133 (accessed on 6 April 2022).
- Petre, C.E.; Dittmer, D.P. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 277–288. [Google Scholar]
- Fassas, A.; Anagnostopoulos, A. The Use of Liposomal Daunorubicin (DaunoXome) in Acute Myeloid Leukemia. Leuk. Lymphoma 2005, 46, 795–802. [Google Scholar] [CrossRef]
- Kaposi Sarcoma Treatment (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/kaposi-treatment-pdq (accessed on 28 February 2022).
- Vyxeos liposomal1 (daunorubicin / cytarabine). An Overview of Vyxeos Liposomal and Why It Is Authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/vyxeos-liposomal-epar-medicine-overview_en.pdf (accessed on 5 March 2022).
- Tardi, P.; Johnstone, S.; Harasym, N.; Xie, S.; Harasym, T.; Zisman, N.; Harvie, P.; Bermudes, D.; Mayer, L. In Vivo Maintenance of Synergistic Cytarabine: Daunorubicin Ratios Greatly Enhances Therapeutic Efficacy. Leuk. Res. 2009, 33, 129–139. [Google Scholar] [CrossRef]
- Lancet, J.E.; Cortes, J.E.; Hogge, D.E.; Tallman, M.S.; Kovacsovics, T.J.; Damon, L.E.; Komrokji, R.; Solomon, S.R.; Kolitz, J.E.; Cooper, M.; et al. Phase 2 Trial of CPX-351, a Fixed 5:1 Molar Ratio of Cytarabine/Daunorubicin, vs Cytarabine/Daunorubicin in Older Adults with Untreated AML. Blood 2014, 123, 3239–3246. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (Cytarabine and Daunorubicin) Liposome for Injection versus Conventional Cytarabine plus Daunorubicin in Older Patients with Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Zolsketil Pegylated Liposomal-Ema.Europa.Eu. (n.d.) Retrieved 7 April 2022. Available online: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-opinion-zolsketil-pegylated-liposomal_en.pdf (accessed on 6 April 2022).
- Efficacy and Safety of Paclitaxel Liposome and S-1 as First-Line Therapy in Advanced Pancreatic Cancer Patients. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04217096 (accessed on 28 February 2022).
- Evaluation of PLD Combined with Carboplatin Versus Paclitaxel Plus Carboplatin in the First-Line Treatment of Epithelial Ovarian Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03794778 (accessed on 28 February 2022).
- Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma. Available online: https://clinicaltrials.gov/ct2/show/NCT01906385 (accessed on 28 February 2022).
- Study of C6 Ceramide NanoLiposome (CNL) in Patients with Relapsed/Refractory Acute Myeloid Leukemia. Available online: https://clinicaltrials.gov/ct2/show/NCT04716452 (accessed on 28 February 2022).
- Kester, M.; Bassler, J.; Fox, T.E.; Carter, C.J.; Davidson, J.A.; Parette, M.R. Preclinical Development of a C6-Ceramide Nanoliposome, a Novel Sphingolipid Therapeutic. Biol. Chem. 2015, 396, 737–747. [Google Scholar] [CrossRef]
- Liposomal SN-38 in Treating Patients with Metastatic Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00311610 (accessed on 28 February 2022).
- Ocean, A.J.; Niedzwiecki, D.; Atkins, J.N.; Parker, B.; O’Neil, B.H.; Lee, J.W.; Wadler, S.; Goldberg, R.M. Le-sn38 for Metastatic Colorectal Cancer after Progression on Oxaliplatin: Results of CALGB 80402. J. Clin. Oncol. 2008, 26 (Suppl. S15), 4109. [Google Scholar] [CrossRef]

| Product Name | Active Ingredient | Approved Indication | Effective Dosage | Side-Effects (May Affect More Than 1 in 5 or 1 in 10 People) | Marketing Status | Regulatory Organism | Ref (Agency’s Website) |
|---|---|---|---|---|---|---|---|
| Marqibo | Vincristine sulphate | Acute lymphoid leukaemia | Every 7 days by infusion at a dose of 2.25 mg/m2 | Constipation, nausea, pyrexia, fatigue, peripheral neuropathy, febrile neutropenia, diarrhea, anemia, decreased appetite, and insomnia | Discontinued | FDA | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202497Orig1s000SumR.pdf (accessed on 6 April 2022) |
| Doxil | Doxorubicin hydrochloride | Breast and ovarian, multiple myeloma and Kaposi’s sarcoma | Dose depends on the condition it is used for and is calculated on the basis of the patient’s weight and height | Asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia | Prescription | FDA | https://www.accessdata.fda.gov/drugsatfda_docs/summary_review/2008/050718se7-033_SUMR.pdf (accessed on 6 April 2022) |
| Caelyx pegylated liposomal | Doxorubicin hydrochloride | Breast and ovarian, multiple myeloma and Kaposi’s sarcoma. | Dose depends on the condition it is used for and is calculated on the basis of the patient’s weight and height | Side-effects depend on the type of cancer being treated. The most common is nausea, palmar-plantar erythrodysesthesia syndrome, vomiting, stomatitis, rash, weakness, low blood cell counts, loss of appetite, hair loss, tiredness, diarrhea, constipation, and mucositis | Prescription | EMA | ema.europa.eu/medicines/human/EPAR/caelyx (accessed on 6 April 2022) |
| Myocet liposomal | Doxorubicin hydrochloride | Breast neoplasms | Every 3 weeks by infusion. Dose is calculated on the basis of the woman’s weight and height | Neutropenic fever, infection, neutropenia, thrombocytopenia, anemia, leucopenia, loss of appetite, nausea, vomiting, stomatitis, mucositis, diarrhea, hair loss, weakness, fever, pain, and rigors (shaking chills) | Prescription | EMA | ema.europa.eu/medicines/human/EPAR/myocet-liposomal (accessed on 6 April 2022) |
| Onivyde pegylated liposomal | Irinotecan | Pancreatic neoplasms | Every 2 weeks by infusion together with fluorouracil and leucovorin. Dose is calculated on the basis of the patient’s weight and height | Diarrhea, nausea, vomiting, loss of appetite, neutropenia, tiredness, weakness, anemia, stomatitis, and fever Onivyde pegylated liposomal must not be given to patients who had a severe hypersensitivity reaction to irinotecan in the past and to breastfeeding women | Prescription | FDA EMA | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207793Orig1s000TOC.cfm ema.europa.eu/medicines/human/EPAR/onivyde-pegylated-liposomal (accessed on 6 April 2022) |
| Daunoxome | Daunorubicin citrate | Acute myeloid leukaemia, Kaposi’s sarcoma | Every 2 weeks by infusion Dose is calculated on the basis of the patient’s weight and height | Myelosuppression, cardiotoxicity, alopecia, neuropathy | Discontinued Prescription | FDA EMA | ema.europa.eu/en/medicines /human/orphan-designations/eu308585 ema.europa.eu/en/documents/psusa/daunorubicin-list-nationally-authorised-medicinal-products-psusa/00000936/201506_en.pdf (accessed on 6 April 2022) |
| Vyxeos liposomal | Cytarabine + daunorubicin hydrochloride | Acute myeloid leukaemia | On days 1, 3, and 5 of the first treatment course. Further courses on days 1 and 3 by infusion Dose is calculated using the patient’s height and weight | Hypersensitivity, febrile neutropenia, oedema, diarrhea, colitis, mucositis, tiredness, muscle and bone pain, belly pain, decreased appetite, cough, headache, chills, arrhythmias, fever, sleep disorders, and hypotension | Prescription | EMA | ema.europa.eu/medicines/human/epar/vyxeos-liposomal (accessed on 6 April 2022) |
| Zolsketil pegylated liposomal | doxorubicin hydrochloride | Breast and ovarian, multiple myeloma and Kaposi’s sarcoma | Initial authorization (positive opinion 24 March 2022) | EMA Pending EC decision | ema.europa.eu/en/medicines/human/summaries-opinion/zolsketil-pegylated-liposomal (accessed on 6 April 2022) |
| NCT Number | NCT04217096 | NCT03794778 | NCT01906385 | NCT04716452 | NCT00311610 |
|---|---|---|---|---|---|
| Title of the study | Efficacy and safety of paclitaxel liposome and S-1 as first-line therapy in advanced pancreatic cancer patients | Evaluation of PLD Combined With Carboplatin Versus Paclitaxel Plus Carboplatin in the First-line Treatment of Epithelial Ovarian Cancer | Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma | Study of C6 Ceramide NanoLiposome in Patients With Relapsed/Refractory Acute Myeloid Leukemia | Liposomal SN-38 in Treating Patients With Metastatic Colorectal Cancer |
| Conditions | Advanced Pancreatic Cancer | Efficacy and Safety | Glioma | Acute Myeloid Leukemia, in Relapse|Acute Myeloid Leukemia, Refractory | Colorectal Cancer |
| Interventions | Drug: Paclitaxel liposome|Drug: S-1 | Drug: pegylated liposomal doxorubicin|Drug: paclitaxel|Drug: Carboplatin | Drug: Rhenium Liposome Treatment | Drug: Ceramide NanoLiposome (Ceraxa) | Drug: SN-38 liposome |
| Outcome Measures | Progression free survival|Overall Response Rate|overall survival|Disease control rate|Quality of life|Adverse events | PFS|OS|ORR|DCR|the incidence and severity of adverse reactions|quality of life assessment | Maximum Tolerated Dose|Dose Distribution|Response rate|Survival | Number of Patients with Dose-Limiting Toxicities, and with Adverse Events|Severity and Duration of Adverse Events|Dose Levels achieved during study|Half Life|Clearance|Other | Objective response rate|Toxicity|Progression-free survival|Overall survival |
| Gender | All | Female | All | All | All |
| Age | 18 Years to 75 Years (Adult, Older Adult) | 18 Years to 75 Years (Adult, Older Adult) | 18 Years and older (Adult, Older Adult) | 18 Years and older (Adult, Older Adult) | 18 Years to 120 Years (Adult, Older Adult) |
| Phase | 4 | 4 | 1|2 | 1 | 2 |
| Enrolment | 40 | 396 | 55 | 18 | 30 |
| Sponsor | Fudan University | Women’s Hospital School Of Medicine Zhejiang University | Plus Therapeutics|National Cancer Institute (NCI) | Keystone Nano, Inc.|University of Virginia|Memorial Sloan Kettering Cancer Center|Milton S. Hershey Medical Center | Alliance for Clinical Trials in Oncology|National Cancer Institute (NCI) |
| Study Designs | Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment | Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment | Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment | Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment | Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taléns-Visconti, R.; Díez-Sales, O.; de Julián-Ortiz, J.V.; Nácher, A. Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials. Int. J. Mol. Sci. 2022, 23, 4249. https://doi.org/10.3390/ijms23084249
Taléns-Visconti R, Díez-Sales O, de Julián-Ortiz JV, Nácher A. Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials. International Journal of Molecular Sciences. 2022; 23(8):4249. https://doi.org/10.3390/ijms23084249
Chicago/Turabian StyleTaléns-Visconti, Raquel, Octavio Díez-Sales, Jesus Vicente de Julián-Ortiz, and Amparo Nácher. 2022. "Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials" International Journal of Molecular Sciences 23, no. 8: 4249. https://doi.org/10.3390/ijms23084249
APA StyleTaléns-Visconti, R., Díez-Sales, O., de Julián-Ortiz, J. V., & Nácher, A. (2022). Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials. International Journal of Molecular Sciences, 23(8), 4249. https://doi.org/10.3390/ijms23084249







