Direct Reprograming of Mouse Fibroblasts into Dermal Papilla Cells via Small Molecules
Abstract
1. Introduction
2. Results
2.1. Three-Compound Combination Efficiently Induces L929 Mouse Fibroblast Cell Lines to DPC-LCs
2.2. TTNPB Induces Primary Mouse Fibroblast Conversion into DPC-LCs
2.3. DPC-LCs Converted from Mouse Fibroblasts by TTNPB Acquired Hair-Inducing Capacity
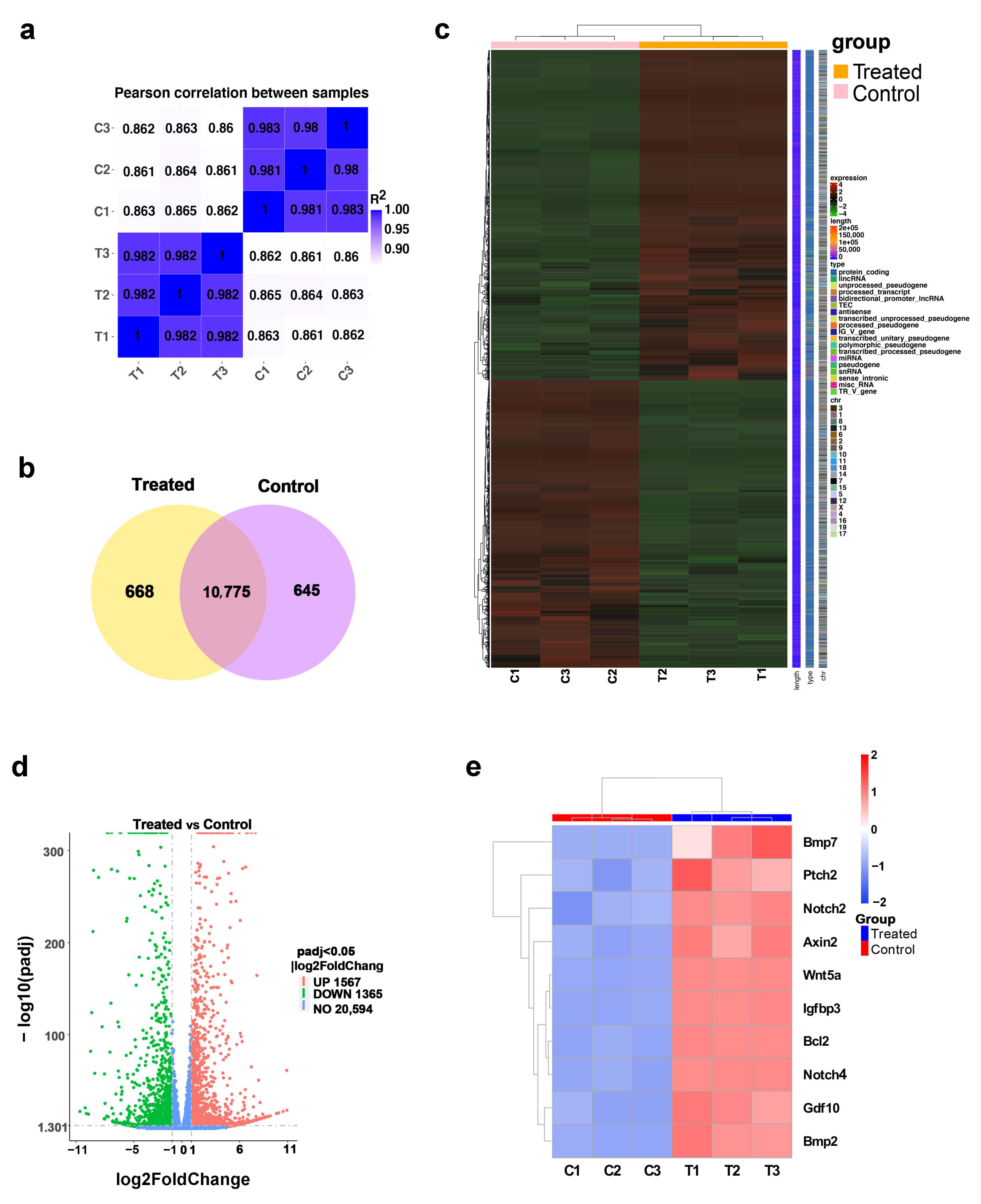
2.4. TTNPB Induced Dramatic Changes in Gene Expression Profile in Adult Mouse Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cells Isolation and Culture
4.3. Small-Molecule Compounds
4.4. Chemical Induction of DPC-LCs from Mice Fibroblasts
4.5. ALP Staining
4.6. Immunofluorescence-Staining Analysis
4.7. Quantitative RT-PCR
4.8. In Vivo HF Regeneration
4.9. RNA-Sequencing and Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Wang, Q.; Wang, C.; Shang, L. Living Materials for Regenerative Medicine. Eng. Regen. 2021, 2, 96–104. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Liu, K.; Yu, C.; Xie, M.; Li, K.; Ding, S. Chemical Modulation of Cell Fate in Stem Cell Therapeutics and Regenerative Medicine. Cell Chem. Biol. 2016, 23, 893–916. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Wei, W.; Ding, S. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell 2013, 13, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tang, S.; Ding, S. Chemical approaches to cell reprogramming. Curr. Opin. Genet. Dev. 2014, 28, 50–56. [Google Scholar] [CrossRef]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Yao, X.; Yu, Y.; Tian, T.; Xu, W.; Zhou, Y.; Ouyang, H. Pharmaceutical therapeutics for articular regeneration and restoration: State-of-the-art technology for screening small molecular drugs. Cell. Mol. Life Sci. 2021, 78, 8127–8155. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Sia, J.; Soto, J.; Wang, P.; Li, L.; Hsueh, Y.; Sun, R.; Faull, K.F.; Tidball, J.G.; Li, S. Skeletal muscle regeneration via the chemical induction and expansion of myogenic stem cells in situ or in vitro. Nat. Biomed. Eng. 2021, 5, 864–879. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, J.C.; Yeh, H.; Ma, N.X.; Lee, G.; Chen, X.A.; Wang, Y.; Lin, L.; Chen, L.; Jin, P. Small Molecules Efficiently Reprogram Human Astroglial Cells into Functional Neurons. Cell Stem Cell 2015, 17, 735–747. [Google Scholar] [CrossRef]
- Pan, S.H.; Zhao, N.; Feng, X.; Jie, Y.; Jin, Z.B. Conversion of mouse embryonic fibroblasts into neural crest cells and functional corneal endothelia by defined small molecules. Sci. Adv. 2021, 7, eabg5749. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Do, J.T.; Desponts, C.; Hahm, H.S.; Schöler, H.R.; Ding, S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell 2008, 2, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.H.; Norris, M.J.; Dover, R.; Busuttil, N. Causes of hair loss and the developments in hair rejuvenation. Int. J. Cosmet. Sci. 2002, 24, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kollar, E.J. The induction of hair follicles by embryonic dermal papillae. J. Investig. Dermatol. 1970, 55, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The transplantation of individual rat and guineapig whisker papillae. J. Embryol. Exp. Morphol. 1961, 9, 117–127. [Google Scholar]
- Oliver, R.F. The experimental induction of whisker growth in the hooded rat by implantation of dermal papillae. J. Embryol. Exp. Morphol. 1967, 18, 43–51. [Google Scholar] [CrossRef]
- Oliver, R.F. The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. J. Embryol. Exp. Morphol. 1970, 23, 219–236. [Google Scholar] [CrossRef]
- Pisansarakit, P.; Moore, G.P. Induction of hair follicles in mouse skin by rat vibrissa dermal papillae. J. Embryol. Exp. Morphol. 1986, 94, 113–119. [Google Scholar] [CrossRef]
- Jahoda, C.A. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: Vibrissa-type fibres are specified. Development 1992, 115, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Cerqueira, M.T.; Pirraco, R.P.; Gasperini, L.; Reis, R.L.; Marques, A.P. Rescuing key native traits in cultured dermal papilla cells for human hair regeneration. J. Adv. Res. 2021, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, J.; Lee, D.; Baden, H.P.; Brissette, J.; Dotto, G.P. Primary mouse keratinocyte cultures contain hair follicle progenitor cells with multiple differentiation potential. J. Investig. Dermatol. 1997, 109, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, W.C.; Goodman, L.V.; George, C.; Morgan, D.L.; Ledbetter, S.; Yuspa, S.H.; Lichti, U. Reconstitution of hair follicle development in vivo: Determination of follicle formation, hair growth, and hair quality by dermal cells. J. Investig. Dermatol. 1993, 100, 229–236. [Google Scholar] [CrossRef]
- Fan, S.M.Y.; Tsai, C.F.; Yen, C.M.; Lin, M.H.; Wang, W.H.; Chan, C.C.; Chen, C.L.; Phua, K.K.L.; Pan, S.H.; Plikus, M.V. Inducing hair follicle neogenesis with secreted proteins enriched in embryonic skin. Biomaterials 2018, 167, 121–131. [Google Scholar] [CrossRef]
- Rendl, M.; Lewis, L.; Fuchs, E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005, 3, e331. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Yang, Q.; Yang, J.; Wang, H.; Xu, H.; Gao, W.Q. Chemical conversion of mouse fibroblasts into functional dopaminergic neurons. Exp. Cell Res. 2016, 347, 283–292. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, C.; Xu, X.; Gu, H.; Ye, Y.; Jiang, C.; Qiu, Z.; Xie, X. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res. 2015, 25, 1013–1024. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, N.; Zhang, H.; Lei, X.; Cao, Y.; Xia, G.; Duan, E.; Liu, S. Chemically induced transformation of human dermal fibroblasts to hair-inducing dermal papilla-like cells. Cell Prolif. 2019, 52, e12652. [Google Scholar] [CrossRef]
- Ichida, J.K.; Blanchard, J.; Lam, K.; Son, E.Y.; Chung, J.E.; Egli, D.; Loh, K.M.; Carter, A.C.; Giorgio, F.P.D.; Koszka, K. A small-molecule inhibitor of tgf-β signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell 2009, 5, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Desponts, C.; Do, J.T.; Hahm, H.S.; Schöler, H.R.; Ding, S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 2008, 3, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Chen, S.; Muhlestein, W.; Melton, D.A. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 2008, 26, 1269–1275. [Google Scholar] [CrossRef]
- Mali, P.; Chou, B.K.; Yen, J.; Ye, Z.; Zou, J.; Dowey, S.; Brodsky, R.A.; Ohm, J.E.; Yu, W.; Baylin, S.B. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 2010, 28, 713–720. [Google Scholar] [CrossRef]
- McElwee, K.J.; Kissling, S.; Wenzel, E.; Huth, A.; Hoffmann, R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. Investig. Dermatol. 2003, 121, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmueller, U.; Mann, M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell. Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef]
- Seluanov, A.; Vaidya, A.; Gorbunova, V. Establishing primary adult fibroblast cultures from rodents. J. Vis. Exp. 2010, 44, 2033. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, J.; Cai, T.; Guo, L.; Wang, S.; Wang, J.; Gao, Y.; Ge, J.; Jiang, Y. Hair Follicle and Sebaceous Gland De Novo Regeneration with Cultured Epidermal Stem Cells and Skin-Derived Precursors. Stem Cells Transl. Med. 2016, 5, 1695–1706. [Google Scholar] [CrossRef]
- Harn, H.I.C.; Wang, S.P.; Lai, Y.C.; Handel, B.V.; Liang, Y.C.; Tsai, S.; Schiessl, I.M.; Sarkar, A.; Xi, H.; Hughes, M.; et al. Symmetry breaking of tissue mechanics in wound induced hair follicle regeneration of laboratory and spiny mice. Nat. Commun. 2021, 12, 2595. [Google Scholar] [CrossRef]
- Phan, Q.M.; Sinha, S.; Biernaskie, J.; Driskell, R.R. Single-Cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp. Dermatol. 2021, 30, 92–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Fu, D.; Liu, Z.; Wang, H.; Wang, J.; Qu, Q.; Li, K.; Fan, Z.; Hu, Z. Transcriptome Analysis Reveals an Inhibitory Effect of Dihydrotestosterone-Treated 2D- and 3D-Cultured Dermal Papilla Cells on Hair Follicle Growth. Front. Cell Dev. Biol. 2021, 9, 724310. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Fliniaux, I.; Schneider, P.; Mikkola, M. Identification of Ectodysplasin Target Genes Reveals the Involvement of Chemokines in Hair Development. J. Investig. Dermatol. 2012, 132, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dihydrotestosterone-Inducible IL-6 Inhibits Elongation of Human Hair Shafts by Suppressing Matrix Cell Proliferation and Promotes Regression of Hair Follicles in Mice. J. Investig. Dermatol. 2012, 132, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Aoi, N.; Yamauchi, Y.; Sato, T.; Suga, H.; Eto, H.; Kato, H.; Tabata, Y.; Yoshimura, K. TGF-β2 is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J. Cell. Mol. Med. 2009, 13, 4643–4656. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, M.L. TNF superfamily in skin appendage development. Cytokine Growth Factor Rev. 2008, 19, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Gnedeva, K.; Vorotelyak, E.; Cimadamore, F.; Cattarossi, G.; Giusto, E.; Terskikh, V.V. Derivation of hair-inducing cell from human pluripotent stem cells. PLoS ONE 2015, 10, e0116892. [Google Scholar] [CrossRef]
- Toyoshima, K.E.; Asakawa, K.; Ishibashi, N.; Toki, H.; Ogawa, M.; Hasegawa, T.; Irié, T.; Tachikawa, T.; Sato, A.; Takeda, A. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat. Commun. 2012, 3, 784. [Google Scholar] [CrossRef]
- Wu, M.; Sun, Q.; Guo, X.; Liu, H. hMSCs possess the potential to differentiate into DP cells in vivo and in vitro. Cell Biol. Int. Rep. 2012, 19, e00019. [Google Scholar] [CrossRef]
- Rompolas, P.; Greco, V. Stem cell dynamics in the hair follicle niche. Semin. Cell Dev. Biol. 2014, 25–26, 34–42. [Google Scholar] [CrossRef]
- Veraitch, O.; Kobayashi, T.; Imaizumi, Y.; Akamatsu, W.; Sasaki, T.; Yamanaka, S.; Amagai, M.; Okano, H.; Ohyama, M. Human induced pluripotent stem cell-derived ectodermal precursor cells contribute to hair follicle morphogenesis in vivo. J. Investig. Dermatol. 2013, 133, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Naujok, O.; Lentes, J.; Diekmann, U.; Davenport, C.; Lenzen, S. Cytotoxicity and activation of the Wnt/β-catenin pathway in mouse embryonic stem cells treated with four GSK3 inhibitors. BMC Res. Notes 2014, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Reddy, S.T.; Gaddapara, R.T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Yoshida, Y.; Soma, T.; Matsuzaki, T.; Kishimoto, J. Wnt activator CHIR99021-stimulated human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle-enriched human skin. Biochem. Biophys. Res. Commun. 2019, 516, 599–605. [Google Scholar] [CrossRef]
- Takahashi, T.; Kamimura, A.; Shirai, A.; Yokoo, Y. Several selective protein kinase C inhibitors including procyanidins promote hair growth. Skin Pharmacol. Appl. Skin Physiol. 2000, 13, 133–142. [Google Scholar] [CrossRef]
- Zile, M.H. Vitamin a requirement for early cardiovascular morphogenesis specification in the vertebrate embryo: Insights from the avian embryo. Exp. Biol. Med. 2004, 229, 598–606. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Wu, W.K.K.; Wang, X.; Liang, J.; Qiu, G.; Liu, J. Vitamin A deficiency induces congenital spinal deformities in rats. PLoS ONE 2012, 7, e46565. [Google Scholar] [CrossRef]
- Glover, J.C.; Renaud, J.S.; Rijli, F.M. Retinoic acid and hindbrain patterning. J. Neurobiol. 2006, 66, 705–725. [Google Scholar] [CrossRef]
- Pourjafar, M.; Saidijam, M.; Mansouri, K.; Ghasemibasir, H.; Karimi Dermani, F.; Najafi, R. All-Trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo. Cell Prolif. 2017, 50, e12315. [Google Scholar] [CrossRef]
- Saito, A.; Sugawara, A.; Uruno, A.; Kudo, M.; Kagechika, H.; Sato, Y.; Owada, Y.; Kondo, H.; Sato, M.; Kurabayashi, M. All-Trans retinoic acid induces in vitro angiogenesis via retinoic acid receptor: Possible involvement of paracrine effects of endogenous vascular endothelial growth factor signaling. Endocrinology 2007, 148, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, G.; Terezakis, N.; Attia, H.; Bazzano, A.; Dover, R.; Fenton, D.; Mandir, N.; Celleno, L.; Tamburro, M.; Jaconi, S. Effect of retinoids on follicular cells. J. Investig. Dermatol. 1993, 101 (Suppl. 1), 138s–142s. [Google Scholar] [CrossRef]
- Shin, H.S.; Won, C.H.; Lee, S.H.; Kwon, O.S.; Kim, K.H.; Eun, H.C. Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss: A randomized, double-blind, comparative clinical trial. Am. J. Clin. Dermatol. 2007, 8, 285–290. [Google Scholar] [CrossRef]
- Bazzano, G.S.; Terezakis, N.; Galen, W. Topical tretinoin for hair growth promotion. J. Am. Acad. Dermatol. 1986, 15, 880–883, 890–893. [Google Scholar] [CrossRef]
- Cole, B.J.; Seroyer, S.T.; Filardo, G.; Bajaj, S.; Fortier, L.A. Platelet-rich plasma: Where are we now and where are we going? Sports Health 2010, 2, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Kong, D.; Tian, R.; Pang, M.; Mo, M.; Chen, Y.; Yang, G.; Cheng, L.H.; Lei, X.; Fang, K. Platelet sonicates activate hair follicle stem cells and mediate enhanced hair follicle regeneration. J. Cell. Mol. Med. 2020, 24, 1786–1794. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, L.H.H.; Cui, X.; Lei, X.X.; Tang, J.B.; Cheng, B. Application of standardized platelet-rich plasma in elderly patients with complex wounds. Wound Repair Regen. 2019, 27, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Trink, A.; Sorbellini, E.; Bezzola, P.; Rodella, L.; Rezzani, R.; Ramot, Y.; Rinaldi, F. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br. J. Dermatol. 2013, 169, 690–694. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, J.; Li, J.; Jia, M.; Wang, Y.; Shen, H. Platelet-Rich plasma stimulates angiogenesis in mice which may promote hair growth. Eur. J. Med. Res. 2017, 22, 39. [Google Scholar] [CrossRef]
- De Groot, S.C.; Ulrich, M.M.W.; Gho, C.G.; Huisman, M.A. Back to the Future: From Appendage Development Toward Future Human Hair Follicle Neogenesis. Front. Cell Dev. Biol. 2021, 9, 661787. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Bulfone-Paus, S.; Handjiski, B.; Welker, P.; Sundberg, J.P.; McKay, I.A.; Vladimir, A. Botchkarev, Ralf Paus. Intercellular adhesion molecule-1 and hair follicle regression. J. Histochem. Cytochem. 2000, 48, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Peters, E.J.M.; Botchkarev, V.A.; Panteleyev, A. Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J. Histochem. Cytochem. 1998, 46, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.; Rein, S.; Pförringer, D.; Thor, D. Molecular Mechanisms of Hair Growth and Regeneration: Current Understanding and Novel Paradigms. Dermatology 2020, 236, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lichti, U.; Anders, J.; Yuspa, S.H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 2008, 3, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Osada, A.; Iwabuchi, T.; Kishimoto, J.; Hamazaki, T.S.; Okochi, H. Long-Term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007, 13, 975–982. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, X.; Wang, W.; Boucher, M.; Parimoo, S.; Stenn, K.S. Organogenesis from dissociated cells: Generation of mature cycling hair follicles from skin-derived cells. J. Investig. Dermatol. 2005, 124, 867–876. [Google Scholar] [CrossRef]




| No. | Full Name | Function | Concentration (μM) |
|---|---|---|---|
| 1 | SB431542 | TGFβ inhibitor | 2 |
| 2 | CHIR99021 | GSK3 inhibition | 10 |
| 3 | Forskolin | PKA activation | 10 |
| 4 | RG108 | DNA methyltransferase inhibition | 10 |
| 5 | Tranylcypromine/Parnate | LSD1 histone demethylase inhibition | 2 |
| 6 | TTNPB | Retinoic acid receptor agonist | 1 |
| 7 | RepSox | TGFβ inhibitor | 10 |
| 8 | BayK8644 | l-type Ca2+ channel activation | 2 |
| 9 | BIX01294 | Histone methyltransferase inhibition | 1 |
| 10 | Valproic acid (VPA) | Histone deacetylase inhibition | 500 |
| 11 | Sodium butyrate (NaB) | Histone deacetylase inhibition | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Lin, Y.; Huang, W.; Wang, X. Direct Reprograming of Mouse Fibroblasts into Dermal Papilla Cells via Small Molecules. Int. J. Mol. Sci. 2022, 23, 4213. https://doi.org/10.3390/ijms23084213
Ma Y, Lin Y, Huang W, Wang X. Direct Reprograming of Mouse Fibroblasts into Dermal Papilla Cells via Small Molecules. International Journal of Molecular Sciences. 2022; 23(8):4213. https://doi.org/10.3390/ijms23084213
Chicago/Turabian StyleMa, Yihe, Yumiao Lin, Wenting Huang, and Xusheng Wang. 2022. "Direct Reprograming of Mouse Fibroblasts into Dermal Papilla Cells via Small Molecules" International Journal of Molecular Sciences 23, no. 8: 4213. https://doi.org/10.3390/ijms23084213
APA StyleMa, Y., Lin, Y., Huang, W., & Wang, X. (2022). Direct Reprograming of Mouse Fibroblasts into Dermal Papilla Cells via Small Molecules. International Journal of Molecular Sciences, 23(8), 4213. https://doi.org/10.3390/ijms23084213







