Fluorine-Containing Drug Administration in Rats Results in Fluorination of Selected Proteins in Liver and Brain Tissue
Abstract
1. Introduction
2. Results
3. Discussion
4. Study Limitations
5. Materials and Methods
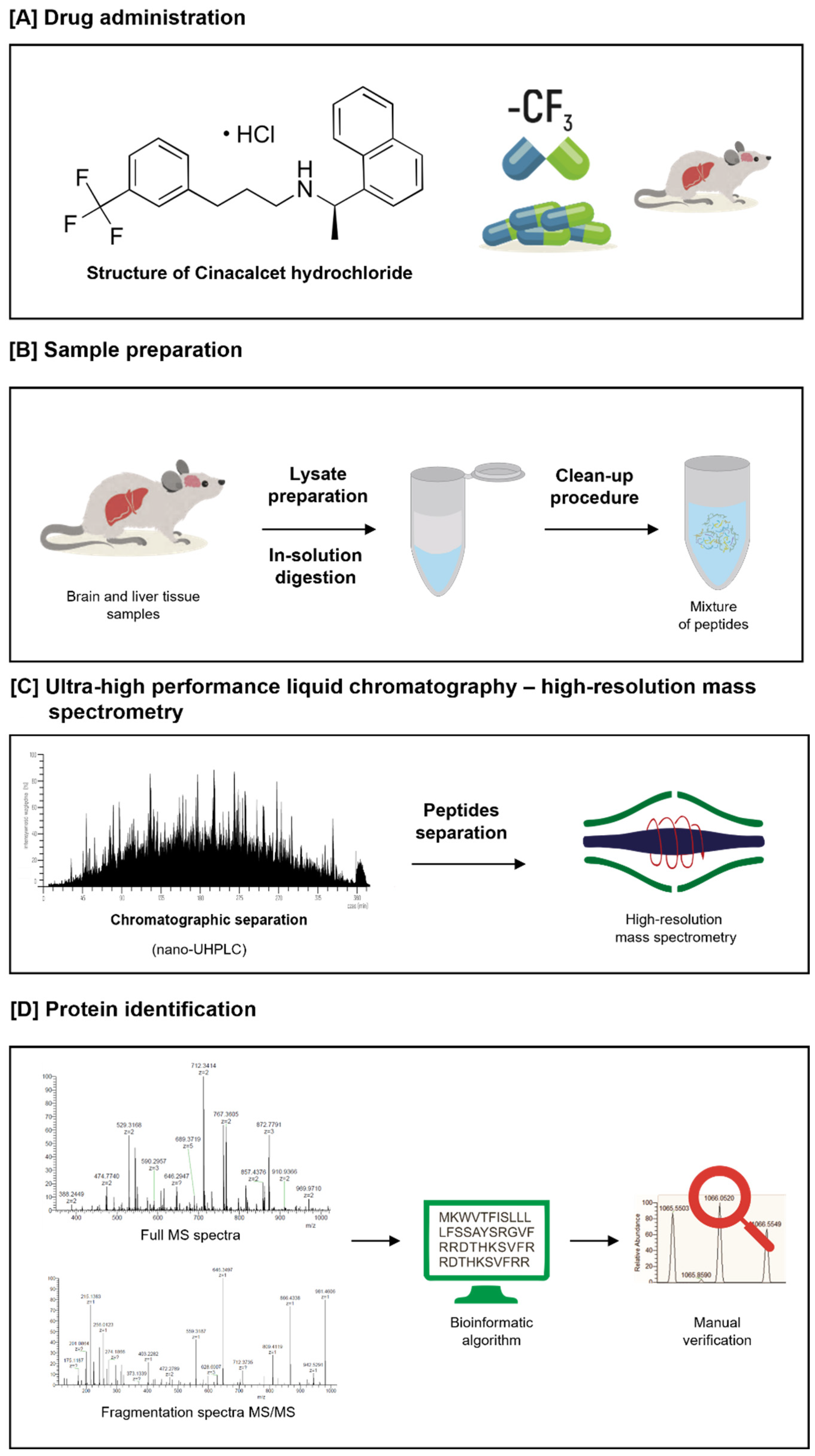
5.1. Chemicals, Reagents, and Instrumentation
5.2. Sample Preparation and Liquid Chromatography–MS/MS Analysis
5.3. Data Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Calero, P.; Volke, D.C.; Lowe, P.T.; Gotfredsen, C.H.; O’Hagan, D.; Nike, P.I. A fluoride-responsive genetic circuit enables in vivo biofluorination in engineered Pseudomonas putida. Nat. Commun. 2020, 11, 5045. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, C.; Chaudhary, M.; De Oliveira, R.N.; Borbas, A.; Kempaiah, P.; Singh, P.; Rathi, B. Fluorinated scaffolds for antimalarial drug discovery. Expert Opin. Drug Discov. 2020, 15, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, S.; Nyakatura, E.K.; Rohrhofer, A.; Moschner, J.; Schmidt, B.; Eichler, J.; Roth, C.; Koksch, B. Systematic Evaluation of Fluorination as Modification for Peptide-Based Fusion Inhibitors against HIV-1 Infection. ChemBioChem 2021, 22, 3443–3451. [Google Scholar] [CrossRef]
- Park, B.K.; Kitteringham, N.R.; O’Neill, P.M. Metabolism of fluorine-containing drugs. Annu. Rev. Pharm. Toxicol. 2001, 41, 443–470. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Johnson, B.M.; Shu, Y.Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef]
- Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/mimpara-epar-product-information_en.pdf (accessed on 6 December 2021).
- Bover, J.; Ureña, P.; Ruiz-García, C.; DaSilva, I.; Lescano, P.; del Carpio, J.; Ballarín, J.; Cozzolino, M. Clinical and Practical Use of Calcimimetics in Dialysis Patients with Secondary Hyperparathyroidism. Clin. J. Am. Soc. Nephrol. 2016, 11, 161–174. [Google Scholar] [CrossRef]
- Thompson, D.C.; Perera, K.; London, R. Spontaneous Hydrolysis of 4-Trifluoromethylphenol to a Quinone Methide and Subsequent Protein Alkylation. Chem. Biol. Interact. 2000, 126, 1–14. [Google Scholar] [CrossRef]
- Khojasteh, S.C.; Argikar, U.A.; Driscoll, J.P.; Heck, C.J.S.; King, L.; Jackson, K.D.; Jian, W.; Kalgutkar, A.S.; Miller, G.P.; Kramlinger, V.; et al. Novel advances in biotransformation and bioactivation research—2020 year in review. Drug Metab. Rev. 2021, 53, 384–433. [Google Scholar] [CrossRef]
- Ekstrand., J.; Alván, G.; Boréus, L.O.; Norlin, A. Pharmacokinetics of fluoride in man after single and multiple oral doses. Eur. J. Clin. Pharm. 1977, 12, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Liang, C.; Yang, W.; Zhu, Q.; Luo, H.; Liu, X.; Wang, J.; Zhang, J. Potential Protective Effect of Riboflavin Against Pathological Changes in the Main Organs of Male Mice Induced by Fluoride Exposure. Biol. Trace Elem. Res. 2021, 200, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Li, C.; Yin, Z.; Hu, D.; Ge, Y.; Chen, L. Fluoride exposure decreased neurite formation on cerebral cortical neurons of SD rats in vitro. Environ. Sci. Pollut. Res. Int. 2021, 28, 50975–50982. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, L.; Govindarajan, V.; Ameeramja, J.; Nair, H.R.; Perumal, E. Single oral acute fluoride exposure causes changes in cardiac expression of oxidant and antioxidant enzymes, apoptotic and necrotic markers in male rats. Biochimie 2015, 119, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gawor, A.; Konopka, A.; Ruszczyńska, A.; Pączek, L.; Gajewski, Z.; Bulska, E. Molecular Absorption and Mass Spectrometry for Complementary Analytical Study of Fluorinated Drugs in Animal Organisms. J. Anal. Spectrom. 2020, 35, 1840–1847. [Google Scholar] [CrossRef]
- Eriksson, J.; Chait, B.T.; Fenyö, D. A statistical basis for testing the significance of mass spectrometric protein identification results. Anal. Chem. 2000, 72, 999–1005. [Google Scholar] [CrossRef]
- Resing, K.A.; Meyer-Arendt, K.; Mendoza, A.M.; Aveline-Wolf, L.D.; Jonscher, K.R.; Pierce, K.G.; Old, W.M.; Cheung, H.T.; Russell, S.; Wattawa, J.L.; et al. Improving reproducibility and sensitivity in identifying human proteins by shotgun proteomics. Anal. Chem. 2004, 76, 3556–3568. [Google Scholar] [CrossRef]
- Padhi, D.; Harris, R. Clinical pharmacokinetic and pharmacodynamic profile of cinacalcet hydrochloride. Clin. Pharmacokinet. 2009, 48, 303–311. [Google Scholar] [CrossRef]
- Buer, B.C.; Meagher, J.L.; Stuckey, J.A.; Marsh, E.N. Structural basis for the enhanced stability of highly fluorinated proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 4810–4815. [Google Scholar] [CrossRef]
- Kumar, G.H.; Sproul, C.; Poppe, L.; Turner, S.; Gohdes, M.; Ghoborah, H.; Padhi, D.; Roskos, L. Metabolism and disposition of calcimimetic agent cinacalcet HCl in humans and animal models. Drug Metab. Dispos. 2004, 32, 1491–1500. [Google Scholar] [CrossRef]
- Buer, B.C.; Marsh, E.N. Fluorine: A new element in protein design. Protein Sci. 2012, 21, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Robalo, J.R.; Vila Verde, A. Unexpected trends in the hydrophobicity of fluorinated amino acids reflect competing changes in polarity and conformation. Phys. Chem. Chem. Phys. 2019, 21, 2029–2038. [Google Scholar] [CrossRef]
- Marsh, E.N. Fluorinated proteins: From design and synthesis to structure and stability. Acc. Chem. Res. 2014, 47, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.H.; Link, A.J.; Tirrell, D.A. Evolution of a fluorinated green fluorescent protein. Proc. Natl. Acad. Sci. USA 2007, 104, 13887–13890. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Liu, Z.; Doenen, R.; Nash, M.A. Influence of Fluorination on Single-Molecule Unfolding and Rupture Pathways of a Mechanostable Protein Adhesion Complex. Nano Lett. 2020, 20, 8940–8950. [Google Scholar] [CrossRef]
- Naarmann, N.; Bilgiçer, B.; Meng, H.; Kumar, K.; Steinem, C. Fluorinated interfaces drive self-association of transmembrane alpha helices in lipid bilayers. Angew. Chem. Int. Ed. Engl. 2006, 45, 2588–2591. [Google Scholar] [CrossRef]
- Welte, H.; Zhou, T.; Mihajlenko, X.; Mayans, O.; Kovermann, M. What does fluorine do to a protein? Thermodynamic, and highly-resolved structural insights into fluorine-labelled variants of the cold shock protein. Sci. Rep. 2020, 10, 2640. [Google Scholar] [CrossRef]
- Sun, X.; Dyson, H.J.; Wright, P.E. Fluorotryptophan Incorporation Modulates the Structure and Stability of Transthyretin in a Site-Specific Manner. Biochemistry 2017, 56, 5570–5581. [Google Scholar] [CrossRef]
- Meng, H.; Krishnaji, S.T.; Beinborn, M.; Kumar, K. Influence of selective fluorination on the biological activity and proteolytic stability of glucagon-like peptide-1. J. Med. Chem. 2008, 51, 7303–7307. [Google Scholar] [CrossRef][Green Version]
- Tang, H.C.; Lin, Y.J.; Horng, J.C. Modulating the folding stability and ligand binding affinity of Pin1 WW domain by proline ring puckering. Proteins 2014, 82, 67–76. [Google Scholar] [CrossRef]
- Dominguez, M.A., Jr.; Thornton, K.C.; Melendez, M.G.; Dupureur, C.M. Differential effects of isomeric incorporation of fluorophenylalanines into PvuII endonuclease. Proteins 2001, 45, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Uniprot. Available online: https://www.uniprot.org (accessed on 30 October 2021).
- Salwiczek, M.; Nyakatura, E.K.; Gerling, U.I.; Ye, S.; Koksch, B. Fluorinated amino acids: Compatibility with native protein structures and effects on protein-protein interactions. Chem. Soc. Rev. 2012, 41, 2135–2171. [Google Scholar] [CrossRef] [PubMed]
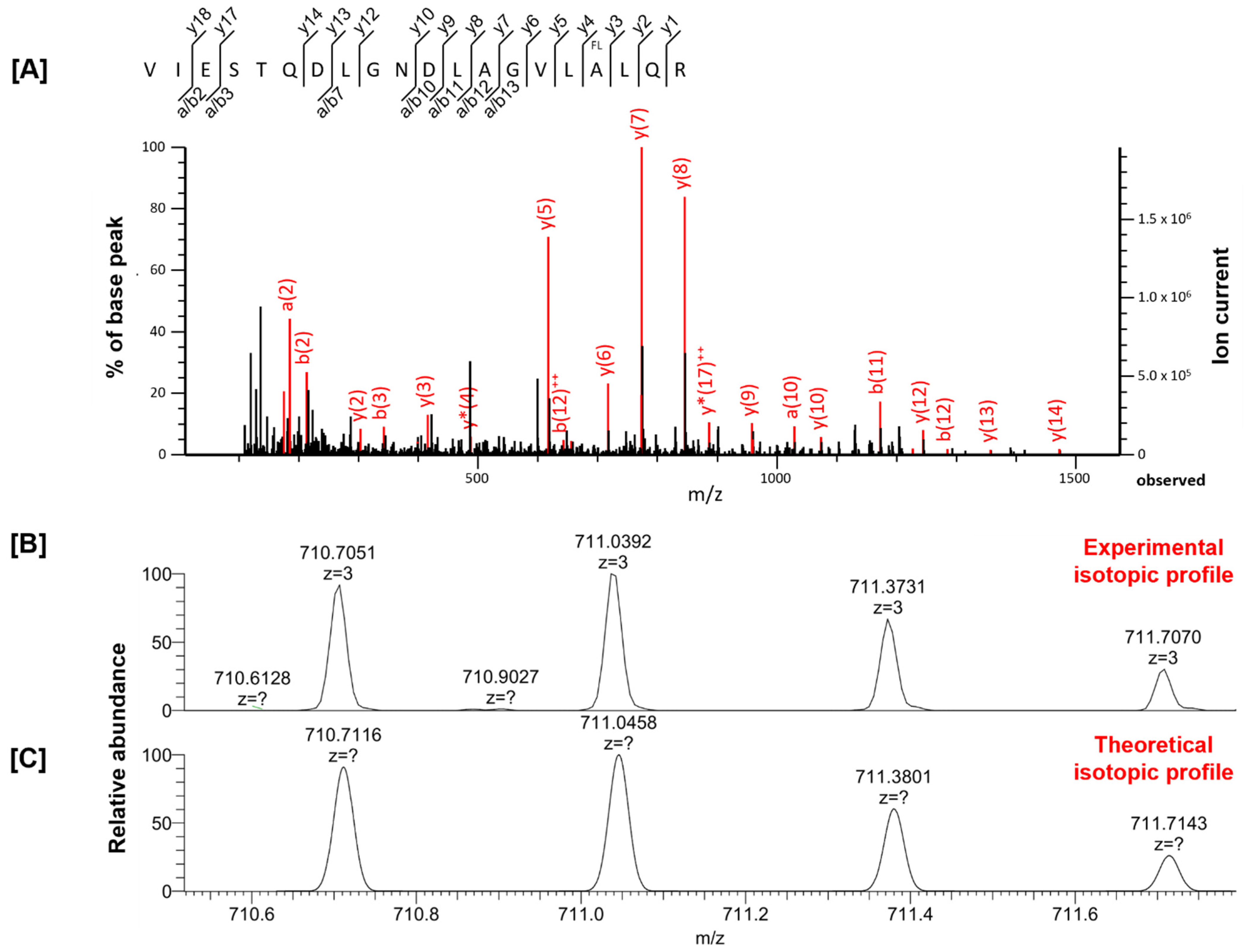
| Examined Tissue | Peptide Sequence | Variable Modification | Experimental Mass | Theoretical Mass | Δ Mass [ppm] | Peptide Score | Protein Name | Gene Name |
|---|---|---|---|---|---|---|---|---|
| Liver | GASIPQFTNSPTMVIMVGLPAR | fluorination (A) | 2304.1958 | 2304.1770 | 8.15 | 120 | 6-phosphofructo-2-kinase/ fructose-2,6-bisphosphatase 1 (Fragment) | Pfkfb1 |
| VIESTQDLGNDLAGVLALQR | fluorination (A) | 2129.0930 | 2129.1128 | −9.29 | 112 | Spectrin beta chain | Sptbn2 | |
| LFATEATSDWLNANNVPATPV AWPSQEGQNPSLSSIR | fluorination (A) | 3985.9498 | 3985.9246 | 6.31 | 79 | Carbamoyl-phosphate synthase [ammonia], mitochondrial | Cps1 | |
| Brain | GVVVFGEPITASLGTDGSHYWSK | fluorination (F) | 2424.1777 | 2424.1762 | 0.61 | 73 | Dihydropyrimidinase-related protein 4 (Fragment) | Dpysl4 |
| DLDLLNPAAR | fluorination (A) | 1114.5835 | 1114.5782 | 4.49 | 47 | Prominin-2 | Prom2 | |
| VIESTQDLGNDLAGVLALQR | fluorination (A) | 2129.1112 | 2129.1128 | −0.78 | 43 | Spectrin beta chain | Sptbn2 | |
| ALSYALR | fluorination (A) | 810.4402 | 810.4399 | 0.37 | 36 | tRNA phosphotransferase 1 | Trpt1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawor, A.; Gajewski, Z.; Paczek, L.; Czarkowska-Paczek, B.; Konopka, A.; Wryk, G.; Bulska, E. Fluorine-Containing Drug Administration in Rats Results in Fluorination of Selected Proteins in Liver and Brain Tissue. Int. J. Mol. Sci. 2022, 23, 4202. https://doi.org/10.3390/ijms23084202
Gawor A, Gajewski Z, Paczek L, Czarkowska-Paczek B, Konopka A, Wryk G, Bulska E. Fluorine-Containing Drug Administration in Rats Results in Fluorination of Selected Proteins in Liver and Brain Tissue. International Journal of Molecular Sciences. 2022; 23(8):4202. https://doi.org/10.3390/ijms23084202
Chicago/Turabian StyleGawor, Andrzej, Zdzislaw Gajewski, Leszek Paczek, Bozena Czarkowska-Paczek, Anna Konopka, Grzegorz Wryk, and Ewa Bulska. 2022. "Fluorine-Containing Drug Administration in Rats Results in Fluorination of Selected Proteins in Liver and Brain Tissue" International Journal of Molecular Sciences 23, no. 8: 4202. https://doi.org/10.3390/ijms23084202
APA StyleGawor, A., Gajewski, Z., Paczek, L., Czarkowska-Paczek, B., Konopka, A., Wryk, G., & Bulska, E. (2022). Fluorine-Containing Drug Administration in Rats Results in Fluorination of Selected Proteins in Liver and Brain Tissue. International Journal of Molecular Sciences, 23(8), 4202. https://doi.org/10.3390/ijms23084202






