Development of Viral-Vectored Vaccines and Virus Replicon Particle-Based Neutralisation Assay against Mayaro Virus
Abstract
:1. Introduction
2. Results
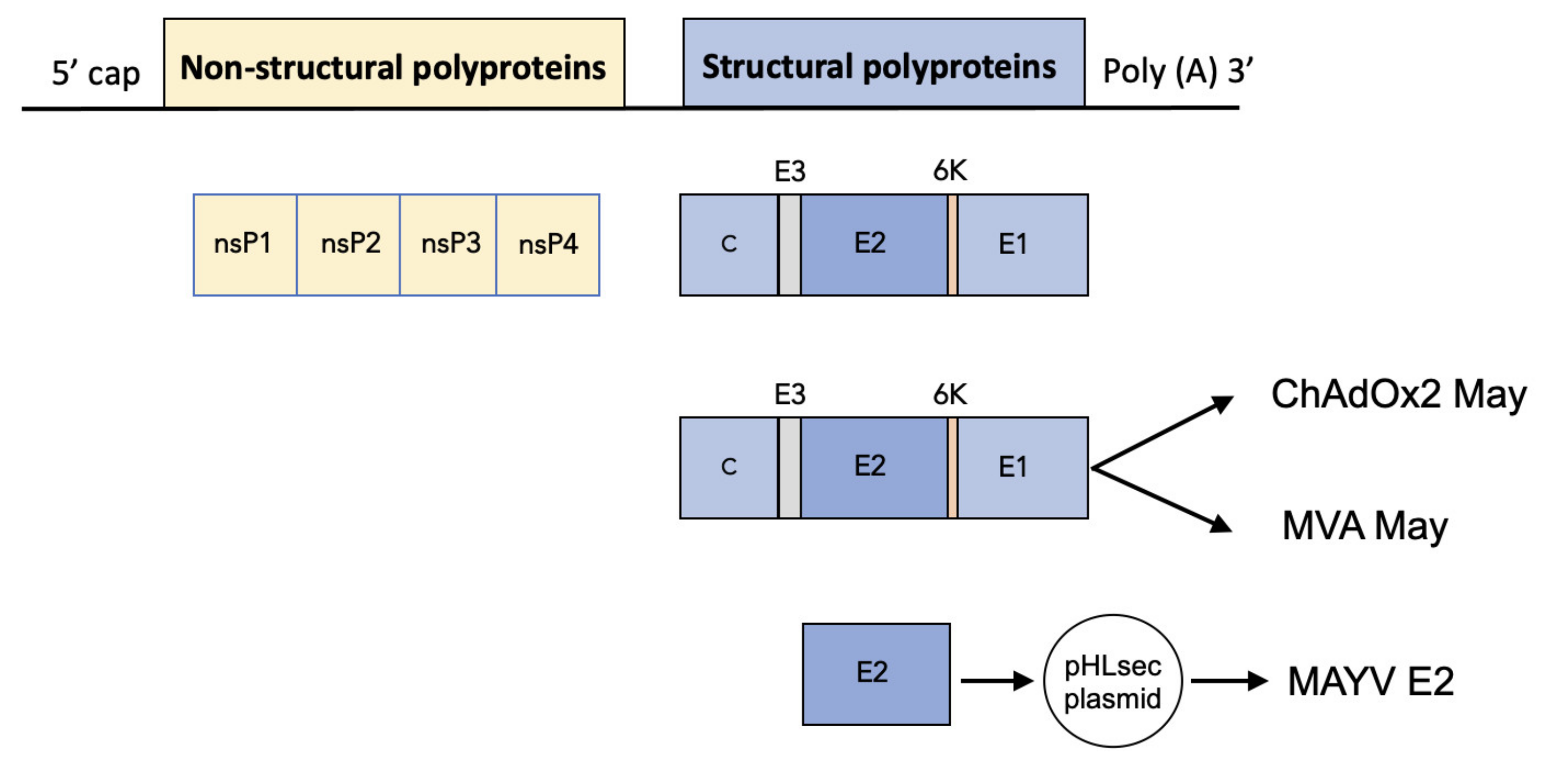
2.1. Construction of ChAdOx2 and MVA May
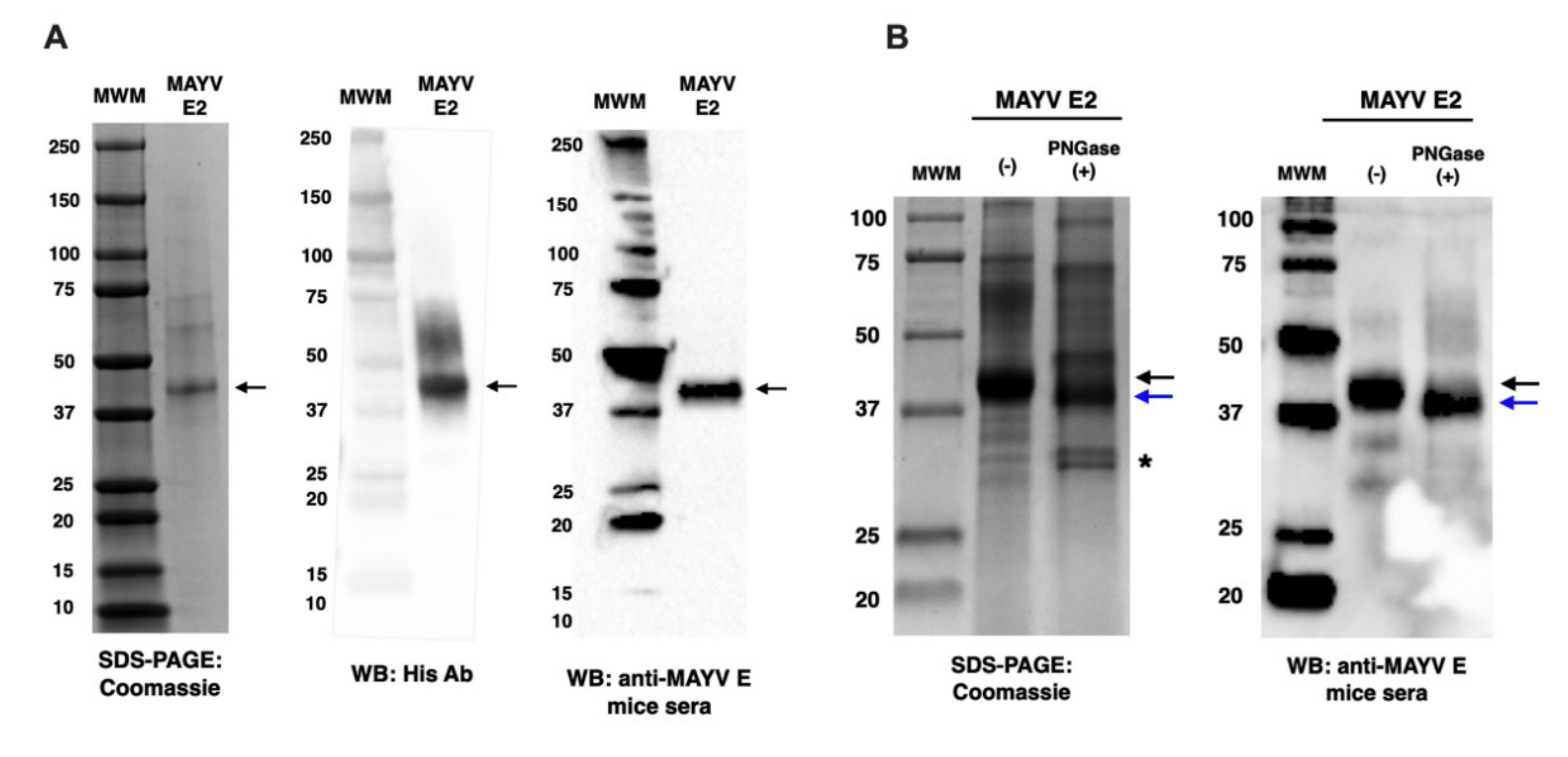
2.2. Characterisation of MAYV E2
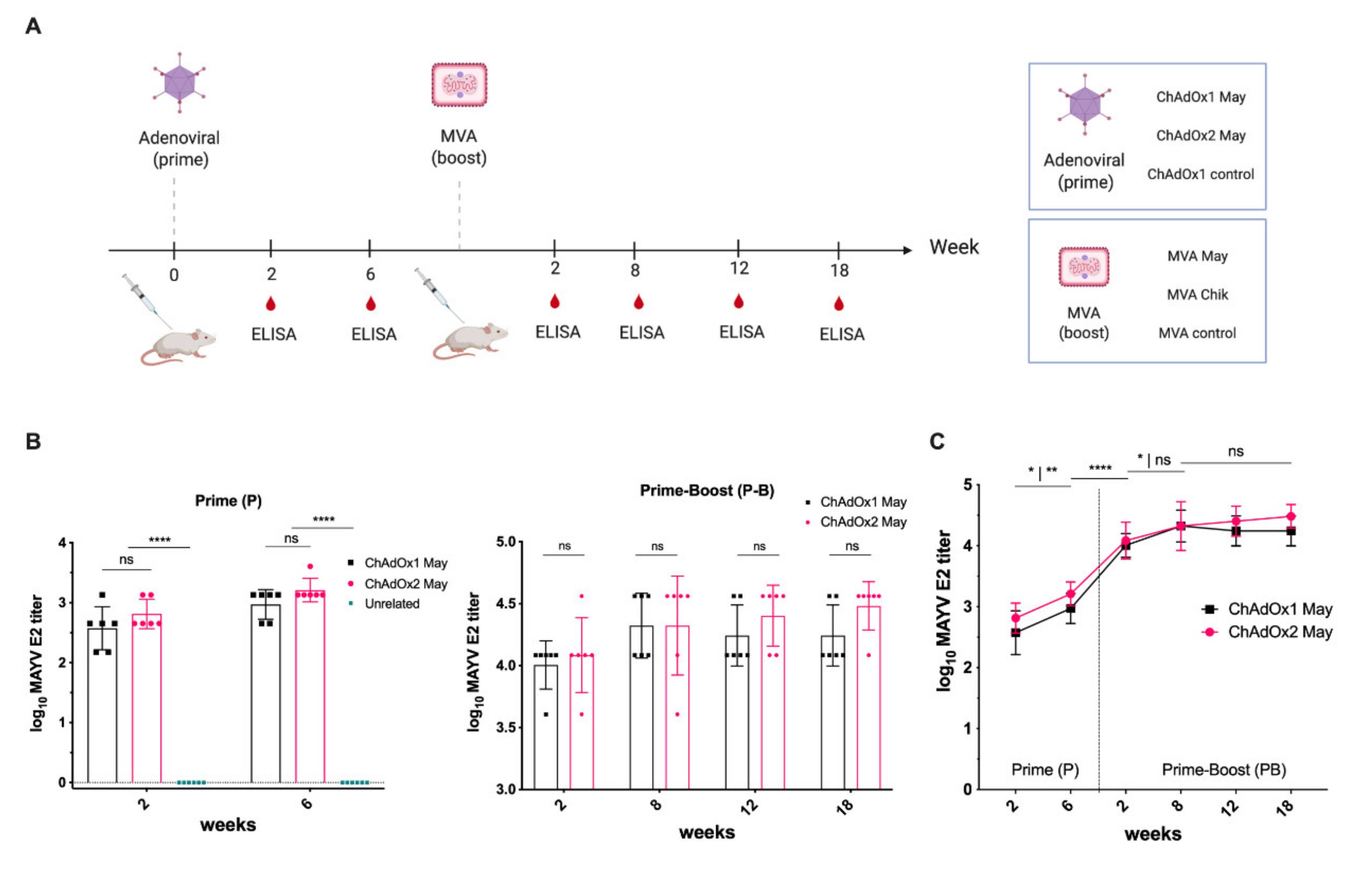
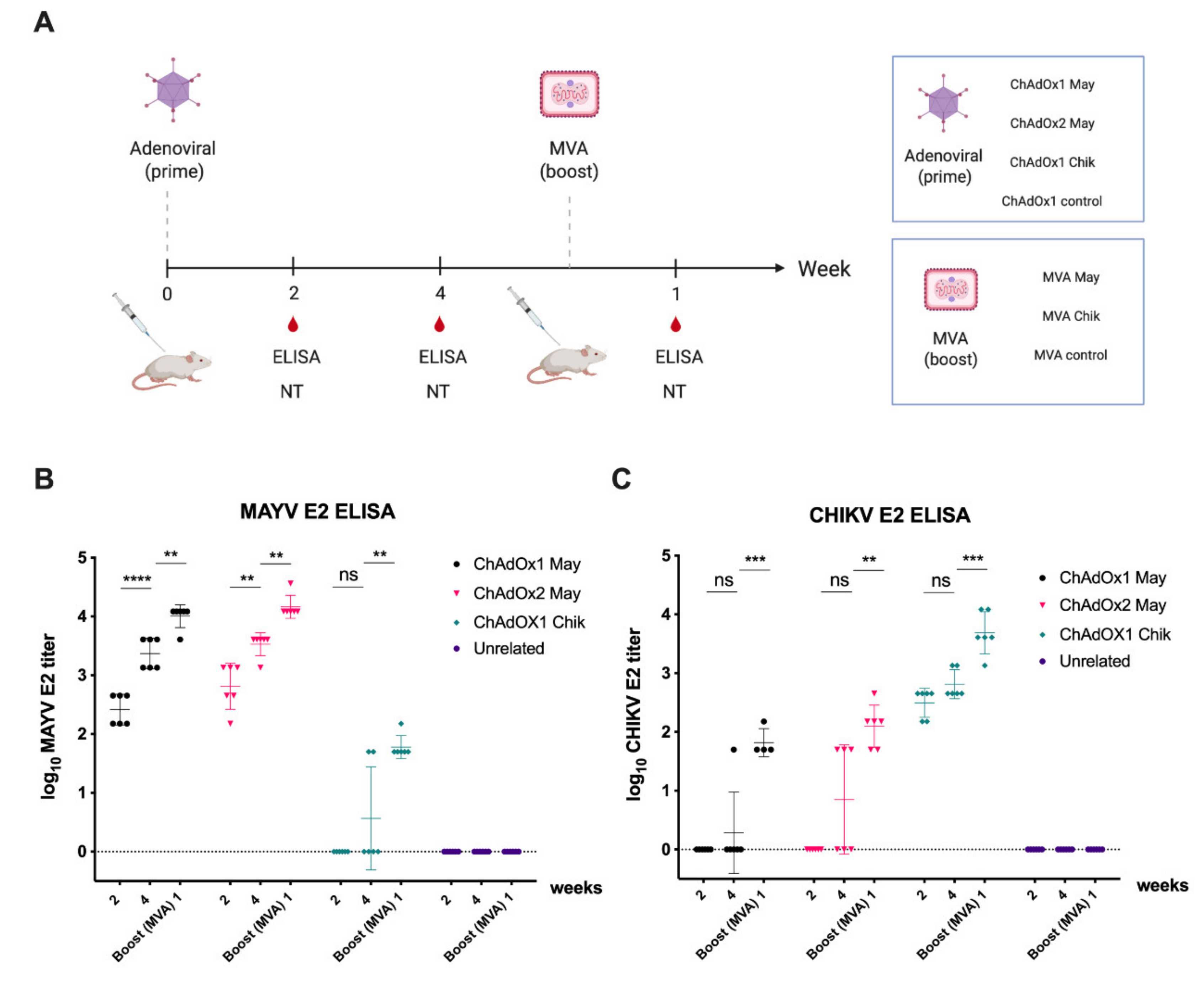
2.3. Immunogenicity of ChAdOx2 and MVA May Vaccines
2.4. Immunogenicity of ChAdOx2 and MVA May Vaccines
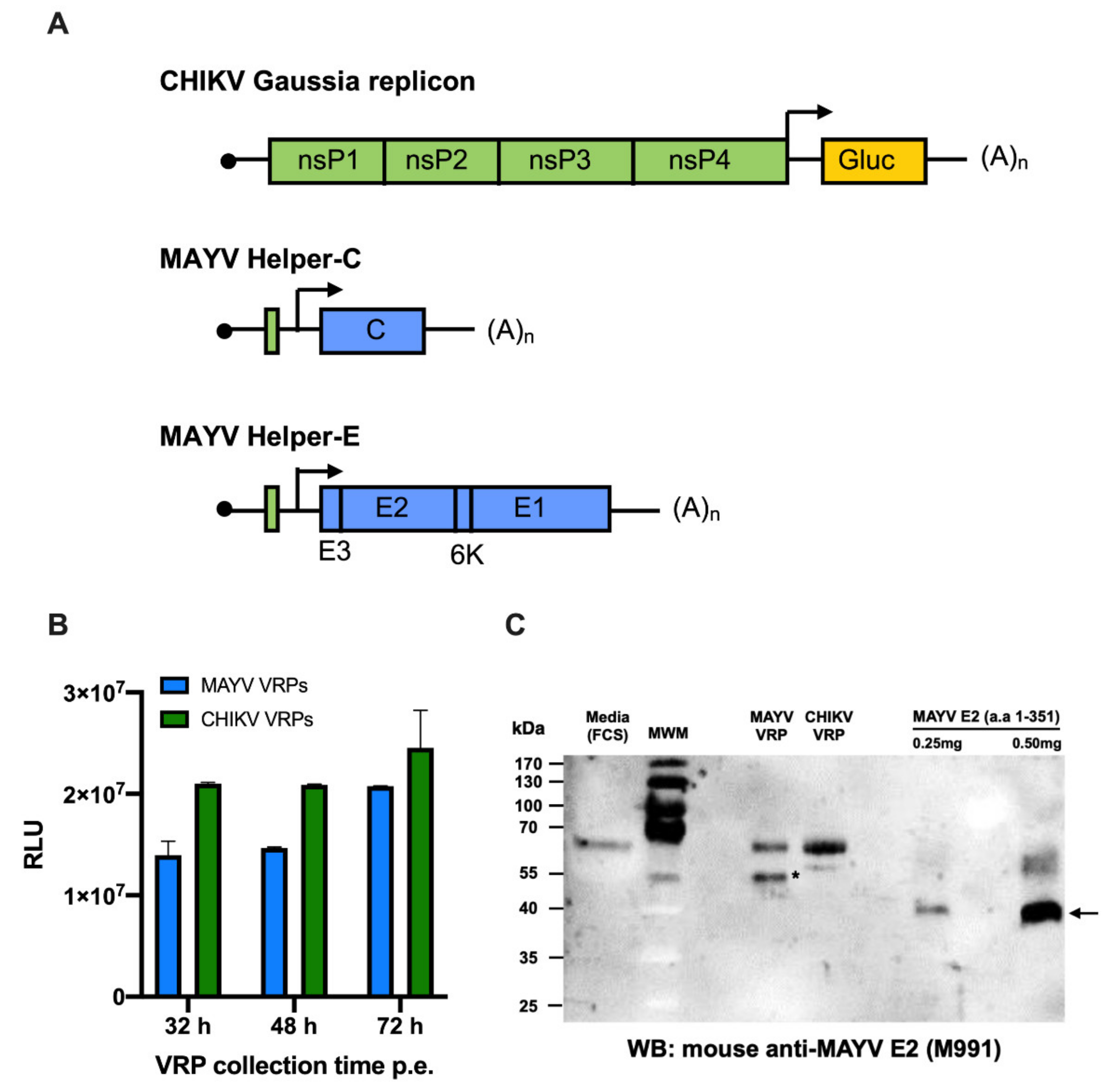
2.5. Establishment of MAYV VRP-Based Neutralisation Assay
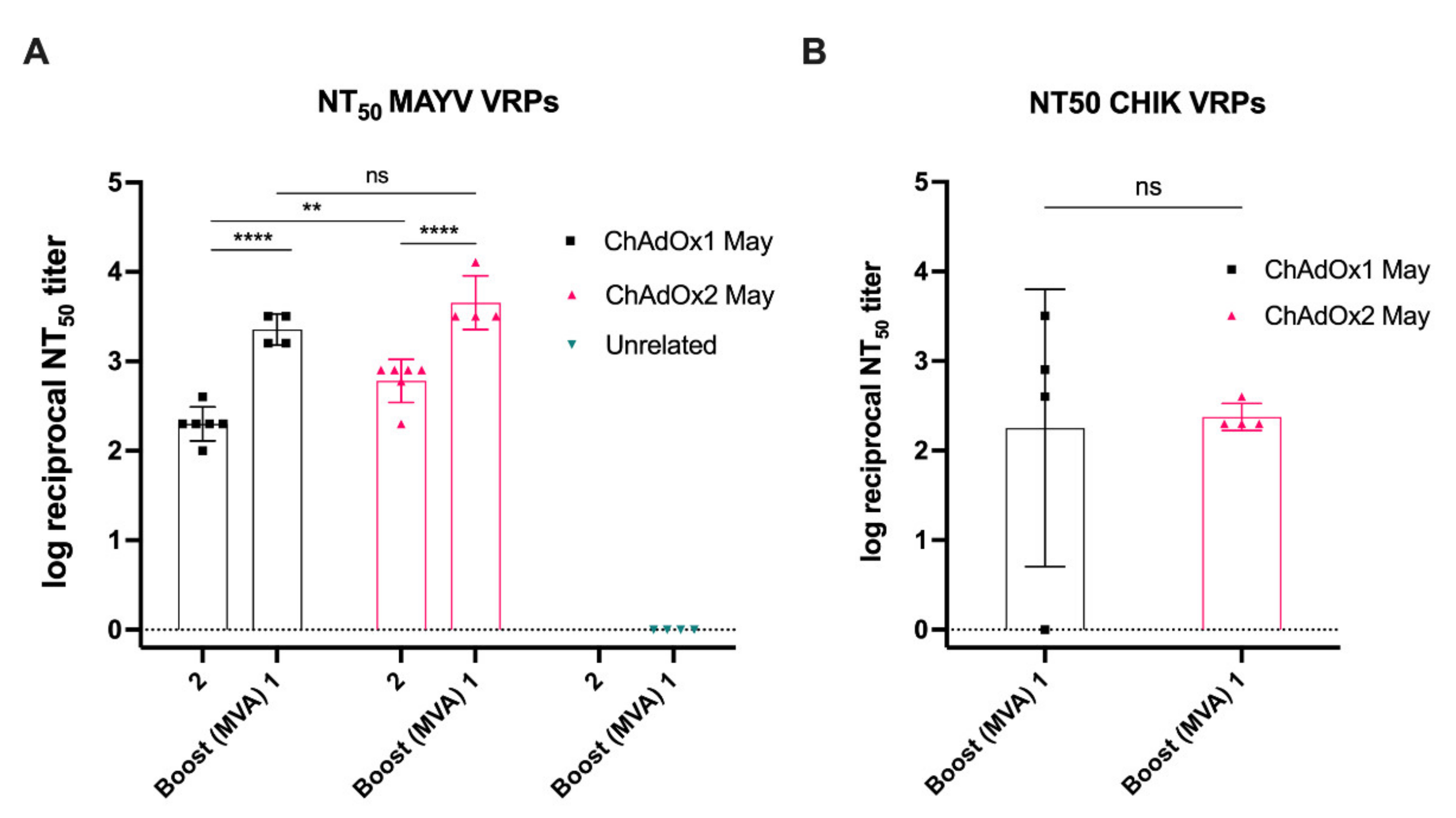
2.6. MAYV Neutralisation—Neutralising Ability in Vaccinated Mice Sera
3. Discussion
4. Materials and Methods
4.1. Transgene Design
4.2. Viral-Vectored Vaccine Production
4.3. Design and Production of the ChAdOx1 Chik and ChAdOx1 May
4.4. Animals
4.5. Vaccination
4.6. Production of Recombinant MAYV E2
4.7. SDS-PAGE and Western Blot Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Establishment of MAYV VRP-Based Neutralisation Assay
4.10. CHIKV VRP-Based Neutralisation Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Murray, K.O. Dengue, West Nile Virus, Chikungunya, Zika—And Now Mayaro? PLoS Negl. Trop. Dis. 2017, 11, e0005462. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.-M.; Ramírez-Santana, C. Mayaro: An Emerging Viral Threat? Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Halsey, E.S.; Siles, C.; Guevara, C.; Vilcarromero, S.; Jhonston, E.J.; Ramal, C.; Aguilar, P.V.; Ampuero, J.S. Mayaro Virus Infection, Amazon Basin Region, Peru, 2010–2013. Emerging Infect. Dis. 2013, 19, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Mackay, I.M.; Arden, K.E. Mayaro Virus: A Forest Virus Primed for a Trip to the City? Microbes Infect. 2016, 18, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.T.D.O.; Ribeiro, M.R.; Vedovello, D.; Nogueira, M.L. Mayaro Virus: A Neglected Arbovirus of the Americas. Future Virol. 2015, 10, 1109–1122. [Google Scholar] [CrossRef]
- Weaver, S.C.; Forrester, N.L. Chikungunya: Evolutionary History and Recent Epidemic Spread. Antiviral Res. 2015, 120, 32–39. [Google Scholar] [CrossRef]
- Weaver, S.C. Urbanization and Geographic Expansion of Zoonotic Arboviral Diseases: Mechanisms and Potential Strategies for Prevention. Trends Microbiol. 2013, 21, 360–363. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Bozza, F.; Merino Merino, X.J.; Pedroso, C.; de Oliveira Filho, E.F.; Moreira-Soto, A.; Schwalb, A.; de Lamballerie, X.; Netto, E.M.; Bozza, P.T.; et al. Robustness of Serologic Investigations for Chikungunya and Mayaro Viruses Following Coemergence. mSphere 2020, 5, e00915-19. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.M.; Cole, F.E.; Mcmanus, A.T.; Pedersen, C.E. Inactivated Mayaro vaccine produced in human diploid cell cultures. Mil. Med. 1976, 141, 163–166. [Google Scholar] [CrossRef]
- Weise, W.J.; Hermance, M.E.; Forrester, N.; Adams, A.P.; Langsjoen, R.; Gorchakov, R.; Wang, E.; Alcorn, M.D.H.; Tsetsarkin, K.; Weaver, S.C. A Novel Live-Attenuated Vaccine Candidate for Mayaro Fever. PLoS Negl. Trop. Dis. 2014, 8, e2969. [Google Scholar] [CrossRef]
- Choi, H.; Kudchodkar, S.B.; Reuschel, E.L.; Asija, K.; Borole, P.; Ho, M.; Wojtak, K.; Reed, C.; Ramos, S.; Bopp, N.E.; et al. Protective Immunity by an Engineered DNA Vaccine for Mayaro Virus. PLoS Negl. Trop. Dis. 2019, 13, e0007042. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Haese, N.N.; Denton, M.; Ando, T.; Kreklywich, C.; Bonin, K.; Streblow, C.E.; Kreklywich, N.; Smith, P.; Broeckel, R.; et al. Non-Replicating Adenovirus Based Mayaro Virus Vaccine Elicits Protective Immune Responses and Cross Protects against Other Alphaviruses. PLoS Negl. Trop. Dis. 2021, 15, e0009308. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The Alphaviruses: Gene Expression, Replication, and Evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.L.; Klose, T.; Urakami, A.; Hasan, S.S.; Akahata, W.; Rossmann, M.G. Structural Studies of Chikungunya Virus Maturation. Proc. Natl. Acad. Sci. USA 2017, 114, 13703–13707. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Filho, H.V.; Coimbra, L.D.; Cassago, A.; Rocha, R.P.F.; Guerra, J.V.D.S.; de Felicio, R.; Carnieli, C.M.; Leme, L.; Padilha, A.C.; Paes Leme, A.F.; et al. Cryo-EM Structure of the Mature and Infective Mayaro Virus at 4.4 Å Resolution Reveals Features of Arthritogenic Alphaviruses. Nat. Commun. 2021, 12, 3038. [Google Scholar] [CrossRef]
- Sun, S.; Xiang, Y.; Akahata, W.; Holdaway, H.; Pal, P.; Zhang, X.; Diamond, M.S.; Nabel, G.J.; Rossmann, M.G. Structural Analyses at Pseudo Atomic Resolution of Chikungunya Virus and Antibodies Show Mechanisms of Neutralization. eLife 2013, 2, e00435. [Google Scholar] [CrossRef]
- Basore, K.; Kim, A.S.; Nelson, C.A.; Zhang, R.; Smith, B.K.; Uranga, C.; Vang, L.; Cheng, M.; Gross, M.L.; Smith, J.; et al. Cryo-EM Structure of Chikungunya Virus in Complex with the Mxra8 Receptor. Cell 2019, 177, 1725–1737.e16. [Google Scholar] [CrossRef]
- Earnest, J.T.; Basore, K.; Roy, V.; Bailey, A.L.; Wang, D.; Alter, G.; Fremont, D.H.; Diamond, M.S. Neutralizing Antibodies against Mayaro Virus Require Fc Effector Functions for Protective Activity. J. Exp. Med. 2019, 216, 2282–2301. [Google Scholar] [CrossRef]
- Zhou, Q.F.; Fox, J.M.; Earnest, J.T.; Ng, T.-S.; Kim, A.S.; Fibriansah, G.; Kostyuchenko, V.A.; Shi, J.; Shu, B.; Diamond, M.S.; et al. Structural Basis of Chikungunya Virus Inhibition by Monoclonal Antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 27637–27645. [Google Scholar] [CrossRef]
- Earnest, J.T.; Holmes, A.C.; Basore, K.; Mack, M.; Fremont, D.H.; Diamond, M.S. The Mechanistic Basis of Protection by Non-Neutralizing Anti-Alphavirus Antibodies. Cell Rep. 2021, 35, 108962. [Google Scholar] [CrossRef]
- Zhang, R.; Earnest, J.T.; Kim, A.S.; Winkler, E.S.; Desai, P.; Adams, L.J.; Hu, G.; Bullock, C.; Gold, B.; Cherry, S.; et al. Expression of the Mxra8 Receptor Promotes Alphavirus Infection and Pathogenesis in Mice and Drosophila. Cell Rep. 2019, 28, 2647–2658.e5. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Long, F.; Edeling, M.A.; Lin, H.; van Duijl-Richter, M.K.S.; Fong, R.H.; Kahle, K.M.; Smit, J.M.; Jin, J.; Simmons, G.; et al. Broadly Neutralizing Alphavirus Antibodies Bind an Epitope on E2 and Inhibit Entry and Egress. Cell 2015, 163, 1095–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 Is a Receptor for Multiple Arthritogenic Alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Kroon Campos, R.; Preciado-Llanes, L.; Azar, S.R.; Kim, Y.C.; Brandon, O.; López-Camacho, C.; Reyes-Sandoval, A.; Rossi, S.L. Adenoviral-Vectored Mayaro and Chikungunya Virus Vaccine Candidates Afford Partial Cross-Protection From Lethal Challenge in A129 Mouse Model. Front. Immunol. 2020, 11, 591885. [Google Scholar] [CrossRef]
- López-Camacho, C.; Kim, Y.C.; Blight, J.; Lazaro Moreli, M.; Montoya-Diaz, E.; Huiskonen, J.T.; Kümmerer, B.M.; Reyes-Sandoval, A. Assessment of Immunogenicity and Neutralisation Efficacy of Viral-Vectored Vaccines Against Chikungunya Virus. Viruses 2019, 11, 322. [Google Scholar] [CrossRef] [Green Version]
- Folegatti, P.M.; Harrison, K.; Preciado-Llanes, L.; Lopez, F.R.; Bittaye, M.; Kim, Y.C.; Flaxman, A.; Bellamy, D.; Makinson, R.; Sheridan, J.; et al. A Single Dose of ChAdOx1 Chik Vaccine Induces Neutralizing Antibodies against Four Chikungunya Virus Lineages in a Phase 1 Clinical Trial. Nat. Commun. 2021, 12, 4636. [Google Scholar] [CrossRef]
- Antrobus, R.D.; Coughlan, L.; Berthoud, T.K.; Dicks, M.D.; Hill, A.V.; Lambe, T.; Gilbert, S.C. Clinical Assessment of a Novel Recombinant Simian Adenovirus ChAdOx1 as a Vectored Vaccine Expressing Conserved Influenza A Antigens. Mol. Ther. 2014, 22, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Folegatti, P.M.; Bellamy, D.; Roberts, R.; Powlson, J.; Edwards, N.J.; Mair, C.F.; Bowyer, G.; Poulton, I.; Mitton, C.H.; Green, N.; et al. Safety and Immunogenicity of a Novel Recombinant Simian Adenovirus ChAdOx2 as a Vectored Vaccine. Vaccines 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, J.S.; Rao, A.; Raghava, G.P.S. In Silico Platform for Prediction of N-, O- and C-Glycosites in Eukaryotic Protein Sequences. PLoS ONE 2013, 8, e67008. [Google Scholar] [CrossRef]
- Reyes-Sandoval, A.; Berthoud, T.; Alder, N.; Siani, L.; Gilbert, S.C.; Nicosia, A.; Colloca, S.; Cortese, R.; Hill, A.V.S. Prime-Boost Immunization with Adenoviral and Modified Vaccinia Virus Ankara Vectors Enhances the Durability and Polyfunctionality of Protective Malaria CD8+ T-Cell Responses. Infect. Immun. 2010, 78, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Campos, R.K.; Preciado-Llanes, L.; Azar, S.R.; Lopez-Camacho, C.; Reyes-Sandoval, A.; Rossi, S.L. A Single and Un-Adjuvanted Dose of a Chimpanzee Adenovirus-Vectored Vaccine against Chikungunya Virus Fully Protects Mice from Lethal Disease. Pathogens 2019, 8, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gläsker, S.; Lulla, A.; Lulla, V.; Couderc, T.; Drexler, J.F.; Liljeström, P.; Lecuit, M.; Drosten, C.; Merits, A.; Kümmerer, B.M. Virus Replicon Particle Based Chikungunya Virus Neutralization Assay Using Gaussia Luciferase as Readout. Virol. J. 2013, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smerdou, C.; Liljeström, P. Two-Helper RNA System for Production of Recombinant Semliki Forest Virus Particles. J. Virol. 1999, 73, 1092–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, M.T.D.O.; Costa, V.V.; Sugimoto, M.A.; Guimarães, G.D.F.; Queiroz-Junior, C.M.; Moreira, T.P.; de Sousa, C.D.; Santos, F.M.; Queiroz, V.F.; Passos, I.; et al. In-Depth Characterization of a Novel Live-Attenuated Mayaro Virus Vaccine Candidate Using an Immunocompetent Mouse Model of Mayaro Disease. Sci. Rep. 2020, 10, 5306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.; Gilbert, S.C.; Blanchard, T.J.; Hanke, T.; Robson, K.J.; Hannan, C.M.; Becker, M.; Sinden, R.; Smith, G.L.; Hill, A.V. Enhanced Immunogenicity for CD8+ T Cell Induction and Complete Protective Efficacy of Malaria DNA Vaccination by Boosting with Modified Vaccinia Virus Ankara. Nat. Med. 1998, 4, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Aricescu, A.R.; Lu, W.; Jones, E.Y. A Time- and Cost-Efficient System for High-Level Protein Production in Mammalian Cells. Acta Crystallogr. Sect. D 2006, 62, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sp6Helper F | GCTGACAGCGCCTTTTTGAA |
| Sp6Helper R | AGGGGTTATTCGGCCCTTG |
| 001F | TAAGAGACACACTGTACATAGCAA |
| 002R | TGTAGCGCTGATTAGTGTTTAGATACTTG |
| 003F | CACTAATCAGCTACAATGGATTTTCTGCCAACACAGG |
| 004R | ACAGTGTGTCTCTTACCATTCCTCGGTGCCCTC |
| 005F | TAAGTATGAAGGTATATGTGTCCCCTA |
| 006R | GGTGGCCTAGGTAGCTGATTAG |
| 007F | GCTACCTAGGCCACCATGGCTGCTCCTACAGTGACAGC |
| 008R | ATACCTTCATACTTATCTTCTCAGGGTGATACAGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.C.; Lücke, A.-C.; López-Camacho, C.; Kümmerer, B.M.; Reyes-Sandoval, A. Development of Viral-Vectored Vaccines and Virus Replicon Particle-Based Neutralisation Assay against Mayaro Virus. Int. J. Mol. Sci. 2022, 23, 4105. https://doi.org/10.3390/ijms23084105
Kim YC, Lücke A-C, López-Camacho C, Kümmerer BM, Reyes-Sandoval A. Development of Viral-Vectored Vaccines and Virus Replicon Particle-Based Neutralisation Assay against Mayaro Virus. International Journal of Molecular Sciences. 2022; 23(8):4105. https://doi.org/10.3390/ijms23084105
Chicago/Turabian StyleKim, Young Chan, Arlen-Celina Lücke, César López-Camacho, Beate Mareike Kümmerer, and Arturo Reyes-Sandoval. 2022. "Development of Viral-Vectored Vaccines and Virus Replicon Particle-Based Neutralisation Assay against Mayaro Virus" International Journal of Molecular Sciences 23, no. 8: 4105. https://doi.org/10.3390/ijms23084105
APA StyleKim, Y. C., Lücke, A.-C., López-Camacho, C., Kümmerer, B. M., & Reyes-Sandoval, A. (2022). Development of Viral-Vectored Vaccines and Virus Replicon Particle-Based Neutralisation Assay against Mayaro Virus. International Journal of Molecular Sciences, 23(8), 4105. https://doi.org/10.3390/ijms23084105






