Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases
Abstract
1. Introduction
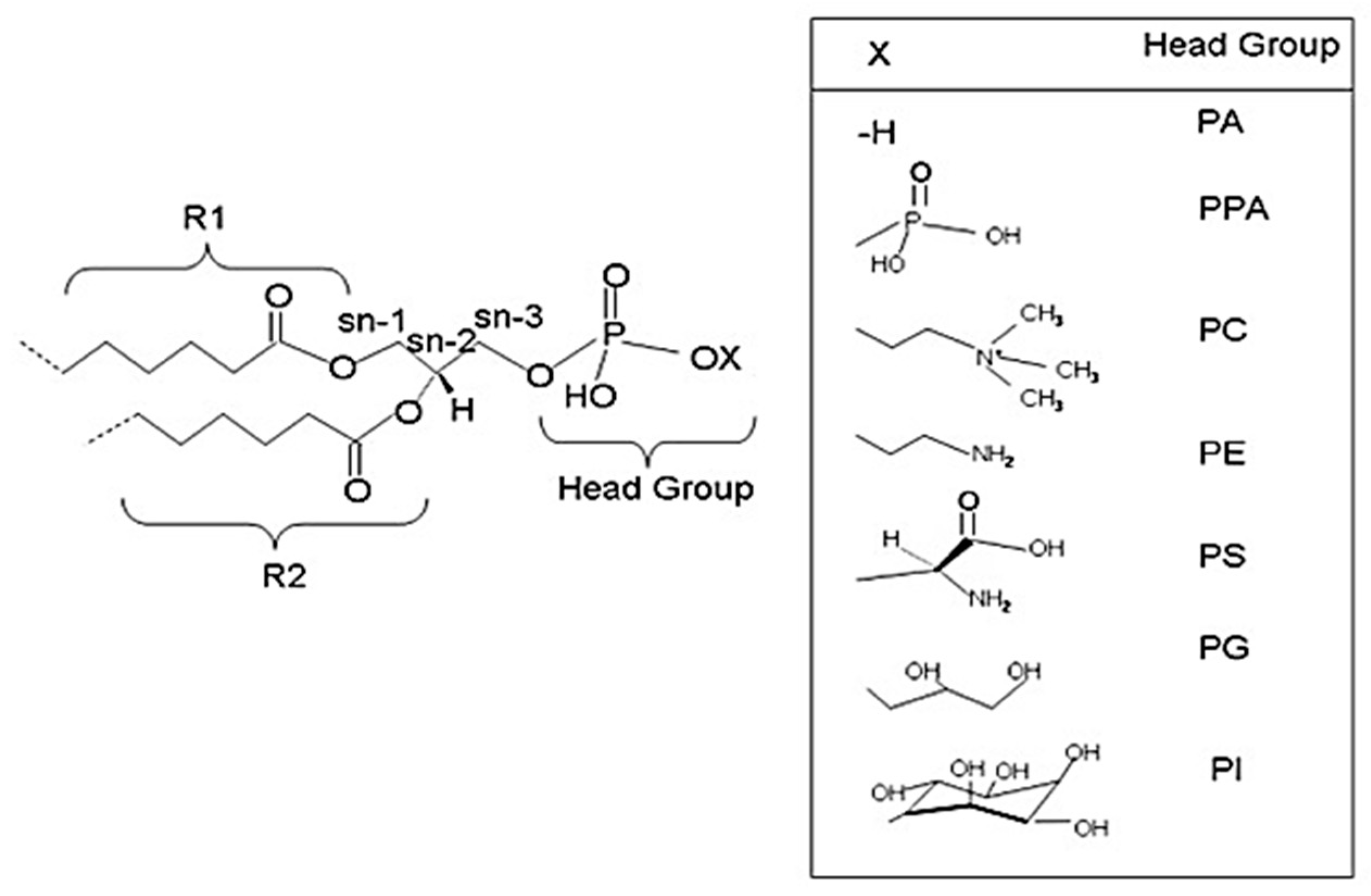
2. Glycerophospholipids
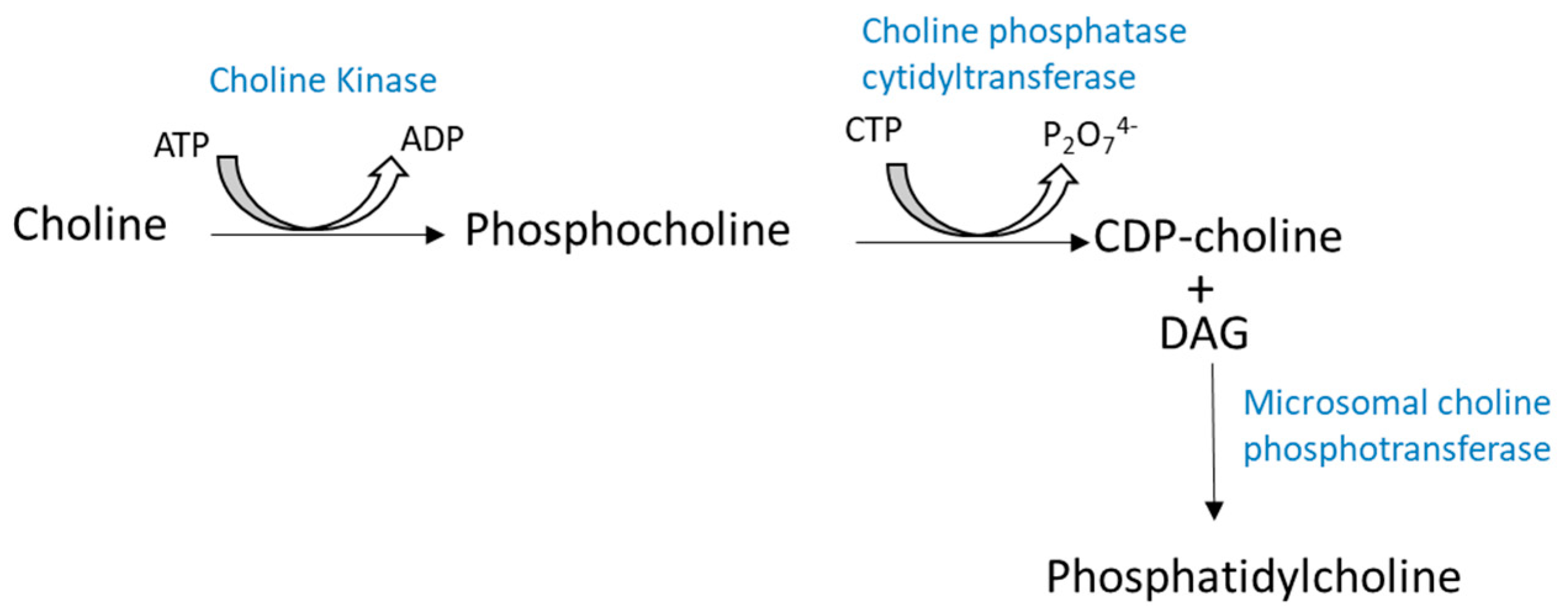
2.1. Biosynthesis of Glycerophospholipids
2.2. Remodeling of Gylcerophospholipids
2.2.1. De-Acylation/re-Acylation Pathways
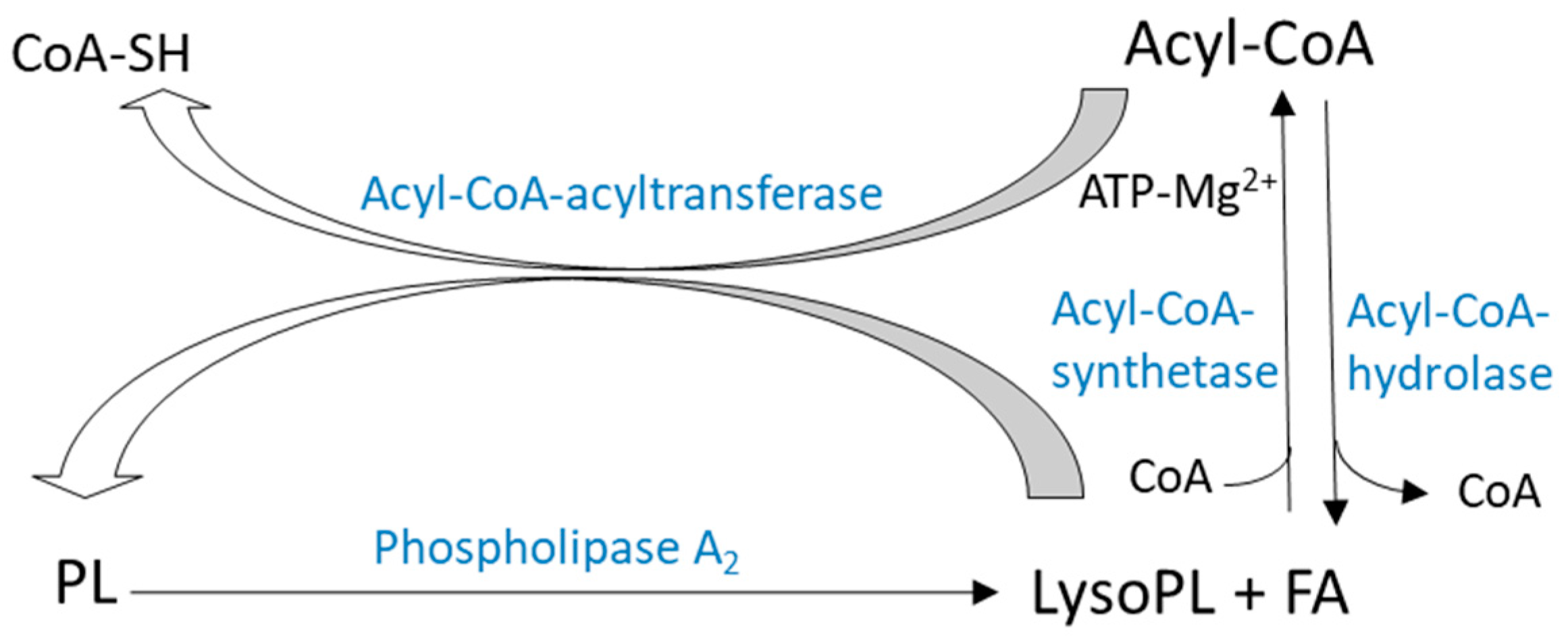
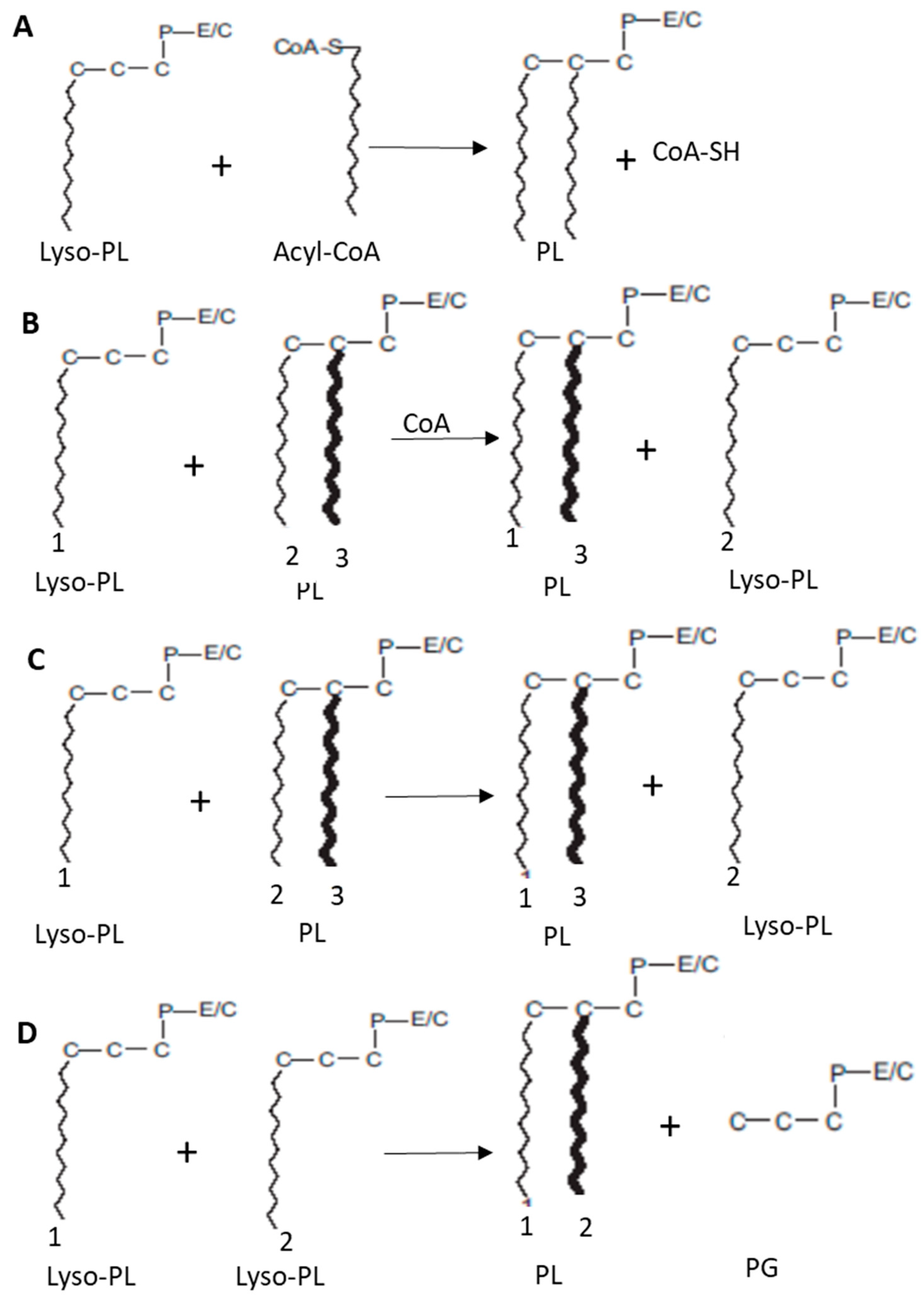
2.2.2. Transacylation Pathways
2.2.3. Remodeling Activities in the Brain
3. Properties and Functions of Phospholipids and Lysophospholipids with Omega-3 PUFA in the Brain
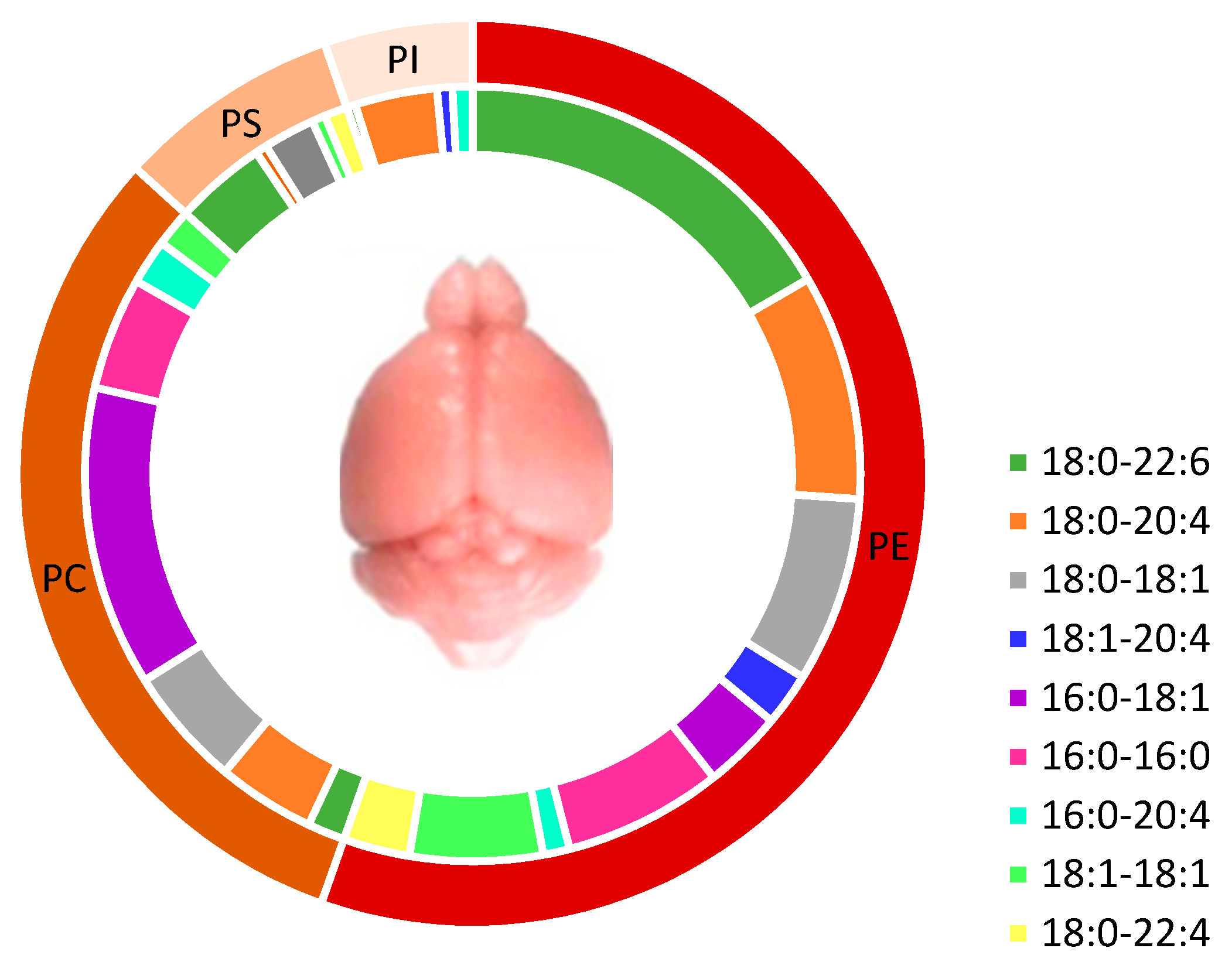
3.1. Brain Phospholipids and Their Fatty Acid Composition
3.2. Cerebral Lysophospholipids with Omega-3 PUFA
3.2.1. Lysophosphatidylcholine
- By hepatic lipase (LH) action which hydrolyzes PLs found in lipoproteins (high density lipoprotein (HDL)) and prefers PE to PCs. LH plays little role in Lyso-PC’s plasma production pool [51].
- By lecithin-cholesterol acyltransferase (LCAT) action, which is secreted by the liver and circulates in plasma mainly linked to lipoproteins, in particular high density lipoprotein (HDL) and in a minority to low density lipoprotein (LDL). LCAT catalyzes FA’s transfer at sn-2 position of a PC found in HDL to the 3-hydroxyl group of cholesterol to form cholesterol ester and Lyso-PC. Researchers suggested that the positional specificity of human LCAT is changed when 16:0-22:6-PC concentration is improved following DHA supply [52,53]. After a diet rich in DHA, sn-2-lysoPC-DHA level in plasma increases 3.5 times and that of 16:0–22:6-PC increases by 12% without any modification of sn-2-lysoPC-16:0.
- Highly unsaturated Lyso-PCs are also secreted by the liver after the ingestion of unsaturated TG [54]. However, exact mechanisms of Lyso-PC secretion by liver are not well established.
- Chen et al., 2007 proposed a plasma synthesis of Lyso-PC due to endothelial lipase (LE) activity [55].
3.2.2. Lysophosphatidylethanolamine
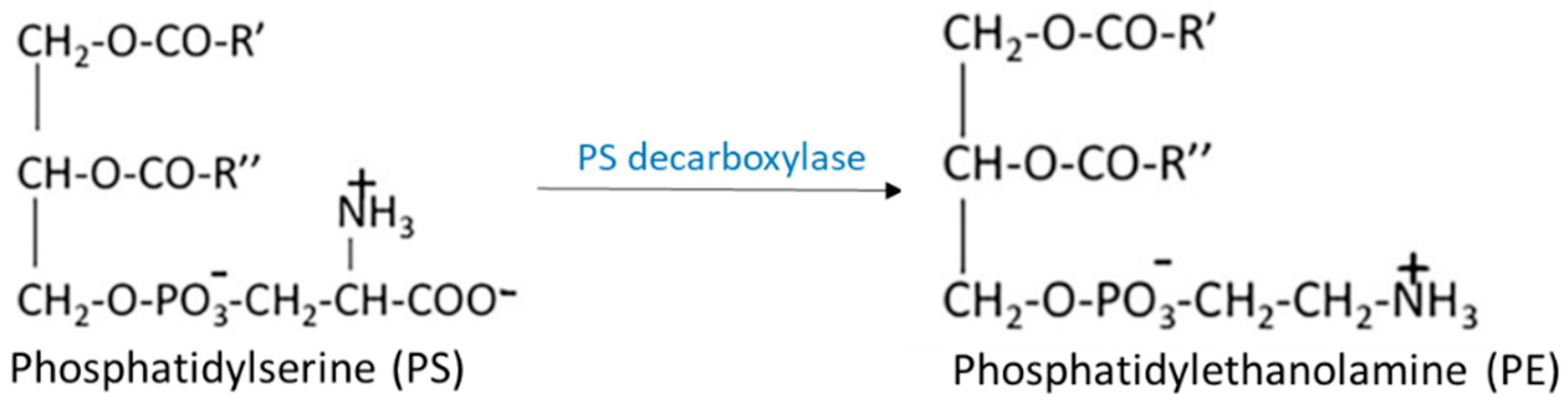
3.2.3. Lysophosphatidylserine
3.2.4. Lysophosphatidic Acid
4. Docosahexaenoic Acid Esterified at sn-2 Position of Phospholipids and Lysophospholipids for Brain Benefits
4.1. Benefits of DHA in Healthy Conditions and Brain Disorders
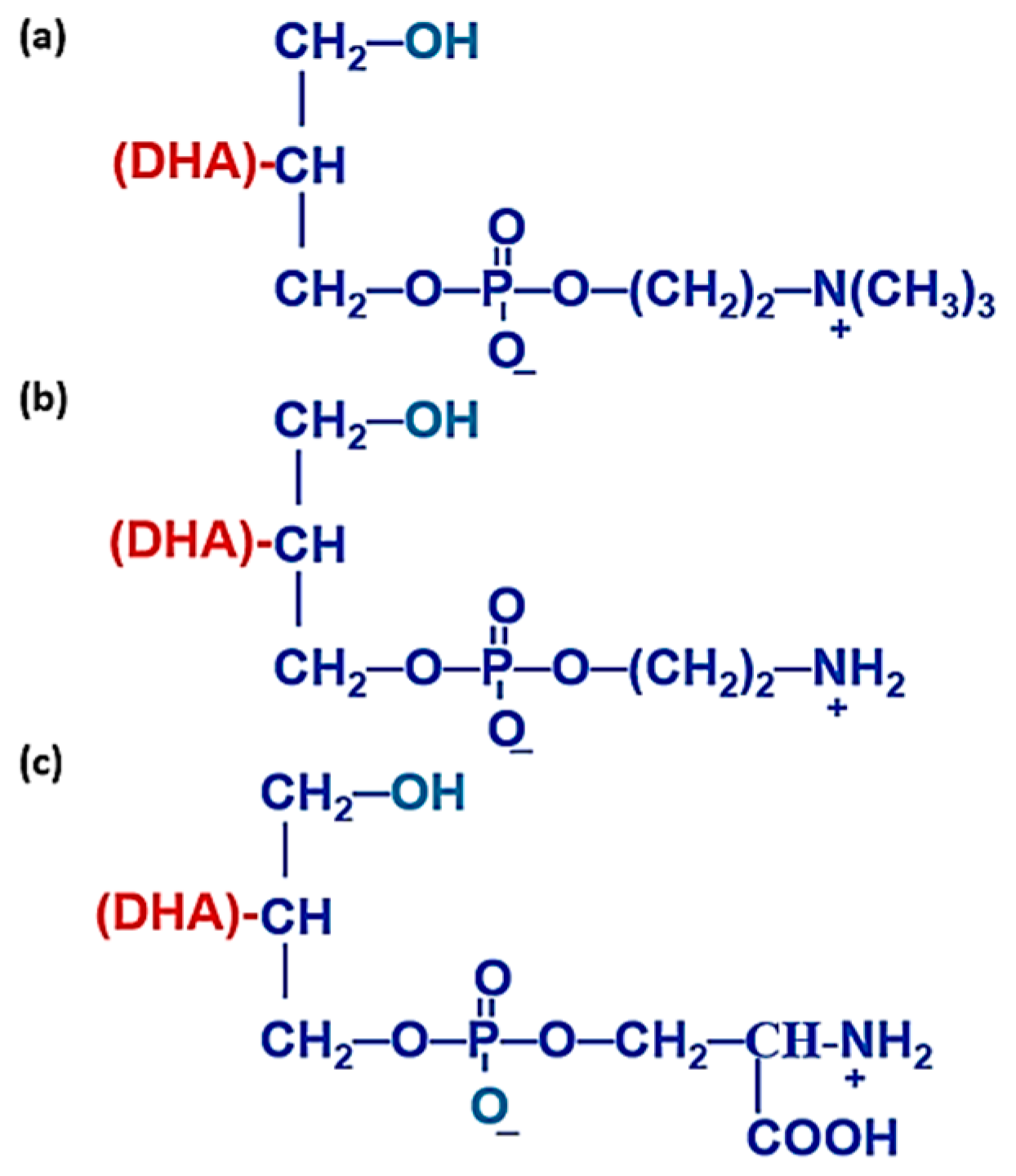
4.2. Chemical, Biochemical, and Nutritional Properties of sn-2 Position
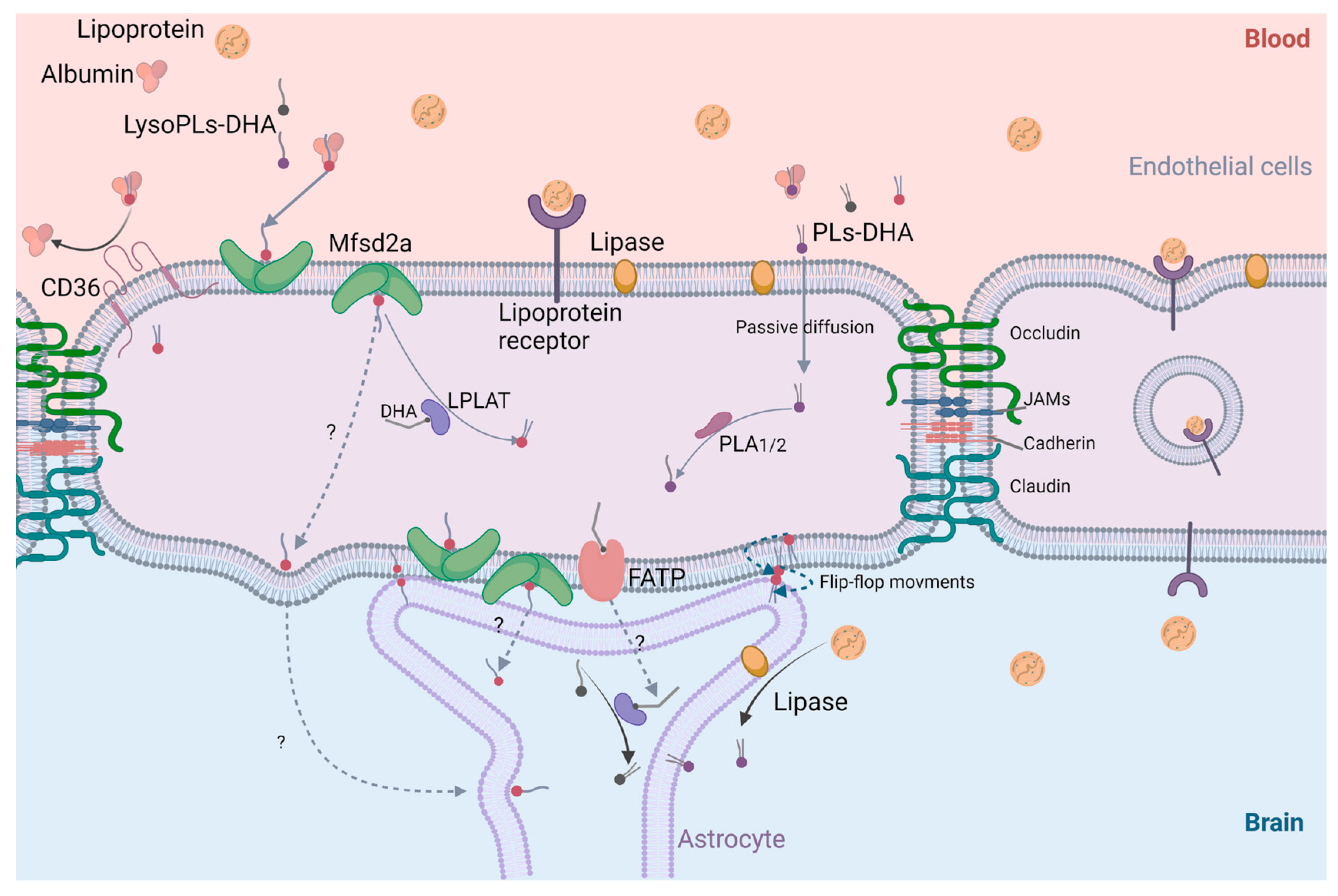
4.3. Cerebral Bioavailability of DHA
5. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Hachem, M.; Belkouch, M.; Van, A.L.; Picq, M.; Bernoud-Hubac, N.; Lagarde, M. Brain targeting with docosahexaenoic acid as a prospective therapy for neurodegenerative diseases and its passage across blood brain barrier. Biochimie 2020, 170, 203–211. [Google Scholar] [CrossRef]
- Bernoud-Hubac, N.; Hachem, M.; Lo Van, A.; Picq, M.; Lagarde, M. Specific uptake of DHA by the brain from a structured phospholipid, AceDoPC®: Captage sélectif du DHA par le cerveau à partir d’un phospholipide structuré, l’AceDoPC®. Oilseeds Fats Crops Lipids 2017, 24, 1–4. [Google Scholar]
- Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®. Nutrients 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, M.; Hachem, M.; Picq, M.; Guichardant, M.; Bernoud-Hubac, N. AceDoPC, a structured phospholipid to target the brain with docosahexaenoic acid. OCL 2015, 23, D102. [Google Scholar] [CrossRef]
- Bernoud, N.; Fenart, L.; Molière, P.; Dehouck, M.-P.; Lagarde, M.; Cecchelli, R.; Lecerf, J. Preferential Transfer of 2-Docosahexaenoyl-1-Lysophosphatidylcholine Through an In Vitro Blood-Brain Barrier Over Unesterified Docosahexaenoic Acid. J. Neurochem. 1999, 72, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Pillon, C.; Moliere, P.; Lagarde, M.; Lecerf, J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. Integr. Comp. Physiol. 1994, 267, R1273–R1279. [Google Scholar] [CrossRef] [PubMed]
- Hachem, M.; Géloën, A.; Van, A.L.; Foumaux, B.; Fenart, L.; Gosselet, F.; Da Silva, P.; Breton, G.; Lagarde, M.; Picq, M.; et al. Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol. Neurobiol. 2015, 53, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Lacombe, R.S.; Metherel, A.H.; Masoodi, M.; Bazinet, R.P. DHA Esterified to Phosphatidylserine or Phosphatidylcholine is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Mol. Nutr. Food Res. 2019, 63, e1801224. [Google Scholar] [CrossRef] [PubMed]
- Yetukuri, L.; Katajamaa, M.; Medina-Gomez, G.; Seppänen-Laakso, T.; Vidal-Puig, A.; Orešič, M. Bioinformatics strategies for lipidomics analysis: Characterization of obesity related hepatic steatosis. BMC Syst. Biol. 2007, 1, 12. [Google Scholar] [CrossRef]
- Kitson, A.P.; Stark, K.D.; Duncan, R.E. Enzymes in brain phospholipid docosahexaenoic acid accretion: A PL-ethora of potential PL-ayers. Prostaglandins, Leukot. Essent. Fat. Acids 2012, 87, 1–10. [Google Scholar] [CrossRef]
- Bishop, W.R.; Bell, R.M. Functions of diacylglycerol in glycerolipid metabolism, signal transduction and cellular transformation. Oncogene Res. 1988, 2, 205–218. [Google Scholar]
- Dennis, E.A.; Kennedy, E.P. Intracellular sites of lipid synthesis and the biogenesis of mitochondria. J. Lipid Res. 1972, 13, 263–267. [Google Scholar] [CrossRef]
- DeLong, C.J.; Shen, Y.-J.; Thomas, M.J.; Cui, Z. Molecular Distinction of Phosphatidylcholine Synthesis between the CDP-Choline Pathway and Phosphatidylethanolamine Methylation Pathway. J. Biol. Chem. 1999, 274, 29683–29688. [Google Scholar] [CrossRef]
- Ross, B.M.; Moszczynska, A.; Blusztajn, J.K.; Sherwin, A.; Lozano, A.; Kish, S.J. Phospholipid biosynthetic enzymes in human brain. Lipids 1997, 32, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Schneider, W.J. Conversion of phosphatidylethanolamine to phosphatidylcholine. Methods Enzym. 1981, 71, 581–588. [Google Scholar] [CrossRef]
- Hirata, F.; Axelrod, J. Phospholipid Methylation and Biological Signal Transmission. Science 1980, 209, 1082–1090. [Google Scholar] [CrossRef]
- Hattori, H.; Bansal, V.S.; Orihel, D.; Kanfer, J.N. Presence of Phospholipid-N-Methyltransferases and Base-Exchange Enzymes in Rat Central Nervous System Axolemma-Enriched Fractions. J. Neurochem. 1984, 43, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Tsvetnitsky, V.; Auchi, L.; Nicolaou, A.; Gibbons, W.A. Characterization of phospholipid methylation in rat brain myelin. Biochem. J. 1995, 307, 239–244. [Google Scholar] [CrossRef]
- Jelsema, C.L.; Morré, D.J. Distribution of phospholipid biosynthetic enzymes among cell components of rat liver. J. Biol. Chem. 1978, 253, 7960–7971. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Exton, J.H. Phospholipid base exchange activity in rat liver plasma membranes. Evidence for regulation by G-protein and P2y-purinergic receptor. J. Biol. Chem. 1992, 267, 5755–5761. [Google Scholar] [CrossRef]
- Grandmaison, P.A.; Nanowski, T.S.; Vance, J.E. Externalization of phosphatidylserine during apoptosis does not specifically require either isoform of phosphatidylserine synthase. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2004, 1636, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kiyasu, J.Y.; Pieringer, R.A.; Paulus, H.; Kennedy, E.P. The biosynthesis of phosphatidylglycerol. J. Biol. Chem. 1963, 238, 2293–2298. [Google Scholar] [CrossRef]
- Schlame, M.; Hostetler, K. Cardiolipin synthase from mammalian mitochondria. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1997, 1348, 207–213. [Google Scholar] [CrossRef]
- Hatch, G.M. Cardiolipin biosynthesis in the isolated heart. Biochem. J. 1994, 297, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Diagne, A.; Fauvel, J.; Record, M.; Chap, H.; Douste-Blazy, L. Studies on ether phospholipids: II. Comparative composition of various tissues from human, rat and guinea pig. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1984, 793, 221–231. [Google Scholar] [CrossRef]
- Lands, W.E.; Merkl, I. Metabolism of glycerolipids. III. Reactivity of various acyl esters of coenzyme A with alpha’-acylglycerophosphorylcholine, and positional specificities in lecithin synthesis. J. Biol. Chem. 1963, 238, 898–904. [Google Scholar] [CrossRef]
- MacDonald, J.I.; Sprecher, H. Phospholipid fatty acid remodeling in mammalian cells. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1991, 1084, 105–121. [Google Scholar] [CrossRef]
- Reddy, T.S.; Bazan, N.G. Activation of polyunsaturated fatty acids by rat tissues in vitro. Lipids 1984, 19, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.; Bazan, N. Synthesis of arachidonoyl coenzyme a and docosahexaenoyl coenzyme a in synaptic plasma membranes of cerebrum and microsomes of cerebrum, cerebellum, and brain stem of rat brain. J. Neurosci. Res. 1985, 13, 381–390. [Google Scholar] [CrossRef]
- Yamashita, A.; Hayashi, Y.; Nemoto-Sasaki, Y.; Ito, M.; Oka, S.; Tanikawa, T.; Waku, K.; Sugiura, T. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 2013, 53, 18–81. [Google Scholar] [CrossRef]
- Sugiura, T.; Masuzawa, Y.; Waku, K. Coenzyme A-dependent transacylation system in rabbit liver microsomes. J. Biol. Chem. 1988, 263, 17490–17498. [Google Scholar] [CrossRef]
- Kramer, R.M.; Deykin, D. Arachidonoyl transacylase in human platelets. Coenzyme A-independent transfer of arachidonate from phosphatidylcholine to lysoplasmenylethanolamine. J. Biol. Chem. 1983, 258, 13806–13811. [Google Scholar] [CrossRef]
- Masuzawa, Y.; Sugiura, T.; Sprecher, H.; Waku, K. Selective acyl transfer in the reacylation of brain glycerophospholipids. Comparison of three acylation systems for 1-alk-1′-enylglycero-3-phosphoethanolamine, 1-acylglycero-3-phosphoethanolamine and 1-acylglycero-3-phosphocholine in rat brain microsomes. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1989, 1005, 1–12. [Google Scholar] [CrossRef]
- Onuma, Y.; Masuzawa, Y.; Ishima, Y.; Waku, K. Selective incorporation of docosahexaenoic acid in rat brain. Biochim. Biophys. Acta 1984, 793, 80–85. [Google Scholar] [PubMed]
- Baker, R.; Chang, H.-Y. A comparison of lysophosphatidylcholine acyltransferase activities in neuronal nuclei and microsomes isolated from immature rabbit cerebral cortex. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1981, 666, 223–229. [Google Scholar] [CrossRef]
- Ross, B.M.; Kish, S.J. Characterization of Lysophospholipid Metabolizing Enzymes in Human Brain. J. Neurochem. 2002, 63, 1839–1848. [Google Scholar] [CrossRef]
- Vaswani, K.K.; Ledeen, R.W. Purified rat brain myelin contains measurable acyl-CoA:lysophospholipid acyltransferase(s) but little, if any, glycerol-3-phosphate acyltransferase. J. Neurochem. 1989, 52, 69–74. [Google Scholar] [CrossRef]
- Choi, J.; Yin, T.; Shinozaki, K.; Lampe, J.W.; Stevens, J.F.; Becker, L.B.; Kim, J. Comprehensive analysis of phospholipids in the brain, heart, kidney, and liver: Brain phospholipids are least enriched with polyunsaturated fatty acids. Mol. Cell. Biochem. 2017, 442, 187–201. [Google Scholar] [CrossRef]
- Brenna, J.T.; Diau, G.-Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins, Leukot. Essent. Fat. Acids 2007, 77, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed Choline Plasmalogen and Phospholipid Fatty Acid Concentrations in Alzheimer’s Disease Prefrontal Cortex. J. Alzheimer’s Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, J.; Sinclair, A.J. Plasmalogens and Alzheimer’s disease: A review. Lipids Heal. Dis. 2019, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Carne, A.; Bunga, S.; Tian, H.; Bekhit, A.E.-D.A. Lipidomic signature of Pacific lean fish species head and skin using gas chromatography and nuclear magnetic resonance spectroscopy. Food Chem. 2021, 365, 130637. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Chen, C.T.; Metherel, A.H.; Lacombe, R.S.; Thies, F.; Masoodi, M.; Bazinet, R.P. Phospholipid class-specific brain enrichment in response to lysophosphatidylcholine docosahexaenoic acid infusion. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1092–1098. [Google Scholar] [CrossRef]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807. [Google Scholar] [CrossRef]
- Hishikawa, D.; Shindou, H.; Kobayashi, S.; Nakanishi, H.; Taguchi, R.; Shimizu, T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Thiés, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem. 1992, 59, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Ojala, P.J.; Hirvonen, T.E.; Hermansson, M.; Somerharju, P.; Parkkinen, J. Acyl chain-dependent effect of lysophosphatidylcholine on human neutrophils. J. Leukoc. Biol. 2007, 82, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azéma, C.; Marques-Vidal, P.; Lespine, A.; Simard, G.; Chap, H.; Perret, B. Kinetic evidence for phosphatidylethanolamine and triacylglycerol as preferential substrates for hepatic lipase in HDL subfractions: Modulation by changes in the particle surface, or in the lipid core. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1990, 1046, 73–80. [Google Scholar] [CrossRef]
- Glomset, J.A. The mechanism of the plasma cholesterol esterification reaction: Plasma fatty acid transferase. Biochim. Biophys. Acta 1962, 65, 128–135. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Sowa, J.M.; Davidson, M.H. Evidence for altered positional specificity of LCAT in vivo: Studies with docosahexaenoic acid feeding in humans. J. Lipid Res. 2004, 45, 2245–2251. [Google Scholar] [CrossRef]
- Brossard, N.; Croset, M.; Normand, S.; Pousin, J.; Lecerf, J.; Laville, M.; Tayot, J.L.; Lagarde, M. Human plasma albumin transports [13C]docosahexaenoic acid in two lipid forms to blood cells. J. Lipid Res. 1997, 38, 1571–1582. [Google Scholar] [CrossRef]
- Chen, S.; Subbaiah, P.V. Phospholipid and fatty acid specificity of endothelial lipase: Potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 1319–1328. [Google Scholar] [CrossRef]
- Gendaszewska-Darmach, E. Lysophosphatidic acids, cyclic phosphatidic acids and autotaxin as promising targets in therapies of cancer and other diseases. Acta Biochim. Pol. 2008, 55, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, J.-H.; Kim, N.-H.; Lee, C.-W.; Kim, M.-J.; Kim, S.-H.; Huh, S.-O. Lysophosphatidylcholine-induced apoptosis in H19-7 hippocampal progenitor cells is enhanced by the upregulation of Fas Ligand. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2009, 1791, 61–68. [Google Scholar] [CrossRef]
- McMurray, H.F.; Parthasarathy, S.; Steinberg, D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. J. Clin. Investig. 1993, 92, 1004–1008. [Google Scholar] [CrossRef]
- Takahara, N.; Kashiwagi, A.; Maegawa, H.; Shigeta, Y. Lysophosphatidylcholine stimulates the expression and production of MCP-1 by human vascular endothelial cells. Metabolism 1996, 45, 559–564. [Google Scholar] [CrossRef]
- Aiyar, N.; Disa, J.; Ao, Z.; Ju, H.; Nerurkar, S.; Willette, R.N.; Macphee, C.H.; Johns, D.G.; Douglas, S.A. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Mol. Cell. Biochem. 2006, 295, 113–120. [Google Scholar] [CrossRef]
- Katz, A.M.; Messineo, F.C. Lipids and Membrane Function: Implications in Arrhythmias. Hosp. Pract. 1981, 16, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Hong, K.H.; Ko, J.; Rhee, K.S.; Hong, M.K.; Kim, J.J.; Kim, Y.H.; Park, S.J. Lysophosphatidylcholine up-regulates CXCR4 chemokine receptor expression in human CD4 T cells. J. Leukoc. Biol. 2004, 76, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-J.; Jung, J.-S.; Lee, J.-E.; Lee, J.; Huh, S.-O.; Kim, H.-S.; Jung, K.C.; Cho, J.-Y.; Nam, J.-S.; Suh, H.-W.; et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004, 10, 161–167. [Google Scholar] [CrossRef]
- Zembowicz, A.; Tang, J.-L.; Wu, K.K. Transcriptional Induction of Endothelial Nitric Oxide Synthase Type III by Lysophosphatidylcholine. J. Biol. Chem. 1995, 270, 17006–17010. [Google Scholar] [CrossRef]
- Hung, N.D.; Sok, D.-E.; Kim, M.R. Prevention of 1-palmitoyl lysophosphatidylcholine-induced inflammation by polyunsaturated acyl lysophosphatidylcholine. Agents Actions 2012, 61, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Alakbarzade, V.; Hameed, A.; Quek, D.Q.Y.; Chioza, B.A.; Baple, E.L.; Cazenave-Gassiot, A.; Nguyen, L.N.; Wenk, M.R.; Ahmad, A.Q.; Sreekantan-Nair, A.; et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat. Genet. 2015, 47, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.P.; Wong, B.H.; Chin, C.F.; Galam, D.L.A.; Foo, J.C.; Wong, L.C.; Ghosh, S.; Wenk, M.R.; Cazenave-Gassiot, A.; Silver, D.L. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol. 2018, 16, e2006443. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB J. 2019, 33, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Scheinman, S.B.; Sugasini, D.; Zayed, M.; Yalagala, P.C.R.; Marottoli, F.M.; Subbaiah, P.V.; Tai, L.M. LPC-DHA/EPA-Enriched Diets Increase Brain DHA and Modulate Behavior in Mice That Express Human APOE4. Front. Neurosci. 2021, 15, 767. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D. Perspective: The Potential Role of Circulating Lysophosphatidylcholine in Neuroprotection against Alzheimer Disease. Adv. Nutr. Int. Rev. J. 2020, 11, 760–772. [Google Scholar] [CrossRef]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thies, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a Preferred Carrier Form of Docosahexaenoic Acid to the Brain. J. Mol. Neurosci. 2001, 16, 201–204. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.H.; Chan, J.P.; Cazenave-Gassiot, A.; Poh, R.W.; Foo, J.C.; Galam, D.L.A.; Ghosh, S.; Nguyen, L.N.; Barathi, V.A.; Yeo, S.W.; et al. Mfsd2a Is a Transporter for the Essential ω-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem. 2016, 291, 10501–10514. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.; Subbaiah, P. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 2020, 12, 3114. [Google Scholar] [CrossRef] [PubMed]
- Yalagala, P.C.; Sugasini, D.; Dasarathi, S.; Pahan, K.; Subbaiah, P.V. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: Potential treatment for depression. J. Lipid Res. 2019, 60, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma1. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lee, H.Y.; Lee, S.Y.; Kim, M.-K.; Kim, S.D.; Kim, J.M.; Yun, J.; Im, D.-S.; Bae, Y.-S. Lysophosphatidylethanolamine stimulates chemotactic migration and cellular invasion in SK-OV3 human ovarian cancer cells: Involvement of pertussis toxin-sensitive G-protein coupled receptor. FEBS Lett. 2007, 581, 4411–4416. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, K.-P.; Kang, S.; Chung, H.-Y.; Bae, Y.-S.; Okajima, F.; Im, D.-S. Lysophosphatidylethanolamine utilizes LPA1 and CD97 in MDA-MB-231 breast cancer cells. Cell. Signal. 2013, 25, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Hisano, K.; Yoshida, H.; Kawase, S.; Mimura, T.; Haniu, H.; Tsukahara, T.; Kurihara, T.; Matsuda, Y.; Saito, N.; Uemura, T. Abundant oleoyl-lysophosphatidylethanolamine in brain stimulates neurite outgrowth and protects against glutamate toxicity in cultured cortical neurons. J. Biochem. 2021, 170, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hisano, K.; Kawase, S.; Mimura, T.; Yoshida, H.; Yamada, H.; Haniu, H.; Tsukahara, T.; Kurihara, T.; Matsuda, Y.; Saito, N.; et al. Structurally different lysophosphatidylethanolamine species stimulate neurite outgrowth in cultured cortical neurons via distinct G-protein-coupled receptors and signaling cascades. Biochem. Biophys. Res. Commun. 2020, 534, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.D.; Kim, M.R.; Sok, D.-E. 2-Polyunsaturated Acyl Lysophosphatidylethanolamine Attenuates Inflammatory Response in Zymosan A-Induced Peritonitis in Mice. Lipids 2011, 46, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Shindou, H.; Yamamoto, S.; Tamura-Nakano, M.; Shimizu, T. Lysophosphatidylethanolamine acyltransferase 2 (LPEAT2) incorporates DHA into phospholipids and has possible functions for fatty acid-induced cell death. Biochem. Biophys. Res. Commun. 2020, 526, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Guaqueta, A.M.S.; Villamil-Ortiz, J.G.; Arias-Londoño, J.D.; Cardona-Gomez, G.P. Inverse Phosphatidylcholine/Phosphatidylinositol Levels as Peripheral Biomarkers and Phosphatidylcholine/Lysophosphatidylethanolamine-Phosphatidylserine as Hippocampal Indicator of Postischemic Cognitive Impairment in Rats. Front. Neurosci. 2018, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.K.; Kim, H.-Y. Phosphatidylserine synthase 2: High efficiency for synthesizing phosphatidylserine containing docosahexaenoic acid. J. Lipid Res. 2013, 54, 214–222. [Google Scholar] [CrossRef]
- Shanbhag, K.; Mhetre, A.; Khandelwal, N.; Kamat, S.S. The Lysophosphatidylserines—An Emerging Class of Signalling Lysophospholipids. J. Membr. Biol. 2020, 253, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hasse, S.; Bourgoin, S.G. Phosphatidylserine-specific phospholipase A1: A friend or the devil in disguise. Prog. Lipid Res. 2021, 83, 101112. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.L.; Lee, Y.K.; Han, M.; Sacket, S.J.; Jo, J.Y.; Kim, Y.L.; Im, D.S. Lysophosphatidylserine induces calcium signaling through Ki16425/VPC32183-sensitive GPCR in bone marrow-derived mast cells and in C6 glioma and colon cancer cells. Arch. Pharm. Res. 2008, 31, 310–317. [Google Scholar] [CrossRef]
- Konkel, J.E.; Zhang, D.; Zanvit, P.; Chia, C.; Zangarle-Murray, T.; Jin, W.; Wang, S.; Chen, W. Transforming Growth Factor-β Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 2017, 46, 660–674. [Google Scholar] [CrossRef]
- Okudaira, M.; Inoue, A.; Shuto, A.; Nakanaga, K.; Kano, K.; Makide, K.; Saigusa, D.; Tomioka, Y.; Aoki, J. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res. 2014, 55, 2178–2192. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Makide, K.; Shuto, A.; Ikubo, M.; Inoue, A.; Suzuki, K.; Sato, Y.; Nakamura, S.; Otani, Y.; Ohwada, T.; et al. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J. Biochem. 2012, 151, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Long, J.Z.; Trauger, S.A.; Siuzdak, G.; Cravatt, B.F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci. USA 2013, 110, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Joshi, A.; Kamat, S.S. Mapping the neuroanatomy of ABHD16A–ABHD12 & lysophosphatidylserines provides new insights into the pathophysiology of the human neurological disorder PHARC. Biochemistry 2020, 59, 2299–2311. [Google Scholar] [PubMed]
- Bononi, G.; Tuccinardi, T.; Rizzolio, F.; Granchi, C. α/β-Hydrolase Domain (ABHD) Inhibitors as New Potential Therapeutic Options against Lipid-Related Diseases. J. Med. Chem. 2021, 64, 9759–9785. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, S.; Iijima, H.; Otake, Y.; Amano, T.; Tani, M.; Yoshihara, T.; Tashiro, T.; Tsujii, Y.; Inoue, T.; Hayashi, Y.; et al. Novel mass spectrometry-based comprehensive lipidomic analysis of plasma from patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2020, 35, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ishiguro, J.; Kitamura, H.; Arima, N.; Okutani, M.; Shuto, A.; Higashiyama, S.; Ohwada, T.; Arai, H.; Makide, K.; et al. TGFα shedding assay: An accurate and versatile method for detecting GPCR activation. Nat. Methods 2012, 9, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Fukushima, N.; Ye, X.; Chun, J. Lysophospholipid Receptors: Signaling and Biology. Annu. Rev. Biochem. 2004, 73, 321–354. [Google Scholar] [CrossRef]
- Wang, C.-C.; Guo, Y.; Zhou, M.-M.; Xue, C.-H.; Chang, Y.-G.; Zhang, T.-T.; Wang, Y.-M. Comparative studies of DHA-enriched phosphatidylcholine and recombination of DHA-ethyl ester with egg phosphatidylcholine on ameliorating memory and cognitive deficiency in SAMP8 mice. Food Funct. 2019, 10, 938–950. [Google Scholar] [CrossRef]
- Khandelwal, N.; Shaikh, M.; Mhetre, A.; Singh, S.; Sajeevan, T.; Joshi, A.; Balaji, K.N.; Chakrapani, H.; Kamat, S.S. Fatty acid chain length drives lysophosphatidylserine-dependent immunological outputs. Cell Chem. Biol. 2021, 28, 1169–1179.e6. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, M.; Zhong, S.; Feng, C.; Nwobodo, A.K.; Chen, B.; Song, Y.; Wang, Y. Dihydroartemisinin ameliorates LPS-induced neuroinflammation by inhibiting the PI3K/AKT pathway. Metab. Brain Dis. 2020, 35, 661–672. [Google Scholar] [CrossRef]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 2017, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Richmond, G.S.; Smith, T.K. Phospholipases A1. Int. J. Mol. Sci. 2011, 12, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Inoue, A.; Makide, K.; Saiki, N.; Arai, H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 2007, 89, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Shimbara, S.; Kambe, T.; Kuwata, H.; Winstead, M.V.; Tischfield, J.A.; Kudo, I. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release: Type IIA and type V secretory phospholipase A2s are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 1998, 273, 14411–14423. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, T.; Ohkubo, T.; Onoyama, K.; Linder, M.; Takahashi, K. Lysophosphatidylserine form DHA maybe the most effective as substrate for brain DHA accretion. Biocatal. Agric. Biotechnol. 2013, 3, 303–309. [Google Scholar] [CrossRef]
- Greig, L.C.; Woodworth, M.; Galazo, M.; Padmanabhan, H.; Macklis, J.D. Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 2013, 14, 755–769. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Zhao, E.Y.; Zhuang, W.-X.; Sun, F.-X.; Han, H.-L.; Han, H.-R.; Lin, Z.-J.; Pan, Z.-F.; Qu, M.-H.; Zeng, X.-W.; et al. LPA signaling is required for dopaminergic neuron development and is reduced through low expression of the LPA1 receptor in a 6-OHDA lesion model of Parkinson’s disease. Neurol. Sci. 2015, 36, 2027–2033. [Google Scholar] [CrossRef]
- Roza, C.; Campos-Sandoval, J.A.; Gómez-García, M.C.; Peñalver, A.; Márquez, J. Lysophosphatidic Acid and Glutamatergic Transmission. Front. Mol. Neurosci. 2019, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; Spohr, T.C.L.D.S.; Amaral, R.F.D.; Fonseca, A.C.C.D.; Garcia, C.; Mendes, F.D.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Sig. Transduct. Target. Ther. 2021, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.; Albacete, S.; Schuelert, N.; Mitchell, P.; Lin, C.; Oskins, J.; Bui, H.; Chambers, M. Lysophosphatidic acid provides a missing link between osteoarthritis and joint neuropathic pain. Osteoarthr. Cartil. 2016, 25, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govindarajulu, M.; Suppiramaniam, V.; Moore, T.; Dhanasekaran, M. Autotaxin–Lysophosphatidic Acid Signaling in Alzheimer’s Disease. Int. J. Mol. Sci. 2018, 19, 1827. [Google Scholar] [CrossRef]
- Ueda, H. Lysophosphatidic Acid as the Initiator of Neuropathic Pain. Biol. Pharm. Bull. 2011, 34, 1154–1158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaire, B.P.; Sapkota, A.; Song, M.-R.; Choi, J.W. Lysophosphatidic acid receptor 1 (LPA1) plays critical roles in microglial activation and brain damage after transient focal cerebral ischemia. J. Neuroinflamm. 2019, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Shindou, H.; Shimizu, T. A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids. Biochem. Biophys. Res. Commun. 2013, 443, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Omori, W.; Kano, K.; Hattori, K.; Kajitani, N.; Tsuchioka, M.O.; Boku, S.; Kunugi, H.; Aoki, J.; Takebayashi, M. Reduced cerebrospinal fluid levels of lysophosphatidic acid docosahexaenoic acid (LPA-DHA) in patients with major depressive disorder and schizophrenia. Int. J. Neuropsychopharmacol. 2021, 24, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, P.; Tsofliou, F.; Johnson, A.; Dyall, S.C. Combining a high DHA multi-nutrient supplement with aerobic exercise: Protocol for a randomised controlled study assessing mobility and cognitive function in older women. Prostaglandins Leukot. Essent. Fat. Acids 2019, 143, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- Li, T.; Ruan, D.-G.; Lin, Z.-M.; Liu, T.-Y.; Wang, K.; Xu, X.-Y.; Duan, R. Endurance Training Counteracts the High-Fat Diet-Induced Profiling Changes of ω-3 Polyunsaturated Fatty Acids in Skeletal Muscle of Middle-Aged Rats. Front. Physiol. 2019, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2017, 43, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Albero, M.; Martínez, M.I.; Cauli, O. Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake in Children with Attention Deficit and Hyperactivity Disorder. Brain Sci. 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Cho, T.-H.; Perez, M.; Guichardant, M.; Riou, A.; Aguettaz, P.; Picq, M.; Lagarde, M.; Berthezene, Y.; Nighoghossian, N.; et al. Brain-Targeting Form of Docosahexaenoic Acid for Experimental Stroke Treatment: MRI Evaluation and Anti-Oxidant Impact. Curr. Neurovascular Res. 2011, 8, 95–102. [Google Scholar] [CrossRef]
- Belkouch, M.; Hachem, M.; Elgot, A.; Van, A.L.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef]
- Kamel, F.; Goldman, S.M.; Umbach, D.M.; Chen, H.; Richardson, G.; Barber, M.R.; Meng, C.; Marras, C.; Korell, M.; Kasten, M.; et al. Dietary fat intake, pesticide use, and Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Van, A.L.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Mechanisms of DHA transport to the brain and potential therapy to neurodegenerative diseases. Biochimie 2016, 130, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, M.; Loria, G.; Salvati, S.; Di Biase, A.; Conte, G.; Villella, C.; Righino, E.; Ciciarelli, C.; Bria, P.; La Torre, G.; et al. DHA effects in Parkinson disease depression. Basal Ganglia 2014, 4, 61–66. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabia, M.A.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Mori, M.A.; Delattre, A.M.; Carabelli, B.; Pudell, C.; Bortolanza, M.; Staziaki, P.V.; Visentainer, J.V.; Montanher, P.F.; Del Bel, E.A.; Ferraz, A.C. Neuroprotective effect of omega-3 polyunsaturated fatty acids in the 6-OHDA model of Parkinson’s disease is mediated by a reduction of inducible nitric oxide synthase. Nutr. Neurosci. 2017, 21, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, D.; Xu, J.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. J. Funct. Foods 2018, 45, 417–426. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Plourde, M.; Pifferi, F.; Bégin, M.; Féart, C.; Barberger-Gateau, P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog. Lipid Res. 2009, 48, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.C.M.; Hofman, A.; Breteler, M.M.B. Dietary fat intake and the risk of incident dementia in the Rotterdam study. Ann. Neurol. 1997, 42, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of Fish and n-3 Fatty Acids and Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxen-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Cui, J.-G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Li, Q.; Ding, L.; Shi, H.-H.; Xue, C.-H.; Mao, X.-Z.; Wang, Y.-M.; Zhang, T.-T. A comparative study of the effects of phosphatidylserine rich in DHA and EPA on Aβ-induced Alzheimer’s disease using cell models. Food Funct. 2021, 12, 4411–4423. [Google Scholar] [CrossRef]
- Tassoni, D.; Kaur, G.; Weisinger, R.S.; Sinclair, A.J. The role of eicosanoids in the brain. Asia Pac. J. Clin. Nutr. 2008, 17, 220–228. [Google Scholar]
- Zhang, T.-T.; Xu, J.; Wang, Y.-M.; Xue, C.-H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef]
- Bandarra, N.M.; Lopes, P.A.; Martins, S.V.; Ferreira, J.; Alfaia, C.M.; Rolo, E.A.; Correia, J.J.; Pinto, R.M.; Ramos-Bueno, R.P.; Batista, I.; et al. Docosahexaenoic acid at the sn-2 position of structured triacylglycerols improved n-3 polyunsaturated fatty acid assimilation in tissues of hamsters. Nutr. Res. 2016, 36, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, H.; O’Brien, J.S. Positional distribution of fatty acids in glycerophosphatides of bovine gray matter. J. Lipid Res. 1968, 9, 65–67. [Google Scholar] [CrossRef]
- Winther, B.; Hoem, N.; Berge, K.; Reubsaet, L. Elucidation of Phosphatidylcholine Composition in Krill Oil Extracted from Euphausia superba. Lipids 2010, 46, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Sato, N.; Tsuji, N.; Tsuzuki, M.; Nishida, I. The Significance of C16 Fatty Acids in the sn-2 Positions of Glycerolipids in the Photosynthetic Growth of Synechocystis sp. PCC6803. Plant Physiol. 2006, 141, 546–556. [Google Scholar] [CrossRef]
- Ratnayake, W.N.; Galli, C. Fat and Fatty Acid Terminology, Methods of Analysis and Fat Digestion and Metabolism: A Background Review Paper. Ann. Nutr. Metab. 2009, 55, 8–43. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef]
- Polette, A.; Deshayes, C.; Chantegrel, B.; Croset, M.; Armstrong, J.M.; Lagarde, M. Synthesis of acetyl, docosahexaenoyl-glycerophosphocholine and its characterization using nuclear magnetic resonance. Lipids 1999, 34, 1333–1337. [Google Scholar] [CrossRef]
- Sovic, A.; Panzenboeck, U.; Wintersperger, A.; Kratzer, I.; Hammer, A.; Levak-Frank, S.; Frank, S.; Rader, D.J.; Malle, E.; Sattler, W. Regulated expression of endothelial lipase by porcine brain capillary endothelial cells constituting the blood-brain barrier. J. Neurochem. 2005, 94, 109–119. [Google Scholar] [CrossRef]
- Bernhard, W.; Maas, C.; Shunova, A.; Mathes, M.; Böckmann, K.; Bleeker, C.; Vek, J.; Poets, C.F.; Schleicher, E.; Franz, A.R. Transport of long-chain polyunsaturated fatty acids in preterm infant plasma is dominated by phosphatidylcholine. Eur. J. Nutr. 2017, 57, 2105–2112. [Google Scholar] [CrossRef]
- Metherel, A.H.; Rezaei, K.; Lacombe, R.S.; Bazinet, R.P. Plasma unesterified eicosapentaenoic acid is converted to docosahexaenoic acid (DHA) in the liver and supplies the brain with DHA in the presence or absence of dietary DHA. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158942. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Mesa-Herrera, F.; Marín, R. DHA and Its Elaborated Modulation of Antioxidant Defenses of the Brain: Implications in Aging and AD Neurodegeneration. Antioxidants 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Lo Van, A.; Hachem, M.; Lagarde, M.; Bernoud-Hubac, N. Omega-3 Docosahexaenoic Acid Is a Mediator of Fate-Decision of Adult Neural Stem Cells. Int. J. Mol. Sci. 2019, 20, 4240. [Google Scholar] [CrossRef] [PubMed]
- Pencreac’h, G.; Ergan, F.; Poisson, L. Production of lysophospholipids rich in DHA. Lipid Technol. 2011, 23, 250–252. [Google Scholar] [CrossRef]


| PE | PC | PS | PI | PG | CL | Total | |
|---|---|---|---|---|---|---|---|
| Brain | 53.9 ± 5.3 | 30.5 ± 4.5 | 7.78 ± 0.93 | 5.13 ± 0.76 | 0.02 ± 0.01 | 0.27 ± 0.15 | 97.6 ± 21.6 |
| Heat | 22.0 ± 6.0 | 17.0 ± 5.0 | 0.48 ± 0.14 | 2.74 ± 0.86 | 0.17 ± 0.06 | 2.31 ± 0.66 | 44.7 ± 12.6 |
| Kidney | 24.7 ± 5.5 | 18.0 ± 4.2 | 2.31 ± 0.45 | 5.31 ± 1.3 | 0.02 ± 0.01 | 1.38 ± 0.35 | 51.7 ± 11.3 |
| Liver | 22.8 ± 3.3 | 31.2 ± 3.8 | 1.21 ± 0.32 | 8.52 ± 1.1 | 0.01 ± 0.00 | 1.08 ± 0.21 | 64.9 ± 8.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachem, M.; Nacir, H. Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases. Int. J. Mol. Sci. 2022, 23, 3969. https://doi.org/10.3390/ijms23073969
Hachem M, Nacir H. Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases. International Journal of Molecular Sciences. 2022; 23(7):3969. https://doi.org/10.3390/ijms23073969
Chicago/Turabian StyleHachem, Mayssa, and Houda Nacir. 2022. "Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases" International Journal of Molecular Sciences 23, no. 7: 3969. https://doi.org/10.3390/ijms23073969
APA StyleHachem, M., & Nacir, H. (2022). Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases. International Journal of Molecular Sciences, 23(7), 3969. https://doi.org/10.3390/ijms23073969







