Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(D,L-lactide-co-glycolide) Biphasic Scaffolds for Bone Tissue Regeneration
Abstract
:1. Introduction
2. Results
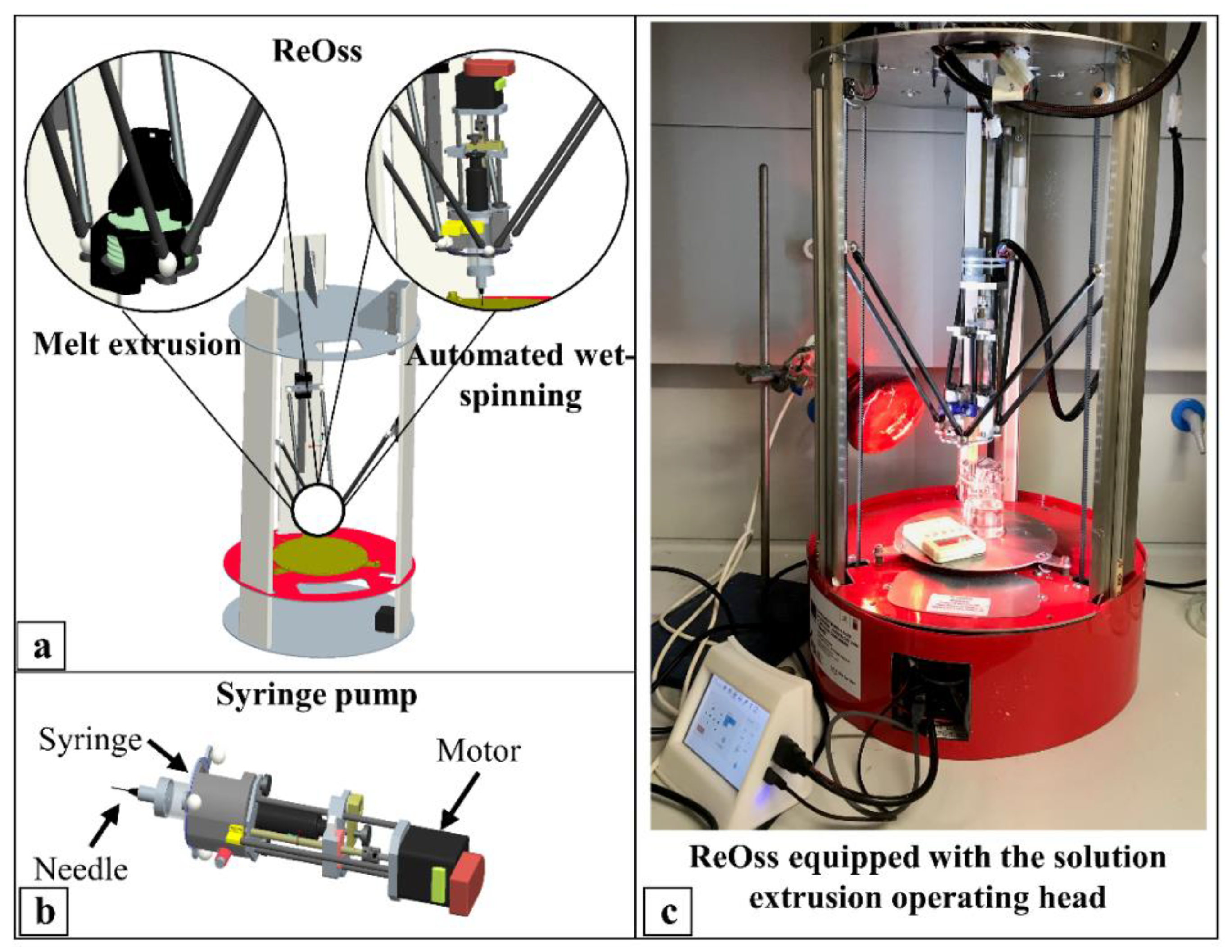
2.1. Scaffold Fabrication
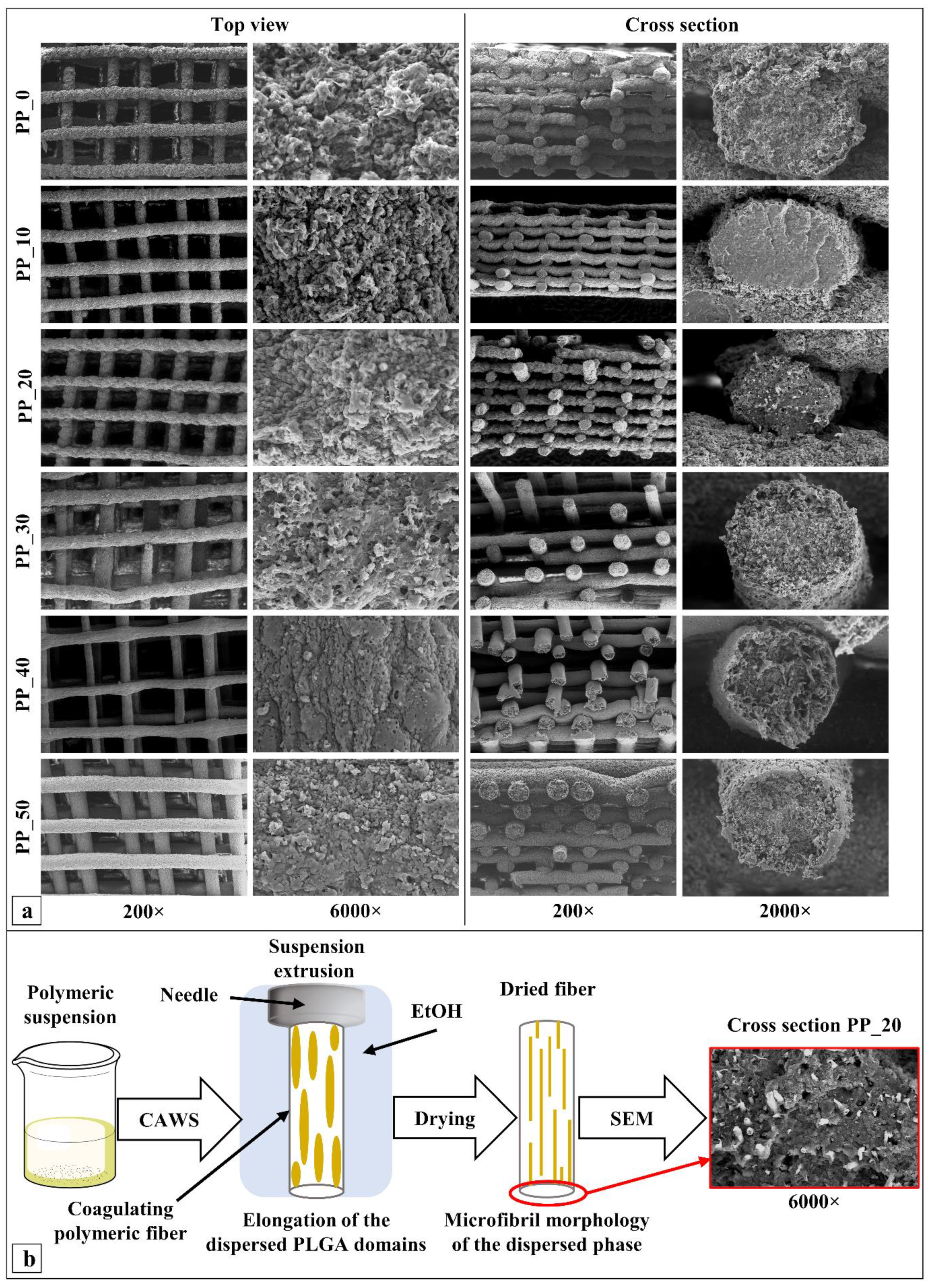
2.2. Morphological Characterization
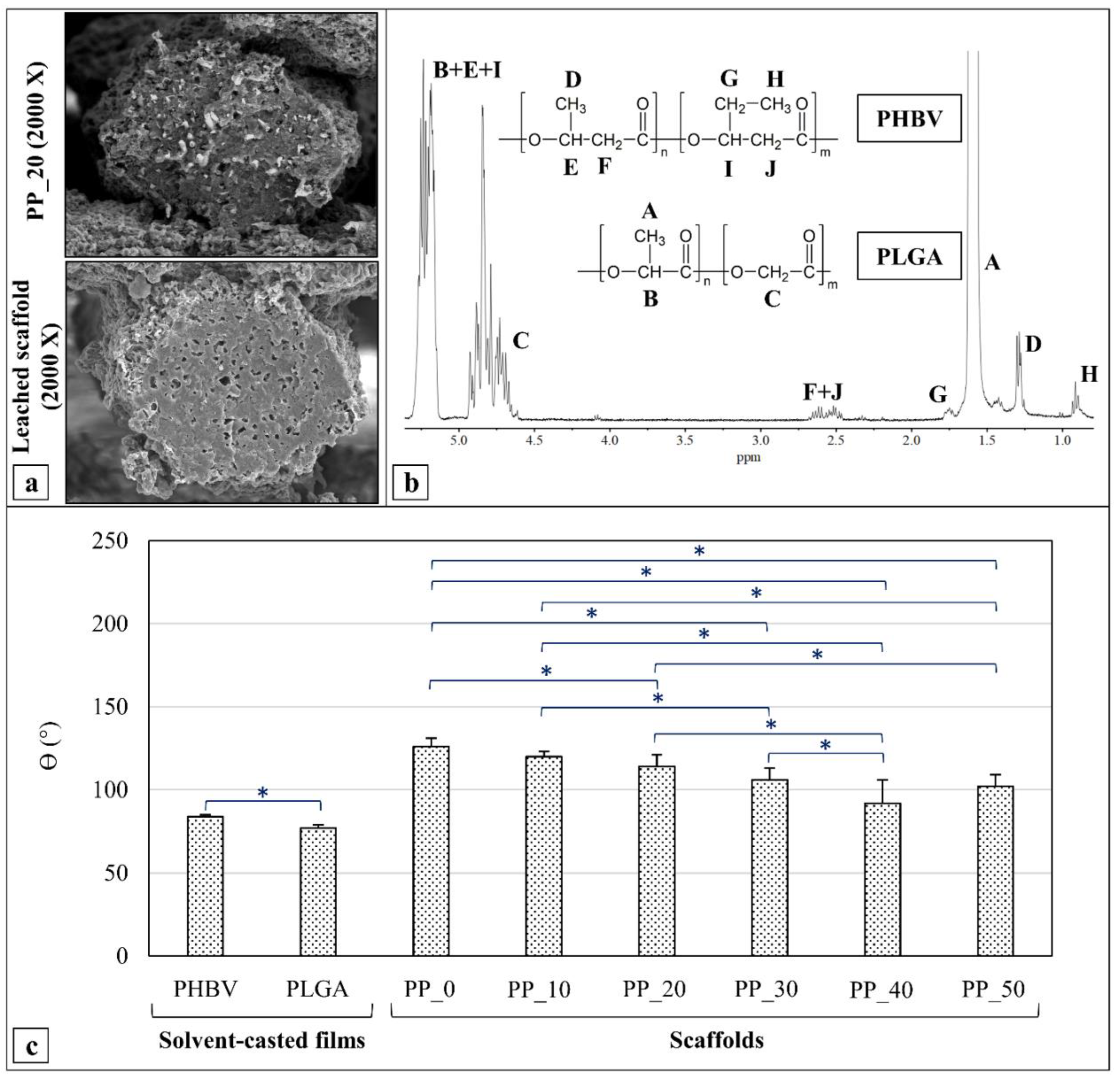
2.3. Acetone Leaching and 1HNMR Analysis
2.4. Contact Angle Measurements
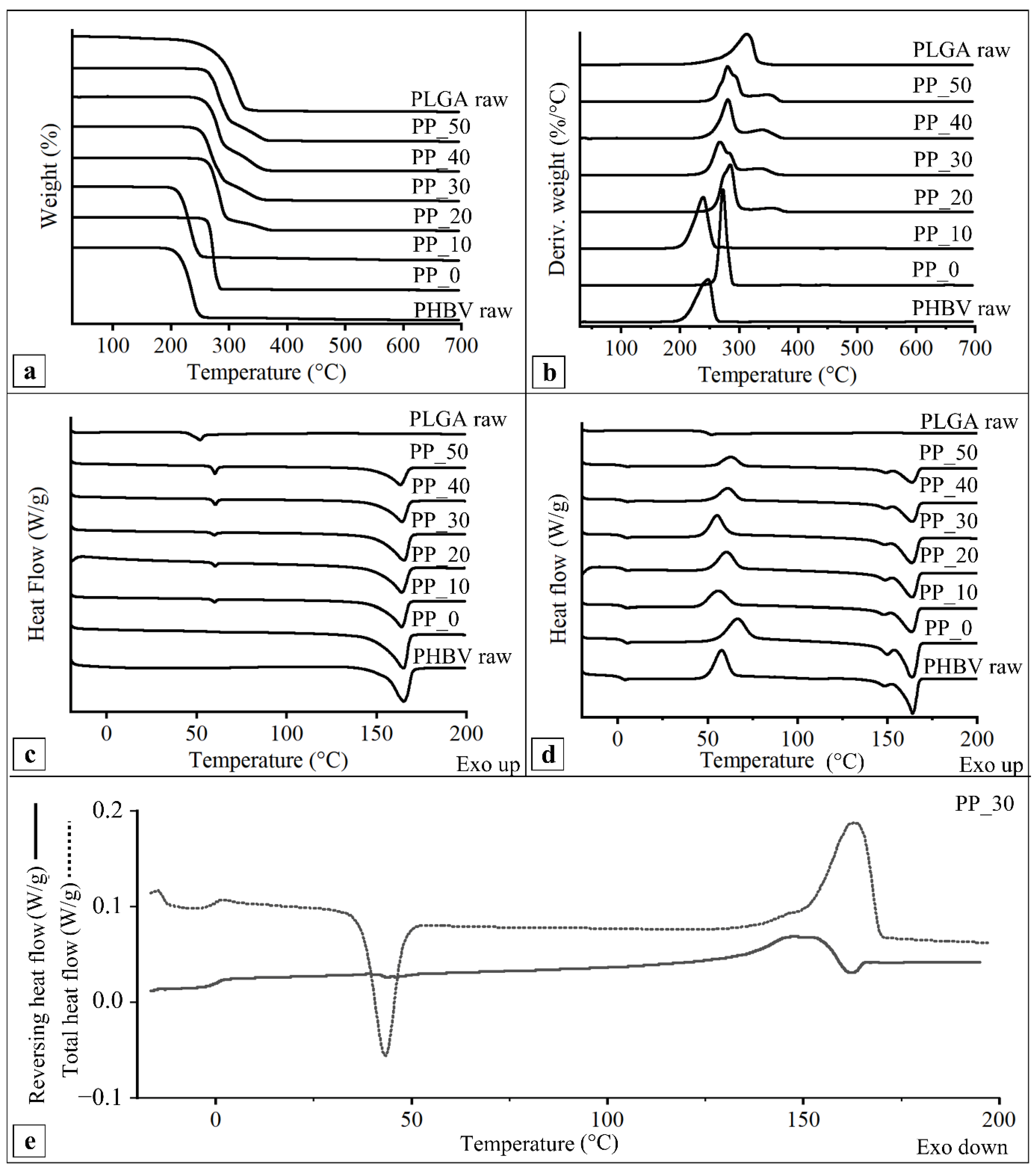
2.5. Thermal Characterization
2.6. Mechanical Characterization
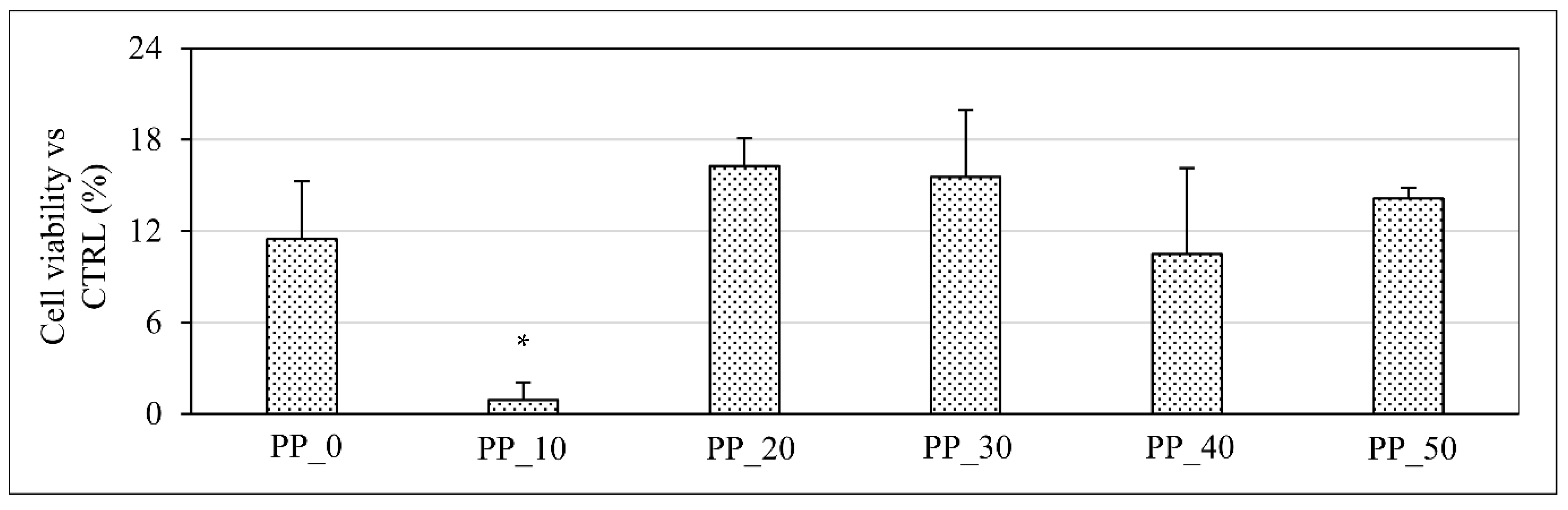
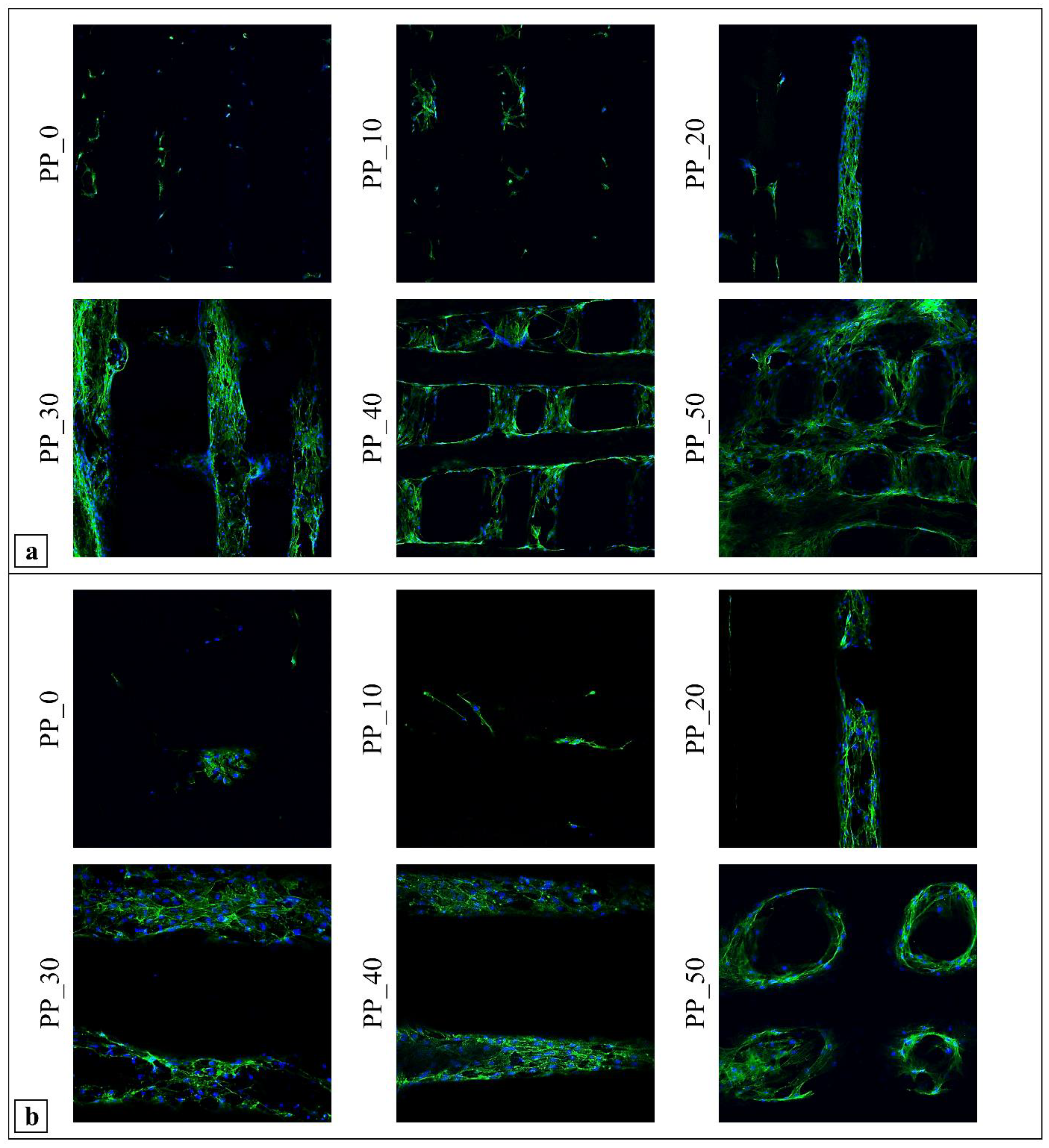
2.7. Biological Characterization
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Scaffold Fabrication
4.3. Film Preparation
4.4. Morphological Characterization
4.5. Viscosity Measurements
4.6. PLGA Extraction and 1HNMR Analysis
4.7. Contact Angle Measurements
4.8. Thermal Characterization
4.9. Mechanical Characterization
4.10. In Vitro Biological Characterization
4.10.1. Cell Culture
4.10.2. Cell Viability and Proliferation
4.10.3. Morphological Characterization by Confocal Scanning Laser Microscopy (CLSM)
4.10.4. Cell Differentiation
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Brouki Milan, P.; Kim, H.W.; Baino, F. Bone Tissue Engineering Using Human Cells: A Comprehensive Review on Recent Trends, Current Prospects, and Recommendations. Appl. Sci. 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Srivastava, J.; Chandel, A.; Sharma, L.; Mallick, N.; Singh, S. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Y.; Zhang, X.Y.; Ramakrishna, S.; He, L.M.; Wu, G. Reactive blends based on polyhydroxyalkanoates: Preparation and biomedical application. Mater. Sci. Eng. C 2017, 70, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Diermann, S.H.; Lu, M.; Edwards, G.; Dargusch, M.; Huang, H. In vitro degradation of a unique porous PHBV scaffold manufactured using selective laser sintering. J. Biomed. Mater. Res. 2019, 107, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Kyrylenko, S.; Kornienko, V.; Gogotsi, O.; Oleshko, O.; Kolesnyk, M.; Mishchenko, O.; Zahorodna, V.; Buranich, V.; Pogrebnjak, A.; Zozulia, Y.; et al. Bio-Functionalization of Electrospun Polymeric Nanofibers by Ti3C2Tx MXene. In Proceedings of the IEEE International Conference on Nanomaterials: Applications & Properties, Sumy, Ukraine, 9–13 November 2020. [Google Scholar]
- Naumenko, E.; Fakhrullin, R. Halloysite Nanoclay/Biopolymers Composite Materials in Tissue Engineering. Biotechnol. J. 2019, 14, e1900055. [Google Scholar] [CrossRef] [PubMed]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef]
- Kosorn, W.; Sakulsumbat, M.; Uppanan, P.; Kaewkong, P.; Chantaweroad, S.; Jitsaard, J.; Sitthiseripratip, K.; Janvikul, W. PCL/PHBV blended three dimensional scaffolds fabricated by fused deposition modeling and responses of chondrocytes to the scaffolds. J. Biomed. Mater. Res. B 2017, 105, 1141–1150. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Nigmatullin, R.; Taylor, C.; Haycock, J.W.; Claeyssens, F.; Knowles, J.C.; Roy, I. Nerve tissue engineering using blends of poly(3-hydroxyalkanoates) for peripheral nerve regeneration. Eng. Life Sci. 2015, 15, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Xu, P.; Chen, M.; Dong, W.; Cai, X.; Schmit, P.; Spoelstra, A.B.; Lemstra, P.J. Structure-property relationships of reactively compatibilized PHB/EVA/starch blends. Carbohydr. Polym. 2014, 108, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavioun, P.; Halley, P.J.; Doherty, W.O.S. Thermophysical properties and rheology of PHB/lignin blends. Ind. Crops Prod. 2013, 50, 270–275. [Google Scholar] [CrossRef]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. The Effects of Process Engineering on the Performance of PLA and PHBV Blends. Macromol. Mater. Eng. 2011, 296, 719–728. [Google Scholar] [CrossRef]
- Rodrigues, J.; Parra, D.; Lug, A. Crystallization of PHB/PEG blends evaluation by DSC. J. Therm. Anal. 2005, 79, 379–381. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.L.; Wang, M.; Wong, S.Y.; Tjiu, W.C.; He, C.B.; Goh, S.H.; Li, J. Improving hydrophilicity, mechanical properties and biocompatibility of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] through blending with poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide). Acta Biomater. 2009, 5, 2002–2012. [Google Scholar] [CrossRef]
- Naznin, S.; Tareef Hayat, K. In VitroDegradation of PHBV Scaffolds and nHA/PHBV Composite Scaffolds Containing Hydroxyapatite Nanoparticles for Bone Tissue Engineering. J. Nanomater. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef]
- Myakinin, A.; Turlybekuly, A.; Pogrebnjak, A.; Mirek, A.; Bechelany, M.; Liubchak, I.; Oleshko, O.; Husak, Y.; Korniienko, V.; Lesniak-Ziolkowska, K.; et al. In vitro evaluation of electrochemically bioactivated Ti6Al4V 3D porous scaffolds. Mater. Sci. Eng. C 2021, 121, 111870. [Google Scholar] [CrossRef]
- Diermann, S.H.; Lu, M.; Dargusch, M.; Grondahl, L.; Huang, H. Akermanite reinforced PHBV scaffolds manufactured using selective laser sintering. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2596–2610. [Google Scholar] [CrossRef] [PubMed]
- Diermann, S.H.; Lu, M.; Zhao, Y.; Vandi, L.J.; Dargusch, M.; Huang, H. Synthesis, microstructure, and mechanical behaviour of a unique porous PHBV scaffold manufactured using selective laser sintering. J. Mech. Behav. Biomed. Mater. 2018, 84, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Cheung, W.; Wang, M. Optimized fabrication of Ca-P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 2011, 3, 015001. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M.; Zhou, W.; Cheung, W.; Li, Z.; Lu, W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Pereira, T.; Silva, M.; Oliveira, M.; Maia, I.; Silva, J.; Costa, M.; Thiré, R. Effect of process parameters on the properties of selective laser sintered Poly(3-hydroxybutyrate) scaffolds for bone tissue engineering. Virtual Phys. Prototyp. 2012, 7, 275–285. [Google Scholar] [CrossRef]
- Saska, S.; Pires, L.C.; Cominotte, M.A.; Mendes, L.S.; De Oliveira, M.F.; Maia, I.A.; Da Silva, J.V.L.; Ribeiro, S.J.L.; Cirelli, J.A. Three-dimensional printing and in vitro evaluation of poly(3-hydroxybutyrate) scaffolds functionalized with osteogenic growth peptide for tissue engineering. Mater. Sci. Eng. C 2018, 89, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Guo, W.; Gao, C.; Yang, Y.; Wu, P.; Feng, P. An nMgO containing scaffold: Antibacterial activity, degradation properties and cell responses. Int. J. Bioprinting 2017, 4, 120. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Wang, M. Customized Ca-P/PHBV nanocomposite scaffolds for bone tissue engineering: Design, fabrication, surface modification and sustained release of growth factor. J. R. Soc. Interface 2010, 7, 615–629. [Google Scholar] [CrossRef] [Green Version]
- Kovalcik, A.; Sangroniz, L.; Kalina, M.; Skopalova, K.; Humpolicek, P.; Omastova, M.; Mundigler, N.; Muller, A.J. Properties of scaffolds prepared by fused deposition modeling of poly(hydroxyalkanoates). Int. J. Biol. Macromol. 2020, 161, 364–376. [Google Scholar] [CrossRef]
- Mencik, P.; Prikryl, R.; Stehnova, I.; Melcova, V.; Kontarova, S.; Figalla, S.; Alexy, P.; Bockaj, J. Effect of Selected Commercial Plasticizers on Mechanical, Thermal, and Morphological Properties of Poly(3-hydroxybutyrate)/Poly(lactic acid)/Plasticizer Biodegradable Blends for Three-Dimensional (3D) Print. Materials 2018, 11, 1893. [Google Scholar] [CrossRef] [Green Version]
- Valentini, F.; Dorigato, A.; Rigotti, D.; Pegoretti, A. Polyhydroxyalkanoates/Fibrillated Nanocellulose Composites for Additive Manufacturing. J. Polym. Environ. 2019, 27, 1333–1341. [Google Scholar] [CrossRef]
- Ye, X.; Li, L.; Lin, Z.; Yang, W.; Duan, M.; Chen, L.; Xia, Y.; Chen, Z.; Lu, Y.; Zhang, Y. Integrating 3D-printed PHBV/Calcium sulfate hemihydrate scaffold and chitosan hydrogel for enhanced osteogenic property. Carbohydr. Polym. 2018, 202, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Wang, S.; Puppi, D.; Gazzarri, M.; Migone, C.; Chiellini, F.; Chen, G.; Chiellini, E. Additive manufacturing of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] scaffolds for engineered bone development. J. Tissue Eng. Regen. Med. 2017, 11, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Braccini, S.; Ranaudo, A.; Chiellini, F. Poly(3-hydroxybutyrate-co-3-hydroxyexanoate) scaffolds with tunable macro- and microstructural features by additive manufacturing. J. Biotechnol. 2020, 308, 96–107. [Google Scholar] [CrossRef]
- Puppi, D.; Morelli, A.; Chiellini, F. Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) Blend Scaffolds for Tissue Engineering. Bioengineering 2017, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Puppi, D.; Pirosa, A.; Morelli, A.; Chiellini, F. Design, fabrication and characterization of tailored poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyexanoate] scaffolds by computer-aided wet-spinning. Rapid Prototyp. J. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Wet-spinning of biomedical polymers: From single-fibre production to additive manufacturing of three-dimensional scaffold. Polym. Int. 2017, 66, 1690–1696. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable polymers-an overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Lu, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Gil-Castell, O.; Badia, J.D.; Ontoria-Oviedo, I.; Castellano, D.; Marco, B.; Rabal, A.; Bou, J.J.; Serra, A.; Monreal, L.; Blanes, M.; et al. In vitro validation of biomedical polyester-based scaffolds: Poly(lactide-co-glycolide) as model-case. Polym. Test. 2018, 66, 256–267. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Chiellini, F.; Chiellini, E.; Martins, A.; Leonor, I.B.; Neves, N.; Reis, R. Optimized electro- and wet-spinning techniques for the production of polymeric fibrous scaffolds loaded with bisphosphonate and hydroxyapatite. J. Tissue Eng. Regen. Med. 2011, 5, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Mota, C.; Gazzarri, M.; Dinucci, D.; Gloria, A.; Myrzabekova, M.; Ambrosio, L.; Chiellini, F. Additive manufacturing of wet-spun polymeric scaffolds for bone tissue engineering. Biomed. Microdevices 2012, 14, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.D.; Cerruti, R.; Fernandes, E.; Peña, L.; Saez, V.; Pinto, J.C.; Ramón, J.A.; Oliveira, G.E.; Souza, F.G. Influence of PLGA and PLGA-PEG on the dissolution profile of oxaliplatin. Polímeros 2016, 26, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Puppi, D.; Morelli, A.; Bello, F.; Valentini, S.; Chiellini, F. Additive Manufacturing of Poly(Methyl Methacrylate) Biomedical Implants with Dual-Scale Porosity. Macromol. Mater. Eng. 2018, 303, 1800247. [Google Scholar] [CrossRef]
- Calvert, J.W.; Chua, W.C.; Gharibjanian, N.A.; Dhar, S.; Evans, G.R. Osteoblastic phenotype expression of MC3T3-E1 cells cultured on polymer surfaces. Plast. Reconstr. Surg. 2005, 116, 567–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Woei, K.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Feinberg, A.W. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioeng. 2021, 5, 010904. [Google Scholar] [CrossRef]
- Hochleitner, G.; Jungst, T.; Brown, T.D.; Hahn, K.; Moseke, C.; Jakob, F.; Dalton, P.D.; Groll, J. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 2015, 7, 035002. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.; Wu, H.; Wei, Y.; Wang, S. Solubility of Polyhydroxyalkanoates by Experiment and Thermodynamic Correlations. AIChE J. 2007, 53, 2704–2714. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, X.; Chen, Y.; Akram, M.Y.; Nie, J.; Zhu, X. Preparation of polymer/calcium phosphate porous composite as bone tissue scaffolds. Mater. Sci. Eng. C 2017, 70, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, M.; Sharrock, P.; Fiallo, M.; Elfeki, H. Hydroxyapatite bioceramic with large porosity. Mater. Sci. Eng. C 2017, 76, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Anuvrat, K.; Garai, S.; Sahu, S.; Chakraborty, J. One pot method to synthesize three-dimensional porous hydroxyapatite nanocomposite for bone tissue engineering. J. Porous Mater. 2020, 27, 225–235. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Castro, N.J.; Cheng, X.; Keidar, M.; Zhang, L.G. Cold Atmospheric Plasma Modified Electrospun Scaffolds with Embedded Microspheres for Improved Cartilage Regeneration. PLoS ONE 2015, 10, e0134729. [Google Scholar] [CrossRef] [Green Version]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Della Volpe, C.; Hołysz, L.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2020, 8, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Davoudinejad, A.; Cai, Y.; Pedersen, D.; Luo, X.; Tosello, G. Fabrication of micro-structured surfaces by additive manufacturing, with simulation of dynamic contact angle. Mater. Des. 2019, 176, 107839. [Google Scholar] [CrossRef]
- Liu, Q.S.; Zhu, M.F.; Wu, W.H.; Qin, Z.Y. Reducing the formation of six-membered ring ester during thermal degradation of biodegradable PHBV to enhance its thermal stability. Polym. Degrad. Stab. 2009, 94, 18–24. [Google Scholar] [CrossRef]
- Thiré, R.; Arruda, L.C.; Barreto, L.S. Morphology and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/attapulgite nanocomposites. Mater. Res. 2011, 14, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Palacios, J.; Albano, C.; González, G.; Castillo, R.V.; Karam, A.; Covis, M. Characterization and thermal degradation of poly(d,l-lactide-co-glycolide) composites with nanofillers. Polym. Eng. 2013, 53, 1414–1429. [Google Scholar] [CrossRef]
- Silva, M.F.; Hechenleitner, A.A.W.; Irache, J.M.; Oliveira, A.J.A.; Pineda, E.A.G. Study of Thermal Degradation of PLGA, PLGA Nanospheres and PLGA/Maghemite Superparamagnetic Nanospheres. Mater. Res. 2015, 18, 1400–1406. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Luo, T. Polymer Nanofibers with Outstanding Thermal Conductivity and Thermal Stability: Fundamental Linkage between Molecular Characteristics and Macroscopic Thermal Properties. J. Phys. Chem. C 2014, 118, 21148–21159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Luo, L.; Lyu, S.; Schley, J.; Pudil, B.; Benz, M.; Buckalew, A.; Chaffin, K.; Hobot, C.; Sparer, R. Spontaneous transesterification reactions between poly(lactide-co-glycolide) and poly(trimethylene carbonate) at the interface. J. Appl. Polym. Sci. 2010, 117, 2153–2158. [Google Scholar] [CrossRef]
- Arcos-Hernández, M.V.; Laycock, B.; Donose, B.C.; Pratt, S.; Halley, P.; Al-Luaibi, S.; Werker, A.; Lant, P.A. Physicochemical and mechanical properties of mixed culture polyhydroxyalkanoate (PHBV). Eur. Polym. J. 2013, 49, 904–913. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Lagaron, J.M. Stabilized nanosilver based antimicrobial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites of interest in active food packaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Laycock, B.; Arcos-Hernandez, M.V.; Langford, A.; Pratt, S.; Werker, A.; Halley, P.J.; Lant, P.A. Crystallisation and fractionation of selected polyhydroxyalkanoates produced from mixed cultures. New Biotechnol. 2014, 31, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Fei, Q.; Zhang, Y.H.; Wang, X.Z.; Fan, D.D.; Chang, H.N. Thermal Properties and Biodegradability Studies of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Polym. Environ. 2011, 20, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.; Pirosa, A.; Sandreschi, S.; Chiellini, F. Levofloxacin-loaded star poly(epsilon-caprolactone) scaffolds by additive manufacturing. J. Mater. Sci. Mater. Med. 2016, 27, 44. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Brickley, M.; Ives, R. Background to Bone Biology and Mineral Metabolism. In The Bioarcheology of Metabolic Bone Disease, 1st ed.; Press, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 21–40. [Google Scholar]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, C.; Cantini, M.; Ballester-Beltran, J.; Altankov, G.; Salmeron-Sanchez, M. The strength of the protein-material interaction determines cell fate. Acta Biomater. 2018, 77, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Tamjid, E.; Simchi, A. Fabrication of a highly ordered hierarchically designed porous nanocomposite via indirect 3D printing: Mechanical properties and in vitro cell responses. Mater. Des. 2015, 88, 924–931. [Google Scholar] [CrossRef]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.; Van Dongen, J.T.; Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Fenchel, W. Uber Kriimmung und Windung gesehlossener Raumkurven. Math. Ann. 1929, 101, 1432–1807. [Google Scholar] [CrossRef]
- Huang, W.; Shi, X.; Ren, L.; Du, C.; Wang, Y. PHBV microspheres--PLGA matrix composite scaffold for bone tissue engineering. Biomaterials 2010, 31, 4278–4285. [Google Scholar] [CrossRef]
- Zhu, X.H.; Wang, C.H.; Tong, Y.W. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J. Biomed. Mater. Res. A 2009, 89, 411–423. [Google Scholar] [CrossRef]
- Hassan, M.I.; Sultana, N. In vitro cell viability of PHBV/PLGA nanofibrous membrane for tissue engineering. Mal. J. Fund. Appl. Sci. 2019, 15, 522–527. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Kim, H.W. Core-shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater. 2015, 21, 2–19. [Google Scholar] [CrossRef]



| Scaffold | PHBV/PLGA [w/w%] | F [mL/h] | Vtransl [mm/min] | Tcoag [°C] | Post-Processing |
|---|---|---|---|---|---|
| PP_0 | 100:0 | 2.1 | 1200 | 38–39 | - EtOH immersion (6 days) - Kept under a fume hood (48 h) - Vacuum drying at RT (6 h) |
| PP_10 | 90:10 | 2.3 | 1200 | 41–42 | |
| PP_20 | 80:20 | 2.3 | 1200 | 42–43 | - Pressure applied on scaffold’s top surface during drying under a fume hood (48 h) - Vacuum drying at RT (6 h) |
| PP_30 | 70:30 | 2.3 | 1200 | 42–43 | |
| PP_40 | 60:40 | 2.3 | 1200 | 43–44 | |
| PP_50 | 50:50 | 2.3 | 600 | 46–47 |
| Sample | Fiber Diameter (µm) | Pore Size (µm) | Porosity (%) |
|---|---|---|---|
| PP_0 | 112 ± 8 | 268 ± 34 | 75 ± 14 |
| PP_10 | 110 ± 12 | 249 ± 40 | 73 ± 10 |
| PP_20 | 110 ± 13 | 237 ± 37 * | 70 ± 8 |
| PP_30 | 130 ± 22 a | 296 ± 72 *,** | 68 ± 10 |
| PP_40 | 88 ± 9 a | 360 ± 96 a | 70 ± 12 |
| PP_50 | 150 ± 13 a | 227 ± 64 ** | 70 ± 13 |
| Sample | DTG | |
|---|---|---|
| Tmax1 (°C) | Tmax2 (°C) | |
| Raw PHBV | 244 ± 6 * | - |
| Raw PLGA | - | 309 ± 8 a |
| PP_0 | 271 ± 4 | - |
| PP_10 | 236 ± 3 * | - |
| PP_20 | 276 ± 5 | 348 ± 8 |
| PP_30 | 273 ± 4 | 334 ± 15 |
| PP_40 | 274 ± 6 | 338 ± 4 |
| PP_50 | 283 ± 4 | 337 ± 7 |
| Sample | First Heating | Second Heating | Sample | First Heating | Second Heating | Sample | First Heating | Second Heating |
|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | Tg (°C) | Tm (°C) | Tg (°C) | Tm (°C) | |||
| Raw PHBV | - | 164.0 ± 1.0 | 76.0 ± 4.4 | 2.4 ± 0.1 a | 58.0 ± 1.0 | 43.8 ± 1.0 * | 163.0 ± 1.0 | 64.0 ± 1.0 * |
| Raw PLGA | 50.3 ± 0.4 | - | - | 50.1 ± 0.2 | - | - | - | - |
| PP_0 | - | 165.4 ± 0.5 | 78.9 ± 0.1 a | 3.2 ± 0.7 | 64.6 ± 2.0 | 44.7 ± 1.0 * | 164.5 ± 1.0 | 63.9 ± 0.6 * |
| PP_10 | 59.3 ± 0.4 | 164.6 ± 0.7 | 58.4 ± 3.1 | 3.8 ± 0.2 | 57.0 ± 3.0 | 30.9 ± 0.6 | 163.9 ± 0.4 | 49.0 ± 0.3 |
| PP_20 | 59.3 ± 0.1 | 163.6 ± 0.3 | 57.8 ± 1.5 | 3.6 ± 0.1 | 59.0 ± 2.0 | 31.5 ± 0.3 | 163.5 ± 0.2 | 48.3 ± 1.0 |
| PP_30 | 59.0 ± 0.2 | 165.1 ± 0.7 | 60.9 ± 3.0 | 3.6 ± 0.3 | 56.0 ± 1.0 | 30.1 ± 2.3 | 164.1 ± 0.4 | 52.9 ± 1.0 |
| PP_40 | 59.5 ± 0.1 | 163.9 ± 0.3 | 45.2 ± 0.7 | 3.6 ± 0.1 | 61.2 ± 0.4 | 21.7 ± 0.4 a | 163.7 ± 0.2 | 35.5 ± 2.0 a |
| PP_50 | 58.0 ± 3.0 | 163.2 ± 0.1 | 38.0 ± 0.7 | 3.1 ± 0.3 | 61.0 ± 1.0 | 16.9 ± 0.1 a | 163.3 ± 0.3 | 27.8 ± 0.3 a |
| Sample | Dry | Wet | ||||
|---|---|---|---|---|---|---|
| E (MPa) | σ50 (MPa) | HRec (%) | E (MPa) | σ50 (MPa) | HRec (%) | |
| PP_0 | 1.1 ± 0.4 | 0.4 ± 0.1 | 30.4 ± 3.9 | 1.0 ± 0.3 | 0.3 ± 0.2 | 28.2 ± 4.0 |
| PP_10 | 1.0 ± 0.4 * | 0.4 ± 0.1 | 32.7 ± 8.6 | 2.1 ± 0.6 * | 0.4 ± 0.1 | 35.6 ± 4.6 |
| PP_20 | 1.4 ± 0.3 | 0.5 ± 0.2 | 36.2 ± 10.9 | 1.5 ± 0.3 | 0.4 ± 0.1 | 30.1 ± 5.1 |
| PP_30 | 1.0 ± 0.4 | 0.5 ± 0.2 | 34.4 ± 8.3 | 1.7 ± 0.8 | 0.6 ± 0.3 | 33.9 ± 9.5 |
| PP_40 | 1.1 ± 0.4 | 0.7 ± 0.4 | 31.0 ± 3.0 | 1.3 ± 0.4 | 0.5 ± 0.1 | 38.2 ± 9.8 |
| PP_50 | 3.4 ± 2.1 a | 2.2 ± 1.9 | 36.1 ± 9.2 | 3.7 ± 1.1 a | 1.8 ± 1.0 | 51.0 ± 17.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecorini, G.; Braccini, S.; Parrini, G.; Chiellini, F.; Puppi, D. Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(D,L-lactide-co-glycolide) Biphasic Scaffolds for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 3895. https://doi.org/10.3390/ijms23073895
Pecorini G, Braccini S, Parrini G, Chiellini F, Puppi D. Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(D,L-lactide-co-glycolide) Biphasic Scaffolds for Bone Tissue Regeneration. International Journal of Molecular Sciences. 2022; 23(7):3895. https://doi.org/10.3390/ijms23073895
Chicago/Turabian StylePecorini, Gianni, Simona Braccini, Gianluca Parrini, Federica Chiellini, and Dario Puppi. 2022. "Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(D,L-lactide-co-glycolide) Biphasic Scaffolds for Bone Tissue Regeneration" International Journal of Molecular Sciences 23, no. 7: 3895. https://doi.org/10.3390/ijms23073895
APA StylePecorini, G., Braccini, S., Parrini, G., Chiellini, F., & Puppi, D. (2022). Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(D,L-lactide-co-glycolide) Biphasic Scaffolds for Bone Tissue Regeneration. International Journal of Molecular Sciences, 23(7), 3895. https://doi.org/10.3390/ijms23073895








