PEDOT-Carbon Nanotube Counter Electrodes and Bipyridine Cobalt (II/III) Mediators as Universally Compatible Components in Bio-Sensitized Solar Cells Using Photosystem I and Bacteriorhodopsin
Abstract
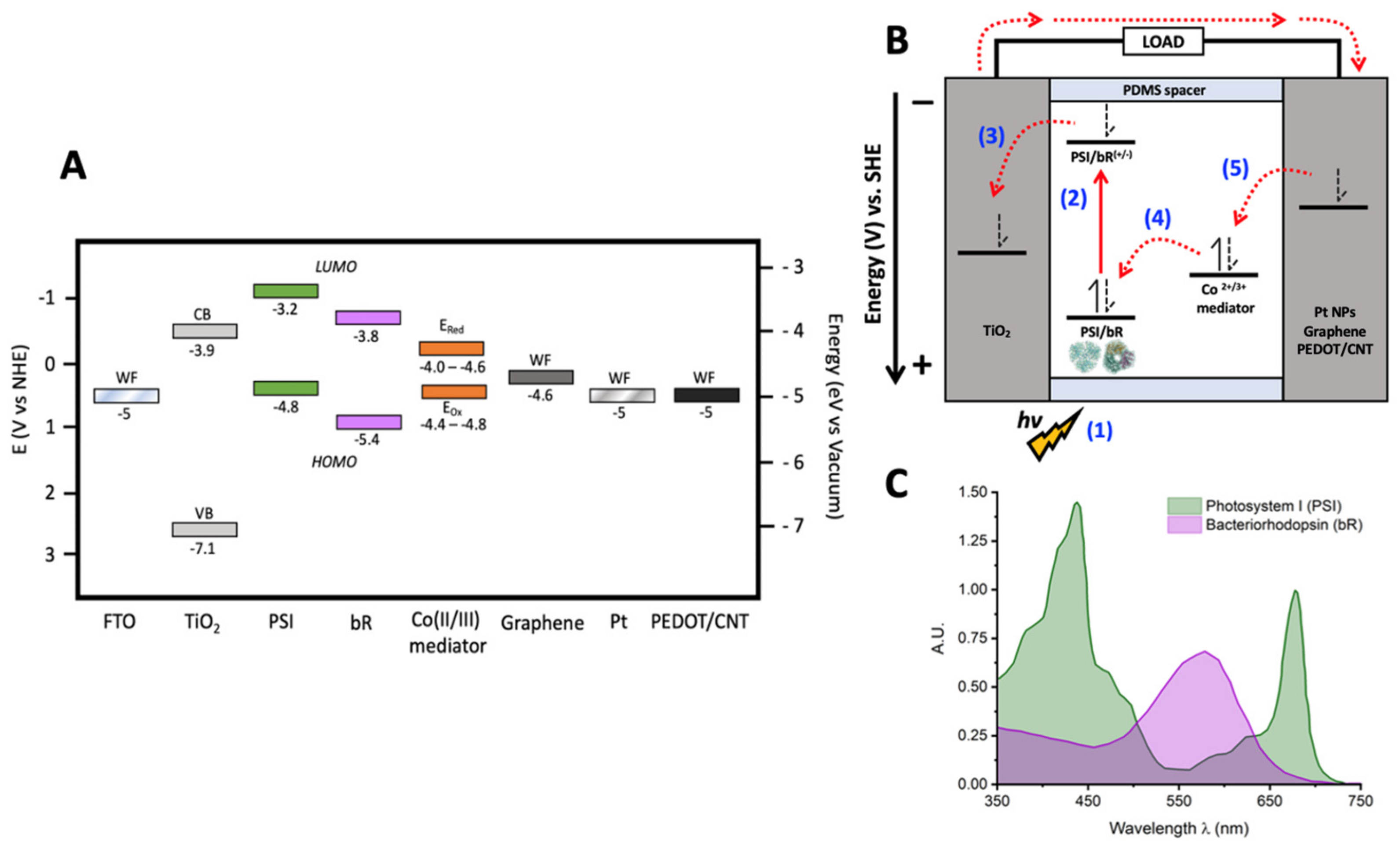
1. Introduction
2. Results
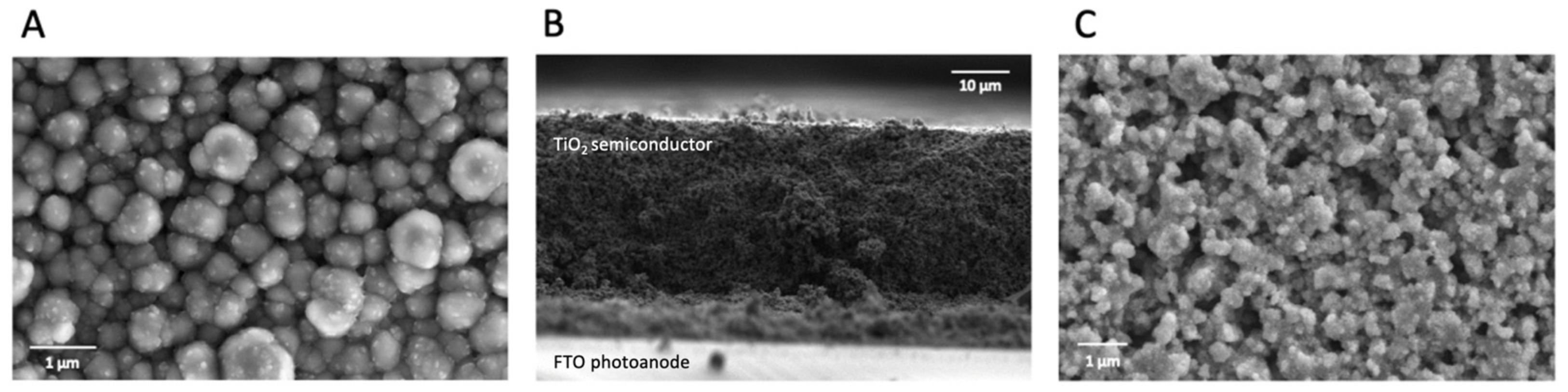
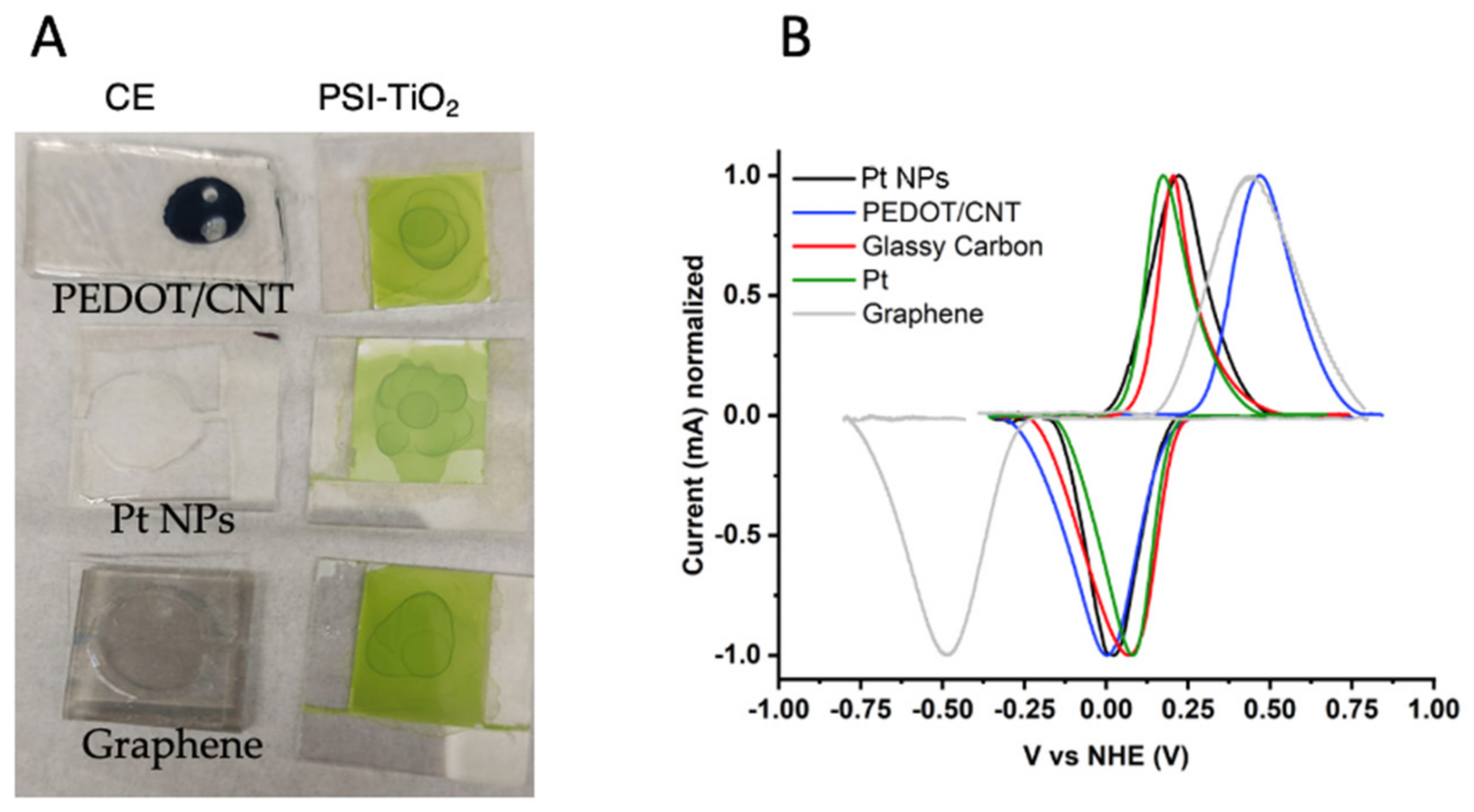
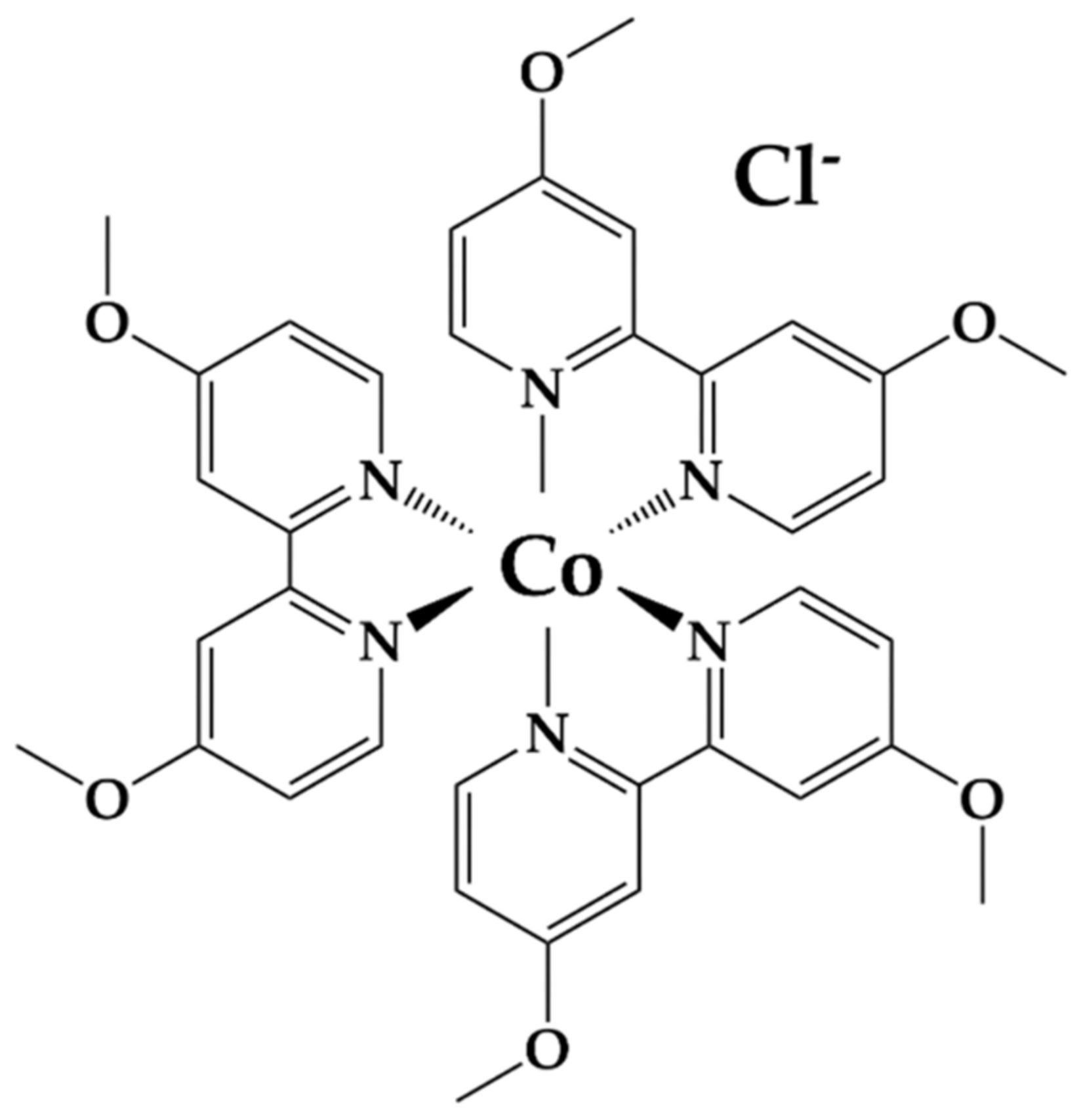
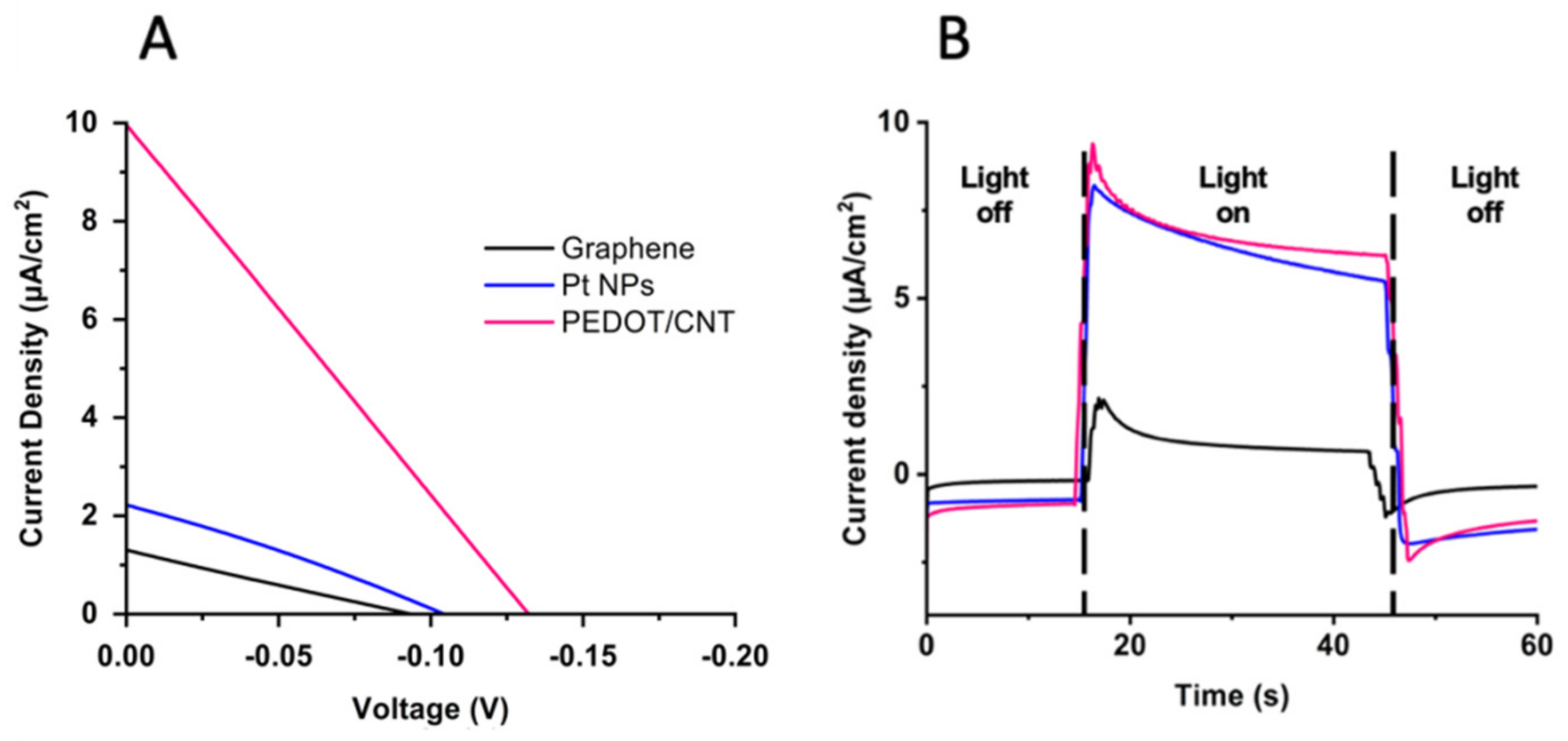
2.1. Counter Electrode Characterization
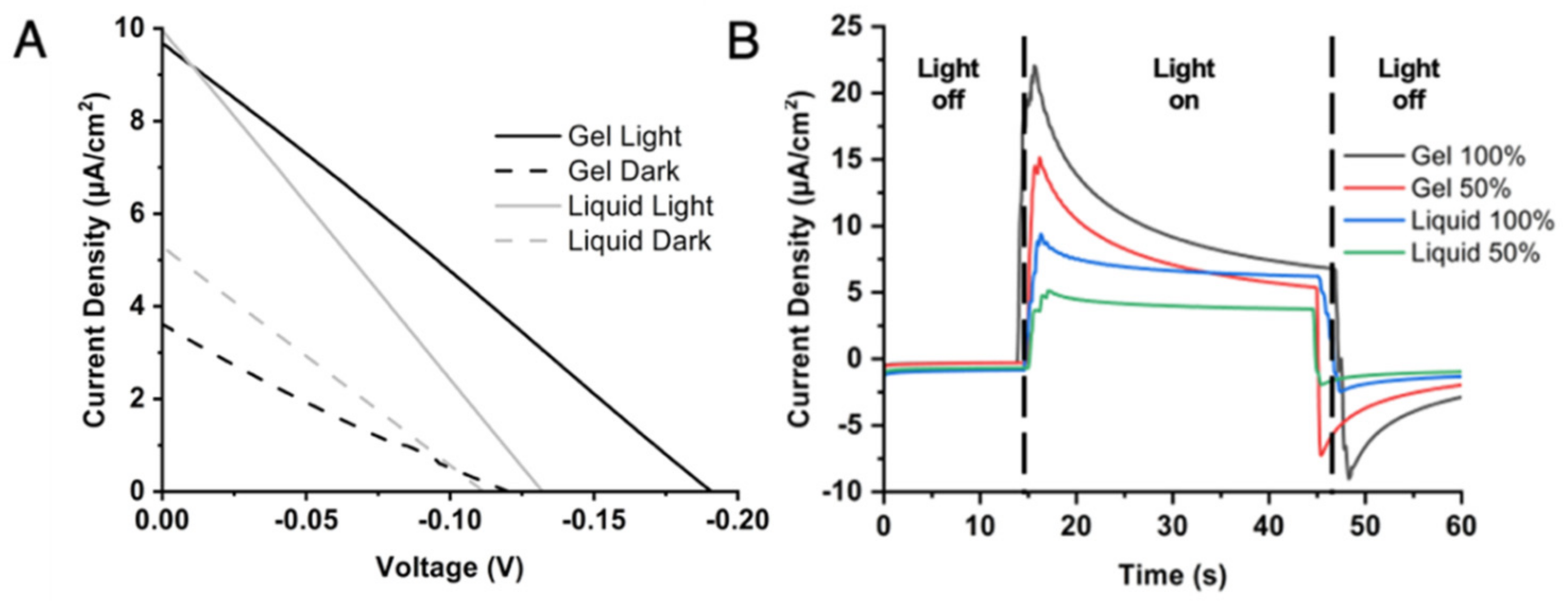
2.2. Comparison of Liquid and Gel Electrolyte Composition on PSI-SSC Output and Efficiency
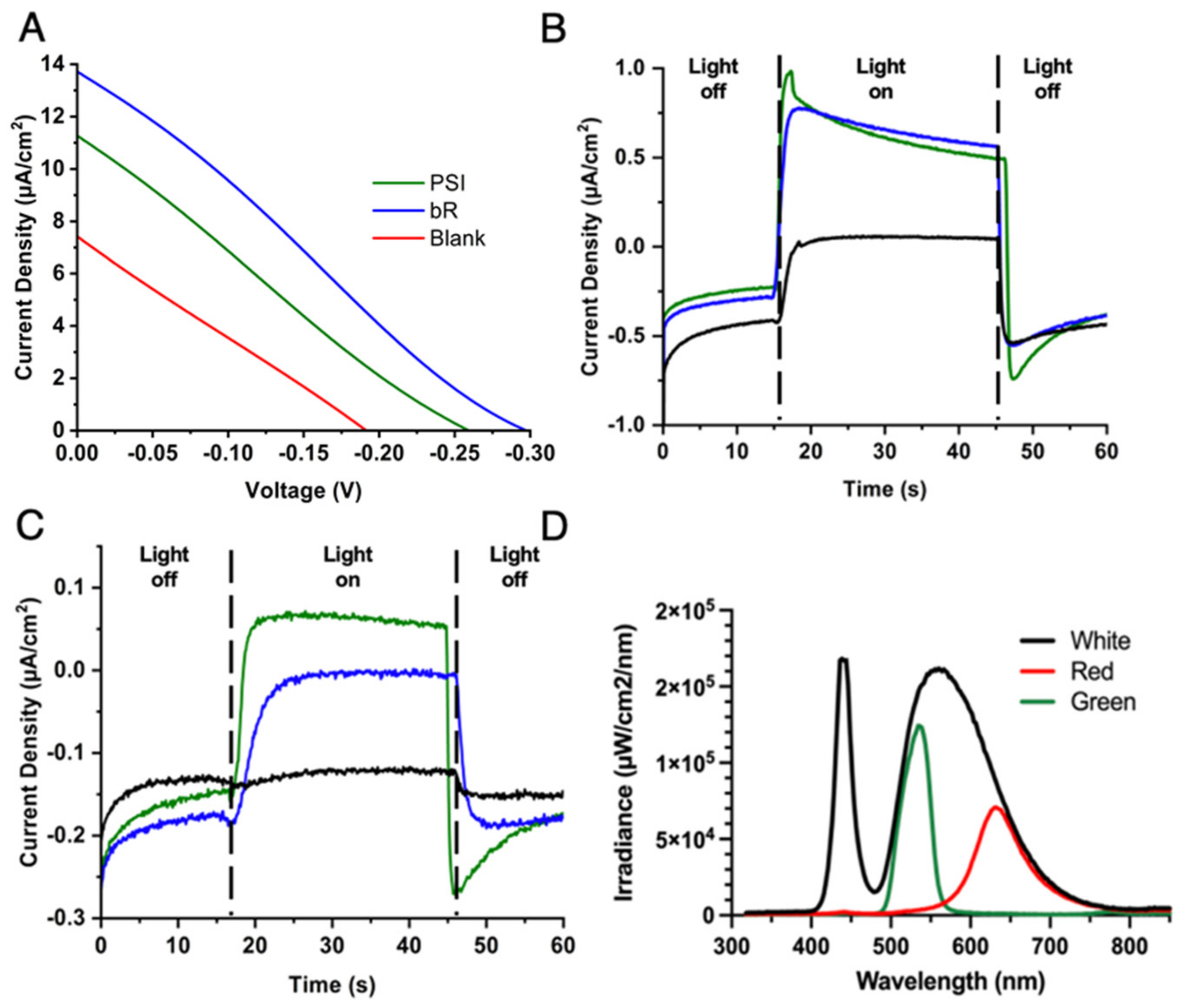
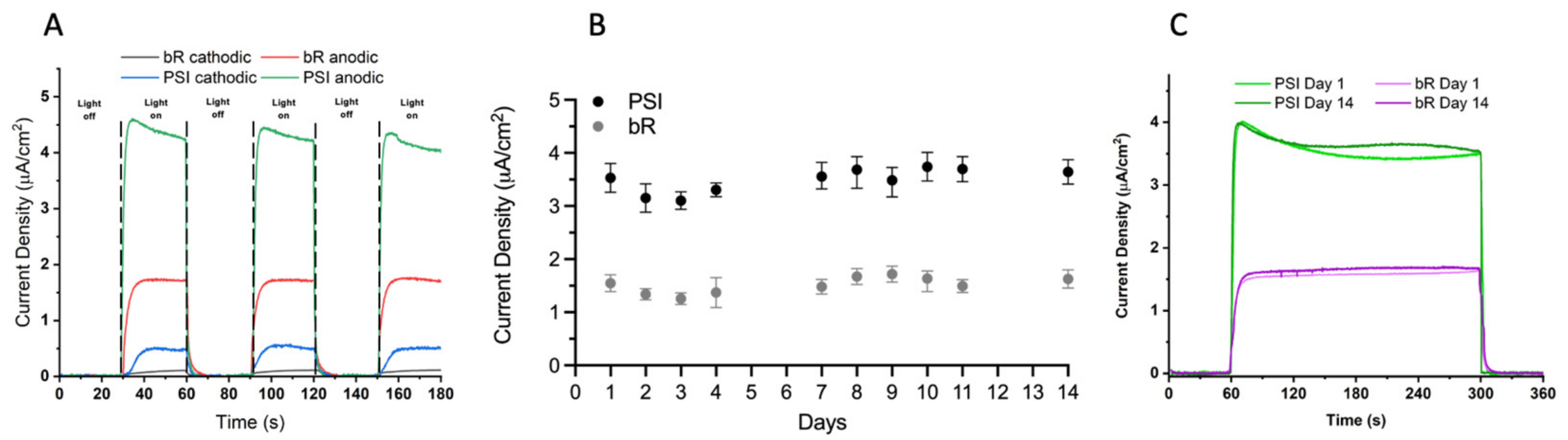
2.3. Aqueous CoII/III Redox Mediator Gel Electrolytes and PEDOT/CNT Counter Electrodes Are Compatible with Multiple Proteins
3. Discussion
4. Materials and Methods
4.1. Counter Electrode Fabrication
4.2. Protein Isolation
4.3. Cobalt Redox Mediator Synthesis
4.4. Cyclic Voltammetry of Co Redox Mediator with Variable Electrodes
4.5. Scanning Electron Microscopy
4.6. Device Fabrication
4.7. Device Testing
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cronshaw, I. World Energy Outlook 2014 projections to 2040: Natural gas and coal trade, and the role of China. Aust. J. Agric. Resour. Econ. 2015, 59, 571–585. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2007, 104, 20142. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.D.; Vaughn, M.D.; Bergkamp, J.J.; Gust, D.; Moore, A.L.; Moore, T.A. Evolution of reaction center mimics to systems capable of generating solar fuel. Photosynth. Res. 2014, 120, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An overview of solar photovoltaic panels’ end-of-life material recycling. Energy Strategy Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]
- Oregan, B.; Gratzel, M. A Low-Cost, High-Efficiency Solar-Cell Based on Dye-Sensitized Colloidal Tio2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Stieger, K.R.; Feifel, S.C.; Lokstein, H.; Hejazi, M.; Zouni, A.; Lisdat, F. Biohybrid architectures for efficient light-to-current conversion based on photosystem I within scalable 3D mesoporous electrodes. J. Mater. Chem. A 2016, 4, 17009–17017. [Google Scholar] [CrossRef]
- Vanselow, C.; Weber, A.P.M.; Krause, K.; Fromme, P. Genetic analysis of the Photosystem I subunits from the red alga, Galdieria sulphuraria. Bba-Bioenergetics 2009, 1787, 46–59. [Google Scholar] [CrossRef]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauss, N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef]
- Li, M.; Calteau, A.; Semchonok, D.A.; Witt, T.A.; Nguyen, J.T.; Sassoon, N.; Boekema, E.J.; Whitelegge, J.; Gugger, M.; Bruce, B.D. Physiological and evolutionary implications of tetrameric photosystem I in cyanobacteria. Nat. Plants 2019, 5, 1309–1319. [Google Scholar] [CrossRef]
- Caspy, I.; Nelson, N. Structure of the plant photosystem I. Biochem. Soc. Trans. 2018, 46, 285–294. [Google Scholar] [CrossRef]
- Netzer-El, S.Y.; Caspy, I.; Nelson, N. Crystal Structure of Photosystem I Monomer From Synechocystis PCC 6803. Front. Plant. Sci. 2018, 9, 1865. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 2nd ed.; Wiley/Blackwell: Chichester, UK, 2014. [Google Scholar]
- Hasegawa, N.; Jonotsuka, H.; Miki, K.; Takeda, K. X-ray structure analysis of bacteriorhodopsin at 1.3 A resolution. Sci. Rep. 2018, 8, 13123. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S. The structure of bacteriorhodopsin: An emerging consensus. Curr. Opin. Struct. Biol. 1999, 9, 462–468. [Google Scholar] [CrossRef]
- Lanyi, J.K. Bacteriorhodopsin. Annu. Rev. Physiol. 2004, 66, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Teodor, A.H.; Bruce, B.D. Putting Photosystem I to Work: Truly Green Energy. Trends Biotechnol. 2020, 38, 1329–1342. [Google Scholar] [CrossRef]
- Amao, Y.; Tadokoro, A.; Nakamura, M.; Shuto, N.; Kuroki, A. Artificial photosynthesis by using chloroplasts from spinach adsorbed on a nanocrystalline TiO2 electrode for photovoltaic conversion. Res. Chem. Intermediat. 2014, 40, 3257–3265. [Google Scholar] [CrossRef]
- Badura, A.; Guschin, D.; Kothe, T.; Kopczak, M.J.; Schuhmann, W.; Rogner, M. Photocurrent generation by photosystem 1 integrated in crosslinked redox hydrogels. Energ. Environ. Sci. 2011, 4, 2435–2440. [Google Scholar] [CrossRef]
- Baker, D.R.; Simmerman, R.F.; Sumner, J.J.; Bruce, B.D.; Lundgren, C.A. Photoelectrochemistry of photosystem I bound in nafion. Langmuir 2014, 30, 13650–13655. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Pan, R.L.; Gross, E.L. A Photosystem-I-Phenosafranine Solar-Cell. Photochem. Photobiol. 1981, 34, 215–222. [Google Scholar] [CrossRef]
- Carmeli, I.; Frolov, L.; Carmeli, C.; Richter, S. Photovoltaic activity of photosystem I-based self-assembled monolayer. J. Am. Chem. Soc. 2007, 129, 12352–12353. [Google Scholar] [CrossRef]
- Carter, J.R.; Baker, D.R.; Witt, T.A.; Bruce, B.D. Enhanced photocurrent from Photosystem I upon in vitro truncation of the antennae chlorophyll. Photosynth. Res. 2016, 127, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Gross, E.L.; Pan, R.L. A Photoelectrochemical Cell Using Electrodes Modified by Photosystem-I Particles of Spinach. Bot. Bull. Acad. Sin. 1992, 33, 9–15. [Google Scholar]
- Ciesielski, P.N.; Hijazi, F.M.; Scott, A.M.; Faulkner, C.J.; Beard, L.; Emmett, K.; Rosenthal, S.J.; Cliffel, D.; Jennings, G.K. Photosystem I—Based biohybrid photoelectrochemical cells. Bioresour. Technol. 2010, 101, 3047–3053. [Google Scholar] [CrossRef]
- Ciornii, D.; Riedel, M.; Stieger, K.R.; Feifel, S.C.; Hejazi, M.; Lokstein, H.; Zouni, A.; Lisdat, F. Bioelectronic Circuit on a 3D Electrode Architecture: Enzymatic Catalysis Interconnected with Photosystem I. J. Am. Chem. Soc. 2017, 139, 16478–16481. [Google Scholar] [CrossRef] [PubMed]
- Teodor, A.H.; Sherman, B.D.; Ison, Z.Y.; Ooi, E.-J.; Bergkamp, J.J.; Bruce, B.D. Green Catalysts: Applied and Synthetic Photosynthesis. Catalysts 2020, 10, 1016. [Google Scholar] [CrossRef]
- Terasaki, N.; Yamamoto, N.; Tamada, K.; Hattori, M.; Hiraga, T.; Tohri, A.; Sato, I.; Iwai, M.; Iwai, M.; Taguchi, S.; et al. Bio-photosensor: Cyanobacterial photosystem I coupled with transistor via molecular wire. Biochim. Biophys. Acta 2007, 1767, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.K.; Yen, C.-W.; Near, R.D.; El-Sayed, M.A. Bacteriorhodopsin/TiO2 nanotube arrays hybrid system for enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2011, 4, 2909–2914. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Wang, P.; Schaller, R.D.; Rajh, T.; Rozhkova, E.A. High-Performance Bioassisted Nanophotocatalyst for Hydrogen Production. Nano Lett. 2013, 13, 3365–3371. [Google Scholar] [CrossRef]
- Chu, L.K.; Yen, C.W.; El-Sayed, M.A. Bacteriorhodopsin-based photo-electrochemical cell. Biosens. Bioelectron. 2010, 26, 620–626. [Google Scholar] [CrossRef]
- Das, S.; Wu, C.; Song, Z.; Hou, Y.; Koch, R.; Somasundaran, P.; Priya, S.; Barbiellini, B.; Venkatesan, R. Bacteriorhodopsin Enhances Efficiency of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 30728–30734. [Google Scholar] [CrossRef]
- Hampp, N.A. Bacteriorhodopsin: Mutating a biomaterial into an optoelectronic material. Appl. Microbiol. Biotechnol. 2000, 53, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.D.; Honig, T.; Ron, I.; Friedman, N.; Sheves, M.; Cahen, D. Bacteriorhodopsin as an electronic conduction medium for biomolecular electronics. Chem. Soc. Rev. 2008, 37, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Gakhar, S.; Risbud, S.H.; Longo, M.L. Development and Characterization of Titanium Dioxide Gel with Encapsulated Bacteriorhodopsin for Hydrogen Production. Langmuir 2018, 34, 7488–7496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Tian, Y.; Tian, H.; Tu, T.; Gou, G.Y.; Wang, Q.; Qiao, Y.C.; Yang, Y.; Ren, T.L. A Review on Bacteriorhodopsin-Based Bioelectronic Devices. Sensors 2018, 18, 1368. [Google Scholar] [CrossRef]
- Renugopalakrishnan, V.; Barbiellini, B.; King, C.; Molinari, M.; Mochalov, K.; Sukhanova, A.; Nabiev, I.; Fojan, P.; Tuller, H.L.; Chin, M.; et al. Engineering a Robust Photovoltaic Device with Quantum Dots and Bacteriorhodopsin. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 16710–16717. [Google Scholar] [CrossRef]
- Thavasi, V.; Lazarova, T.; Filipek, S.; Kolinski, M.; Querol, E.; Kumar, A.; Ramakrishna, S.; Padros, E.; Renugopalakrishnan, V. Study on the feasibility of bacteriorhodopsin as bio-photosensitizer in excitonic solar cell: A first report. J. Nanosci. Nanotechnol. 2009, 9, 1679–1687. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Liu, Z.Y.; Zhai, J. Photocurrent generation in a light-harvesting system with multifunctional artificial nanochannels. Chem. Commun. 2015, 51, 12286–12289. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, P.; Xu, X.; Sheves, M.; Jin, Y. Bacteriorhodopsin/Ag nanoparticle-based hybrid nano-bio electrocatalyst for efficient and robust H2 evolution from water. J. Am. Chem. Soc. 2015, 137, 2840–2843. [Google Scholar] [CrossRef]
- Ghernaout, D.; Boudjemline, A.; Elboughdiri, N. Electrochemical Engineering in the Core of the Dye-Sensitized Solar Cells (DSSCs). Open Access Libr. J. 2020, 7, e6178. [Google Scholar] [CrossRef]
- Kumara, N.T.R.N.; Lim, A.; Lim, C.M.; Petra, M.I.; Ekanayake, P. Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 78, 301–317. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. Dye-Sensitized Solar Cell for Indoor Applications: A Mini-Review. J. Electron. Mater. 2021, 50, 3187–3206. [Google Scholar] [CrossRef]
- Abisharani, J.M.; Balamurugan, S.; Thomas, A.; Devikala, S.; Arthanareeswari, M.; Ganesan, S.; Prakash, M. Incorporation of organic additives with electron rich donors (N, O, S) in gelatin gel polymer electrolyte for dye sensitized solar cells. Sol. Energy 2021, 218, 552–562. [Google Scholar] [CrossRef]
- Teodor, A.H.; Ooi, E.-J.; Medina, J.; Alarcon, M.; Vaughn, M.D.; Bruce, B.D.; Bergkamp, J.J. Aqueous-soluble bipyridine cobalt(ii/iii) complexes act as direct redox mediators in photosystem I-based biophotovoltaic devices. RSC Adv. 2021, 11, 10434–10450. [Google Scholar] [CrossRef]
- Trasatti, S. The Absolute Electrode Potential—An Explanatory Note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Kawano, H. Effective Work Functions of the Elements. Prog. Surf. Sci. 2021, 97, 100583. [Google Scholar]
- Nakamura, A.; Suzawa, T.; Kato, Y.; Watanabe, T. Species dependence of the redox potential of the primary electron donor p700 in photosystem I of oxygenic photosynthetic organisms revealed by spectroelectrochemistry. Plant. Cell. Physiol. 2011, 52, 815–823. [Google Scholar] [CrossRef]
- Ueno, S.; Shibata, A.; Yorimitsu, A.; Baba, Y.; Kamo, N. Redox potentials of the oriented film of the wild-type, the E194Q-, E204Q- and D96N-mutated bacteriorhodopsins. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1609, 109–114. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; de Kok, M.M.; Vinken, E.; Maturova, K.; Janssen, R.A.J. Conductivity, work function, and environmental stability of PEDOT:PSS thin films treated with sorbitol. Org. Electron. 2008, 9, 727–734. [Google Scholar] [CrossRef]
- Garg, R.; Dutta, N.K.; Choudhury, N.R. Work Function Engineering of Graphene. Nanomaterials 2014, 4, 267–300. [Google Scholar] [CrossRef]
- Helander, M.G.; Greiner, M.T.; Wang, Z.B.; Tang, W.M.; Lu, Z.H. Work function of fluorine doped tin oxide. J. Vacuum Sci. Technol. A 2011, 29, 011019. [Google Scholar] [CrossRef]
- Ngidi, N.P.D.; Ollengo, M.A.; Nyamori, V.O. Heteroatom-doped graphene and its application as a counter electrode in dye-sensitized solar cells. Int. J. Energy Res. 2019, 43, 1702–1734. [Google Scholar] [CrossRef]
- Yoon, C.H.; Vittal, R.; Lee, J.; Chae, W.S.; Kim, K.J. Enhanced performance of a dye-sensitized solar cell with an electrodeposited-platinum counter electrode. Electrochim. Acta 2008, 53, 2890–2896. [Google Scholar] [CrossRef]
- Ghanem, M.A. Electrocatalytic activity and simultaneous determination of catechol and hydroquinone at mesoporous platinum electrode. Electrochem. Commun. 2007, 9, 2501–2506. [Google Scholar] [CrossRef]
- Kumarasinghe, K.D.M.S.P.K.; Kumara, G.R.A.; Rajapakse, R.M.G.; Liyanage, D.N.; Tennakone, K. Activated coconut shell charcoal based counter electrode for dye-sensitized solar cells. Org. Electron. 2019, 71, 93–97. [Google Scholar] [CrossRef]
- Balraju, P.; Kumar, M.; Roy, M.S.; Sharma, G.D. Dye sensitized solar cells (DSSCs) based on modified iron phthalocyanine nanostructured TiO2 electrode and PEDOT:PSS counter electrode. Synthetic Met. 2009, 159, 1325–1331. [Google Scholar] [CrossRef]
- Chen, J.G.; Wei, H.Y.; Ho, K.C. Using modified poly(3,4-ethylene dioxythiophene): Poly(styrene sulfonate) film as a counter electrode in dye-sensitized solar cells. Sol. Energy Mater. Sol. C 2007, 91, 1472–1477. [Google Scholar] [CrossRef]
- Ellis, H.; Jiang, R.; Ye, S.; Hagfeldt, A.; Boschloo, G. Development of high efficiency 100% aqueous cobalt electrolyte dye-sensitised solar cells. Phys. Chem. Chem. Phys. 2016, 18, 8419–8427. [Google Scholar] [CrossRef]
- Mukherjee, S.; Singh, R.; Gopinathan, S.; Murugan, S.; Gawali, S.; Saha, B.; Biswas, J.; Lodha, S.; Kumar, A. Solution-Processed Poly(3,4-ethylenedioxythiophene) Thin Films as Transparent Conductors: Effect of p-Toluenesulfonic Acid in Dimethyl Sulfoxide. ACS Appl. Mater. Int. 2014, 6, 17792–17803. [Google Scholar] [CrossRef]
- Ravi, S.K.; Yu, Z.M.; Swainsbury, D.J.K.; Ouyang, J.Y.; Jones, M.R.; Tan, S.C. Enhanced Output from Biohybrid Photoelectrochemical Transparent Tandem Cells Integrating Photosynthetic Proteins Genetically Modified for Expanded Solar Energy Harvesting. Adv. Energy Mater. 2017, 7, 1601821. [Google Scholar] [CrossRef]
- Robinson, M.T.; Simons, C.E.; Cliffel, D.E.; Jennings, G.K. Photocatalytic photosystem I/PEDOT composite films prepared by vapor-phase polymerization. Nanoscale 2017, 9, 6158–6166. [Google Scholar] [CrossRef]
- Wei, W.; Wang, H.; Hu, Y.H. A review on PEDOT-based counter electrodes for dye-sensitized solar cells. Int. J. Energy Res. 2014, 38, 1099–1111. [Google Scholar] [CrossRef]
- Rudd, S.; Evans, D. Recent advances in the aqueous applications of PEDOT. Nanoscale Adv. 2022, 4, 733–741. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Subramanyam, B.V.R.S.; Mahakul, P.C.; Sa, K.; Raiguru, J.; Alam, I.; Das, S.; Mondal, M.; Subudhi, S.; Mahanandia, P. Improved stability and performance of organic photovoltaic cells by application of carbon nanostructures and PEDOT:PSS composites as additional transparent electrodes. Sol. Energy 2019, 186, 146–155. [Google Scholar] [CrossRef]
- Feifel, S.C.; Lokstein, H.; Hejazi, M.; Zouni, A.; Lisdat, F. Unidirectional Photocurrent of Photosystem I on pi-System-Modified Graphene Electrodes: Nanobionic Approaches for the Construction of Photobiohybrid Systems. Langmuir 2015, 31, 10590–10598. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.H. Graphene as a counter electrode material for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 8182–8188. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Gerbaldi, C.; Barolo, C.; Gratzel, M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3431–3473. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Gratzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Gregg, B.A.; Pichot, F.; Ferrere, S.; Fields, C.L. Interfacial recombination processes in dye-sensitized solar cells and methods to passivate the interfaces. J. Phys. Chem. B 2001, 105, 1422–1429. [Google Scholar] [CrossRef]
- Nusbaumer, H.; Zakeeruddin, S.M.; Moser, J.E.; Gratzel, M. An alternative efficient redox couple for the dye-sensitized solar cell system. Chemistry 2003, 9, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Nusbaumer, H.M.; Moser, J.E.; Zakeeruddin, S.; Nazeeruddin, M.; Gratzel, M. CoII((dbbip)2)2+ Complex Rivals Tri-iodide/Iodide Redox Mediator in Dye-Sensitized Photovoltaic Cells. J. Phys. Chem. B 2001, 105, 10461–10464. [Google Scholar] [CrossRef]
- Mosconi, E.; Yum, J.H.; Kessler, F.; Gomez Garcia, C.J.; Zuccaccia, C.; Cinti, A.; Nazeeruddin, M.K.; Gratzel, M.; De Angelis, F. Cobalt electrolyte/dye interactions in dye-sensitized solar cells: A combined computational and experimental study. J. Am. Chem. Soc. 2012, 134, 19438–19453. [Google Scholar] [CrossRef] [PubMed]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal Coordination Complexes as Redox Mediators in Regenerative Dye-Sensitized Solar Cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef]
- Sapp, S.A.; Elliott, C.M.; Contado, C.; Caramori, S.; Bignozzi, C.A. Substituted polypyridine complexes of cobalt(II/III) as efficient electron-transfer mediators in dye-sensitized solar cells. J. Am. Chem. Soc. 2002, 124, 11215–11222. [Google Scholar] [CrossRef] [PubMed]
- Sonai, G.G.; Tiihonen, A.; Miettunen, K.; Lund, P.D.; Nogueira, A.F. Long-Term Stability of Dye-Sensitized Solar Cells Assembled with Cobalt Polymer Gel Electrolyte. J. Phys. Chem. C 2017, 121, 17577–17585. [Google Scholar] [CrossRef]
- Passantino, J.M.; Wolfe, K.D.; Simon, K.T.; Cliffel, D.E.; Jennings, G.K. Photosystem I Enhances the Efficiency of a Natural, Gel-Based Dye-Sensitized Solar Cell. ACS Appl. Bio Mater. 2020, 3, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Vaughn, M.; Frymier, P.; Bruce, B.D. In vitro kinetics of P700 + reduction of Thermosynechococcus elongatus trimeric Photosystem I complexes by recombinant cytochrome c 6 using a Joliot-type LED spectrophotometer. Photosynth. Res. 2017, 131, 79–91. [Google Scholar] [CrossRef]
- Toth-Boconadi, R.; Der, A.; Keszthelyi, L. Buffer effects on electric signals of light-excited bacteriorhodopsin. Biophys. J. 2000, 78, 3170–3177. [Google Scholar] [CrossRef][Green Version]
- Liu, S.Y.; Kono, M.; Ebrey, T.G. Effect of pH buffer molecules on the light-induced currents from oriented purple membrane. Biophys. J. 1991, 60, 204–216. [Google Scholar] [CrossRef]
- Al-Mohsin, H.A.; Mineart, K.P.; Armstrong, D.P.; Spontak, R.J. Tuning the performance of aqueous photovoltaic elastomer gels by solvent polarity and nanostructure development. J. Polym. Sci. Pol. Phys. 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Nakamura, A.; Kuroiwa, Y.; Kato, Y.; Watanabe, T. Spectroelectrochemistry of P700 in native photosystem I particles and diethyl ether-treated thylakoid membranes from spinach and Thermosynechococcus elongatus. FEBS Lett. 2008, 582, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- MacKinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Huang, K.-T.; Chen, C.-P.; Jiang, B.-H.; Jeng, R.-J.; Chen, W.-C. Green poly-lysine as electron-extraction modified layer with over 15% power conversion efficiency and its application in bio-based flexible organic solar cells. Org. Electron. 2020, 87, 105924. [Google Scholar] [CrossRef]
- Han, J.; Bao, F.; Huang, D.; Wang, X.; Yang, C.; Yang, R.; Jian, X.; Wang, J.; Bao, X.; Chu, J. A Universal Method to Enhance Flexibility and Stability of Organic Solar Cells by Constructing Insulating Matrices in Active Layers. Adv. Funct. Mater. 2020, 30, 2003654. [Google Scholar] [CrossRef]
- Villarreal, C.C.; Monge, S.; Aguilar, D.; Tames, A.; Araya, N.; Aguilar, M.; Ramakrishna, S.; Thavasi, V.; Song, Z.; Mulchandani, A.; et al. Bio-sensitized solar cells built from renewable carbon sources. Mater. Today Energy 2022, 23, 100910. [Google Scholar] [CrossRef]
- Zamora, R.; Masís-Meléndez, F.; Phillips, H.; Alvarado-Marchena, L.A.; Starbird, R. Development of Poly(3,4- ethylenedioxythiophene(PEDOT)/carbon Nanotube Electrodes for Electrochemical Detection of Mancozeb in Water. Int. J. Electrochem. Sci. 2018, 13, 1931–1944. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Alvarado-Hidalgo, F.; Robles-Chaves, D.; Saenz-Arce, G.; Avendano-Soto, E.D.; Sanchez-Kopper, A.; Starbird-Perez, R. Electrochemical Characterization of Mancozeb Degradation for Wastewater Treatment Using a Sensor Based on Poly (3,4-ethylenedioxythiophene) (PEDOT) Modified with Carbon Nanotubes and Gold Nanoparticles. Polymers 2019, 11, 1449. [Google Scholar] [CrossRef]
- Martin, D.C.; Wu, J.H.; Shaw, C.M.; King, Z.; Spanninga, S.A.; Richardson-Burns, S.; Hendricks, J.; Yang, J.Y. The Morphology of Poly(3,4-Ethylenedioxythiophene). Polym. Rev. 2010, 50, 340–384. [Google Scholar] [CrossRef]
- Marchini, E.; Caramori, S.; Bignozzi, C.A.; Carli, S. On the Use of PEDOT as a Catalytic Counter Electrode Material in Dye-Sensitized Solar Cells. Appl. Sci. 2021, 11, 3795. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, Q.; Zhou, W.; Xia, X.; Yang, F.; Zhang, N.; Xiao, S.; Li, K.; Gu, X.; Xiao, Z.; et al. Novel approach to enhance efficiency of hybrid silicon-based solar cells via synergistic effects of polymer and carbon nanotube composite film. Nano Energy 2017, 33, 436–444. [Google Scholar] [CrossRef]
- Tsao, H.N.; Burschka, J.; Yi, C.; Kessler, F.; Nazeeruddin, M.K.; Grätzel, M. Influence of the interfacial charge-transfer resistance at the counter electrode in dye-sensitized solar cells employing cobalt redox shuttles. Energy Environ. Sci. 2011, 4, 4921–4924. [Google Scholar] [CrossRef]
- Kouhnavard, M.; Yifan, D.; D’Arcy, J.M.; Mishra, R.; Biswas, P. Highly conductive PEDOT films with enhanced catalytic activity for dye-sensitized solar cells. Sol. Energy 2020, 211, 258–264. [Google Scholar] [CrossRef]
- Beitollahi, H.; Movahedifar, F.; Tajik, S.; Jahani, S. A Review on the Effects of Introducing CNTs in the Modification Process of Electrochemical Sensors. Electroanalysis 2018, 31, 1195–1203. [Google Scholar] [CrossRef]
- Yang, L.; Luo, Y.; Yang, L.; Luo, S.; Luo, X.; Dai, W.; Li, T.; Luo, Y. Enhanced photocatalytic activity of hierarchical titanium dioxide microspheres with combining carbon nanotubes as “e-bridge”. J. Hazard. Mater. 2019, 367, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Tang, Z.; Liu, Q.; Ishikawa, R.; Ueno, K.; Shirai, H. Green-tea modified multiwalled carbon nanotubes for efficient poly(3,4-ethylenedioxythiophene):poly(stylenesulfonate)/n-silicon hybrid solar cell. Appl. Phys. Lett. 2013, 102, 063508. [Google Scholar] [CrossRef]
- Hölzl, J.; Schulte, F.K. Solid Surface Physics; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Lin, Y.; Xie, Y.; Wei, Y. Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 2017, 46, 5975–6023. [Google Scholar] [CrossRef]
- Villarreal, C.C.; Pham, T.; Ramnani, P.; Mulchandani, A. Carbon allotropes as sensors for environmental monitoring. Curr. Opin. Electrochem. 2017, 3, 106–113. [Google Scholar] [CrossRef]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Mandal, P.; Manna, J.S.; Das, D.; Mitra, M.K. Energy transfer cascade in bio-inspired chlorophyll-a/polyacrylamide hydrogel: Towards a new class of biomimetic solar cells. Rsc. Adv. 2016, 6, 90280–90289. [Google Scholar] [CrossRef]
- Chellamuthu, J.; Nagaraj, P.; Chidambaram, S.G.; Sambandam, A.; Muthupandian, A. Enhanced photocurrent generation in bacteriorhodopsin based bio-sensitized solar cells using gel electrolyte. J. Photochem. Photobiol. B 2016, 162, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Iwuchukwu, I.J.; Vaughn, M.; Myers, N.; O’Neill, H.; Frymier, P.; Bruce, B.D. Self-organized photosynthetic nanoparticle for cell-free hydrogen production. Nat. Nanotechnol. 2010, 5, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.A.; Smeigh, A.L.; Le Pleux, L.; Hammarstrom, L.; Odobel, F.; Boschloo, G.; Hagfeldt, A. Cobalt Polypyridyl-Based Electrolytes for p-Type Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 9772–9779. [Google Scholar] [CrossRef]
- Fourmond, V.; Hoke, K.; Heering, H.A.; Baffert, C.; Leroux, F.; Bertrand, P.; Leger, C. SOAS: A free program to analyze electrochemical data and other one-dimensional signals. Bioelectrochemistry 2009, 76, 141–147. [Google Scholar] [CrossRef] [PubMed]
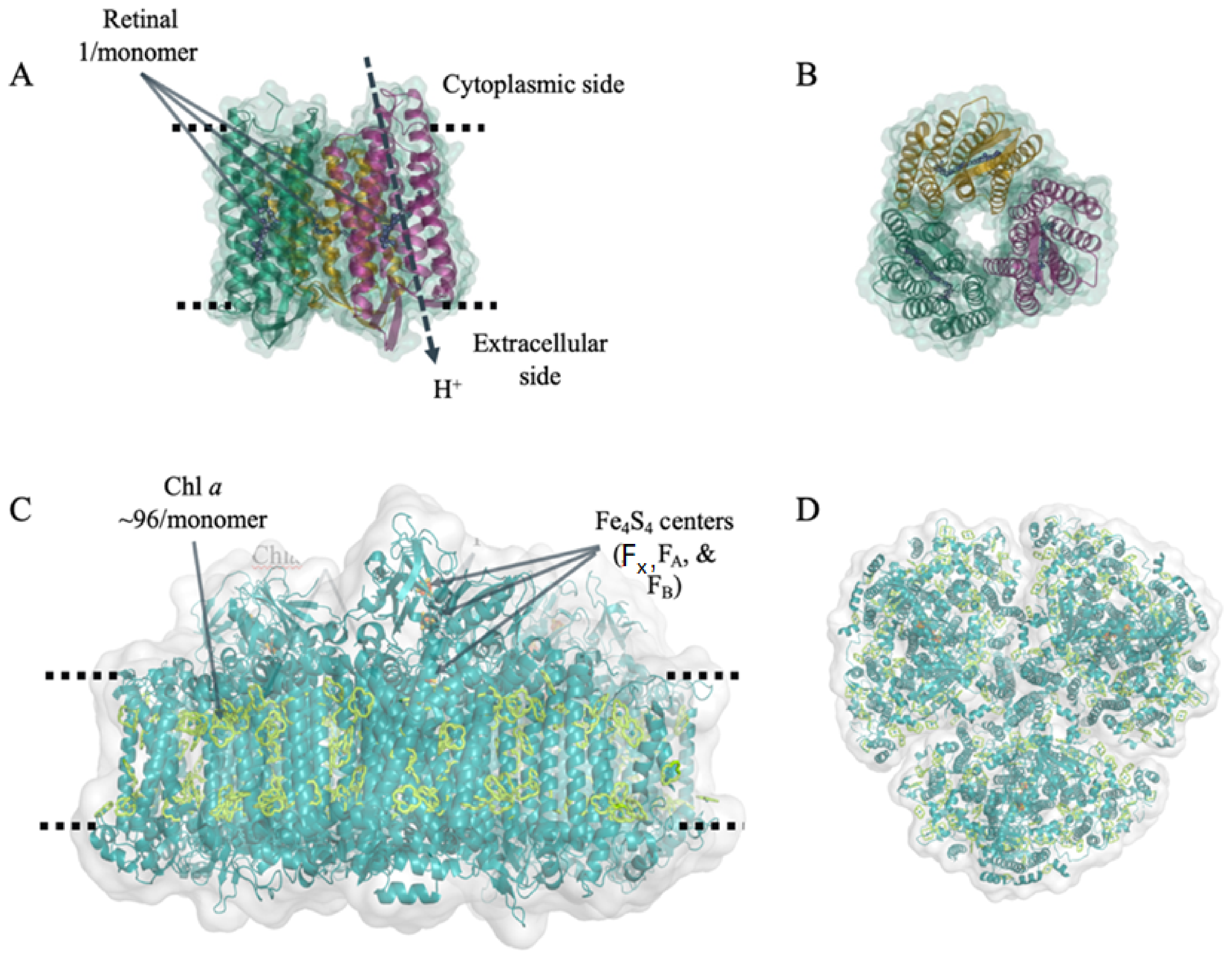
| Protein | Pigment | # Pigments per Monomer | MW per Monomer | Abs. Max. (nm) | Ext. Coeff. at Abs. Max. (mM−1 cm−1) | Organism Source | In Vivo Function |
|---|---|---|---|---|---|---|---|
| Bacteriorhodopsin (bR) | Retinal | 1 | 27 kDa | 560 (green) | 63 | Halobacterium salinarum S9 | unidirectional H+ pump |
| Photosystem I (PSI) | Chlorophyll a | ~100 | ~400 kDa | 680 (red) | 57 | Themosynechococcus elongatus BP-1 | unidirectional e− transfer |
| Electrode Material | Eox (V vs. NHE) | Ered (V vs. NHE) | Em (V vs. NHE) |
|---|---|---|---|
| Graphene | 0.448 | −0.493 | −0.045 |
| PEDOT/CNT | 0.468 | 0.003 | 0.236 |
| Pt NPs | 0.221 | 0.023 | 0.122 |
| Pt wire | 0.173 | 0.081 | 0.127 |
| Glassy Carbon | 0.205 | 0.069 | 0.137 |
| VOC (mV) | JSC (µA/cm2) | FF % | PCE % | |
|---|---|---|---|---|
| PEDOT/CNTs | −132 | 10.00 | 25 | 0.33 |
| Pt NPs | −104 | 2.22 | 28 | 0.06 |
| Graphene | −93 | 1.30 | 24 | 0.03 |
| VOC (mV) | JSC (µA/cm2) | FF % | PCE % | |
|---|---|---|---|---|
| Gel | −190 | 9.67 | 26 | 0.48 |
| Liquid | −132 | 9.94 | 25 | 0.33 |
| VOC (mV) | JSC (µA/cm2) | FF % | PCE % | |
|---|---|---|---|---|
| bR | −298 | 13.67 | 26 | 1.04 |
| PSI | −259 | 11.25 | 24 | 0.70 |
| Blank | −192 | 7.44 | 25 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodor, A.H.; Monge, S.; Aguilar, D.; Tames, A.; Nunez, R.; Gonzalez, E.; Rodríguez, J.J.M.; Bergkamp, J.J.; Starbird, R.; Renugopalakrishnan, V.; et al. PEDOT-Carbon Nanotube Counter Electrodes and Bipyridine Cobalt (II/III) Mediators as Universally Compatible Components in Bio-Sensitized Solar Cells Using Photosystem I and Bacteriorhodopsin. Int. J. Mol. Sci. 2022, 23, 3865. https://doi.org/10.3390/ijms23073865
Teodor AH, Monge S, Aguilar D, Tames A, Nunez R, Gonzalez E, Rodríguez JJM, Bergkamp JJ, Starbird R, Renugopalakrishnan V, et al. PEDOT-Carbon Nanotube Counter Electrodes and Bipyridine Cobalt (II/III) Mediators as Universally Compatible Components in Bio-Sensitized Solar Cells Using Photosystem I and Bacteriorhodopsin. International Journal of Molecular Sciences. 2022; 23(7):3865. https://doi.org/10.3390/ijms23073865
Chicago/Turabian StyleTeodor, Alexandra H., Stephanie Monge, Dariana Aguilar, Alexandra Tames, Roger Nunez, Elaine Gonzalez, Juan J. Montero Rodríguez, Jesse J. Bergkamp, Ricardo Starbird, Venkatesan Renugopalakrishnan, and et al. 2022. "PEDOT-Carbon Nanotube Counter Electrodes and Bipyridine Cobalt (II/III) Mediators as Universally Compatible Components in Bio-Sensitized Solar Cells Using Photosystem I and Bacteriorhodopsin" International Journal of Molecular Sciences 23, no. 7: 3865. https://doi.org/10.3390/ijms23073865
APA StyleTeodor, A. H., Monge, S., Aguilar, D., Tames, A., Nunez, R., Gonzalez, E., Rodríguez, J. J. M., Bergkamp, J. J., Starbird, R., Renugopalakrishnan, V., Bruce, B. D., & Villarreal, C. (2022). PEDOT-Carbon Nanotube Counter Electrodes and Bipyridine Cobalt (II/III) Mediators as Universally Compatible Components in Bio-Sensitized Solar Cells Using Photosystem I and Bacteriorhodopsin. International Journal of Molecular Sciences, 23(7), 3865. https://doi.org/10.3390/ijms23073865







