Negative Elongation Factor (NELF) Inhibits Premature Granulocytic Development in Zebrafish
Abstract
1. Introduction
2. Results
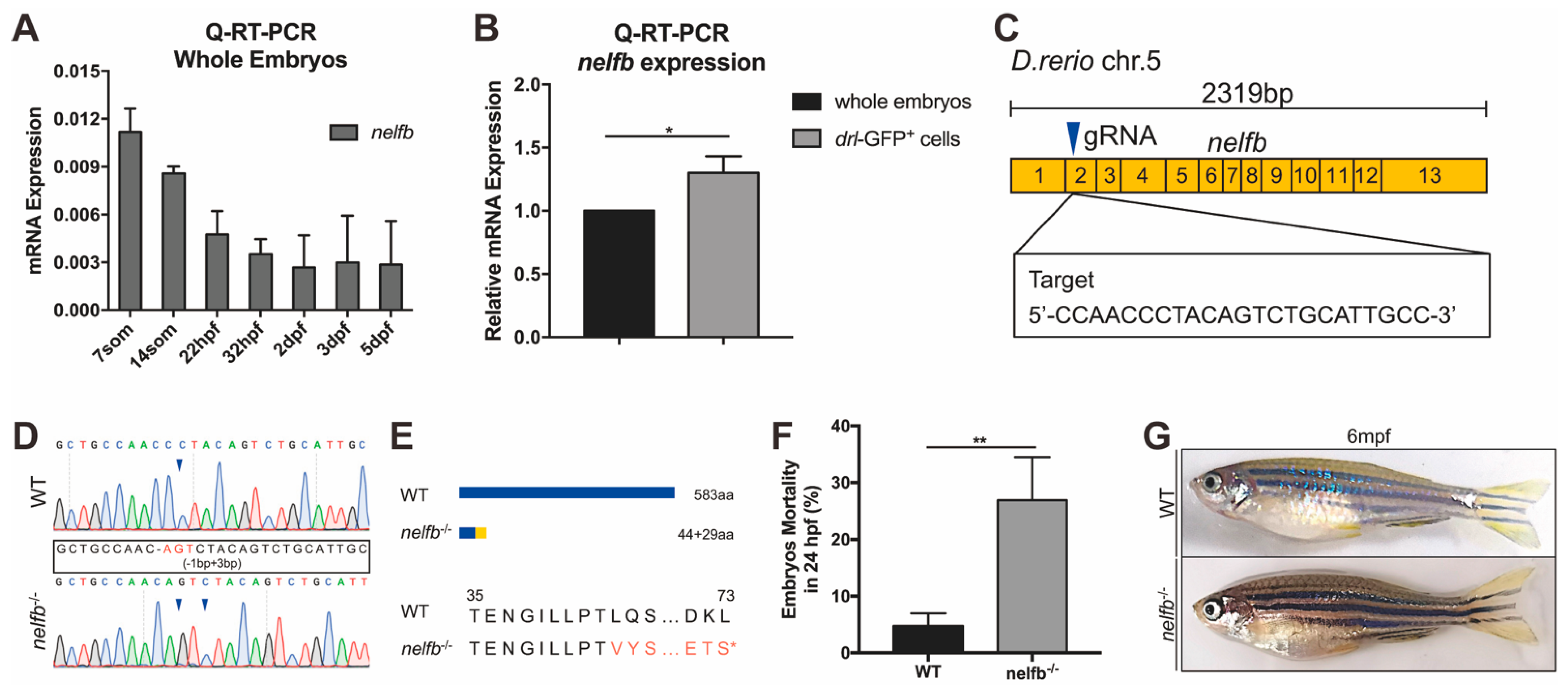
2.1. Expression of nelfb during Development and Generation of Zebrafish nelfb Mutants
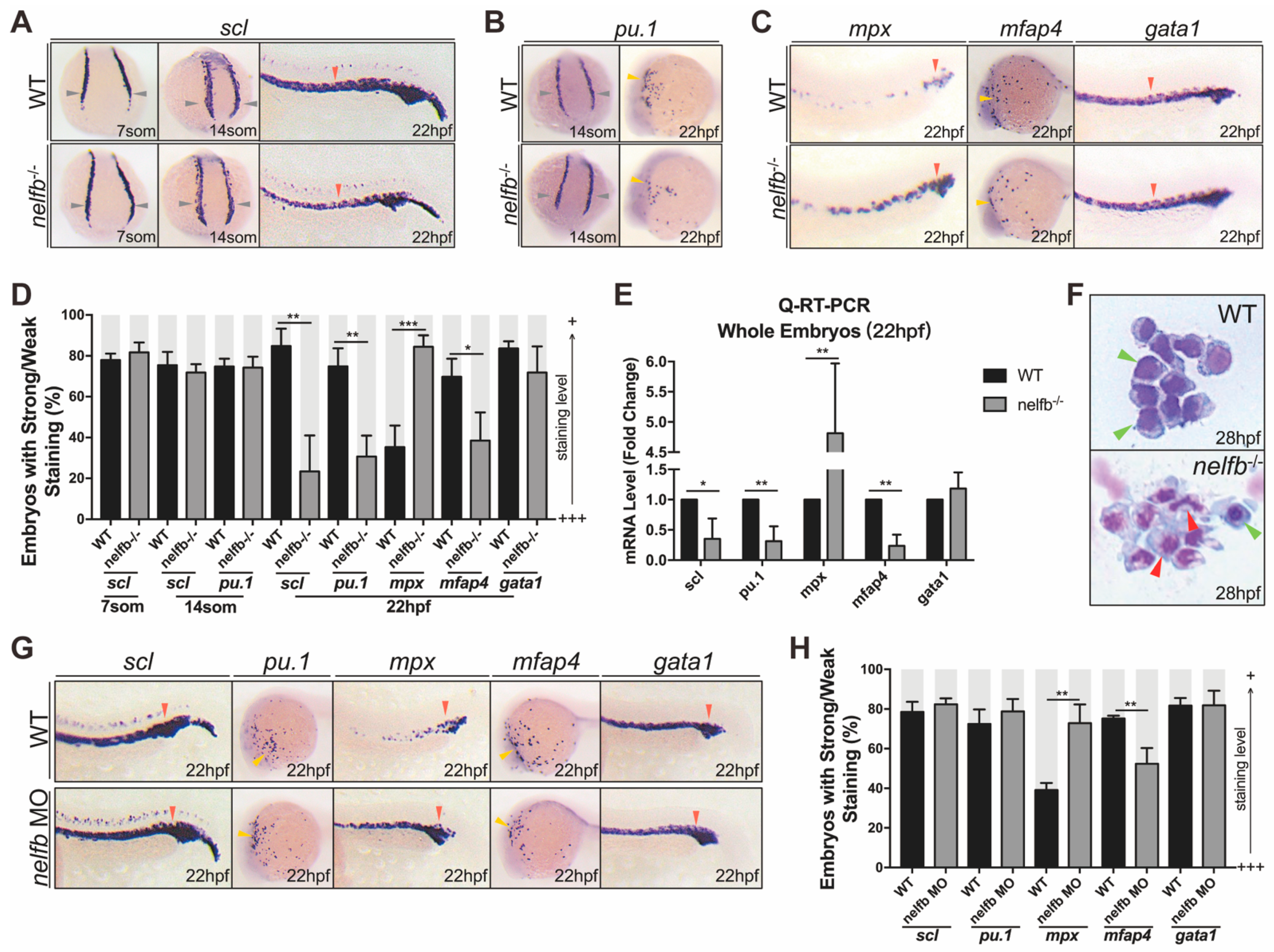
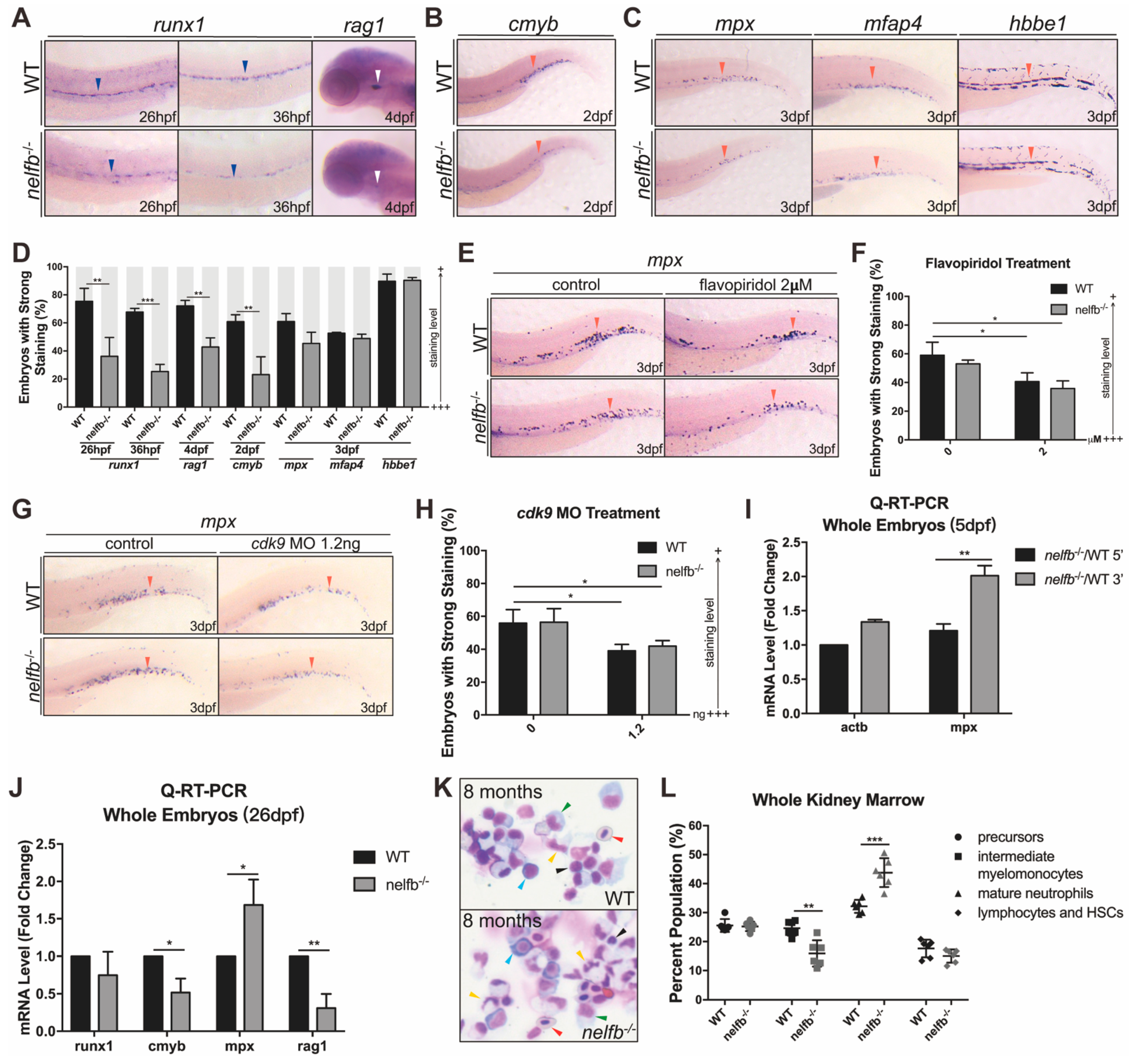
2.2. nelfb Deficiency Leads to Accelerated Granulocytic Development during Primitive Hematopoiesis
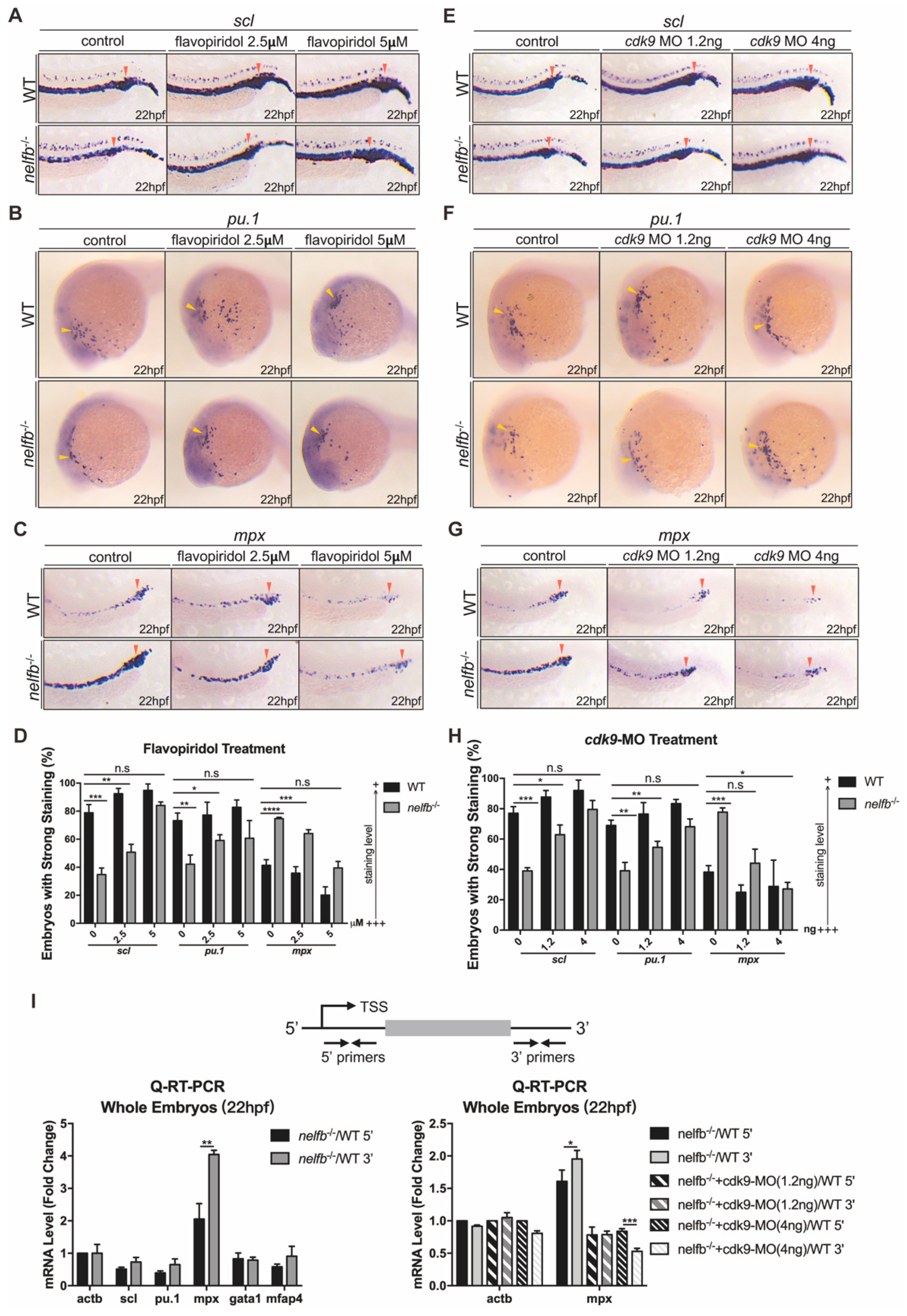
2.3. Inhibition of Pol II Elongation Rescues Primitive Hematopoiesis in nelfb−/− Embryos
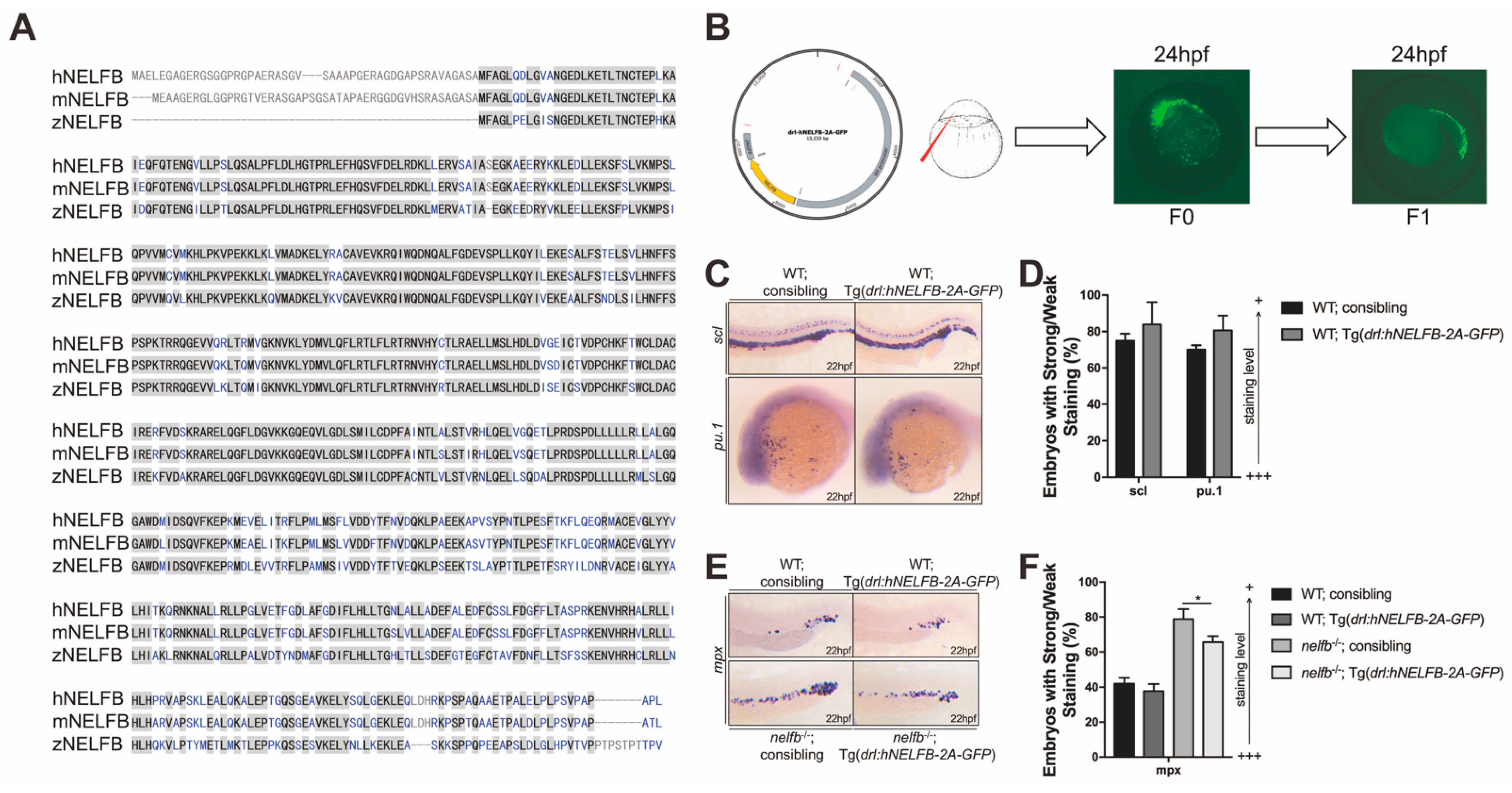
2.4. Expression of Human NELFB in Hematopoietic Cells Partially Rescues Granulopoiesis in nelfb−/− Embryos
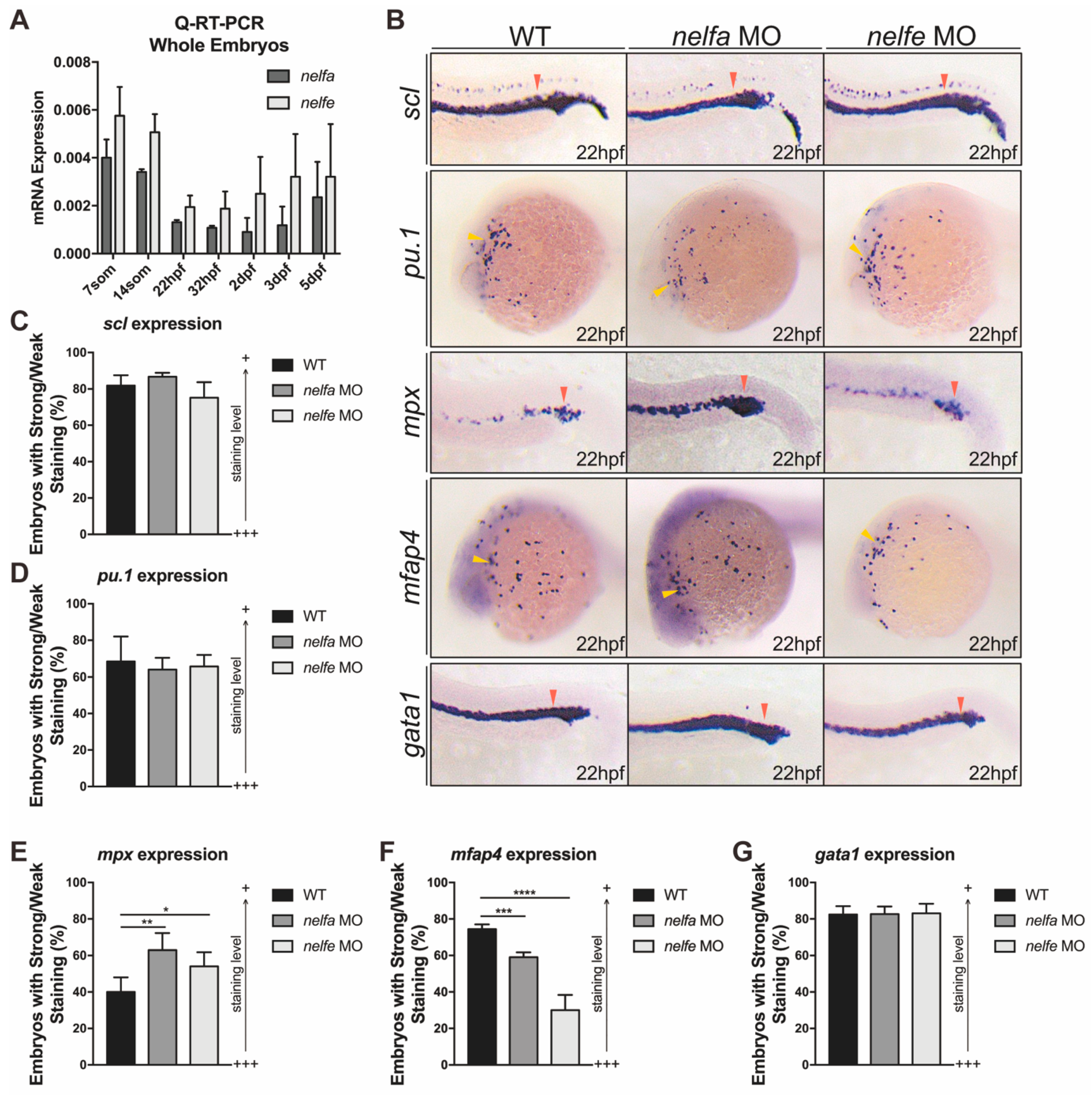
2.5. Other NELF Subunits Play Similar Roles in Primitive Granulocytic Development
2.6. Granulocytic Differentiation at Late Developmental Stages and Adulthood Also Shows Defects in nelfb−/− Zebrafish
3. Discussion
4. Materials and Methods
4.1. Zebrafish Maintenance and Embryo Handling
4.2. Generation of nelfb Knockout Mutants
4.3. Generation of NELFB Overexpression Lines
4.4. Whole-Mount In Situ Hybridization (WISH)
4.5. May–Grünwald–Giemsa Staining of Embryo Blood Cells and Adult Whole Kidney Marrow Cells
4.6. Flow Cytometry and Cell Sorting
4.7. Gene Expression Tested by Real-Time qPCR
4.8. Flavopiridol Treatment
4.9. Morpholino Microinjection
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jing, L.; Zon, L.I. Zebrafish as a model for normal and malignant hematopoiesis. Dis. Model. Mech. 2011, 4, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P. Organization and regulation of gene transcription. Nature 2019, 573, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tunnacliffe, E.; Chubb, J.R. What Is a Transcriptional Burst? Trends Genet. 2020, 36, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Roeder, R.G. 50+ years of eukaryotic transcription: An expanding universe of factors and mechanisms. Nat. Struct. Mol. Biol. 2019, 26, 783–791. [Google Scholar] [CrossRef]
- Abuhashem, A.; Garg, V.; Hadjantonakis, A.-K. RNA polymerase II pausing in development: Orchestrating transcription. Open Biol. 2022, 12, 210220. [Google Scholar] [CrossRef]
- Chen, F.X.; Smith, E.R.; Shilatifard, A. Born to run: Control of transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2018, 19, 464–478. [Google Scholar] [CrossRef]
- Min, I.M.; Waterfall, J.J.; Core, L.J.; Munroe, R.J.; Schimenti, J.; Lis, J.T. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes Dev. 2011, 25, 742–754. [Google Scholar] [CrossRef]
- Williams, L.H.; Fromm, G.; Gokey, N.G.; Henriques, T.; Muse, G.W.; Burkholder, A.; Fargo, D.C.; Hu, G.; Adelman, K. Pausing of RNA Polymerase II Regulates Mammalian Developmental Potential through Control of Signaling Networks. Mol. Cell 2015, 58, 311–322. [Google Scholar] [CrossRef]
- Chen, F.; Gao, X.; Shilatifard, A. Stably paused genes revealed through inhibition of transcription initiation by the TFIIH inhibitor triptolide. Genes Dev. 2015, 29, 39–47. [Google Scholar] [CrossRef]
- Core, L.J.; Waterfall, J.J.; Gilchrist, D.A.; Fargo, D.C.; Kwak, H.; Adelman, K.; Lis, J.T. Defining the Status of RNA Polymerase at Promoters. Cell Rep. 2012, 2, 1025–1035. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Takagi, T.; Wada, T.; Yano, K.; Furuya, A.; Sugimoto, S.; Hasegawa, J.; Handa, H. NELF, a Multisubunit Complex Containing RD, Cooperates with DSIF to Repress RNA Polymerase II Elongation. Cell 1999, 97, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.M.; Farnung, L.; Urlaub, H.; Cramer, P. Structure of paused transcription complex Pol II-DSIF-NELF. Nature 2018, 560, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, D.A.; Nechaev, S.; Lee, C.; Ghosh, S.K.B.; Collins, J.B.; Li, L.; Gilmour, D.S.; Adelman, K. NELF-mediated stalling of Pol II can enhance gene expression by blocking promoter-proximal nucleosome assembly. Genes Dev. 2008, 22, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Qin, K.; Guo, Z.; Ma, Y.; April, C.; Gao, X.; Andrews, T.G.; Bokov, A.; Zhang, J.; Chen, Y.; et al. Negative Elongation Factor Controls Energy Homeostasis in Cardiomyocytes. Cell Rep. 2014, 7, 79–85. [Google Scholar] [CrossRef]
- Wang, X.; Lee, C.; Gilmour, D.S.; Gergen, J.P. Transcription elongation controls cell fate specification in the Drosophila embryo. Genes Dev. 2007, 21, 1031–1036. [Google Scholar] [CrossRef][Green Version]
- Lagha, M.; Bothma, J.P.; Esposito, E.; Ng, S.; Stefanik, L.; Tsui, C.; Johnston, J.; Chen, K.; Gilmour, D.S.; Zeitlinger, J.; et al. Paused Pol II Coordinates Tissue Morphogenesis in the Drosophila Embryo. Cell 2013, 153, 976–987. [Google Scholar] [CrossRef]
- Liu, X.; Gogate, A.A.; Tastemel, M.; Malladi, V.S.; Yao, H.; Nguyen, K.; Huang, L.J.-S.; Bai, X. Dynamic change of transcription pausing through modulating NELF protein stability regulates granulocytic differentiation. Blood Adv. 2017, 1, 1358–1367. [Google Scholar] [CrossRef]
- Peng, J.; Marshall, N.F.; Price, D.H. Identification of a Cyclin Subunit Required for the Function ofDrosophila P-TEFb. J. Biol. Chem. 1998, 273, 13855–13860. [Google Scholar] [CrossRef]
- Fujinaga, K.; Irwin, D.; Huang, Y.; Taube, R.; Kurosu, T.; Peterlin, B.M. Dynamics of Human Immunodeficiency Virus Transcription: P-TEFb Phosphorylates RD and Dissociates Negative Effectors from the Transactivation Response Element. Mol. Cell. Biol. 2004, 24, 787–795. [Google Scholar] [CrossRef]
- Wu, C.H.; Yamaguchi, Y.; Benjamin, L.R.; Horvat-Gordon, M.; Washinsky, J.; Enerly, E.; Larsson, J.; Lambertsson, A.; Handa, H.; Gilmour, D. NELF and DSIF cause promoter proximal pausing on the hsp70 promoter in Drosophila. Genes Dev. 2003, 17, 1402–1414. [Google Scholar] [CrossRef]
- Yamada, T.; Yamaguchi, Y.; Inukai, N.; Okamoto, S.; Mura, T.; Handa, H. P-TEFb-Mediated Phosphorylation of hSpt5 C-Terminal Repeats Is Critical for Processive Transcription Elongation. Mol. Cell 2006, 21, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Paik, E.J.; Zon, L.I. Hematopoietic development in the zebrafish. Int. J. Dev. Biol. 2010, 54, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Detrich, H.W.; Kieran, M.W.; Chan, F.Y.; Barone, L.M.; Yee, K.; Rundstadler, J.A.; Pratt, S.; Ransom, D.; Zon, L.I. Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl. Acad. Sci. USA 1995, 92, 10713–10717. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Zon, L.I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef]
- Hsia, N.; Zon, L.I. Transcriptional regulation of hematopoietic stem cell development in zebrafish. Exp. Hematol. 2005, 33, 1007–1014. [Google Scholar] [CrossRef]
- Jin, H.; Li, L.; Xu, J.; Zhen, F.; Zhu, L.; Liu, P.P.; Zhang, M.; Zhang, W.; Wen, Z. Runx1 regulates embryonic myeloid fate choice in zebrafish through a negative feedback loop inhibiting Pu.1 expression. Blood 2012, 119, 5239–5249. [Google Scholar] [CrossRef]
- Warga, R.M.; Kane, D.A.; Ho, R.K. Fate Mapping Embryonic Blood in Zebrafish: Multi- and Unipotential Lineages Are Segregated at Gastrulation. Dev. Cell 2009, 16, 744–755. [Google Scholar] [CrossRef]
- Narita, T.; Yamaguchi, Y.; Yano, K.; Sugimoto, S.; Chanarat, S.; Wada, T.; Kim, D.-K.; Hasegawa, J.; Omori, M.; Inukai, N.; et al. Human Transcription Elongation Factor NELF: Identification of Novel Subunits and Reconstitution of the Functionally Active Complex. Mol. Cell. Biol. 2003, 23, 1863–1873. [Google Scholar] [CrossRef]
- Herbomel, P.; Thisse, B.; Thisse, C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 1999, 126, 3735–3745. [Google Scholar] [CrossRef]
- Mosimann, C.; Panakova, D.; Werdich, A.A.; Musso, G.; Burger, A.; Lawson, K.; Carr, L.A.; Nevis, K.R.; Sabeh, M.K.; Zhou, Y.; et al. Chamber identity programs drive early functional partitioning of the heart. Nat. Commun. 2015, 6, 8146. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Oates, A.C.; Crowhurst, M.O.; Ward, A.C.; Layton, J.E. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 2001, 98, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, K.; Chi, Y.; Jin, H.; Li, L.; Zhang, W.; Xu, J.; Zhang, Y. Runx1 regulates zebrafish neutrophil maturation via synergistic interaction with c-Myb. J. Biol. Chem. 2021, 296, 100272. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, X.; Zhou, T.; Cook, J.; Nguyen, K.; Bai, X. RNA polymerase II pausing modulates hematopoietic stem cell emergence in zebrafish. Blood 2016, 128, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; D’Orso, I. CDK9: A signaling hub for transcriptional control. Transcription 2019, 10, 57–75. [Google Scholar] [CrossRef]
- Bai, X.; Kim, J.; Yang, Z.; Jurynec, M.J.; Akie, T.E.; Lee, J.; LeBlanc, J.; Sessa, A.; Jiang, H.; DiBiase, A.; et al. TIF1γ Controls Erythroid Cell Fate by Regulating Transcription Elongation. Cell 2010, 142, 133–143. [Google Scholar] [CrossRef]
- Rieger, M.A.; Schroeder, T. Hematopoiesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008250. [Google Scholar] [CrossRef]
- Rhodes, J.; Hagen, A.; Hsu, K.; Deng, M.; Liu, T.X.; Look, A.; Kanki, J.P. Interplay of Pu.1 and Gata1 Determines Myelo-Erythroid Progenitor Cell Fate in Zebrafish. Dev. Cell 2005, 8, 97–108. [Google Scholar] [CrossRef]
- Core, L.; Adelman, K. Promoter-proximal pausing of RNA polymerase II: A nexus of gene regulation. Genes Dev. 2019, 33, 960–982. [Google Scholar] [CrossRef]
- Gilchrist, D.A.; Fromm, G.; dos Santos, G.; Pham, L.N.; McDaniel, I.E.; Burkholder, A.; Fargo, D.C.; Adelman, K. Regulating the regulators: The pervasive effects of Pol II pausing on stimulus-responsive gene networks. Genes Dev. 2012, 26, 933–944. [Google Scholar] [CrossRef]
- Amleh, A.; Nair, S.J.; Sun, J.; Sutherland, A.; Hasty, P.; Li, R. Mouse Cofactor of BRCA1 (Cobra1) Is Required for Early Embryogenesis. PLoS ONE 2009, 4, e5034. [Google Scholar] [CrossRef]
- Dzierzak, E.; Philipsen, S. Erythropoiesis: Development and Differentiation. Cold Spring Harb. Perspect. Med. 2013, 3, a011601. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Loeb, D.; Soede-Bobok, A.; Touw, I.; Friedman, A. Regulation of granulopoiesis by transcription factors and cytokine signals. Leukemia 2000, 14, 973–990. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Riffelmacher, T.; Clarke, A.; Richter, F.C.; Stranks, A.; Pandey, S.; Danielli, S.; Hublitz, P.; Yu, Z.; Johnson, E.; Schwerd, T.; et al. Autophagy-Dependent Generation of Free Fatty Acids Is Critical for Normal Neutrophil Differentiation. Immunity 2017, 47, 466–480.e5. [Google Scholar] [CrossRef]
- Wilkinson, D.G. In situ hybridization: A practical approach. Methods Cell Biol. 1992, 35, 259–262. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Chen, W.; Lian, F.; Lang, L.; Huang, Y.; Xu, Y.; Zhang, N.; Chen, Y.; Liu, M.; et al. Pharmacological inhibition of dihydroorotate dehydrogenase induces apoptosis and differentiation in acute myeloid leukemia cells. Haematologica 2018, 103, 1472–1483. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, T.; Jia, D.; Han, Y.; Liu, F.; Huang, Y.; Qu, Z.; Zhao, Y.; Tu, J.; Lv, Y.; et al. BCAS2 is essential for hematopoietic stem and progenitor cell maintenance during zebrafish embryogenesis. Blood 2019, 133, 805–815. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Ahmed, A.; Wang, W.; Wang, X.; Ma, C.; Jiang, H.; Li, W.; Jing, L. Negative Elongation Factor (NELF) Inhibits Premature Granulocytic Development in Zebrafish. Int. J. Mol. Sci. 2022, 23, 3833. https://doi.org/10.3390/ijms23073833
Huang M, Ahmed A, Wang W, Wang X, Ma C, Jiang H, Li W, Jing L. Negative Elongation Factor (NELF) Inhibits Premature Granulocytic Development in Zebrafish. International Journal of Molecular Sciences. 2022; 23(7):3833. https://doi.org/10.3390/ijms23073833
Chicago/Turabian StyleHuang, Mengling, Abrar Ahmed, Wei Wang, Xue Wang, Cui Ma, Haowei Jiang, Wei Li, and Lili Jing. 2022. "Negative Elongation Factor (NELF) Inhibits Premature Granulocytic Development in Zebrafish" International Journal of Molecular Sciences 23, no. 7: 3833. https://doi.org/10.3390/ijms23073833
APA StyleHuang, M., Ahmed, A., Wang, W., Wang, X., Ma, C., Jiang, H., Li, W., & Jing, L. (2022). Negative Elongation Factor (NELF) Inhibits Premature Granulocytic Development in Zebrafish. International Journal of Molecular Sciences, 23(7), 3833. https://doi.org/10.3390/ijms23073833





