Integrated OMICs Approach for the Group 1 Protease Mite-Allergen of House Dust Mite Dermatophagoides microceras
Abstract
1. Introduction
2. Results
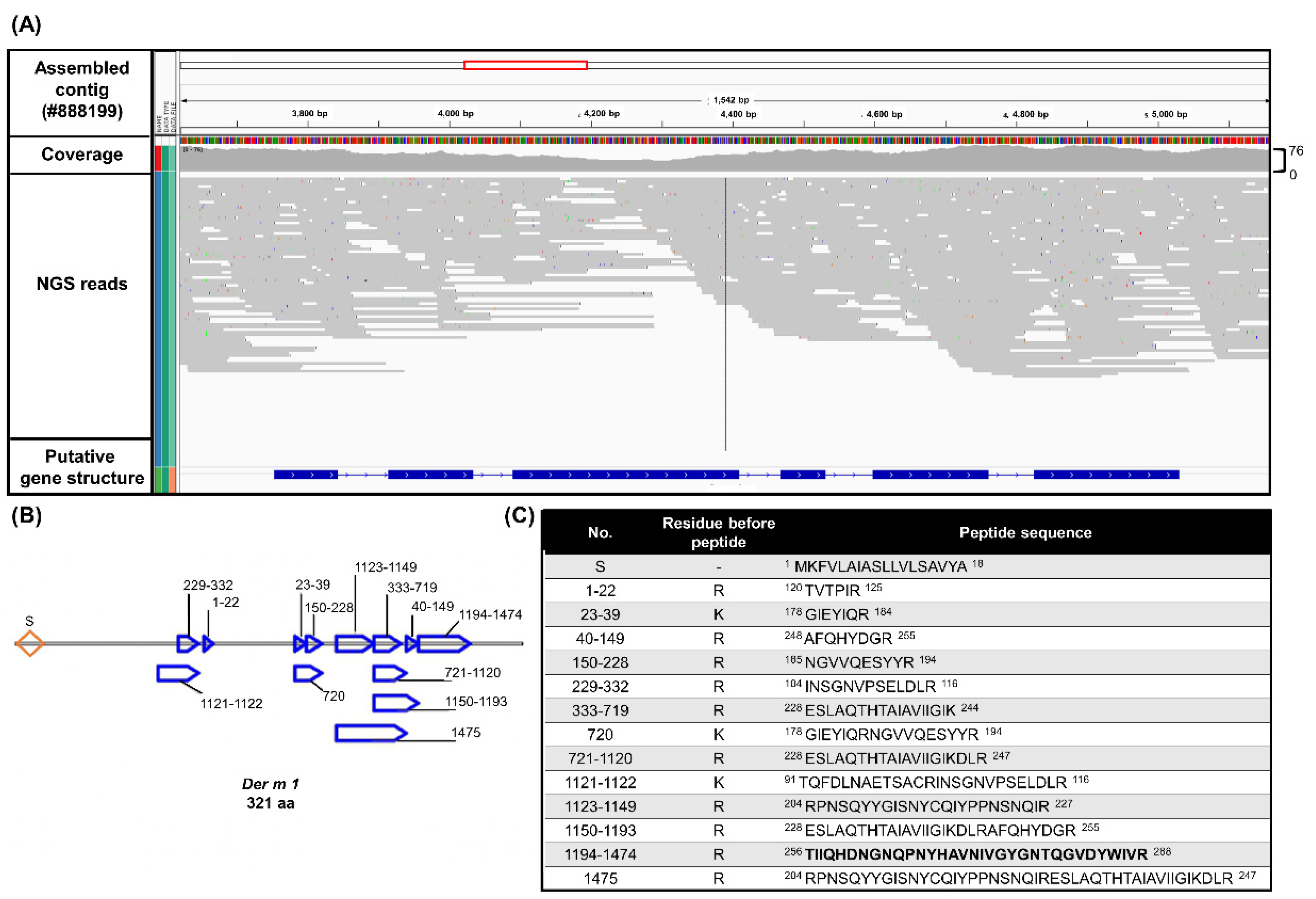
2.1. Integrated OMICs Analysis of the Potential Der m 1 HDMs Major Allergens from D. microceras
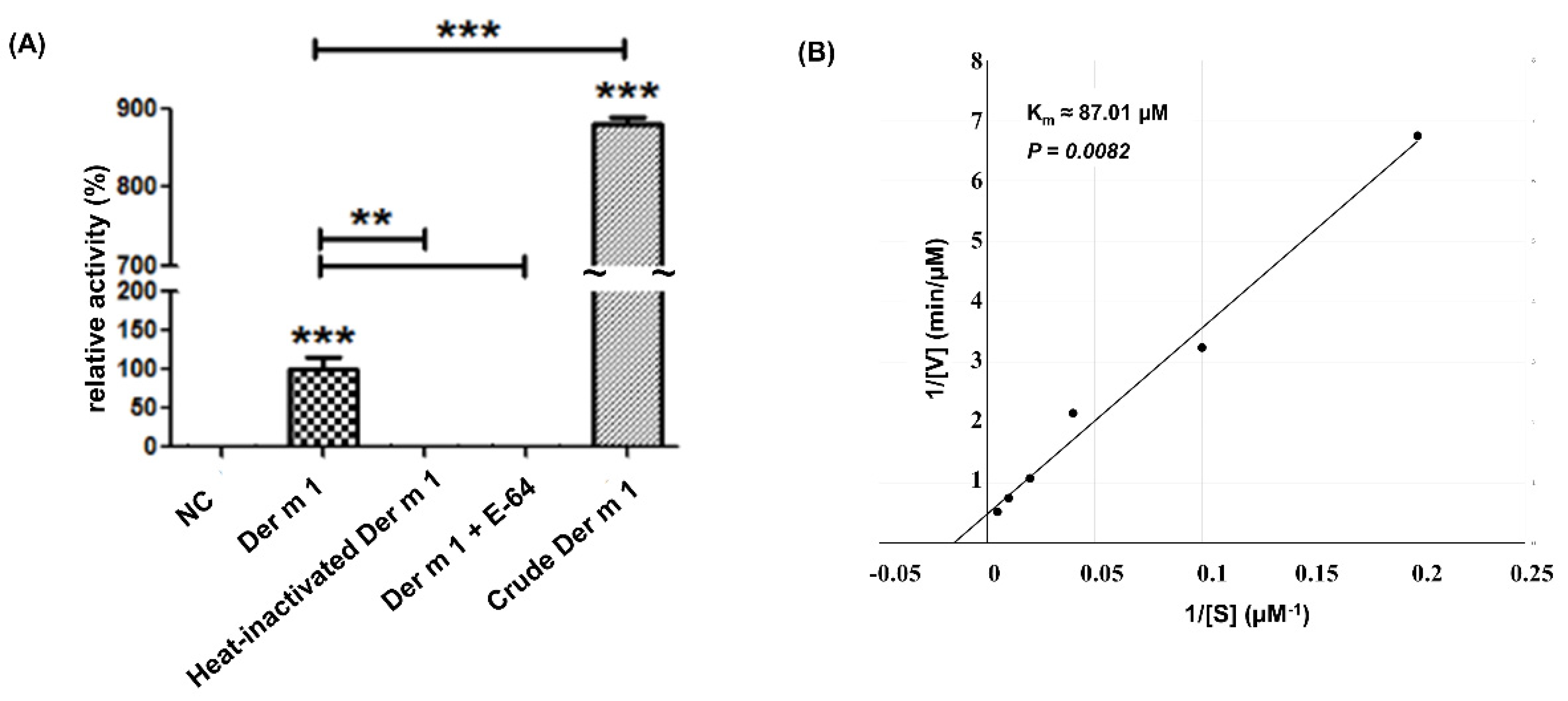
2.2. Cysteine Protease Activity of Recombinant Truncated Der m 1 Allergen
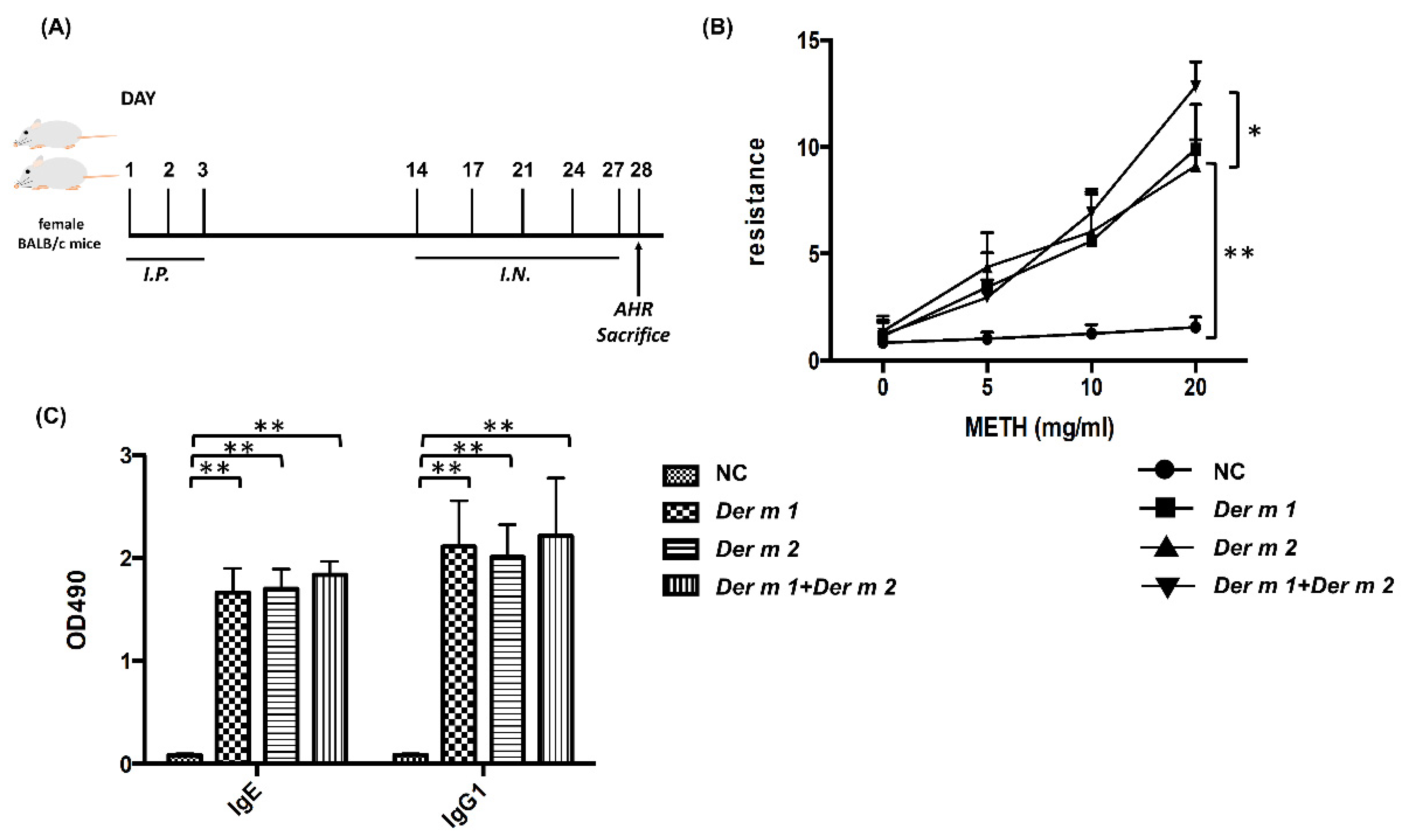
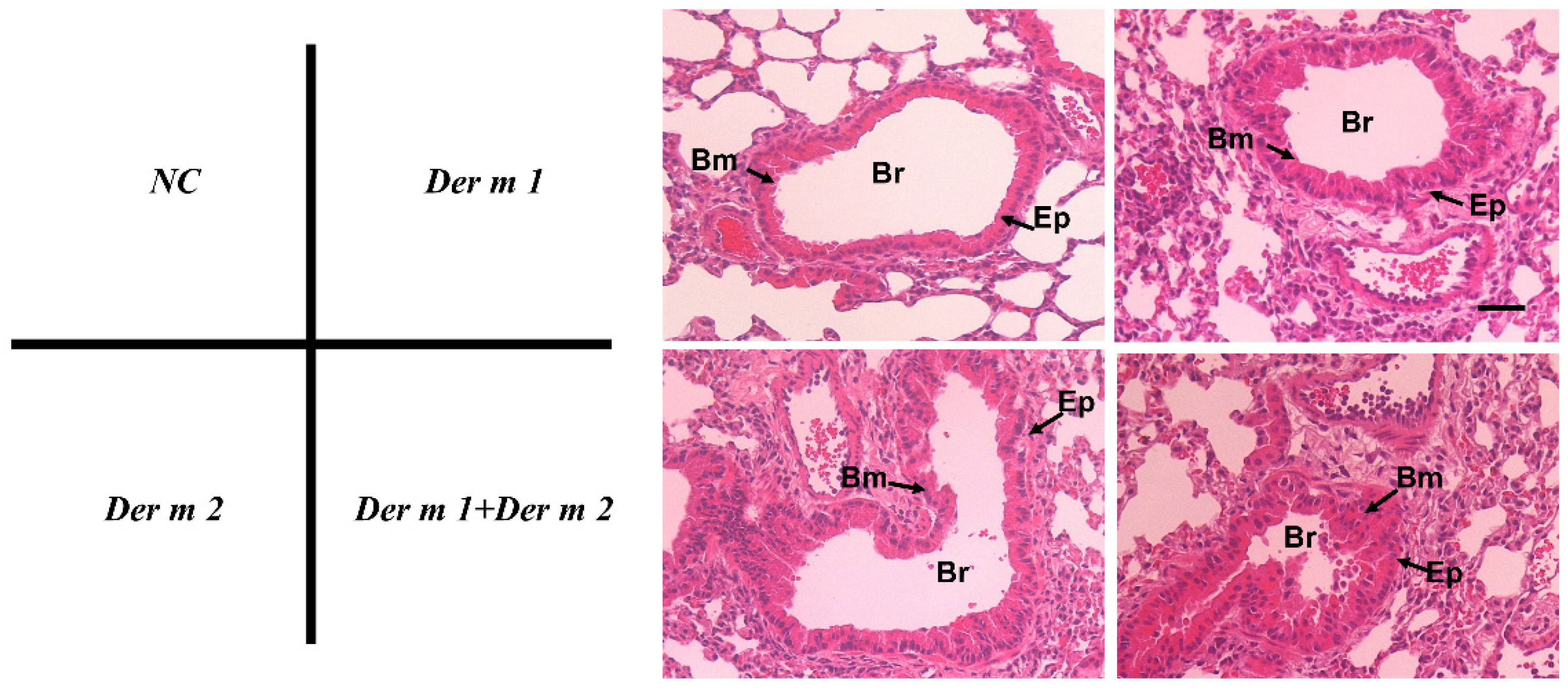
2.3. Pathophysiological Features of Asthma Using Recombinant Der m 1 Allergen-Sensitized Mice Models
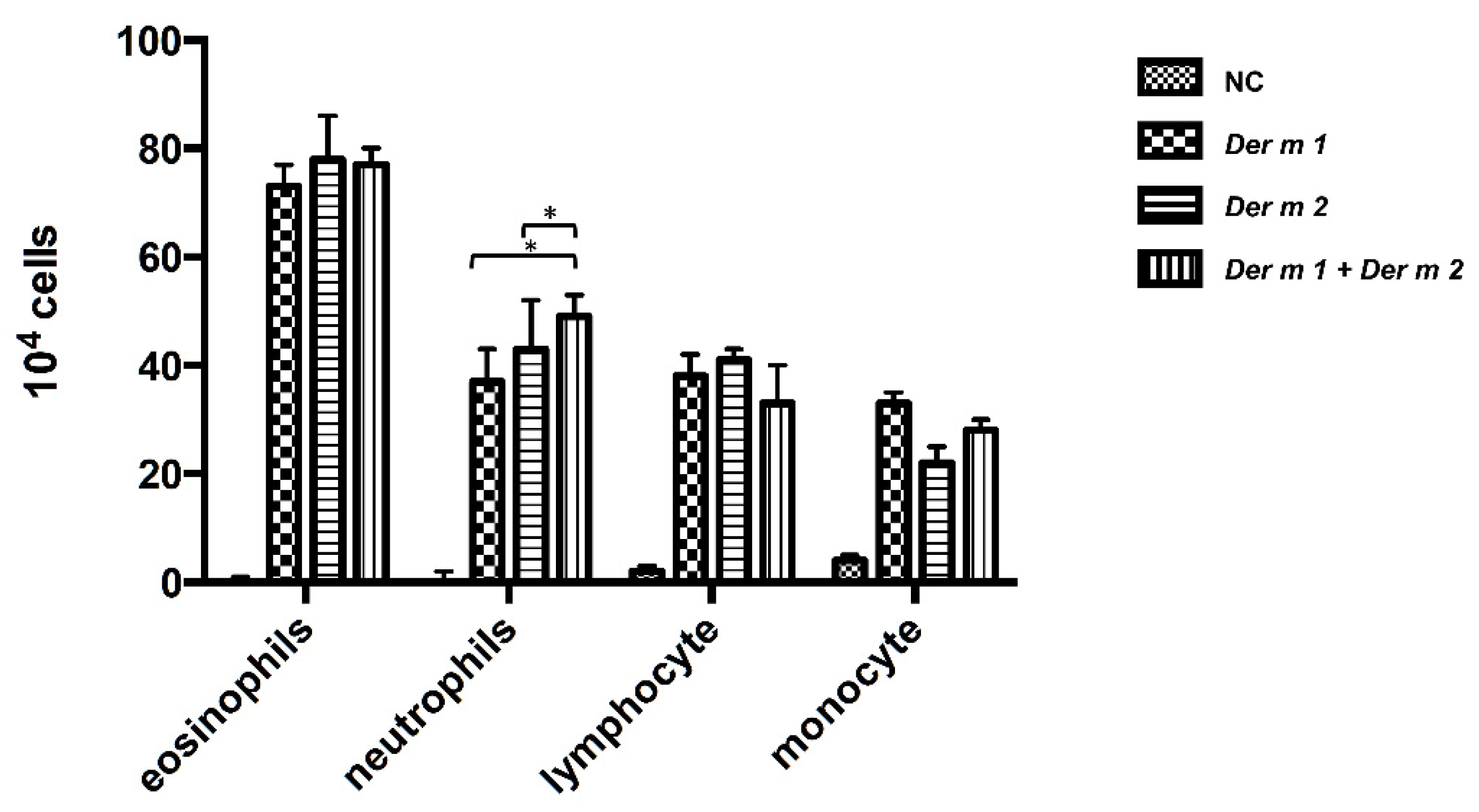
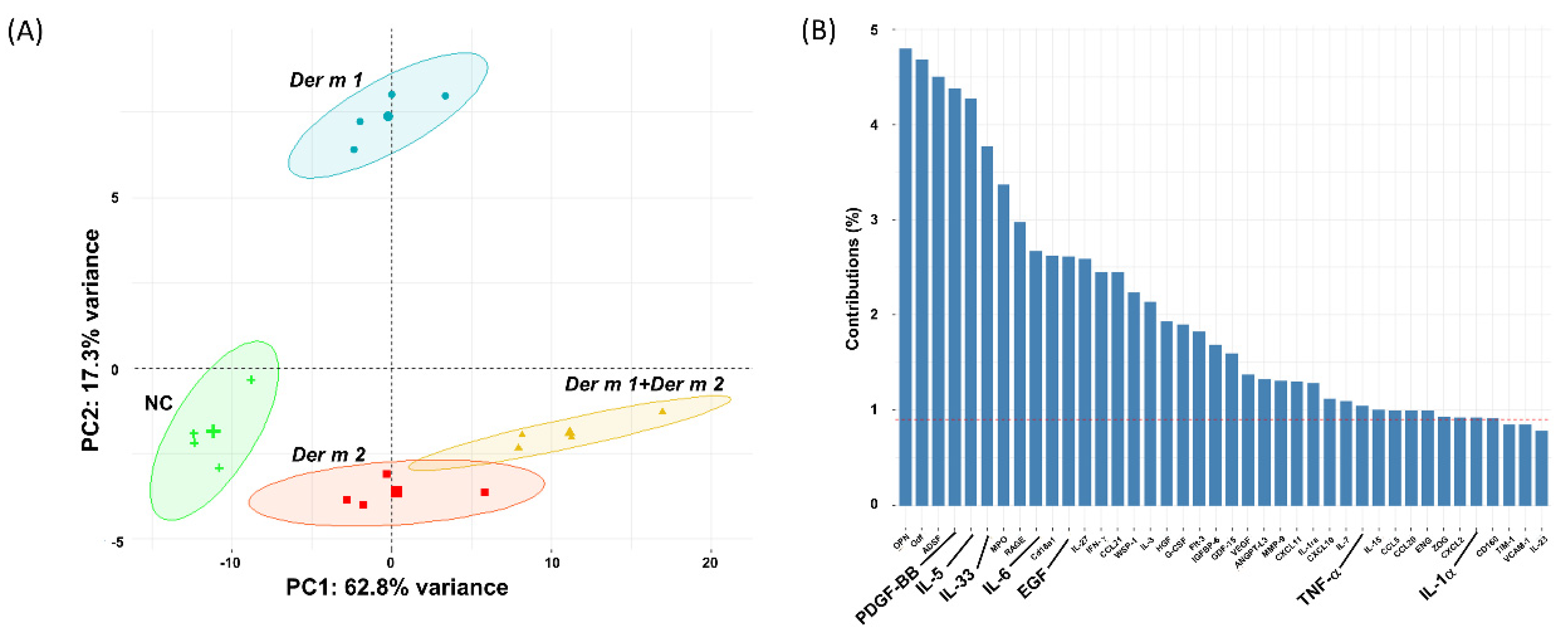
2.4. Synergistic Effect in the BALF of Allergen-Sensitized Mice Models with Der m 1 + Der m 2 Combined Inhalation
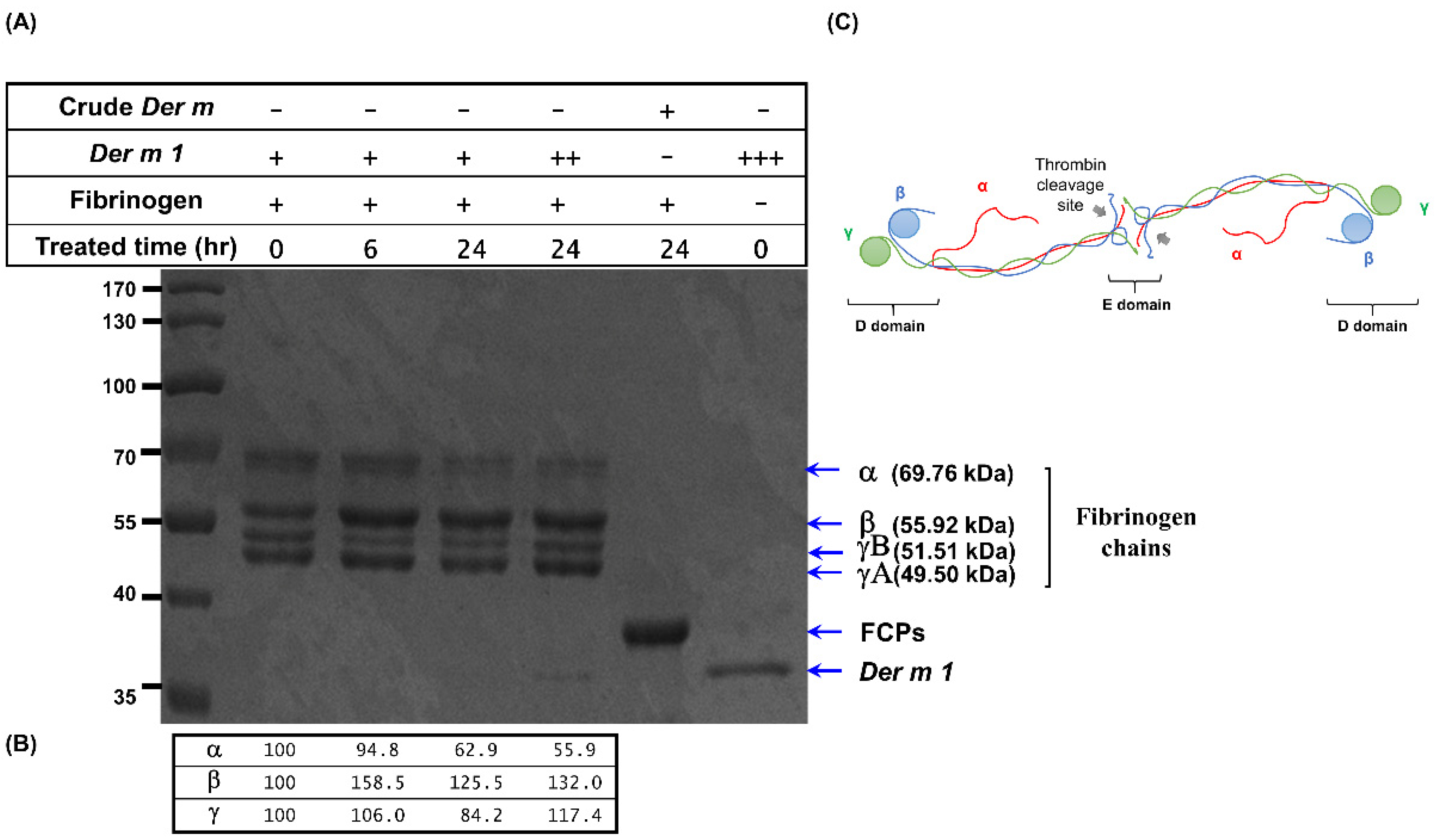
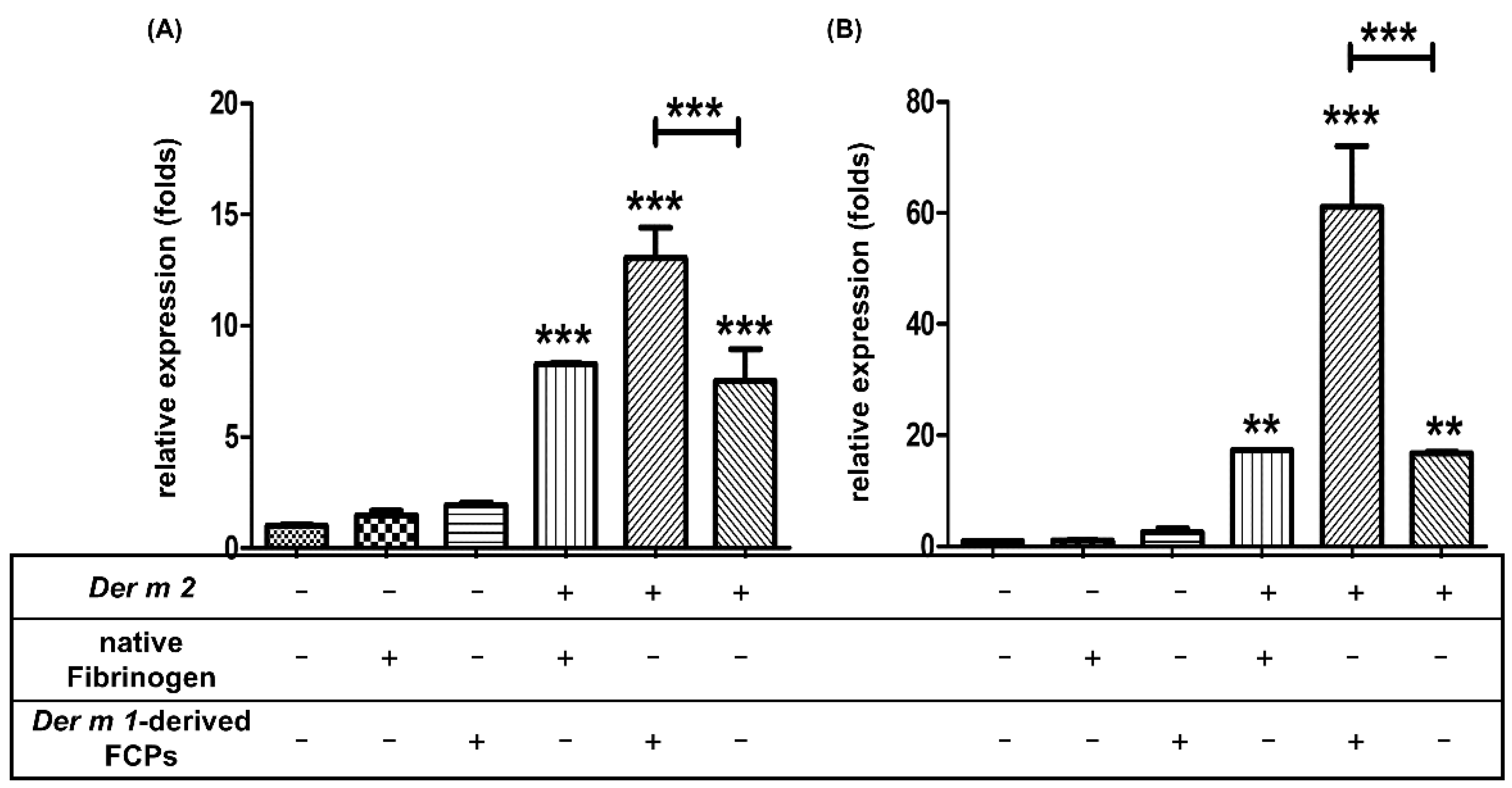
2.5. Der m 1 Is a Cysteine Proteinase That Digested the α Chain of Fibrinogen, and the Combination of Fibrinogen Cleavage Products Could Induce the Expression of Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Next-Generation Sequencing and De Novo Assembly
4.3. Expression and Purification of Recombinant Proteins
4.4. Cysteine Protease Activity and Fibrinogen Cleavage
4.5. Cell Culture
4.6. qPCR (Real-Time PCR)
4.7. Mice
4.7.1. Protocol for Mice Allergen Immunization and Challenge
4.7.2. Airway Hyper-Responsiveness and Bronchoalveolar Lavage Fluid Collection
4.7.3. The Specific Antibodies in Mice Serum
4.7.4. Mouse Cytokine Array
4.7.5. Histograms
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Disease, G.B.D.; Injury, I. Prevalence C: Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar]
- Andiappan, A.K.; Puan, K.J.; Lee, B.; Nardin, A.; Poidinger, M.; Connolly, J.; Chew, F.T.; Wang, D.Y. Rotzschke O: Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy 2014, 69, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Lue, K.H.; Wong, R.H.; Sun, H.L.; Sheu, J.N.; Lu, K.H. Distribution of allergens in children with different atopic disorders in central Taiwan. Acta Paediatr. Taiwan 2006, 47, 127–134. [Google Scholar] [PubMed]
- Pan, H.H.; Hsiao, Y.P.; Chen, P.J.; Kang, Y.T.; Chao, Y.H.; Sheu, J.N.; Lue, K.H.; Ko, J.L. Epithelial growth factor receptor tyrosine kinase inhibitors alleviate house dust mite allergen Der p2-induced IL-6 and IL-8. Environ. Toxicol. 2019, 34, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, Q.; Chen, F.; Chen, D.; Xu, G.; Li, H. Induction of IL-6 and IL-8 by house dust mite allergen Der p1 in cultured human nasal epithelial cells is associated with PAR/PI3K/NFkappaB signaling. ORL J. Otorhinolaryngol. Relat. Spec. 2010, 72, 256–265. [Google Scholar] [CrossRef]
- Douwes, J.; Gibson, P.; Pekkanen, J.; Pearce, N. Non-eosinophilic asthma: Importance and possible mechanisms. Thorax 2002, 57, 643–648. [Google Scholar] [CrossRef]
- Jatakanon, A.; Uasuf, C.; Maziak, W.; Lim, S.; Chung, K.F.; Barnes, P.J. Neutrophilic inflammation in severe persistent asthma. Am. J. Respir. Crit. Care Med. 1999, 160 Pt 1, 1532–1539. [Google Scholar] [CrossRef]
- Kikuchi, S.; Nagata, M.; Kikuchi, I.; Hagiwara, K.; Kanazawa, M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int. Arch. Allergy Immunol. 2005, 137 (Suppl. S1), 7–11. [Google Scholar] [CrossRef]
- Duvoix, A.; Dickens, J.; Haq, I.; Mannino, D.; Miller, B.; Tal-Singer, R.; Lomas, D.A. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax 2013, 68, 670–676. [Google Scholar] [CrossRef]
- Wagers, S.S.; Norton, R.J.; Rinaldi, L.M.; Bates, J.H.; Sobel, B.E.; Irvin, C.G. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J. Clin. Investig. 2004, 114, 104–111. [Google Scholar] [CrossRef]
- Cho, M.; Lee, J.E.; Lim, H.; Shin, H.W.; Khalmuratova, R.; Choi, G.; Kim, H.S.; Choi, W.S.; Park, Y.J.; Shim, I.; et al. Fibrinogen cleavage products and Toll-like receptor 4 promote the generation of programmed cell death 1 ligand 2-positive dendritic cells in allergic asthma. J. Allergy Clin. Immunol. 2018, 142, 530–541.e536. [Google Scholar] [CrossRef] [PubMed]
- Millien, V.O.; Lu, W.; Shaw, J.; Yuan, X.; Mak, G.; Roberts, L.; Song, L.Z.; Knight, J.M.; Creighton, C.J.; Luong, A.; et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 2013, 341, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Landers, C.T.; Tung, H.Y.; Knight, J.M.; Madison, M.C.; Wu, Y.; Zeng, Z.; Porter, P.C.; Rodriguez, A.; Flick, M.J.; Kheradmand, F.; et al. Selective cleavage of fibrinogen by diverse proteinases initiates innate allergic and antifungal immunity through CD11b. J. Biol. Chem. 2019, 294, 8834–8847. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Takeda, H.; Tokura, T.; Suzuki, M.; Inui, K.; Hara, M.; Matsuda, H.; Matsuda, A.; Oboki, K.; Ohno, T.; et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J. Immunol. 2013, 190, 4489–4499. [Google Scholar] [CrossRef]
- Hu, R.H.; Wu, C.T.; Wu, T.S.; Yu, F.Y.; Ko, J.L.; Lue, K.H.; Liu, Y.F. Systematic Characterization of the Group 2 House Dust Mite Allergen in Dermatophagoides microceras. Front. Cell. Infect. Microbiol. 2022, 11, 793559. [Google Scholar] [CrossRef]
- Ichikawa, S.; Takai, T.; Yashiki, T.; Takahashi, S.; Okumura, K.; Ogawa, H.; Kohda, D.; Hatanaka, H. Lipopolysaccharide binding of the mite allergen Der f 2. Genes Cells 2009, 14, 1055–1065. [Google Scholar] [CrossRef]
- Reginald, K.; Chew, F.T. The major allergen Der p 2 is a cholesterol binding protein. Sci. Rep. 2019, 9, 1556. [Google Scholar] [CrossRef]
- Chua, K.Y.; Stewart, G.A.; Thomas, W.R.; Simpson, R.J.; Dilworth, R.J.; Plozza, T.M.; Turner, K.J. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J. Exp. Med. 1988, 167, 175–182. [Google Scholar] [CrossRef]
- Asokananthan, N.; Graham, P.T.; Stewart, D.J.; Bakker, A.J.; Eidne, K.A.; Thompson, P.J.; Stewart, G.A. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: The cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J. Immunol. 2002, 169, 4572–4578. [Google Scholar] [CrossRef]
- Takai, T.; Kato, T.; Sakata, Y.; Yasueda, H.; Izuhara, K.; Okumura, K.; Ogawa, H. Recombinant Der p 1 and Der f 1 exhibit cysteine protease activity but no serine protease activity. Biochem. Biophys. Res. Commun. 2005, 328, 944–952. [Google Scholar] [CrossRef]
- Walker, P.P.; Hadcroft, J.; Costello, R.W.; Calverley, P.M. Lung function changes following methacholine inhalation in COPD. Respir. Med. 2009, 103, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Brannan, J.D.; Lougheed, M.D. Airway hyperresponsiveness in asthma: Mechanisms, clinical significance, and treatment. Front. Physiol. 2012, 3, 460. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C.; Gressler, A.E.; Schubert, K.; Schulze, B.; Muller, U.; Brombacher, F.; von Bergen, M.; Alber, G. Identification of T helper (Th)1- and Th2-associated antigens of Cryptococcus neoformans in a murine model of pulmonary infection. Sci. Rep. 2018, 8, 2681. [Google Scholar] [CrossRef] [PubMed]
- de Boer, J.D.; Majoor, C.J.; van’t Veer, C.; Bel, E.H.; van der Poll, T. Asthma and coagulation. Blood 2012, 119, 3236–3244. [Google Scholar] [CrossRef]
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef]
- Winter, M.C.; Shasby, S.S.; Ries, D.R.; Shasby, D.M. PAR2 activation interrupts E-cadherin adhesion and compromises the airway epithelial barrier: Protective effect of beta-agonists. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L628–L635. [Google Scholar] [CrossRef]
- Deb, R.; Shakib, F.; Reid, K.; Clark, H. Major house dust mite allergens Dermatophagoides pteronyssinus 1 and Dermatophagoides farinae 1 degrade and inactivate lung surfactant proteins A and D. J. Biol. Chem. 2007, 282, 36808–36819. [Google Scholar] [CrossRef]
- Florsheim, E.; Yu, S.; Bragatto, I.; Faustino, L.; Gomes, E.; Ramos, R.N.; Barbuto, J.A.; Medzhitov, R.; Russo, M. Integrated innate mechanisms involved in airway allergic inflammation to the serine protease subtilisin. J. Immunol. 2015, 194, 4621–4630. [Google Scholar] [CrossRef]
- Hirota, J.A.; Ask, K.; Farkas, L.; Smith, J.A.; Ellis, R.; Rodriguez-Lecompte, J.C.; Kolb, M.; Inman, M.D. In vivo role of platelet-derived growth factor-BB in airway smooth muscle proliferation in mouse lung. Am. J. Respir. Cell Mol. Biol. 2011, 45, 566–572. [Google Scholar] [CrossRef]
- Kardas, G.; Daszynska-Kardas, A.; Marynowski, M.; Brzakalska, O.; Kuna, P.; Panek, M. Role of Platelet-Derived Growth Factor (PDGF) in Asthma as an Immunoregulatory Factor Mediating Airway Remodeling and Possible Pharmacological Target. Front. Pharmacol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Balenga, N.A.; Klichinsky, M.; Xie, Z.; Chan, E.C.; Zhao, M.; Jude, J.; Laviolette, M.; Panettieri, R.A., Jr.; Druey, K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015, 6, 6763. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Cheng, C.C.; Su, H.H.; Huang, N.C.; Chen, J.J.; Kang, H.Y.; Chang, T.H. Lipopolysaccharide Attenuates Induction of Proallergic Cytokines, Thymic Stromal Lymphopoietin, and Interleukin 33 in Respiratory Epithelial Cells Stimulated with PolyI:C and Human Parechovirus. Front. Immunol. 2016, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H.; Brightling, C.E.; Woltmann, G.; Parker, D.; Wardlaw, A.J.; Pavord, I.D. Analysis of induced sputum in adults with asthma: Identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002, 57, 875–879. [Google Scholar] [CrossRef]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563.e1555. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Park, J.; Crews, A.L.; Li, Y.; Adler, K.B. Protease-activated receptor-2 (PAR-2) is a weak enhancer of mucin secretion by human bronchial epithelial cells in vitro. Int. J. Biochem. Cell Biol. 2008, 40, 1379–1388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.C.; Ho, Y.N.; Hu, R.H.; Yen, Y.T.; Wang, Z.C.; Lee, Y.C.; Hsu, Y.H.; Meng, M. The interaction between bamboo mosaic virus replication protein and coat protein is critical for virus movement in plant hosts. J. Virol. 2011, 85, 12022–12031. [Google Scholar] [CrossRef]
- Wu, T.S.; Lin, Y.T.; Huang, Y.T.; Cheng, Y.C.; Yu, F.Y.; Liu, B.H. Disruption of liver development and coagulation pathway by ochratoxin A in embryonic zebrafish. Toxicol. Appl. Pharmacol. 2018, 340, 1–8. [Google Scholar] [CrossRef]
- Chu, P.Y.; Sun, H.L.; Ko, J.L.; Ku, M.S.; Lin, L.J.; Lee, Y.T.; Liao, P.F.; Pan, H.H.; Lu, H.L.; Lue, K.H. Oral fungal immunomodulatory protein-Flammulina velutipes has influence on pulmonary inflammatory process and potential treatment for allergic airway disease: A mouse model. J. Microbiol. Immunol. Infect. 2017, 50, 297–306. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lee, S.S.; Sun, H.L.; Lu, K.H.; Ku, M.S.; Sheu, J.N.; Ko, J.L.; Lue, K.H. Effect of the fungal immunomodulatory protein FIP-fve on airway inflammation and cytokine production in mouse asthma model. Cytokine 2013, 61, 237–244. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.-H.; Cheng, C.-W.; Wu, C.-T.; Ko, J.-L.; Lue, K.-H.; Liu, Y.-F. Integrated OMICs Approach for the Group 1 Protease Mite-Allergen of House Dust Mite Dermatophagoides microceras. Int. J. Mol. Sci. 2022, 23, 3810. https://doi.org/10.3390/ijms23073810
Hu R-H, Cheng C-W, Wu C-T, Ko J-L, Lue K-H, Liu Y-F. Integrated OMICs Approach for the Group 1 Protease Mite-Allergen of House Dust Mite Dermatophagoides microceras. International Journal of Molecular Sciences. 2022; 23(7):3810. https://doi.org/10.3390/ijms23073810
Chicago/Turabian StyleHu, Rei-Hsing, Chun-Wen Cheng, Chia-Ta Wu, Jiunn-Liang Ko, Ko-Huang Lue, and Yu-Fan Liu. 2022. "Integrated OMICs Approach for the Group 1 Protease Mite-Allergen of House Dust Mite Dermatophagoides microceras" International Journal of Molecular Sciences 23, no. 7: 3810. https://doi.org/10.3390/ijms23073810
APA StyleHu, R.-H., Cheng, C.-W., Wu, C.-T., Ko, J.-L., Lue, K.-H., & Liu, Y.-F. (2022). Integrated OMICs Approach for the Group 1 Protease Mite-Allergen of House Dust Mite Dermatophagoides microceras. International Journal of Molecular Sciences, 23(7), 3810. https://doi.org/10.3390/ijms23073810






