Enrofloxacin—The Ruthless Killer of Eukaryotic Cells or the Last Hope in the Fight against Bacterial Infections?
Abstract
1. Introduction
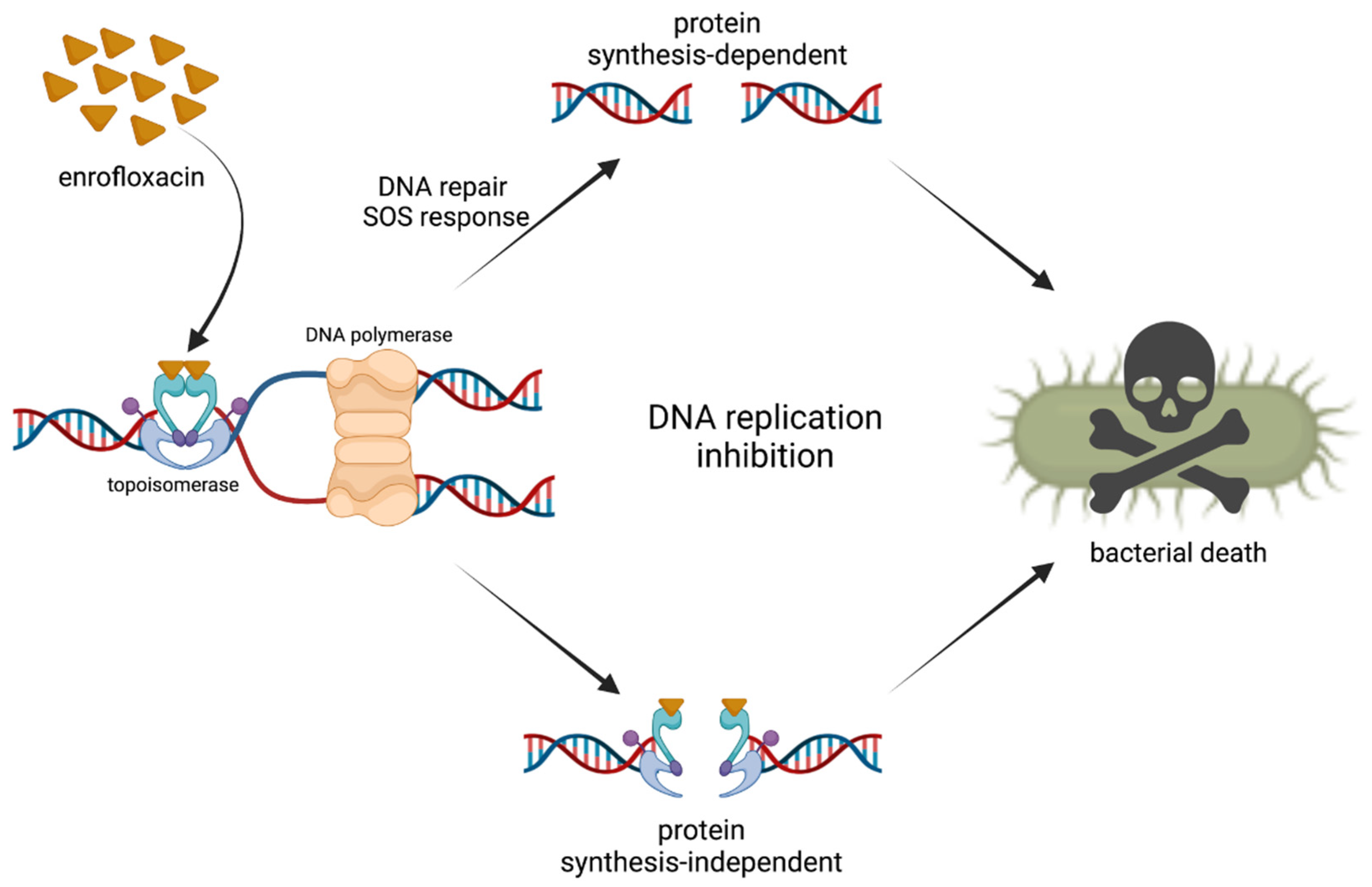
2. Mechanism of Action
3. Pharmacokinetics of Enrofloxacin
3.1. Absorption
3.2. Distribution
3.3. Metabolism
3.4. Elimination
4. Efficacy of Enrofloxacin in Veterinary Medicine
5. Mechanisms of Resistance to Enrofloxacin
6. The Safety of Enrofloxacin Use
6.1. Adverse Effects in Farm Animals
6.1.1. Skeletal System
6.1.2. Reproductive System
6.1.3. Retinopathies
6.1.4. Hepatotoxicity
6.1.5. Immune System
6.2. Other Adverse Effects in Veterinary Medicine
6.3. Environmental Adverse Effects
7. Interactions with Metal Ions
7.1. Effects on Microbial Populations
7.2. Use of Metal–Enrofloxacin Interactions in Laboratory Diagnostics
7.3. Exploitation of Metal–Enrofloxacin Interaction in Antibiotic Degradation
7.4. Translational Implications of Metal–Enrofloxacin Interactions
8. Concluding Remarks and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfson, J.S.; Hooper, D.C. The Fluoroquinolones: Structures, Mechanisms of Action and Resistance, and Spectra of Activity in Vitro. Antimicrob. Agents Chemother. 1985, 28, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Altreuhter, A. Data on chemistry and toxicology of Baytril. Vet.-Med. Nachr. 1987, 2, 87–89. [Google Scholar]
- Trouchon, T.; Lefebvere, A. A Review of Enrofloxacin for Veterinary Use. Open J. Vet. Med. 2016, 6, 40–58. [Google Scholar] [CrossRef]
- European Medicines Agency. Opinion Following Article 35 Referral Baytril 2.5% Injectable, Baytril 5% Injectable, Baytril 10% Injectable and Associated Names, and Related Veterinary Medicinal Products. 2014, EMEA/V/A/097. Available online: https://www.ema.europa.eu/en/documents/referral/opinion-following-article-35-referral-baytril-25-injectable-baytril-5-injectable-baytril-10_en.pdf (accessed on 25 March 2022).
- PubChem Enrofloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/71188 (accessed on 22 February 2022).
- Vancutsem, P.M.; Babish, J.G.; Schwark, W.S. The Fluoroquinolone Antimicrobials: Structure, Antimicrobial Activity, Pharmacokinetics, Clinical Use in Domestic Animals and Toxicity. Cornell Vet. 1990, 80, 173–186. [Google Scholar]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Wang, J.C. DNA Topoisomerases. Annu. Rev. Biochem. 1996, 65, 635–692. [Google Scholar] [CrossRef]
- Tretter, E.M.; Berger, J.M. Mechanisms for Defining Supercoiling Set Point of DNA Gyrase Orthologs. J. Biol. Chem. 2012, 287, 18645–18654. [Google Scholar] [CrossRef]
- Hawkey, P.M. Mechanisms of Quinolone Action and Microbial Response. J. Antimicrob. Chemother. 2003, 51, 29–35. [Google Scholar] [CrossRef]
- Vélez-Cruz, R.; Osheroff, N. DNA Topoisomerases: Type II. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 806–811. ISBN 978-0-12-443710-4. [Google Scholar]
- Zhao, X.; Xu, C.; Domagala, J.; Drlica, K. DNA Topoisomerase Targets of the Fluoroquinolones: A Strategy for Avoiding Bacterial Resistance. Proc. Natl. Acad. Sci. USA 1997, 94, 13991–13996. [Google Scholar] [CrossRef]
- Chen, C.R.; Malik, M.; Snyder, M.; Drlica, K. DNA Gyrase and Topoisomerase IV on the Bacterial Chromosome: Quinolone-Induced DNA Cleavage. J. Biol. Mol. 1996, 258, 627–737. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of Drug Resistance: Quinolone Resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Malik, M.; Drlica, K. Contribution of Reactive Oxygen Species to Pathways of Quinolone-Mediated Bacterial Cell Death. J. Antimicrob. Chemother. 2010, 65, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.S.; Fisher, L.M. Targeting of DNA Gyrase in Streptococcus Pneumoniae by Sparfloxacin: Selective Targeting of Gyrase or Topoisomerase IV by Quinolones. Antimicrob. Agents Chemother. 1997, 41, 471–474. [Google Scholar] [CrossRef]
- Nikaido, H. Antibiotic Resistance Caused by Gram-Negative Multidrug Efflux Pumps. Clin. Infect. Dis. 1998, 27, 32–41. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How Antibiotics Kill Bacteria: From Targets to Networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- López-Cadenas, C.; Sierra-Vega, M.; García-Vieitez, J.J.; Diez-Liébana, M.J.; Sahagún-Prieto, A.; Fernández-Martínez, N. Enrofloxacin: Pharmacokinetics and Metabolism in Domestic Animal Species. Curr. Drug Metab. 2013, 14, 1042–1058. [Google Scholar] [CrossRef]
- Helmick, K.E.; Papich, M.G.; Vliet, K.A.; Bennett, R.A.; Jacobson, E.R. Pharmacokinetics of Enrofloxacin after Single-Dose Oral and Intravenous Administration in the American Alligator (Alligator Mississippiensis). J. Zoo Wildl. Med. 2004, 35, 333–340. [Google Scholar] [CrossRef]
- Nielsen, P.; Gyrd-Hansen, N. Bioavailability of Enrofloxacin after Oral Administration to Fed and Fasted Pigs. Pharmacol. Toxicol. 1997, 80, 246–250. [Google Scholar] [CrossRef]
- Ziółkowski, H.; Jaroszewski, J.J.; Maślanka, T.; Grabowski, T.; Katolik, K.; Pawęska, J.; Siemianowska, M.; Jasiecka, A.; Markiewicz, W.; Spodniewska, A. Influence of Oral Co-Administration of a Preparation Containing Calcium and Magnesium and Food on Enrofloxacin Pharmacokinetics. Res. Vet. Sci. 2014, 97, 99–104. [Google Scholar] [CrossRef]
- Sumano, L.H.; Gutierrez, O.L.; Aguilera, R.; Rosiles, M.R.; Bernard, B.M.J.; Gracia, M.J. Influence of Hard Water on the Bioavailability of Enrofloxacin in Broilers. Poult. Sci. 2004, 83, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Phani, A.R.; Prasad, R.G.S.V.; Sanganal, J.S.; Manali, N.; Gupta, R.; Rashmi, N.; Prabhakara, G.S.; Salins, C.P.; Sandeep, K.; et al. Polyvinylpyrrolidone Oral Films of Enrofloxacin: Film Characterization and Drug Release. Int. J. Pharm. 2014, 471, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, I.; Ozaltin, K.; Vargun, E.; Kucerova, L.; Suly, P.; Harea, E.; Minařík, A.; Štěpánková, K.; Lehocky, M.; Humpolícek, P.; et al. Controlled Release of Enrofloxacin by Vanillin-Crosslinked Chitosan-Polyvinyl Alcohol Blends. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112125. [Google Scholar] [CrossRef] [PubMed]
- Onetto, A.J.; Sharif, S. Drug Distribution. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Messenger, K.M.; Papich, M.G.; Blikslager, A.T. Distribution of Enrofloxacin and Its Active Metabolite, Using an in Vivo Ultrafiltration Sampling Technique after the Injection of Enrofloxacin to Pigs. J. Vet. Pharmacol. Ther. 2012, 35, 452–459. [Google Scholar] [CrossRef]
- Atef, M.; El-Banna, H.A.; Elzorba, H.Y.; Soliman, A.M. Pharmacokinetics and Tissue Residue of Enrofloxacin in Healthy, Eimeria-Infected Broiler Chickens and Those Pre-Treated with Amprolium and Toltrazuril. Int. J. Vet. Sci. Med. 2020, 8, 31–38. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, J.; Zheng, G.; Zhu, X.; Yang, Y.; Ma, L.; Zhao, C.; Li, L.; Yin, Y. Pharmacokinetics and Tissue Residues of Enrofloxacin in the Largemouth Bass (Micropterus Salmoides) after Oral Administration. J. Vet. Pharmacol. Ther. 2020, 43, 147–152. [Google Scholar] [CrossRef]
- Liang, J.P.; Li, J.; Li, J.T.; Liu, P.; Chang, Z.Q.; Nie, G.X. Accumulation and Elimination of Enrofloxacin and Its Metabolite Ciprofloxacin in the Ridgetail White Prawn Exopalaemon Carinicauda Following Medicated Feed and Bath Administration. J. Vet. Pharmacol. Ther. 2014, 37, 508–514. [Google Scholar] [CrossRef]
- Urzúa, N.; Messina, M.J.; Prieto, G.; Lüders, C.; Errecalde, C. Pharmacokinetics and Tissue Disposition of Enrofloxacin in Rainbow Trout after Different Routes of Administration. Xenobiotica 2020, 50, 1236–1241. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, C.-S.; Duan, M.-H.; Wang, H.; Song, Z.-W.; Shao, H.-T.; Ma, K.-L.; Yang, F. Pharmacokinetics and Tissue Distribution of Enrofloxacin Following Single Oral Administration in Yellow River Carp (Cyprinus Carpio Haematoperus). Front. Vet. Sci. 2022, 9, 822032. [Google Scholar] [CrossRef]
- Mitchell, M.A. Enrofloxacin. J. Exot. Pet Med. 2006, 15, 66–69. [Google Scholar] [CrossRef]
- Poapolathep, S.; Chomcheun, T.; Giorgi, M.; Jualaong, S.; Klangkaew, N.; Phaochoosak, N.; Udomkusonsri, P.; Marin, P.; Poapolathep, A. Enrofloxacin and Its Major Metabolite Ciprofloxacin in Green Sea Turtles (Chelonia Mydas): An Explorative Pharmacokinetic Study. J. Vet. Pharmacol. Ther. 2021, 44, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Poapolathep, S.; Giorgi, M.; Chaiyabutr, N.; Chokejaroenrat, C.; Klangkaew, N.; Phaochoosak, N.; Wongwaipairote, T.; Poapolathep, A. Pharmacokinetics of Enrofloxacin and Its Metabolite Ciprofloxacin in Freshwater Crocodiles (Crocodylus Siamensis) after Intravenous and Intramuscular Administration. J. Vet. Pharmacol. Ther. 2020, 43, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Agius, J.E.; Kimble, B.; Govendir, M.; Rose, K.; Pollard, C.-L.; Phalen, D.N. Pharmacokinetic Profile of Enrofloxacin and Its Metabolite Ciprofloxacin in Asian House Geckos (Hemidactylus Frenatus) after Single-Dose Oral Administration of Enrofloxacin. Vet. Anim. Sci. 2020, 9, 100116. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, J.A.; Lewbart, G.A.; Papich, M.G. Population Pharmacokinetics of Enrofloxacin and Its Metabolite Ciprofloxacin in Clinically Diseased or Injured Eastern Box Turtles (Terrapene Carolina Carolina), Yellow-Bellied Sliders (Trachemys Scripta Scripta), and River Cooters (Pseudemys Concinna). J. Vet. Pharmacol. Ther. 2020, 43, 222–230. [Google Scholar] [CrossRef]
- Eshar, D.; Wright, L.T.; McCullough, C.E.; Kukanich, B. Pharmacokinetics of Enrofloxacin and Its Metabolite Ciprofloxacin Following Single-Dose Subcutaneous Injection in Black-Tailed Prairie Dogs (Cynomys Ludovicianus). Am. J. Vet. Res. 2018, 79, 658–663. [Google Scholar] [CrossRef]
- Phillips, B.E.; Harms, C.A.; Lewbart, G.A.; Lahner, L.L.; Haulena, M.; Rosenberg, J.F.; Papich, M.G. Population Pharmacokinetics of Enrofloxacin and Its Metabolite Ciprofloxacin in the Green Sea Urchin (Strongylocentrotus Droebachiensis) Following Intracoelomic and Immersion Administration. J. Zoo Wildl. Med. 2016, 47, 175–186. [Google Scholar] [CrossRef]
- Rantala, M.; Kaartinen, L.; Välimäki, E.; Stryrman, M.; Hiekkaranta, M.; Niemi, A.; Saari, L.; Pyörälä, S. Efficacy and Pharmacokinetics of Enrofloxacin and Flunixin Meglumine for Treatment of Cows with Experimentally Induced Escherichia Coli Mastitis. J. Vet. Pharmacol. Ther. 2002, 25, 251–258. [Google Scholar] [CrossRef]
- Küng, K.; Wanner, M. Pharmacokinetics of baytril (enrofloxacin) in dogs. Schweiz. Arch. Tierheilkd. 1994, 136, 329–334. [Google Scholar]
- Otero, J.L.; Mestorino, N.; Errecalde, J.O. Pharmacokinetics of Enrofloxacin after Single Intravenous Administration in Sheep. Rev. Sci. Tech. 2009, 28, 1129–1142. [Google Scholar] [CrossRef]
- Kordick, D.L.; Papich, M.G.; Breitschwerdt, E.B. Efficacy of Enrofloxacin or Doxycycline for Treatment of Bartonella Henselae or Bartonella Clarridgeiae Infection in Cats. Antimicrob. Agents Chemother. 1997, 41, 2448–2455. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Díaz, M.J.; Fernández-Cruz, M.L.; Martínez, M.A.; Frejo, M.T.; Martínez, M.; Iturbe, J.; Tafur, M. Pharmacokinetic Variables and Tissue Residues of Enrofloxacin and Ciprofloxacin in Healthy Pigs. Am. J. Vet. Res. 1999, 60, 1377–1382. [Google Scholar] [PubMed]
- Kaartinen, L.; Panu, S.; Pyörälä, S. Pharmacokinetics of Enrofloxacin in Horses after Single Intravenous and Intramuscular Administration. Equine Vet. J. 1997, 29, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hossain, M.A.; Park, H.; Kim, Y.; Lee, K.; Park, S. Pharmacokinetic and Pharmacodynamic Integration of Enrofloxacin against Salmonella Enteritidis after Administering to Broiler Chicken by Per-Oral and Intravenous Routes. J. Vet. Sci. 2019, 20, e15. [Google Scholar] [CrossRef] [PubMed]
- Wack, A.N.; KuKanich, B.; Bronson, E.; Denver, M. Pharmacokinetics of Enrofloxacin after Single Dose Oral and Intravenous Administration in the African Penguin (Spheniscus Demersus). J. Zoo Wildl. Med. 2012, 43, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.; Lewbart, G.A.; Hancock-Ronemus, A.; Papich, M.G. Pharmacokinetics of Enrofloxacin and Ciprofloxacin in Atlantic Horseshoe Crabs (Limulus Polyphemus) after Single Injection. J. Vet. Pharmacol. Ther. 2018, 41, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Wanke, M.M.; Delpino, M.V.; Baldi, P.C. Use of Enrofloxacin in the Treatment of Canine Brucellosis in a Dog Kennel (Clinical Trial). Theriogenology 2006, 66, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Dowers, K.L.; Olver, C.; Radecki, S.V.; Lappin, M.R. Use of Enrofloxacin for Treatment of Large-Form Haemobartonella Felis in Experimentally Infected Cats. J. Am. Vet. Med. Assoc. 2002, 221, 250–253. [Google Scholar] [CrossRef]
- Crosby, S.; Credille, B.; Giguère, S.; Berghaus, R. Comparative Efficacy of Enrofloxacin to That of Tulathromycin for the Control of Bovine Respiratory Disease and Prevalence of Antimicrobial Resistance in Mannheimia Haemolytica in Calves at High Risk of Developing Bovine Respiratory Disease. J. Anim. Sci. 2018, 96, 1259–1267. [Google Scholar] [CrossRef]
- Luan, Y.; Chen, K.; Zhao, J.; Cheng, L. Comparative Study on Synergistic Toxicity of Enrofloxacin Combined with Three Antibiotics on Proliferation of THLE-2 Cell. Antibiotics 2022, 11, 394. [Google Scholar] [CrossRef]
- Russo, E.; Lucatello, L.; Giovanardi, D.; Cagnardi, P.; Ortali, G.; Di Leva, V.; Montesissa, C. Approved Medication of Water with Enrofloxacin to Treat Turkey Colibacillosis: Assessment of Efficacy Using a PK/PD Approach. Vet. Microbiol. 2012, 161, 206–212. [Google Scholar] [CrossRef]
- Flammer, K.; Aucoin, D.P.; Whitt, D.A. Intramuscular and Oral Disposition of Enrofloxacin in African Grey Parrots Following Single and Multiple Doses. J. Vet. Pharmacol. Ther. 1991, 14, 359–366. [Google Scholar] [CrossRef]
- Waxman, S.; Prados, A.P.; de Lucas, J.J.; San Andrés, M.I.; Regner, P.; de Oliveira, V.C.; de Roodt, A.; Rodríguez, C. Pharmacokinetic Behavior of Enrofloxacin and Its Metabolite Ciprofloxacin in Urutu Pit Vipers (Bothrops Alternatus) after Intramuscular Administration. J. Zoo Wildl. Med. 2014, 45, 78–85. [Google Scholar] [CrossRef]
- Curry, P.T.; Kropko, M.L.; Garvin, J.R.; Fiedler, R.D.; Theiss, J.C. In Vitro Induction of Micronuclei and Chromosome Aberrations by Quinolones: Possible Mechanisms. Mutat. Res. 1996, 352, 143–150. [Google Scholar] [CrossRef]
- de Boer, M.; Heuer, C.; Hussein, H.; McDougall, S. Minimum Inhibitory Concentrations of Selected Antimicrobials against Escherichia Coli and Trueperella Pyogenes of Bovine Uterine Origin. J. Dairy Sci. 2015, 98, 4427–4438. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Fan, Y.; Du, Y.; Lei, L.; Wang, D.; Liu, Y. Pharmacokinetics and Pharmacodynamics of Enrofloxacin Treatment of Escherichia Coli in a Murine Thigh Infection Modeling. BMC Vet. Res. 2021, 17, 212. [Google Scholar] [CrossRef]
- Pasquali, F.; Manfreda, G. Mutant Prevention Concentration of Ciprofloxacin and Enrofloxacin against Escherichia Coli, Salmonella Typhimurium and Pseudomonas Aeruginosa. Vet. Microbiol. 2007, 119, 304–310. [Google Scholar] [CrossRef]
- Wentzel, J.M.; Biggs, L.J.; Van Vuuren, M. Comparing the Minimum Inhibitory and Mutant Prevention Concentrations of Selected Antibiotics against Animal Isolates of Pasteurella Multocida and Salmonella Typhimurium. Onderstepoort J. Vet. Res. 2022, 89, e1–e7. [Google Scholar] [CrossRef]
- Sasaki, H.; Kawamoto, E.; Kunita, S.; Yagami, K.-I. Comparison of the in Vitro Susceptibility of Rodent Isolates of Pseudomonas Aeruginosa and Pasteurella Pneumotropica to Enrofloxacin. J. Vet. Diagn. Investig. 2007, 19, 557–560. [Google Scholar] [CrossRef]
- Westropp, J.L.; Sykes, J.E.; Irom, S.; Daniels, J.B.; Smith, A.; Keil, D.; Settje, T.; Wang, Y.; Chew, D.J. Evaluation of the Efficacy and Safety of High Dose Short Duration Enrofloxacin Treatment Regimen for Uncomplicated Urinary Tract Infections in Dogs. J. Vet. Intern. Med. 2012, 26, 506–512. [Google Scholar] [CrossRef]
- Reddy, B.S.; Kumari, K.N.; Rao, V.V.; Rayulu, V.C.; Sivajothi, S. Efficacy of Enrofloxacin in the Treatment of Recurrent Pyoderma in Dogs. J. Adv. Vet. Res. 2014, 4, 108–112. [Google Scholar]
- Gerhardt, N.; Schulz, B.S.; Werckenthin, C.; Hartmann, K. Pharmacokinetics of Enrofloxacin and Its Efficacy in Comparison with Doxycycline in the Treatment of Chlamydophila Felis Infection in Cats with Conjunctivitis. Vet. Rec. 2006, 159, 591–594. [Google Scholar] [CrossRef]
- Suojala, L.; Simojoki, H.; Mustonen, K.; Kaartinen, L.; Pyörälä, S. Efficacy of Enrofloxacin in the Treatment of Naturally Occurring Acute Clinical Escherichia Coli Mastitis. J. Dairy Sci. 2010, 93, 1960–1969. [Google Scholar] [CrossRef]
- Persson, Y.; Katholm, J.; Landin, H.; Mörk, M.J. Efficacy of Enrofloxacin for the Treatment of Acute Clinical Mastitis Caused by Escherichia Coli in Dairy Cows. Vet. Rec. 2015, 176, 673. [Google Scholar] [CrossRef]
- Alfonseca-Silva, E.; Cruz-Villa, J.C.; Gutiérrez, L.; Sumano, H. Successful Treatment of Recurrent Subclinical Mastitis in Cows Caused by Enrofloxacin Resistant Bacteria by Means of the Sequential Intramammary Infusion of Enrofloxacin HCl-2H2O and Ceftiofur HCl: A Clinical Trial. J. Vet. Sci. 2021, 22, e78. [Google Scholar] [CrossRef]
- Marien, M.; Decostere, A.; Duchateau, L.; Chiers, K.; Froyman, R.; Nauwynck, H. Efficacy of Enrofloxacin, Florfenicol and Amoxicillin against Ornithobacterium Rhinotracheale and Escherichia Coli O2:K1 Dual Infection in Turkeys Following APV Priming. Vet. Microbiol. 2007, 121, 94–104. [Google Scholar] [CrossRef]
- Garmyn, A.; Martel, A.; Froyman, R.; Nauwynck, H.; Duchateau, L.; Haesebrouck, F.; Pasmans, F. Efficacy of Four Enrofloxacin Treatment Regimens against Experimental Infection in Turkey Poults with Avian Pneumovirus and Ornithobacterium Rhinotracheale. Avian Pathol. 2009, 38, 287–292. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.; Cheng, G.; Wang, X.; Ahmed, S.; Shabbir, M.A.B.; Liu, Z.; Dai, M.; Yuan, Z. The Effects of Different Enrofloxacin Dosages on Clinical Efficacy and Resistance Development in Chickens Experimentally Infected with Salmonella Typhimurium. Sci. Rep. 2017, 7, 11676. [Google Scholar] [CrossRef]
- Agius, J.E.; Rose, K.; Emery, J.-P.; Phalen, D.N. Experimental Infection of Asian House Geckos with Enterococcus Lacertideformus Demonstrates Multiple Disease Transmission Routes and the In-Vivo Efficacy of Antibiotics. Sci. Rep. 2021, 11, 13858. [Google Scholar] [CrossRef]
- Ciccarelli, S.; Valastro, C.; Di Bello, A.; Paci, S.; Caprio, F.; Corrente, M.L.; Trotta, A.; Franchini, D. Diagnosis and Treatment of Pulmonary Disease in Sea Turtles (Caretta Caretta). Animals 2020, 10, 1355. [Google Scholar] [CrossRef]
- Pees, M.; Kiefer, I.; Oechtering, G.; Krautwald-Junghanns, M.-E. Computed Tomography for the Diagnosis and Treatment Monitoring of Bacterial Pneumonia in Indian Pythons (Python Molurus). Vet. Rec. 2008, 163, 152–156. [Google Scholar] [CrossRef]
- Morales-Barrera, E.; Calhoun, N.; Lobato-Tapia, J.L.; Lucca, V.; Prado-Rebolledo, O.; Hernandez-Velasco, X.; Merino-Guzman, R.; Petrone-García, V.M.; Latorre, J.D.; Mahaffey, B.D.; et al. Risks Involved in the Use of Enrofloxacin for Salmonella Enteritidis or Salmonella Heidelberg in Commercial Poultry. Front. Vet. Sci. 2016, 3, 72. [Google Scholar] [CrossRef]
- Janssen, P.; Barton, G.; Kietzmann, M.; Meißner, J. Treatment of Pigs with Enrofloxacin via Different Oral Dosage Forms—Environmental Contaminations and Resistance Development of Escherichia Coli. J. Vet. Sci. 2022, 23, e23. [Google Scholar] [CrossRef]
- Lin, D.; Chen, K.; Xie, M.; Ye, L.; Chan, E.W.-C.; Chen, S. Effect of Ceftiofur and Enrofloxacin on E. Coli Sub-Population in Pig Gastrointestinal Tract. J. Glob. Antimicrob. Resist. 2017, 10, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kaspersen, H.; Urdahl, A.M.; Grøntvedt, C.A.; Gulliksen, S.M.; Tesfamichael, B.; Slettemeås, J.S.; Norström, M.; Sekse, C. Actinobacillus Pleuropneumoniae Eradication with Enrofloxacin May Lead to Dissemination and Long-Term Persistence of Quinolone Resistant Escherichia Coli in Pig Herds. Antibiotics 2020, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mól, M.; Czyżewska-Dors, E.; Kwit, K.; Rachubik, J.; Lipowski, A.; Pejsak, Z. Immune Response in Pigs Treated with Therapeutic Doses of Enrofloxacin at the Time of Vaccination against Aujeszky’s Disease. Res. Vet. Sci. 2015, 100, 68–74. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- FDA. Withdrawal of Enrofloxacin for Poultry. 2005. Available online: http://www.fda.gov/animalveterinary/safetyhealth/recallswithdrawals/ucm042004.html (accessed on 25 March 2022).
- Chantziaras, I.; Smet, A.; Haesebrouck, F.; Boyen, F.; Dewulf, J. Studying the Effect of Administration Route and Treatment Dose on the Selection of Enrofloxacin Resistance in Commensal Escherichia Coli in Broilers. J. Antimicrob. Chemother. 2017, 72, 1991–2001. [Google Scholar] [CrossRef][Green Version]
- JETACAR. The Use of Antibiotics in Food-Producing Animals: Antibiotic-Resistant Bacteria in Animals and Humans; Report of the Joint Expert Technical Advisory Committee on Antibiotic Resistance; Biotext: Canberra, Austrilia, 2009. [Google Scholar]
- Ingram, P.R.; Rogers, B.A.; Sidjabat, H.E.; Gibson, J.S.; Inglis, T.J.J.Y. 2013 Co-Selection May Explain High Rates of Ciprofloxacin Non-Susceptible Escherichia Coli from Retail Poultry Reared without Prior Fluoroquinolone Exposure. J. Med. Microbiol. 2013, 62, 1743–1746. [Google Scholar] [CrossRef]
- European Medicines Agency. Questions and Answers on Use of Enrofloxacin-Containing Veterinary Medicines Administered via Drinking Water to Chickens and Turkeys. 2018. Available online: https://www.ema.europa.eu/en/documents/referral/enrofloxacin-article-35-referral-questions-answers-use-enrofloxacin-containing-veterinary-medicines_en.pdf (accessed on 25 March 2022).
- Piddock, L.J. Mechanisms of Fluoroquinolone Resistance: An Update 1994–1998. Drugs 1999, 58, 11–18. [Google Scholar] [CrossRef]
- Pazhani, G.P.; Chakraborty, S.; Fujihara, K.; Yamasaki, S.; Ghosh, A.; Nair, G.B.; Ramamurthy, T. QRDR Mutations, Efflux System & Antimicrobial Resistance Genes in Enterotoxigenic Escherichia Coli Isolated from an Outbreak of Diarrhoea in Ahmedabad, India. Indian J. Med. Res. 2011, 134, 214–223. [Google Scholar]
- Ruiz, J.; Pons, M.J.; Gomes, C. Transferable Mechanisms of Quinolone Resistance. Int. J. Antimicrob. Agents 2012, 40, 196203. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Mechanisms of Fluoroquinolone Resistance. Drug Resist. Updat. 1999, 2, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, L.; Pascual, A.; García, I.; Tran, J.; Jacoby, G.A. Interaction of Plasmid and Host Quinolone Resistance. J. Antimicrob. Chemother. 2003, 51, 1037–1039. [Google Scholar] [CrossRef][Green Version]
- Wong, M.H.; Chan, E.W.; Liu, L.Z.; Chen, S. PMQR Genes OqxAB and Aac(6′)Ib-Cr Accelerate the Development of Fluoroquinolone Resistance in Salmonella Typhimurium. Front. Microbiol. 2014, 5, 521. [Google Scholar] [CrossRef]
- Jacoby, G.A. Mechanisms of Resistance to Quinolones. Clin. Infect. Dis. 2005, 41, S120–S126. [Google Scholar] [CrossRef]
- Robicsek, A.; Jacoby, G.A.; Hooper, D.C. The Worldwide Emergence of Plasmid-Mediated Quinolone Resistance. Lancet Infect. Dis. 2006, 6, 629–640. [Google Scholar] [CrossRef]
- Kurnia, R.S.; Indrawati, A.; Mayasari, N.L.P.I.; Priadi, A. Molecular Detection of Genes Encoding Resistance to Tetracycline and Determination of Plasmid-Mediated Resistance to Quinolones in Avian Pathogenic Escherichia Coli in Sukabumi, Indonesia. Vet. World 2018, 11, 1581–1586. [Google Scholar] [CrossRef]
- Yaqoob, M.; Wang, L.P.; Memon, J.; Kashif, J.; Umar, S.; Naseer, Z.; Iqbal, M.F.; Fiaz, M.; Lu, C.P. Role of Topoisomerase Mutations, Plasmid Mediated Resistance (Qnr) and AcrAB Efflux Pump in Fluoroquinolone Resistant Clinical Isolates of Avian Escherichia Coli. Mol. Genet. Microbiol. Virol. 2017, 32, 49–54. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Z.; Cui, L.; Hu, T.; Guo, K.; Zhang, F.; Wang, X.; Peng, Z.; Liu, Q.; Dai, M. Enrofloxacin Promotes Plasmid-Mediated Conjugation Transfer of Fluoroquinolone-Resistance Gene QnrS. Front. Microbiol. 2022, 12, 773664. [Google Scholar] [CrossRef]
- Nishino, K.; Latifi, T.; Groisman, E.A. Virulence and Drug Resistance Roles of Multidrug Efflux Systems of Salmonella Enterica Serovar Typhimurium. Mol. Microbiol. 2006, 59, 126–141. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, L.; Wu, C.; Huang, J.; Hao, H.; Yuan, Z.; Cheng, G. The Evolution of Fluoroquinolone Resistance in Salmonella under Exposure to Sub-Inhibitory Concentration of Enrofloxacin. Int. J. Mol. Sci. 2021, 22, 12218. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yokota, S.; Okubo, T.; Ishihara, K.; Ueno, H.; Muramatsu, Y.; Fujii, N.; Tamura, Y. Contribution of the AcrAB-TolC Efflux Pump to High-Level Fluoroquinolone Resistance in Escherichia Coli Isolated from Dogs and Humans. J. Vet. Med. Sci. 2013, 75, 407–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huguet, A.; Pensec, J.; Soumet, C. Resistance in Escherichia Coli: Variable Contribution of Efflux Pumps with Respect to Different Fluoroquinolones. J. Appl. Microbiol. 2013, 114, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhai, Y.-J.; Wu, H.; Sun, H.-R.; He, Z.-P.; Wang, Y.-B.; Pan, Y.-S.; Kuang, N.-N.; Hu, G.-Z. Identification and Prevalence of RND Family Multidrug Efflux Pump OqxAB Genes in Enterococci Isolates from Swine Manure in China. J. Med. Microbiol. 2018, 67, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V.; Nordmann, P. Plasmid-Mediated Quinolone Resistance in Gram-Negative Bacterial Species: An Update. CMC 2009, 16, 1028–1046. [Google Scholar] [CrossRef]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of Aac(6′)-Ib-Cr Encoding a Ciprofloxacin-Modifying Enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef]
- Mattioni Marchetti, V.; Bitar, I.; Mercato, A.; Nucleo, E.; Marchesini, F.; Mancinelli, M.; Prati, P.; Scarsi, G.S.; Hrabak, J.; Pagani, L.; et al. Deadly Puppy Infection Caused by an MDR Escherichia Coli O39 BlaCTX–M–15, BlaCMY–2, BlaDHA–1, and Aac(6)-Ib-Cr—Positive in a Breeding Kennel in Central Italy. Front. Microbiol. 2020, 11, 584. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, H.; Liu, Y.; Zhou, X.; Wang, J.; Liu, T.; Beier, R.C.; Hou, X. Characterization of Quinolone Resistance in Salmonella Enterica Serovar Indiana from Chickens in China. Poult. Sci. 2015, 94, 454–460. [Google Scholar] [CrossRef]
- Park, Y.; Oh, J.; Park, S.; Sum, S.; Song, W.; Chae, J.; Park, H. Antimicrobial Resistance and Novel Mutations Detected in the GyrA and ParC Genes of Pseudomonas Aeruginosa Strains Isolated from Companion Dogs. BMC Vet. Res. 2020, 16, 111. [Google Scholar] [CrossRef]
- Pereira, R.V.; Siler, J.D.; Ng, J.C.; Davis, M.A.; Grohn, Y.T.; Warnick, L.D. Effect of on-farm use of antimicrobial drugs on resistance in fecal Escherichia coli of preweaned dairy calves. J. Diary Sci. 2014, 97, 7644–7654. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.; Dai, M.; Zhang, H.; Ning, J.; Cheng, G.; Shabbir, M.A.B.; Sajid, A.; Yuan, Z. Resistance and Virulence Mechanisms of Escherichia Coli Selected by Enrofloxacin in Chicken. Antimicrob. Agents Chemother. 2019, 63, e01824-18. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ishihara, K.; Kojima, A.; Asai, T.; Harada, K.; Tamura, Y. Emergence of Fluoroquinolone Resistance in Campylobacter Jejuni in Chickens Exposed to Enrofloxacin Treatment at the Inherent Dosage Licensed in Japan. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of Quinolone Resistance in Escherichia Coli and Salmonella: Recent Developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, E.J.; Ward, L.R.; Rowe, B. Resistance to Ciprofloxacin in Non-Typhoidal Salmonellas from Humans in England and Wales-the Current Situation. Clin. Microbiol. Infect. 1999, 5, 130–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, D.; Zheng, M.; Xu, J.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Prevalence of Fluoroquinolone Resistance and Mutations in the GyrA, ParC and ParE Genes of Riemerella Anatipestifer Isolated from Ducks in China. BMC Microbiol. 2019, 19, 271. [Google Scholar] [CrossRef] [PubMed]
- Khazaeel, K.; Mazaheri, Y.; Hashemitabar, M.; Najafzadeh, H.; Morovvati, H.; Ghadrdan, A.R. Enrofloxacin Effect on Histomorphologic and Histomorphometric Structure of Lamb Articular Cartilage. Glob. Vet. 2012, 9, 447–453. [Google Scholar] [CrossRef]
- Newman, R.J.; Chow, L.; Goodrich, L.R.; Lambrechts, N.E.; Dow, S.W.; Pezzanite, L.M. Susceptibility of Canine Chondrocytes and Synoviocytes to Antibiotic Cytotoxicity in Vitro. Vet. Surg. 2021, 50, 650–658. [Google Scholar] [CrossRef]
- Lim, S.; Hossain, M.A.; Park, J.; Choi, S.H.; Kim, G. The Effects of Enrofloxacin on Canine Tendon Cells and Chondrocytes Proliferation in Vitro. Vet. Res. Commun. 2008, 32, 243–253. [Google Scholar] [CrossRef]
- Siengdee, P.; Euppayo, T.; Buddhachat, K.; Chomdej, S.; Nganvongpanit, K. Two Fluoroquinolones and Their Combinations with Hyaluronan: Comparison of Effects on Canine Chondrocyte Culture. J. Vet. Pharmacol. Ther. 2016, 39, 439–451. [Google Scholar] [CrossRef]
- Egerbacher, M.; Edinger, J.; Tschulenk, W. Effects of Enrofloxacin and Ciprofloxacin Hydrochloride on Canine and Equine Chondrocytes in Culture. Am. J. Vet. Res. 2001, 62, 704–708. [Google Scholar] [CrossRef]
- Maślanka, T.; Jaroszewski, J.J. Effect of Long-Term Treatment with Therapeutic Doses of Enrofloxacin on Chicken Articular Cartilage. Pol. J. Vet. Sci. 2009, 12, 363–367. [Google Scholar] [PubMed]
- Yoon, J.H.; Brooks, R.L.; Khan, A.; Pan, H.; Bryan, J.; Zhang, J.; Budsberg, S.C.; Mueller, P.O.E.; Halper, J. The Effect of Enrofloxacin on Cell Proliferation and Proteoglycans in Horse Tendon Cells. Cell Biol. Toxicol. 2004, 20, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Joo, H.-G. Determination of the toxicity of enrofloxacin on mouse bone marrow cells. J. Prev. Vet. 2019, 43, 141–145. [Google Scholar] [CrossRef]
- won Seo, K.; Holt, R.; Jung, Y.-S.; Rodriguez, C.O.; Chen, X.; Rebhun, R.B. Fluoroquinolone-Mediated Inhibition of Cell Growth, S-G2/M Cell Cycle Arrest, and Apoptosis in Canine Osteosarcoma Cell Lines. PLoS ONE 2012, 7, e42960. [Google Scholar] [CrossRef]
- Yücel, U.M.; Koşal, V.; Kara, M.; Taşpınar, F.; Uslu, B.A. Adverse Effects of Oxytetracycline and Enrofloxacin on the Fertility of Saanen Bucks. Trop. Anim. Health Prod. 2021, 53, 466. [Google Scholar] [CrossRef] [PubMed]
- Aral, F.; Karaçal, F.; Baba, F. The Effect of Enrofloxacin on Sperm Quality in Male Mice. Res. Vet. Sci. 2008, 84, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Rungsung, S.; Khan, A.M.; Sood, N.K.; Rampal, S.; Singh Saini, S.P. Evaluation of Ameliorative Potential of Supranutritional Selenium on Enrofloxacin-Induced Testicular Toxicity. Chem. Biol. Interact. 2016, 252, 87–92. [Google Scholar] [CrossRef]
- Tkachenko, O.Y.; Scheerer-Bernhard, J.U.; Delimitreva, S.; Wedi, E.; Valle, R.R.; Heistermann, M.; Nayudu, P.L. A Retrospective Analysis of Adverse Effects of an in Vivo Fluoroquinolone Antibiotic Enrofloxacin Treatment on Oocyte Quality in the Common Marmoset. Reprod. Toxicol. 2018, 75, 86–95. [Google Scholar] [CrossRef]
- Anchordoquy, J.P.; Anchordoquy, J.M.; Nikoloff, N.; Gambaro, R.; Padula, G.; Furnus, C.; Seoane, A. Cytotoxic and Genotoxic Effects Induced by Enrofloxacin-Based Antibiotic Formulation Floxagen® in Two Experimental Models of Bovine Cells in Vitro: Peripheral Lymphocytes and Cumulus Cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 2998–3005. [Google Scholar] [CrossRef]
- Hruba, H.; Abdelsalam, E.E.E.; Anisimov, N.; Bandouchova, H.; Havelkova, B.; Heger, T.; Kanova, M.; Kovacova, V.; Nemcova, M.; Piacek, V.; et al. Reproductive Toxicity of Fluoroquinolones in Birds. BMC Vet. Res. 2019, 15, 209. [Google Scholar] [CrossRef]
- Ellerbrock, R.E.; Canisso, I.F.; Roady, P.J.; Rothrock, L.T.; Zhong, L.; Wilkins, P.; Dirikolu, L.; Lima, F.S.; Honoroto, J. Diffusion of Enrofloxacin to Pregnancy Fluids and Effects on Fetal Cartilage after Intravenous Administration to Late Pregnant Mares. Equine Vet. J. 2019, 51, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, K.M.; Beard, L.K.; Sun, X.; Ramsay, E.C. Investigation of Enrofloxacin-associated Retinal Toxicity in Nondomestic Felids. J. Zoo Wildl. Med. 2017, 48, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Gelatt, K.N.; Van Der Woerdt, A.; Ketring, K.L.; Andrew, S.E.; Brooks, D.E.; Biros, D.J.; Denis, H.M.; Cutler, T.J. Enrofloxacin-Associated Retinal Degeneration in Cats. Vet. Ophthalmol. 2001, 4, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.M.; Dubielzig, R.R.; Giuliano, E.A.; Moore, C.P.; Narfström, K.L. Ocular and Systemic Manifestations after Oral Administration of a High Dose of Enrofloxacin in Cats. Am. J. Vet. Res. 2007, 68, 190–202. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Y.; Brown, P.B.; Ge, X.; Xie, J.; Xu, P. Cytotoxic Effects and Apoptosis Induction of Enrofloxacin in Hepatic Cell Line of Grass Carp (Ctenopharyngodon Idellus). Fish Shellfish Immunol. 2015, 47, 639–644. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Zamaratskaia, G. Regulation of Porcine Hepatic Cytochrome P450—Implication for Boar Taint. Comput. Struct. Biotechnol. J. 2014, 11, 106–112. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, M.; Thunders, M.; Ai, X.; Qiu, J. Effect of Enrofloxacin and Roxarsone on CYP450s in Pig. Res. Vet. Sci. 2018, 117, 97–98. [Google Scholar] [CrossRef]
- Vancutsem, P.M.; Babish, J.G. In Vitro and in Vivo Study of the Effects of Enrofloxacin on Hepatic Cytochrome P-450. Potential for Drug Interactions. Vet. Hum. Toxicol. 1996, 38, 254–259. [Google Scholar]
- Vaccaro, E.; Giorgi, M.; Longo, V.; Mengozzi, G.; Gervasi, P.G. Inhibition of Cytochrome P450 Enzymes by Enrofloxacin in the Sea Bass (Dicentrarchus Labrax). Aquat. Toxicol. 2003, 62, 27–33. [Google Scholar] [CrossRef]
- Fu, H.-F.; Gao, Z.-X.; Cheng, Y.-Y. Inhibition of CYP3A MRNA and Protein Expression, and Enzymatic Activity, by Enrofloxacin in Chickens. J. Vet. Pharmacol. Ther. 2010, 33, 546–550. [Google Scholar] [CrossRef]
- Li, Y.; Mou, Y.; Thunders, M.; Wu, Y.; Ai, X.; Zhou, X.; Qiu, J. Effects of Enrofloxacin on Antioxidant System, Microsomal Enzymatic Activity, and Proteomics in Porcine Liver. J. Vet. Pharmacol. Ther. 2018, 41, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Gorla, N.; García Ovando, H.; Larripa, I. Chromosomal Aberrations in Human Lymphocytes Exposed in Vitro to Enrofloxacin and Ciprofloxacin. Toxicol. Lett. 1999, 104, 43–48. [Google Scholar] [CrossRef]
- Qiu, W.; Hu, J.; Magnuson, J.T.; Greer, J.; Yang, M.; Chen, Q.; Fang, M.; Zheng, C.; Schlenk, D. Evidence Linking Exposure of Fish Primary Macrophages to Antibiotics Activates the NF-KB Pathway. Environ. Int. 2020, 138, 105624. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Law, M.; Christiansen, E.F.; Lewbart, G.A.; Harms, C.A. Evaluation of Localized Inflammatory Reactions Secondary to Intramascular Injections of Enrofloxacin in Striped Bass (Morone Saxatilis). J. Zoo Wildl. Med. 2020, 51, 46–52. [Google Scholar] [CrossRef]
- Qin, P.; Pan, X.; Liu, R.; Hu, C.; Dong, Y. Toxic Interaction Mechanism of Two Fluoroquinolones with Serum Albumin by Spectroscopic and Computational Methods. J. Environ. Sci. Health B 2017, 52, 833–841. [Google Scholar] [CrossRef]
- Minta, M.; Wilk, I.; Zmudzki, J. Inhibition of Cell Differentiation by Quinolones in Micromass Cultures of Rat Embryonic Limb Bud and Midbrain Cells. Toxicol. In Vitro 2005, 19, 915–919. [Google Scholar] [CrossRef]
- Spodniewska, A.; Barski, D.; Giżejewska, A. Effect of Enrofloxacin and Chlorpyrifos on the Levels of Vitamins A and E in Wistar Rats. Environ. Toxicol. Pharmacol. 2015, 40, 587–591. [Google Scholar] [CrossRef]
- Rosen, L.E.; Olea-Popelka, F.; Deem, S.L.; Isaza, R.; Schmitt, D.; Miller, M. Survey of Antituberculosis Drug Administration and Adverse Effects in Elephants in North America. J. Zoo Wildl. Med. 2019, 50, 23–32. [Google Scholar] [CrossRef]
- Bauquier, S.H.; Jiang, J.L.; Lai, A.; Cook, M.J. Clonic Seizures in GAERS Rats after Oral Administration of Enrofloxacin. Comp. Med. 2016, 66, 220–224. [Google Scholar]
- Cepiel, A.; Noszczyk-Nowak, A.; Cekiera, A.; Popiel, J.; Pasławska, U. Influence of Long-Term Oral Application of Quinolones on the ECG Curve in Dogs. Pol. J. Vet. Sci. 2017, 20, 567–572. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Li, S.; Lu, T. Rule of Accumulation of Enrofloxacin in Acipenser Baerii and Drug-Induced Damage to the Tissues. Exp. Biol. Med. 2016, 241, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Tokanova, N.; Hodkovicova, N.; Kocour Kroupova, H.; Tumova, J.; Blahova, J.; Marsalek, P.; Plhalova, L.; Doubkova, V.; Dobsikova, R.; et al. Oxidative Stress Induced by Fluoroquinolone Enrofloxacin in Zebrafish (Danio Rerio) Can Be Ameliorated after a Prolonged Exposure. Environ. Toxicol. Pharmacol. 2019, 67, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, J.; Zhu, L.; Wang, J.; Zhu, G. Toxicity of Enrofloxacin, Copper and Their Interactions on Soil Microbial Populations and Ammonia-Oxidizing Archaea and Bacteria. Sci. Rep. 2018, 8, 5828. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-J.; Lee, J.-W.; Lee, E.-S.; Shin, S.-K.; Hwang, S.-R.; Oh, J.-E. Occurrence and Distribution of Pharmaceuticals in Wastewater from Households, Livestock Farms, Hospitals and Pharmaceutical Manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef]
- Vollú, R.E.; Cotta, S.R.; Jurelevicius, D.; de Assis Leite, D.C.; Parente, C.E.T.; Malm, O.; Martins, D.C.; Resende, Á.V.; Marriel, I.E.; Seldin, L. Response of the Bacterial Communities Associated With Maize Rhizosphere to Poultry Litter as an Organomineral Fertilizer. Front. Environ. Sci. 2018, 6, 118. [Google Scholar] [CrossRef]
- Parente, C.E.; Oliveira da Silva, E.; Sales Júnior, S.F.; Hauser-Davis, R.A.; Malm, O.; Correia, F.V.; Saggioro, E.M. Fluoroquinolone-Contaminated Poultry Litter Strongly Affects Earthworms as Verified through Lethal and Sub-Lethal Evaluations. Ecotoxicol. Environ. Saf. 2021, 207, 111305. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, X.; Sun, Z.; Zhao, N.; Li, Y. Toxic Effects of Enrofloxacin on Growth Rate and Catalase Activity in Eisenia Fetida. Environ. Toxicol. Pharmacol. 2008, 26, 177–180. [Google Scholar] [CrossRef]
- Peltzer, P.M.; Lajmanovich, R.C.; Attademo, A.M.; Junges, C.M.; Teglia, C.M.; Martinuzzi, C.; Curi, L.; Culzoni, M.J.; Goicoechea, H.C. Ecotoxicity of Veterinary Enrofloxacin and Ciprofloxacin Antibiotics on Anuran Amphibian Larvae. Environ. Toxicol. Pharmacol. 2017, 51, 114–123. [Google Scholar] [CrossRef]
- Alcaráz, M.R.; Bortolato, S.A.; Goicoechea, H.C.; Olivieri, A.C. A New Modeling Strategy for Third-Order Fast High-Performance Liquid Chromatographic Data with Fluorescence Detection. Quantitation of Fluoroquinolones in Water Samples. Anal. Bioanal. Chem. 2015, 407, 1999–2011. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhuang, H.; Li, X.; Gao, X.; An, Z.; Liu, X.; Yang, H.; Wei, W.; Zhang, X. Excessive Use of Enrofloxacin Leads to Growth Inhibition of Juvenile Giant Freshwater Prawn Macrobrachium Rosenbergii. Ecotoxicol. Environ. Saf. 2019, 169, 344–352. [Google Scholar] [CrossRef]
- Tolosi, R.; De Liguoro, M. Delayed Toxicity of Three Fluoroquinolones and Their Mixtures after Neonatal or Embryonic Exposure, in Daphnia Magna. Ecotoxicol. Environ. Saf. 2021, 225, 112778. [Google Scholar] [CrossRef] [PubMed]
- Dalla Bona, M.; Lizzi, F.; Borgato, A.; De Liguoro, M. Increasing Toxicity of Enrofloxacin over Four Generations of Daphnia Magna. Ecotoxicol. Environ. Saf. 2016, 132, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Dalla Bona, M.; Zounková, R.; Merlanti, R.; Blaha, L.; De Liguoro, M. Effects of Enrofloxacin, Ciprofloxacin, and Trimethoprim on Two Generations of Daphnia Magna. Ecotoxicol. Environ. Saf. 2015, 113, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Giang, C.N.D.; Sebesvari, Z.; Renaud, F.; Rosendahl, I.; Minh, Q.H.; Amelung, W. Occurrence and Dissipation of the Antibiotics Sulfamethoxazole, Sulfadiazine, Trimethoprim, and Enrofloxacin in the Mekong Delta, Vietnam. PLoS ONE 2015, 10, e0131855. [Google Scholar] [CrossRef]
- Ikem, A.; Lin, C.-H.; Broz, B.; Kerley, M.; Thi, H.L. Occurrence of Enrofloxacin in Overflows from Animal Lot and Residential Sewage Lagoons and a Receiving-Stream. Heliyon 2017, 3, e00409. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Chen, M.; Wang, R. Occurrence of Ciprofloxacin, Enrofloxacin, and Florfenicol in Animal Wastewater and Water Resources. J. Environ. Qual. 2012, 41, 1481–1486. [Google Scholar] [CrossRef]
- Yiruhan; Wang, Q.-J.; Mo, C.-H.; Li, Y.-W.; Gao, P.; Tai, Y.-P.; Zhang, Y.; Ruan, Z.-L.; Xu, J.-W. Determination of Four Fluoroquinolone Antibiotics in Tap Water in Guangzhou and Macao. Environ. Pollut. 2010, 158, 2350–2358. [Google Scholar] [CrossRef]
- Babić, S.; Periša, M.; Škorić, I. Photolytic Degradation of Norfloxacin, Enrofloxacin and Ciprofloxacin in Various Aqueous Media. Chemosphere 2013, 91, 1635–1642. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Wang, J.; Zhu, L.; Wang, J.; Yan, S.; Ahmad, Z. Toxicity of Enrofloxacin and Cadmium Alone and in Combination to Enzymatic Activities and Microbial Community Structure in Soil. Environ. Geochem. Health 2019, 41, 2593–2606. [Google Scholar] [CrossRef]
- Yan, H.-T.; Liu, R.-X.; Yang, Q.-Z.; Liu, Y.-C.; Li, H.-C.; Guo, R.-F.; Wu, L.-H.; Liu, L.-M.; Liang, H. A New Calcium(II)-Based Substitute for Enrofloxacin with Improved Medicinal Potential. Pharmaceutics 2022, 14, 249. [Google Scholar] [CrossRef]
- Ftouni, H.; Sayen, S.; Boudesocque, S.; Dechamps-Olivier, I.; Guillon, E. Structural Study of the Copper(II)–Enrofloxacin Metallo-Antibiotic. Inorg. Chim. Acta 2012, 382, 186–190. [Google Scholar] [CrossRef]
- Brauser, A.; Schroeder, I.; Gutsmann, T.; Cosentino, C.; Moroni, A.; Hansen, U.-P.; Winterhalter, M. Modulation of Enrofloxacin Binding in OmpF by Mg2+ as Revealed by the Analysis of Fast Flickering Single-Porin Current. J. Gen. Physiol. 2012, 140, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Graouer-Bacart, M.; Sayen, S.; Guillon, E. Adsorption of Enrofloxacin in Presence of Zn(II) on a Calcareous Soil. Ecotoxicol. Environ. Saf. 2015, 122, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.; Cioatera, N.; Chifiriuc, M.C.; Munteanu, G.; Ganescu, A.; Dabuleanu, I.; Avram, G.; Spinu, C.I.; Rotaru, P. New Biologically Active Mixed-Ligand Co(II) and Ni(II) Complexes of Enrofloxacin. J. Therm. Anal. Calorim. 2018, 134, 527–541. [Google Scholar] [CrossRef]
- Meenongwa, A.; Brissos, R.F.; Soikum, C.; Chaveerach, P.; Trongpanich, Y.; Chaveerach, U. Enhancement of Biological Activities of Copper(II) Complexes Containing Guanidine Derivatives by Enrofloxacin. J. Mol. Struct. 2021, 1241, 130645. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Hu, Y.; Wang, X.; Ai, X.; Tang, L.; Matthew, C.; Cavanagh, J.; Qiu, J. Enrofloxacin at Environmentally Relevant Concentrations Enhances Uptake and Toxicity of Cadmium in the Earthworm Eisenia Fetida in Farm Soils. J. Hazard. Mater. 2016, 308, 312–320. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhu, L.; Wang, J. Individual and Combined Effects of Enrofloxacin and Cadmium on Soil Microbial Biomass and the Ammonia-Oxidizing Functional Gene. Sci. Total Environ. 2018, 624, 900–907. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Zhu, L.; Wang, J.; Yang, L.; Mao, S.; Conkle, J.L.; Chen, Y.; Kim, Y.M. Effects of Interaction between Enrofloxacin and Copper on Soil Enzyme Activity and Evaluation of Comprehensive Toxicity. Chemosphere 2021, 268, 129208. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zhuo, X.; Liu, W.; Wu, J. Synchronous Fluorescence Measurement of Enrofloxacin in the Pharmaceutical Formulation and Its Residue in Milks Based on the Yttrium (III)-Perturbed Luminescence. Talanta 2010, 82, 1858–1863. [Google Scholar] [CrossRef]
- Rezaei, B.; Mokhtari, A. Flow-Injection Chemiluminescence Determination of Enrofloxacin Using the Ru(Phen)3(2+)-Ce(IV) System and Central Composite Design for the Optimization of Chemical Variables. Luminescence 2008, 23, 357–364. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Mucha, A.P.; Almeida, C.M.R.; Gao, W.; Jia, Z.; Carvalho, M.F. Biodegradation of the Veterinary Antibiotics Enrofloxacin and Ceftiofur and Associated Microbial Community Dynamics. Sci. Total Environ. 2017, 581–582, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Tasho, R.P.; Cho, J.Y. Veterinary Antibiotics in Animal Waste, Its Distribution in Soil and Uptake by Plants: A Review. Sci. Total Environ. 2016, 563–564, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Xie, Y.-F.; Li, C.-L.; Zhao, H.-N.; Zhao, H.; Wang, N.; Wang, J.-F. Investigation of Residual Fluoroquinolones in a Soil–Vegetable System in an Intensive Vegetable Cultivation Area in Northern China. Sci. Total Environ. 2014, 468–469, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, L.; Cheng, J.; Jing, C. Mechanistic Insights into TiO2 Thickness in Fe3O4@TiO2-GO Composites for Enrofloxacin Photodegradation. Chem. Eng. J. 2017, 325, 647–654. [Google Scholar] [CrossRef]
- Yang, B.; Kookana, R.S.; Williams, M.; Ying, G.-G.; Du, J.; Doan, H.; Kumar, A. Oxidation of Ciprofloxacin and Enrofloxacin by Ferrate(VI): Products Identification, and Toxicity Evaluation. J. Hazard. Mater. 2016, 320, 296–303. [Google Scholar] [CrossRef]
- Sciscenko, I.; Arques, A.; Varga, Z.; Bouchonnet, S.; Monfort, O.; Brigante, M.; Mailhot, G. Significant Role of Iron on the Fate and Photodegradation of Enrofloxacin. Chemosphere 2021, 270, 129791. [Google Scholar] [CrossRef]
- Carbon, C. Summary. Clin. Microbiol. Infect. 1998, 4, S32–S33. [Google Scholar] [CrossRef]

| Property | Value |
|---|---|
| Molecular formula | C19H22FN3O3 |
| Molecular weight | 359.3 g/mol |
| Chemical safety | Irritant, health hazard, environmental hazard |
| Color/form | Pale yellow crystals |
| Melting point | 220 °C |
| Confirmed Presence of Enrofloxacin in Organs | Volume of Distribution (Vd) (L/kg) | Animal Model | Reference |
|---|---|---|---|
| Serum, liver, kidney, lung, brain, breast muscle, thigh muscle, spleen, and heart | 5.07 | Broiler chicken | [29] |
| Plasma, muscle, skin, and liver | 2.21 | Largemouth bass | [30] |
| Plasma, hepatopancreas, muscle, gill, and ovary | nd | Ridgetail white prawn | [31] |
| Plasma, skin, muscle, liver, kidney, and gut | nd | Rainbow trout | [32] |
| Plasma, skin, muscle, gill, kidney, liver, bile, and gut | nd | Yellow river carp | [33] |
| Applied Dose of Enrofloxacin (mg/kg) | Cmax Ciprofloxacin (μg/mL) | Cmax Enrofloxacin (μg/mL) | Administration Method | Animal Model | Reference |
|---|---|---|---|---|---|
| 7.5 | 0.36 | 2.59 | Intramuscular | Green sea turtles | [35] |
| 5 | no data | 2.33 | Intramuscular | Freshwater crocodiles | [36] |
| 10 | 0.24 | 12.31 | per os | Asian house geckos | [37] |
| 10 | <0.1 | 67.90 | Subcutaneous | Eastern box turtles | [38] |
| 20 | 2.28 | 5.36 | Subcutaneous | Prairie dogs | [39] |
| 10 | <0.1 | 90.92 | Intracelomic | Green sea urchin | [40] |
| Study Type | Animal Model | Observed Effect | Reference |
|---|---|---|---|
| in vitro | Cattle | Toxic interaction with serum albumin | [142] |
| Cattle | Cytotoxicity on embryonic limb bud cells and midbrain cells | [143] | |
| in vivo | Rats | Slight decrease in liver vitamin A and E levels | [144] |
| Elephants | Anorexia, decreased water intake, constipation, depression, ataxia, limb paresis, and tremors | [145] | |
| Genetic Absence Epilepsy Rats from Strasbourg (GAERS) | Induction of clonic seizures | [146] | |
| Dogs | Alteration of cardiac ventricular depolarization and repolarization, as well as increasing the risk of ventricular arrhythmias. | [147] | |
| Acipenser baerii | Structural damage to liver, kidney, and cartilage | [148] | |
| Danio rerio | Changes in the catalytic activity of glutathione peroxidase and glutathione S-transferase | [149] |
| Metal | Observed Effects | Reference |
|---|---|---|
| Co(II) and Ni(II) | (1) Broader spectrum of antibacterial and antifungal activity against: E. coli, S. aureus, P. aeruginosa, and C. albicans (2) No cytotoxic effect of tested complexes on L929 cell line | [171] |
| Cu(II) | (1) Increased antibacterial activity against E. coli and Salmonella (2) Enhanced cytotoxic potential against breast cancer cell line (MCF-7) | [172] |
| Cd | (1) Increased bioaccumulation of Cd caused by enrofloxacin in earthworms (2) Enhancement of oxidative stress induced by Cd | [173] |
| Cd | (1) Increased cytotoxicity of the complex compared to the antibiotic alone (2) Most of the interactions observed were antagonistic reactions | [174] |
| Cu | (1) Application of the complex increased toxicity to soil enzymes | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, Ł.; Gaffke, L.; Pierzynowska, K.; Cyske, Z.; Choszcz, M.; Węgrzyn, G.; Węgrzyn, A. Enrofloxacin—The Ruthless Killer of Eukaryotic Cells or the Last Hope in the Fight against Bacterial Infections? Int. J. Mol. Sci. 2022, 23, 3648. https://doi.org/10.3390/ijms23073648
Grabowski Ł, Gaffke L, Pierzynowska K, Cyske Z, Choszcz M, Węgrzyn G, Węgrzyn A. Enrofloxacin—The Ruthless Killer of Eukaryotic Cells or the Last Hope in the Fight against Bacterial Infections? International Journal of Molecular Sciences. 2022; 23(7):3648. https://doi.org/10.3390/ijms23073648
Chicago/Turabian StyleGrabowski, Łukasz, Lidia Gaffke, Karolina Pierzynowska, Zuzanna Cyske, Marta Choszcz, Grzegorz Węgrzyn, and Alicja Węgrzyn. 2022. "Enrofloxacin—The Ruthless Killer of Eukaryotic Cells or the Last Hope in the Fight against Bacterial Infections?" International Journal of Molecular Sciences 23, no. 7: 3648. https://doi.org/10.3390/ijms23073648
APA StyleGrabowski, Ł., Gaffke, L., Pierzynowska, K., Cyske, Z., Choszcz, M., Węgrzyn, G., & Węgrzyn, A. (2022). Enrofloxacin—The Ruthless Killer of Eukaryotic Cells or the Last Hope in the Fight against Bacterial Infections? International Journal of Molecular Sciences, 23(7), 3648. https://doi.org/10.3390/ijms23073648











