Hypomagnetic Field Induces the Production of Reactive Oxygen Species and Cognitive Deficits in Mice Hippocampus
Abstract
1. Introduction
2. Results
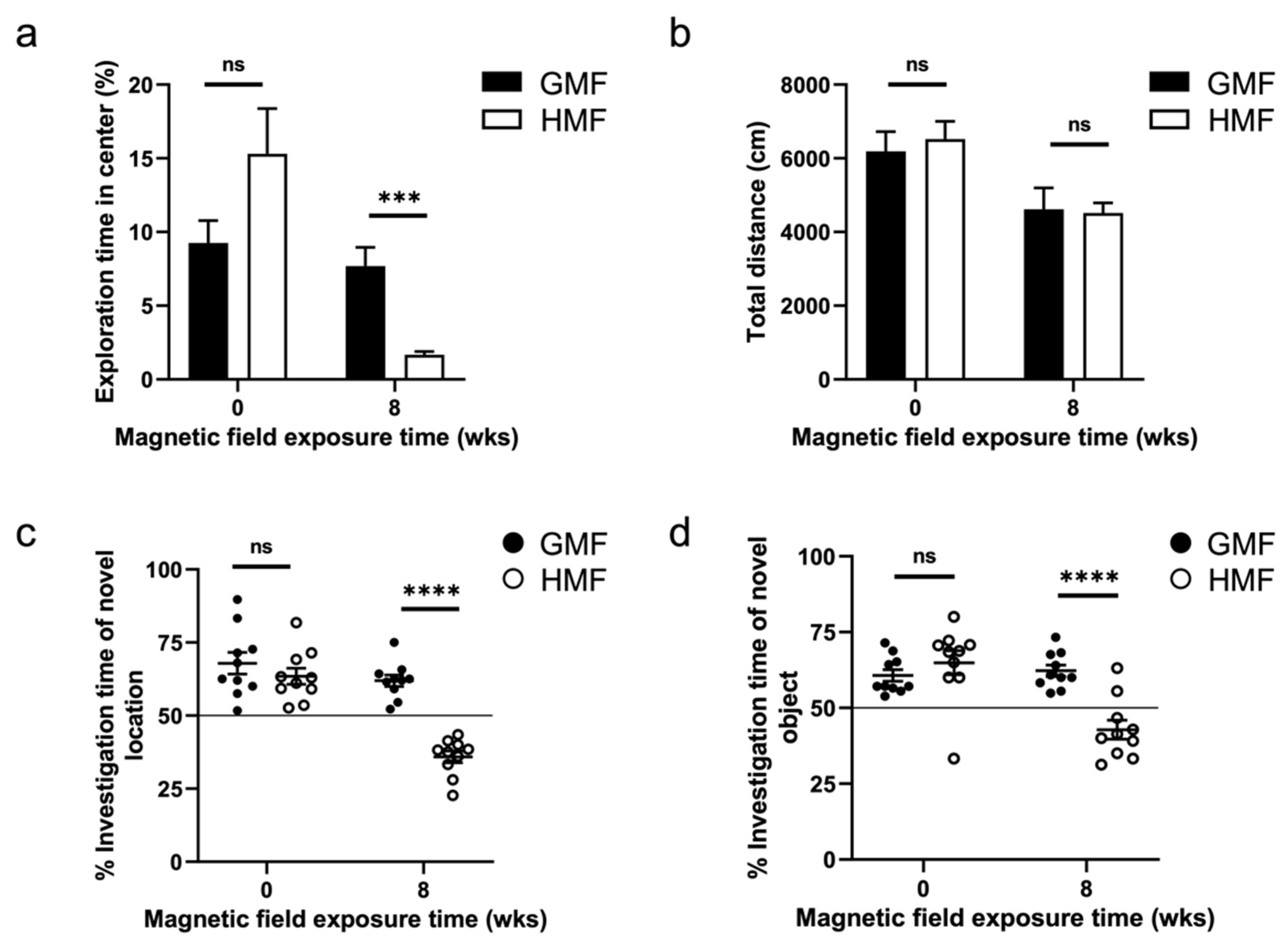
2.1. The Hypomagnetic Field Exposure Impaired Cognitive Function in Mice
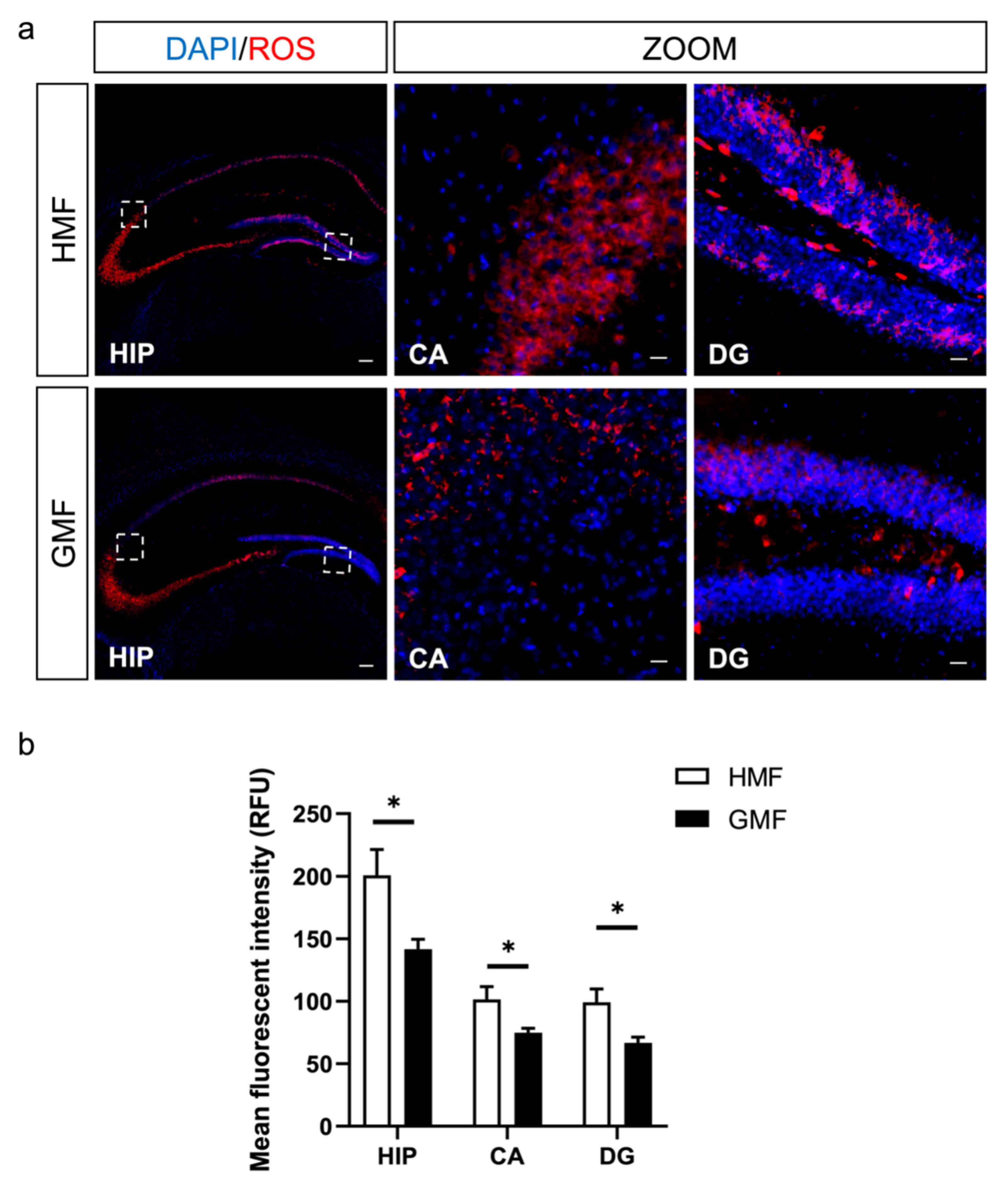
2.2. The Hypomagnetic Field Increased ROS Levels in DG and CA Regions in Hippocampus
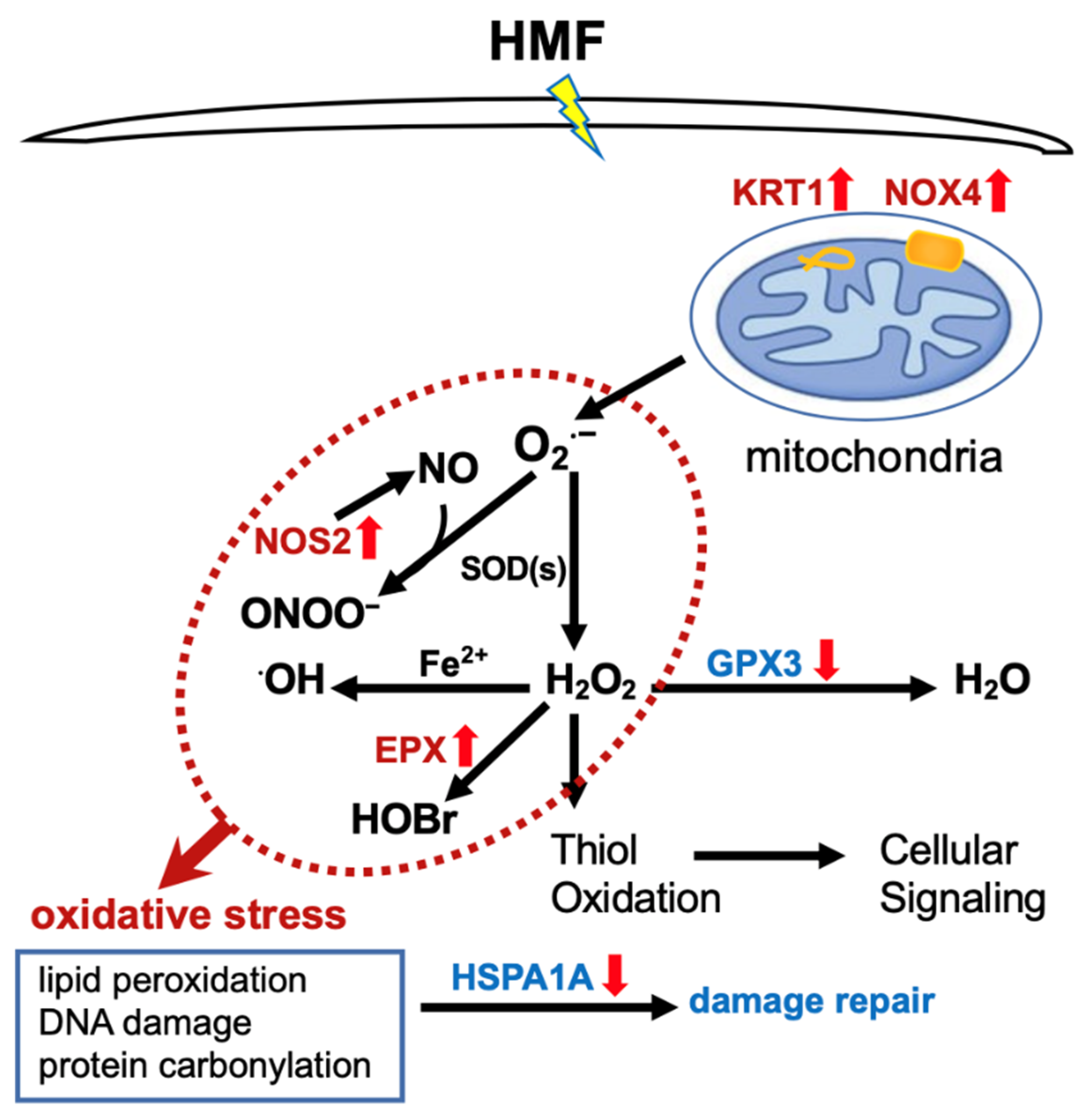
2.3. Differentially Expressed Genes Associated with Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Animals and Magnetic Fields Exposure
4.2. Behavioral Tests
4.2.1. Open-Field Test
4.2.2. OLT or NOR Test
4.3. Immunofluorescence Analysis of the Endogenous ROS Levels
4.4. PCR Arrays Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarduno, J.A.; Cottrell, R.D.; Watkeys, M.K.; Hofmann, A.; Doubrovine, P.V.; Mamajek, E.E.; Liu, D.; Sibeck, D.G.; Neukirch, L.P.; Usui, Y. Geodynamo, solar wind, and magnetopause 3.4 to 3.45 billion years ago. Science 2010, 327, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Gargaud, M. Young Sun, Early Earth and the Origins of Life: Lessons for Astrobiology, 1st ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Kirschvink, J.L.; Jones, D.S.; MacFadden, B.J. Magnetite Biomineralization and Magnetoreception in Organisms: A New Biomagnetism; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Winklhofer, M.; Kirschvink, J.L. A quantitative assessment of torque-transducer models for magnetoreception. J. R. Soc. Interface 2010, 7, S273–S289. [Google Scholar] [CrossRef] [PubMed]
- Wiltschko, W.; Wiltschko, R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 2005, 191, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Eder, S.H.K.; Cadiou, H.; Muhamad, A.; McNaughton, P.A.; Kirschvink, J.L.; Winklhofer, M. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12022–12027. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, K.J.; Endres, C.S.; Putman, N.F.; Ernst, D.A.; Lohmann, C.M.F. Natal Homing and Multi-modal Navigation in Sea Turtles and Salmon. Integr. Comp. Biol. 2017, 57, E330. [Google Scholar]
- Wei, Y.; Pu, Z.Y.; Zong, Q.G.; Wan, W.X.; Ren, Z.P.; Fraenz, M.; Dubinin, E.; Tian, F.; Shi, Q.Q.; Fu, S.Y.; et al. Oxygen escape from the Earth during geomagnetic reversals: Implications to mass extinction. Earth Planet. Sci. Lett. 2014, 394, 94–98. [Google Scholar] [CrossRef]
- Channell, J.E.T.; Vigliotti, L. The Role of Geomagnetic Field Intensity in Late Quaternary Evolution of Humans and Large Mammals. Rev. Geophys. 2019, 57, 709–738. [Google Scholar] [CrossRef]
- Cooper, A.; Turney, C.S.M.; Palmer, J.; Hogg, A.; McGlone, M.; Wilmshurst, J.; Lorrey, A.M.; Heaton, T.J.; Russell, J.M.; McCracken, K.; et al. A global environmental crisis 42,000 years ago. Science 2021, 371, 811–818. [Google Scholar] [CrossRef]
- Mo, W.C.; Liu, Y.; He, R.Q. Hypomagnetic field, an ignorable environmental factor in space? Sci. China Life Sci 2014, 57, 726–728. [Google Scholar] [CrossRef][Green Version]
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytical review of experiments and theories. PLoS ONE 2017, 12, e0179340. [Google Scholar] [CrossRef]
- Binhi, V.N.; Sarimov, R.M. Zero Magnetic Field Effect Observed in Human Cognitive Processes. Electromagn. Biol. Med. 2009, 28, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lu, H.; Xi, W.; Zhou, X.; Xu, S.; Zhang, K.; Jiang, J.; Li, Y.; Guo, A. Exposure to hypomagnetic field space for multiple generations causes amnesia in Drosophila melanogaster. Neurosci. Lett. 2004, 371, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Xu, M.L.; Li, B.; Li, D.F.; Jiang, J.C. Long-term memory was impaired in one-trial passive avoidance task of day-old chicks hatching from hypomagnetic field space. Chin. Sci. Bull. 2003, 48, 2454–2457. [Google Scholar]
- Xavier, G.F.; Costa, V.C. Dentate gyrus and spatial behaviour. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Hainmueller, T.; Bartos, M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci. 2020, 21, 153–168. [Google Scholar] [CrossRef]
- Manns, J.R.; Eichenbaum, H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009, 16, 616–624. [Google Scholar] [CrossRef]
- Burger, T.; Lucova, M.; Moritz, R.E.; Oelschlager, H.H.; Druga, R.; Burda, H.; Wiltschko, W.; Wiltschko, R.; Nemec, P. Changing and shielded magnetic fields suppress c-Fos expression in the navigation circuit: Input from the magnetosensory system contributes to the internal representation of space in a subterranean rodent. J. R. Soc. Interface 2010, 7, 1275–1292. [Google Scholar] [CrossRef]
- Bingman, V.P.; Pemberton, M.L.; Mora, C.V. Avian forebrain processing of magnetic intensity and inclination: Hippocampus, anterior forebrain Wulst and an unexpected double-dissociation. Ethol. Ecol. Evol. 2021, 33, 230–247. [Google Scholar] [CrossRef]
- Bingman, V.P.; Able, K.P. Maps in birds: Representational mechanisms and neural bases. Curr. Opin. Neurobiol. 2002, 12, 745–750. [Google Scholar] [CrossRef]
- Chauhan, P.; Jethwa, K.; Rathawa, A.; Chauhan, G.; Mehra, S. The Anatomy of the Hippocampus. In Cerebral Ischemia; Pluta, R., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef]
- Amaral, D.G.; Scharfman, H.E.; Lavenex, P. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 2007, 163, 3–22. [Google Scholar] [PubMed]
- Zhang, B.F.; Wang, L.; Zhan, A.S.; Wang, M.; Tian, L.X.; Guo, W.X.; Pan, Y.X. Long-term exposure to a hypomagnetic field attenuates adult hippocampal neurogenesis and cognition. Nat. Commun. 2021, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Sritawan, N.; Suwannakot, K.; Naewla, S.; Chaisawang, P.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Effect of metformin treatment on memory and hippocampal neurogenesis decline correlated with oxidative stress induced by methotrexate in rats. Biomed. Pharmacother. 2021, 144, 112280. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch. Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, L. Reactive Oxygen Species: Potential Regulatory Molecules in Response to Hypomagnetic Field Exposure. Bioelectromagnetics 2020, 41, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Calabrese, V.; Guagliano, E.; Sapienza, M.; Mancuso, C.; Butterfield, D.A.; Stella, A.M. Redox regulation of cellular stress response in neurodegenerative disorders. Ital. J. Biochem. 2006, 55, 263–282. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Barnes, P.W.; Robson, T.M.; Neale, P.J.; Williamson, C.E.; Zepp, R.G.; Wilson, S.R.; Madronich, S.; Andrady, A.L.; Heikkila, A.M.; et al. Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2020. Photochem. Photobiol. Sci. 2021, 20, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Gupta, R.; Sahu, M.; Srivastava, D.; Das, A.; Ambasta, R.K.; Kumar, P. Free radical biology in neurological manifestations: Mechanisms to therapeutics interventions. Environ. Sci. Pollut. Res. Int. 2021, 1–48. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Fu, J.P.; Mo, W.C.; Liu, Y.; He, R.Q. Decline of cell viability and mitochondrial activity in mouse skeletal muscle cell in a hypomagnetic field. Bioelectromagnetics 2016, 37, 212–222. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhang, Z.J.; Mo, W.C.; Hu, P.D.; Ding, H.M.; Liu, Y.; Hua, Q.; He, R.Q. Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase. Protein Cell 2017, 8, 527–537. [Google Scholar] [CrossRef]
- Wang, H.Z.; Zhang, X. Magnetic Fields and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 2175. [Google Scholar] [CrossRef]
- Ding, H.M.; Wang, X.; Mo, W.C.; Qin, L.L.; Wong, S.; Fu, J.P.; Tan, Y.; Liu, Y.; He, R.Q.; Hua, Q. Hypomagnetic fields cause anxiety in adult male mice. Bioelectromagnetics 2019, 40, 27–32. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxid. Med. Cell Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Soulimani, R. Evidence that hydrogen peroxide, a component of oxidative stress, induces high-anxiety-related behaviour in mice. Behav. Brain Res. 2019, 359, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid. Med. Cell Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, K.; Nayak, B.K.; Friedrichs, W.E.; Kaushik, D.; Rodriguez, R.; Block, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y.; Abboud, H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 14385–14390. [Google Scholar] [CrossRef] [PubMed]
- Bekhite, M.M.; Figulla, H.R.; Sauer, H.; Wartenberg, M. Static magnetic fields increase cardiomyocyte differentiation of Flk-1+ cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int. J. Cardiol. 2013, 167, 798–808. [Google Scholar] [CrossRef]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef]
- Colon, S.; Luan, H.Y.; Liu, Y.; Meyer, C.; Gewin, L.; Bhave, G. Peroxidasin and eosinophil peroxidase, but not myeloperoxidase, contribute to renal fibrosis in the murine unilateral ureteral obstruction model. Am. J. Physiol.-Renal. 2019, 316, F360–F371. [Google Scholar] [CrossRef]
- Panthi, S.; Manandhar, S.; Gautam, K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl. Neurodegener. 2018, 7, 3. [Google Scholar] [CrossRef]
- Lowenstein, C.J. Metabolism reprogrammed by the nitric oxide signalling molecule. Nature 2019, 565, 33–34. [Google Scholar] [CrossRef]
- Tassorelli, C.; Greco, R.; Morocutti, A.; Costa, A.; Nappi, G. Nitric oxide-induced neuronal activation in the central nervous system as an animal model of migraine: Mechanisms and mediators. Funct. Neurol. 2001, 16 (Suppl. S4), 69–76. [Google Scholar] [PubMed]
- Sun, J.; Cai, X.; Wang, C.; Du, K.; Chen, W.; Feng, F.; Wang, S. Cascade Reactions by Nitric Oxide and Hydrogen Radical for Anti-Hypoxia Photodynamic Therapy Using an Activatable Photosensitizer. J. Am. Chem. Soc. 2021, 143, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Mercatelli, N.; Caporossi, D. Exercise-induced ROS in heat shock proteins response. Free Radic. Biol. Med. 2016, 98, 46–55. [Google Scholar] [CrossRef]
- Bernal, S.D.; Stahel, R.A. Cytoskeleton-associated proteins: Their role as cellular integrators in the neoplastic process. Crit. Rev. Oncol. Hematol. 1985, 3, 191–204. [Google Scholar] [CrossRef]
- Silvander, J.S.G.; Kvarnstrom, S.M.; Kumari-Ilieva, A.; Shrestha, A.; Alam, C.M.; Toivola, D.M. Keratins regulate beta-cell mitochondrial morphology, motility, and homeostasis. Faseb. J. 2017, 31, 4578–4587. [Google Scholar] [CrossRef]
- Na, N.; Chandel, N.S.; Litvan, J.; Ridge, K.M. Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. Faseb. J. 2010, 24, 799–809. [Google Scholar] [CrossRef]
- Vinokurov, A.Y.; Stelmashuk, O.A.; Ukolova, P.A.; Zherebtsov, E.A.; Abramov, A.Y. Brain region specificity in reactive oxygen species production and maintenance of redox balance. Free Radic. Biol. Med. 2021, 174, 195–201. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Finkel, T. Unraveling the truth about antioxidants: ROS and disease: Finding the right balance. Nat. Med. 2014, 20, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, D.; Mevissen, M. Manmade Electromagnetic Fields and Oxidative Stress-Biological Effects and Consequences for Health. Int. J. Mol. Sci. 2021, 22, 3772. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Magnetic Fields and Cancer: Epidemiology, Cellular Biology, and Theranostics. Int. J. Mol. Sci. 2022, 23, 1339. [Google Scholar] [CrossRef]
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar]
- Yang, Y.; Liu, Y.; Zhu, J.; Song, S.; Huang, Y.; Zhang, W.; Sun, Y.; Hao, J.; Yang, X.; Gao, Q.; et al. Neuroinflammation-mediated mitochondrial dysregulation involved in postoperative cognitive dysfunction. Free Radic. Biol. Med. 2022, 178, 134–146. [Google Scholar] [CrossRef]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. JoVE 2018, 141, e58593. [Google Scholar] [CrossRef]
| Gene Symbol | Gene Full Name | Fold Regulation |
|---|---|---|
| Nox4 | NADPH oxidase 4 | 2.55 |
| Epx | eosinophil peroxidase | 1.85 |
| Krt1 | keratin 1 | 1.86 |
| Nos2 | nitric oxide synthase 2 | 1.60 |
| Gpx3 | glutathione peroxidase 3 | −1.70 |
| Hspa1a | heat shock protein 1A | −1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Luo, Y.; Zhan, A.; Ren, J.; Qin, H.; Pan, Y. Hypomagnetic Field Induces the Production of Reactive Oxygen Species and Cognitive Deficits in Mice Hippocampus. Int. J. Mol. Sci. 2022, 23, 3622. https://doi.org/10.3390/ijms23073622
Tian L, Luo Y, Zhan A, Ren J, Qin H, Pan Y. Hypomagnetic Field Induces the Production of Reactive Oxygen Species and Cognitive Deficits in Mice Hippocampus. International Journal of Molecular Sciences. 2022; 23(7):3622. https://doi.org/10.3390/ijms23073622
Chicago/Turabian StyleTian, Lanxiang, Yukai Luo, Aisheng Zhan, Jie Ren, Huafeng Qin, and Yongxin Pan. 2022. "Hypomagnetic Field Induces the Production of Reactive Oxygen Species and Cognitive Deficits in Mice Hippocampus" International Journal of Molecular Sciences 23, no. 7: 3622. https://doi.org/10.3390/ijms23073622
APA StyleTian, L., Luo, Y., Zhan, A., Ren, J., Qin, H., & Pan, Y. (2022). Hypomagnetic Field Induces the Production of Reactive Oxygen Species and Cognitive Deficits in Mice Hippocampus. International Journal of Molecular Sciences, 23(7), 3622. https://doi.org/10.3390/ijms23073622






