Abstract
Iron is a trace metal element necessary to maintain life and is also involved in a variety of biological processes. Aging refers to the natural life process in which the physiological functions of the various systems, organs, and tissues decline, affected by genetic and environmental factors. Therefore, it is imperative to investigate the relationship between iron metabolism and aging-related diseases, including neurodegenerative diseases. During aging, the accumulation of nonheme iron destroys the stability of the intracellular environment. The destruction of iron homeostasis can induce cell damage by producing hydroxyl free radicals, leading to mitochondrial dysfunction, brain aging, and even organismal aging. In this review, we have briefly summarized the role of the metabolic process of iron in the body, then discussed recent developments of iron metabolism in aging and age-related neurodegenerative diseases, and finally, explored some iron chelators as treatment strategies for those disorders. Understanding the roles of iron metabolism in aging and neurodegenerative diseases will fill the knowledge gap in the field. This review could provide new insights into the research on iron metabolism and age-related neurodegenerative diseases.
1. Introduction
Iron is the most abundant transition metal in humans [1], which is distributed in almost all tissues and organs, such as the liver, spleen, kidney, heart, skeletal muscle, and brain. Iron exists in many forms: one is functional iron existing in hemoglobin, myoglobin, enzymes, and cofactors, and another is reserve iron existing in ferritin and hemosiderin [2]. Iron is the auxiliary group of many key enzymes and participates in a series of important physiological processes such as oxygen transport, DNA synthesis and repair, and mitochondrial function maintenance [3]. Iron deficiency and iron overload will cause different degrees of harm to the organism. For example, low concentrations of iron result in consequent anemia [4]. By contrast, high concentrations of labile iron are highly toxic to cells increasing the risk of cancer, diabetes, neurodegenerative diseases, and cardiovascular diseases [5]. Importantly, iron overload can also cause a new type of iron-dependent cell death—Ferroptosis [6]. Thus, iron can act as a double-edged sword, which necessitates accurate regulation of its cellular levels and exquisite equilibrium between iron absorption, circulation, storage, and regulation.
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death [7]. Currently, there are many coexisting theories about the hallmarks of aging, such as the telomere degradation theory, mitochondrial function damage theory, and oxidative damage theory [7]. Among them, the oxidative damage theory holds that reactive oxygen species (ROS) damage biological macromolecules such as lipids, proteins, and DNA, which leads to aging [8]. Mitochondria are the main source of ROS in cells. After reaching mitochondria, iron is mainly used to synthesize heme and iron–sulfur clusters [9]. Excessive iron has strong catalytic potential, which can increase ROS production and cause oxidative damage to cells, eventually leading to cellular senescence. A large number of studies have shown that the loss of mitochondrial iron homeostasis can result in mitochondrial function decline, and then lead to aging [10,11]. Thus, it can be seen that aging has a profound impact on iron homeostasis and it is necessary to elaborate on the complex interactions between iron homeostasis and aging.
Neurodegenerative diseases are caused by the loss of neurons and will worsen over time. As it stands now, iron is indeed a key factor in neurodegenerative diseases [12]. For instance, the imbalance of iron homeostasis in the brain may be at the core of many age-related neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [13,14]. Some less common neurodegenerative diseases, mainly including a series of neurodegeneration with brain iron accumulation (NBIA), have been widely studied and reported [15,16]. Following that, more and more iron chelators are being used to study the treatment of neurodegenerative diseases. This is just another imperative way to explore the potential therapeutic approaches for neurodegenerative diseases through the regulation of iron metabolism.
In this paper, we first describe the metabolic process of iron in the body. Secondly, we review the research advances between iron metabolism and aging and neurodegenerative diseases. Finally, we present some iron chelators as treatment strategies for the cure of age-related neurodegenerative diseases. It is hoped that this review can provide a deep understanding of the role of iron metabolism in aging and age-related neurodegenerative diseases.
2. Iron Metabolism
Maintaining safe iron levels is critical for sustaining iron homeostasis. However, under aerobic conditions, iron also has potential toxicity [17]. Controlling the absorption, circulation, storage, and regulation of iron at both the cellular and systemic levels limits the toxic effects of excessive or abnormal distribution of iron. Specific iron metabolism processes are governed by complex pathways (Figure 1).
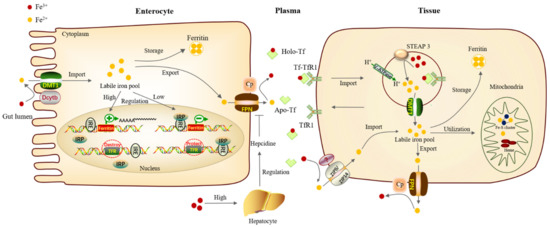
Figure 1.
Iron metabolism process in mammals. The absorption, circulation, storage, and regulation of iron collaborate closely together to maintain the iron homeostasis of organisms. Firstly, Fe3+ in gut lumen is reduced into Fe2+ by Dcytb positioned on the parietal membrane of intestinal epithelial cells, then transported into the iron pool in the cytoplasm through DMT1. A part of iron forms ferritin for storage, and excess iron is transported out of the cells through FPN of the basal lateral membrane, then it is oxidized into Fe3+ by Cp. Fe3+ can combine with transferrin in plasma and transport to other tissues requiring iron through blood circulation. In tissues, after combining with transferrin receptor on the surface of the tissue needing iron, protein complex Tf-TfR1 enters cells through endocytosis. The acidification of early endosomes will change the conformation of Tf-TfR1 and promote the release of Fe3+. This acidification is mainly supported by the V-ATPase on the endosome through proton exchange. Fe3+ is then reduced to Fe2+ by STEAP3 and then enters cytoplasm through DMT1 for utilization. One part of the iron can be stored as ferritin, while other part of iron can enter the mitochondria for the synthesis of iron–sulfur clusters and heme, and the rest of iron is transported out of the cells through FPN. In iron deficiency, the IRPs bind to the IREs, protecting the TfR mRNA from nuclease digestion and preventing the synthesis of ferritin. When iron is abundant, the modified IRP no longer binds to the IREs, making TfR mRNA to be destroyed and allowing the expression of ferritin. Dcytb, Duodenal cytochrome b; DMT1, Divalent metal-ion transporter 1; FPN, Ferroportin; Cp, Ceruloplasmin; V-ATPase, vacuolar H+-ATPase; STEAP, Six-transmembrane epithelial antigen of prostate 3.
In mammals, most of the iron ingested from food exists in the form of Fe3+ which is then reduced to Fe2+ by Duodenal cytochrome b (Dcytb) on the apical surface of intestinal epithelial cells. Fe2+ can be directly exported into the plasma through the Divalent metal-ion transporter 1 (DMT1) [18]. Besides, ZIP8/14 can also mediate iron absorption by acting as a cellular iron importer on the cell surface of a particular tissue [19,20]. Iron imported into the cell forms the cytoplasmic labile iron pool (LIP), which acts as an intermediate between imported, stored, and utilized iron [21]. The excess Fe2+ needs to be oxidized to Fe3+ by Hephaestin (Heph) and Ceruloplasmin (Cp), then be exported through the iron export protein, Ferroportin (FPN) [18].
Iron entering the circulatory system can combine with Transferrin (Tf) in plasma, then be transported to various tissues and organs hungry for iron in mammals. Transferrin carrying iron combines with transferrin receptor 1 (TfR1) on the cell membrane and is then internalized into the cell through endocytosis [22]. The acidification of early endosomes will change the conformation of the protein complex Tf-TfR1 and promote the release of Fe3+. This acidification is mainly supported by the vacuolar H+-ATPase (V-ATPase) on the endosome through proton exchange [23]. The six-transmembrane epithelial antigen of the prostate 3 (STEAP3) will reduce ferric Fe3+ to ferrous Fe2+, and then Fe2+ is released from the endosomes into the cytoplasm via DMT1 for utilization [24].
Some of the iron can be stored in the form of ferritin, while others can enter the mitochondria for the synthesis of iron–sulfur clusters and heme, and the excess iron is transported out of the cells through FPN [18]. In mammals, ferritin mainly exists in the cytoplasm, which is a hollow complex formed by light-chain homo (LCH) and heavy-chain homo (HCH). It can chelate about 4500 iron ions and is an important iron storage protein and detoxification protein [25]. Ferritin plays an important physiological role in maintaining iron homeostasis. For example, in Drosophila, reducing the levels of the ferritin heavy chain in the larval wing discs leads to drastic growth defects [26]. Moreover, in human metastatic melanoma cells, high ferritin expression can enhance cell growth and improve resistance to oxidative stress by interfering with their cellular antioxidant system [27].
In humans, efficient orchestration of iron absorption is critical for maintaining iron homeostasis. Hepcidin secreted by liver parenchymal cells is a key protein and the most important negative regulatory hormone for iron metabolism [28]. An increase in plasma and tissue iron levels stimulates hepcidin production in the liver and the levels of FPN protein on intestinal epithelial cells decrease, which results in inhibiting the absorption of iron in the intestine and a decrease in serum iron concentration [29]. On the other hand, cellular iron homeostasis is also controlled by influencing mRNA translation. Changes in iron status lead to compensating changes in the iron regulatory protein (IRP)/iron regulatory element (IRE) system of iron homeostasis. When iron concentrations are depleted, the IRPs bind to the IREs in the 3′- and 5′- untranslated regions (UTR), protecting the TfR mRNA from nuclease digestion and preventing the synthesis of ferritin in ferritin mRNA. Conversely, when there is surplus iron, the modified IRP no longer binds to the IREs, allowing TfR mRNA to be destroyed and allowing the expression of ferritin [30].
Regulation of iron homeostasis is an intricate process involving multiple modulators at the molecular, cellular, and systemic levels. Cross-talks between different levels are key to sensing iron levels, adjusting absorption, and recycling accordingly, which together maintain the iron homeostasis of the organism.
3. Iron Metabolism and Aging
Aging refers to the natural life process in which the physiological functions of various systems, organs, and tissues decrease due to the aging process, which is influenced by genetic factors and environmental factors [7]. In recent years, people have paid more attention to the role of iron ions in aging. Iron is a transition metal with two main biological oxidation states, and it is also a key intermediate for producing active oxygen [31]. Excessive accumulation of iron ions in aging cells produces excessive reactive oxygen species, which can lead to DNA damage and inhibit its repair function, subsequently accelerating the aging process, which is defined as Ferrosenescence [32].
3.1. Iron and the Production of ROS
Reactive oxygen species (ROS) are chemically reactive substances containing oxygen, including superoxide (O2−), free radicals (HO− and RO−), and peroxides (H2O2 and ROOH) [33]. Iron can participate in the formation of ROS in cells, which eventually leads to cytotoxicity. The Fe2+ and hydrogen peroxide in the organism can oxidize various substrates and cause biological damage, simultaneously producing hydroxyl radicals and a high oxidation state of iron. This reaction is called the Fenton reaction (Figure 2). The ability of iron to exchange single electrons with many substrates can lead to the generation of ROS, oxidative stress, lipid peroxidation, and DNA damage. These results will lead to genome instability and DNA repair defects, which ultimately damage cell vitality and promote programmed cell death [34]. In addition, iron and iron-containing complexes (such as heme or iron–sulfur clusters) are also necessary for ROS-producing enzymes [35]. It can be seen that the iron content in the body is particularly important for maintaining the level of ROS in cells.
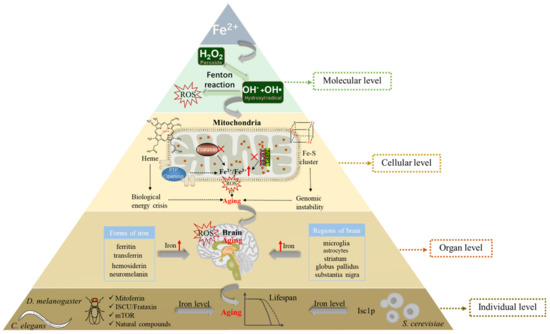
Figure 2.
Relationship between iron and aging at the molecular, cellular, organ, and individual levels. At the molecular level, excessive iron in cells can participate in the Fenton reaction to generate ROS, resulting in damage to biological macromolecules and leading to aging. At the cellular level, defects in heme and iron–sulfur cluster biosynthesis in mitochondria with aging can lead to an energy crisis in organisms and instability of genomes. In addition, decreased Frataxin and Nrp1 levels or increased PTP levels promote the iron content of mitochondria, leading to aging through ROS. At the organ level, the age-associated increase in iron deposition varies between different brain regions and cell types, and then various molecular forms (ferritin, transferrin, hemosiderin, neuromelanin) of iron can deposit in the brain, resulting in brain aging. At the individual level, model organisms (S. cerevisiae, C. elegans, D. melanogaster) can regulate the iron level of the body through different mechanisms (such as mitoferrin, ISCU/Frataxin, mTOR, natural compounds, Isc1p), eventually leading to individual aging. The red fork represents the loss of function; The red upward arrow represents the increase in content. ROS, reactive oxygen species; Nrp1, neuropilin-1; ABCB8, mitochondrial transporter ATP-binding cassette B8; PTP, permeability transition pore; ISCU, iron–sulfur cluster (ISC) assembly machinery central protein; Isc1p, inositolphosphosphingolipid phospholipase C.
3.2. Iron Accumulation in the Mitochondria and Aging
Mitochondria are active participants and are mechanistically linked to the unique biology of senescence [36]. The free radical theory of aging proposes that the cause of aging lies in the production of ROS at the level of the mitochondria, which over time causes extensive mitochondrial and cellular dysfunction [10]. Recent studies in yeast and mammals have shown that iron in mitochondria increases with age, especially under cell stress, which may be the potential cause of age-related mitochondrial dysfunction [37,38].
Iron in mitochondria is mainly used for the synthesis of heme or iron–sulfur clusters [39]. Frataxin is a response gene of Friedreich’s ataxia, that is necessary for the synthesis of heme or iron–sulfur clusters and plays an important role in the iron homeostasis of mitochondria [40]. When Frataxin is insufficient, iron will accumulate in the mitochondria, causing oxidative stress and mitochondrial dysfunction, eventually leading to cell apoptosis or aging [41,42]. In Drosophila, Frataxin-deficient flies are highly sensitive to increased dietary iron uptake, while their mitochondrial function can be restored by inhibiting iron uptake via mitoferrin [43]. New research has found lactoferrin ameliorates pathological cardiac hypertrophy, potentially by improving the mitochondrial quality related to mitochondrial dynamics, thus reducing mitochondria-dependent apoptosis [44].
Altered mechanisms of mitochondrial iron transport cause an accumulation of mitochondrial iron, which causes the decay of mitochondrial structural components, such as proteins, lipids, and nucleic acids. Recent studies have shown that the mitochondrial transmembrane protein Neuropilin-1 (Nrp1) and its interaction factor the mitochondrial transporter ATP-binding cassette B8 (ABCB8) play a critical role in iron accumulation and senescence in endothelial cells. The absence of Nrp1 reduces the protein level of ABCB8, which leads to the accumulation of iron in the mitochondria, inducing iron-dependent oxidative stress and aging [45]. Another study suggests that there is an increase in the susceptibility of the permeability transition pore (PTP) which may cause cellular degeneration via apoptosis or necrosis [46]. In yeast, cysteine-mediated iron deficiency is the main cause of aging-related mitochondrial dysfunction [37]. The loss of vacuolar/lysosomal acidity is an early event during aging that has been linked to mitochondrial dysfunction. Increased iron uptake may salvage the phenotype of mitochondrial dysfunction in yeast vma mutants, which have lost vacuolar acidity due to genetic disruption of the V-ATPase proton pump [47].
Mitochondrial dysfunction during aging is manifested by multiple defects in heme and iron–sulfur cluster biosynthesis in mitochondria with the increase of age, which may lead to an energy crisis of organisms and instability of genomes [48,49]. On the one hand, during the aging process of human fibroblasts, heme deficiency can selectively reduce the expression and activity of cytochrome C oxidase (complex IV), which is the terminal oxidase in the mitochondrial electron transport chain [50]. Therefore, it can be confirmed that age-related heme deficiency leads to impaired mitochondrial energy production by inhibiting complex IV. On the other hand, it was identified that there was a link between the defect of iron–sulfur cluster biosynthesis and age-related genomic instability in yeast [48]. Yeast cells will lose mitochondrial DNA (mt DNA) along with aging, which destroys the stability of the genome and leads to the reduction of mitochondrial membrane potential, finally leading to mitochondrial dysfunction. In aged muscle, cellular and mitochondrial iron homeostasis is perturbed, as reflected by altered levels of mitoferrin and frataxin, which might contribute to loss of mtDNA stability [51]. A recent study has demonstrated that lipoic acid supplementation may be considered as an adjuvant against mitochondrial damage and cognitive decline related to aging and neurodegenerative disorders [52]. Collectively, the above results indicated that iron was closely related to mitochondrial aging, therefore iron regulation may represent a possible target for aging interventions.
3.3. Iron Accumulation and Brain Aging
Ferrosenescence mainly occurs in the nervous system, which is closely related to the excessive deposition of iron ions in the brain [32]. The brain plays a unique role in iron metabolism because of the following characteristics: First, the brain resides behind the vascular barrier, which limits its access to plasma iron. However, little is known about the mechanism of iron release into the brain or the regulation of the transport mechanism. Insights into this transport mechanism could be crucial for understanding how an excess of iron can accumulate in the brain in many neurodegenerative diseases. Second, the iron concentration varies greatly between different brain regions and cell types. Autopsy analysis showed that the total iron concentration in the substantia nigra and globus pallidus of the basal ganglia increased with age [53]. Ashraf and colleagues found that the ratio of iron to microglia in an aging brain is elevated, especially in the basal ganglia, whereas the ratio of iron to astroglia is low in the striatum but elevated in the substantia nigra and globus pallidus. During aging, the astrocytes are vulnerable and susceptible to iron accumulation and oxidative damage [54]. In addition, various molecular forms of iron (ferritin, neuromelanin, transferrin, hemosiderin) between neurons and glial cells can be observed during aging [13,55]. Young individuals had relatively high levels of heavy-chain (H) ferritin compared to light-chain (L) ferritin; However, H and L-ferritin in the frontal cortex, caudate nucleus, putamen, substantia nigra, and globus pallidus are increased with age [56]. Notably, neuromelanin is the most prominent iron storage site in the substantia nigra and locus coeruleus, and its level will increase with age [57]. In short, the accumulation of iron will increase with individual aging in the nervous system.
As the mechanism of brain aging is very complex, it has not been fully understood. Nevertheless, increasing evidence shows that the damage to macromolecules and cell components caused by the destruction of cell redox balance and the imbalance of metal homeostasis is part of the cause of brain aging [58,59]. During brain aging, iron is partially transformed from its stable and soluble form (ferritin) to hemosiderin and other iron-containing hydroxides with higher reactivity, leading to neurons being susceptible to oxidative stress [60]. The transcription factor NF-E2-related factor 2 (Nrf2) is a central regulator of cellular antioxidant and detoxification responses. Studies have shown that the knockout of Nrf2 can reduce the level of iron transporter 1 (FPN1) in brain microvascular endothelial cells, thus reducing brain iron deposition and alleviating age-related motor dysfunction in aging mice [61]. Furthermore, recent evidence has suggested that iron accumulation, as observed in neurodegenerative disorders, hinders autophagy, which might play a part in iron-induced neurotoxicity. Rapamycin, by inducing autophagy, was able to ameliorate iron-induced cognitive impairments. These findings support the use of rapamycin as a potential neuroprotective treatment against the cognitive decline associated with neurodegenerative disorders [62]. Thus, a better understanding of the effect of iron homeostasis on brain aging may provide important insights into a better understanding of age-associated diseases.
3.4. Iron Metabolism and Life Expectancy
Iron content increases significantly with age, resulting in increased ROS production. This harmful process is not only limited to the central nervous system but also reflected in the whole individual, specifically in the length of their lifespan. In many model organisms such as worm, fly, and yeast, increasing antioxidant level [63] or inhibiting iron level by using iron chelators or using genetic manipulation to regulate iron metabolic processes [64,65] has been proved to prolong the lifespan.
The first evidence that inhibiting iron absorption prolongs lifespan was demonstrated in Drosophila [64]. By feeding male flies with a high-iron diet and tea extract, the results show that increasing dietary iron can shorten the lifespan, while dietary tea can prevent age-related iron accumulation. It has been reported that galloyl in tea polyphenols can inhibit iron-binding [66]. In addition, polyphenol compounds in tea extract not only inhibit iron absorption but also have a direct antioxidant effect, both of which may lead to prolonging the lifespan of Drosophila. A new study has shown that green tea may increase the lifespan of fruit flies by regulating mitoferrin and reducing mitochondrial iron [66]. As mitoferrin is required for iron to enter the mitochondria, reducing the mitoferrin level through RNAi treatment in the N2 wild-type strain can extend the lifespan by 50% to 80% in C. elegans [67]. Another finding indicates that iron-starvation-induced mitophagy as a protective mechanism against mitochondrial stress mediates lifespan extension in C. elegans [68]. Similarly, increased dietary iron intake significantly accelerates age-related protein aggregation in the worm and negatively affects organismal longevity [69].
In addition, many natural products and drugs prolong lifespans by seemingly completely different mechanisms, but most of them can chelate iron, so we can reasonably speculate that the life-prolonging mechanism of these compounds can be attributed to the reduction of the iron level in the cell. For example, curcumin is a strong iron-chelating agent. Animals fed curcumin have a decline in liver ferritin [70]. Mice fed 0.2% of curcumin in the diet become iron deficient [71]. It is interesting that curcumin and its metabolite tetrahydrocurcumin increase the average lifespan in at least three model organisms: C. elegans, Drosophila, and mice [72]. Ibuprofen can prolong the lives of many organisms [73]. Similarly, ibuprofen chelates iron, in this way, prevents oxidant lung injury [65]. Moreover, zebrafish have also shown that increased cellular iron concentration has negative health impacts that can be potentially ameliorated by iron chelation [74].
In addition to extending lifespan by chelating iron, genetically manipulating iron-related genes can be applied to alter iron levels. It is known that the inositolphosphosphingolipid phospholipase C (Isc1p) of S. cerevisiae belongs to the neutral sphingomyelinase family. The microarray analysis shows that Isc1p lacks an up-regulated iron regulator, resulting in elevated iron levels. Cells lacking Isc1p also displayed a shortened chronological lifespan which may attribute to the change in iron level [75]. Yeast MET18 is a subunit of the cytosolic iron-sulfur (Fe/S) protein assembly (CIA) machinery which is responsible for the maturation of Fe/S proteins. MET18 deficiency shortens the replicative lifespan of yeast by inhibiting catalase activity [76]. Studies have shown that calorie restriction can improve life expectancy because it can reduce ROS production and cell iron [77]. Apart from this, it was found that ISCU-1, the C. elegans ortholog of the evolutionarily conserved iron–sulfur cluster (ISC) assembly machinery central protein ISCU, suppressed longevity and stress response [78]. Specifically, ISCU-1 can accelerate aging of the intestine. Moreover, the Nrf2 transcription factor SKN-1 and a nuclear hormone receptor NHR-49 as the downstream factors of ISCU-1 have been identified. In addition, a mitochondrial outer membrane protein phosphatase PGAM-5 appears to link ISCU-1 to SKN-1 and NHR-49 in lifespan regulation. Frataxin can participate in the formation of the core complex NSF1/ISD11/ISCU, and its overexpression may contribute to the assembly of iron–sulfur clusters [79], thus inhibiting iron retention. In mice model of frataxin-depleted neurons, administration of fusion protein (TAT-MTScs-FXN) was able to produce a significant lifespan increase [80]. Genetic studies showed that the lack of Frataxin in C. elegans would shorten the lifespan [81], while the overexpression of Frataxin in Drosophila prolonged the lifespan [82], thus establishing the relationship between Frataxin and lifespan.
Moreover, in nematodes, by inhibiting lipid peroxidation or limiting iron retention, age-related cell death can be alleviated, thus the lifespan and healthspan can be significantly prolonged [83]. It is well known that the lifespan of a variety of model animals can be extended by inhibiting the mammalian target of rapamycin (mTOR), the important regulator of cell growth and proliferation. Studies have shown that mTOR plays a vital role in the body’s iron reserve. Specifically, activation of mTOR leads to an increase in body iron levels, which in turn activates mTOR. Therefore, one of the mechanisms inhibiting mTOR in prolonging lifespan may be through inhibiting body iron levels [84]. In addition, ferroptosis is an iron-dependent, nonapoptotic programmed cell death. A recent study indicated that the senolytic drug JQ1 can eliminate senescent cells via ferroptosis, suggesting ferroptosis as a new mechanism of senolytic therapy [85]. The above research implies that inhibiting iron levels may be one of the new ways to delay aging.
In short, at the molecular level, excessive iron in cells can generate a large number of reactive oxygen species (ROS) in the Fenton reaction, finally leading to aging. At the cellular level, mitochondrial iron levels change with aging, which in turn leads to mitochondrial dysfunction, causing further aging. Furthermore, at the organ level, iron concentration can increase in different areas and cell types of the brain, and various molecular forms of iron (ferritin, neuromelanin, transferrin, hemosiderin) can deposit in the brain, which together results in brain aging. Ultimately, at the individual level, model organisms such as S. cerevisiae, C. elegans, and D. melanogaster regulate the iron content of the body through different mechanisms, eventually leading to individual aging (Figure 2).
4. Iron Metabolism and Neurodegenerative Diseases
Currently, many studies have shown that metals are related to the pathogenesis and mechanisms of nervous system diseases [86,87]. There is enough evidence to prove that iron is indeed a key factor in neurodegenerative diseases [12]. In recent years, there has been increasing evidence that many neurodegenerative diseases are related to the excessive iron content in the brain. Progressive iron accumulation in the brain may produce free radicals via the Fenton reaction, promoting the occurrence of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative disorders.
4.1. Iron Metabolism and Alzheimer’s Disease
Alzheimer’s disease (AD) is a slow, progressive neurodegenerative disease involving the loss of cortical and hippocampal neurons, leading to impairment of cognitive functioning, including memory, language, and executive functioning [88]. AD has two pathological features: aggregation of Aβ, a major component of extraneuronal senile plaques (SPS), and hyperphosphorylation of tau, a microtubule-associated protein that forms intracellular neurofibrillary tangles (NFTs) [88]. Metal iron homeostasis disorders with redox activity may be related to the neuropathology of Alzheimer’s disease. With the development of iron quantification technology, especially the magnetic resonance imaging (MRI) method, it has been shown that the iron level in the brain of AD patients increases with age [89]. In addition, the application of other advanced technologies, such as inductively coupled plasma mass spectrometry (ICP-MS) analysis results shows that plasma iron is significantly reduced in patients with Alzheimer’s disease [90], which is speculated to be caused by excessive accumulation of iron in the brain. A recent analysis shows that iron levels and related redox activities in the cortex and cerebellum were increased in patients in the preclinical stage of AD [89]. As the Cisd2 gene encodes the CDGSH iron–sulfur-domain-containing protein 2, Cisd2 overexpression attenuates AD pathogenesis by guaranteeing mitochondrial quality and synaptic functions [91]. Therefore, brain iron accumulation has become a major pathological feature of the early onset of AD. To a certain extent, iron can act as a promising new target in AD for neuroprotection.
In terms of the regulation of iron in the pathogenesis of Alzheimer’s disease, most scholars believe that the homeostasis of zinc, copper, and iron is related to the misfolding process of amyloid (Aβ), amyloid precursor protein (APP), and hyperphosphorylated tau, which finally leads to neuronal oxidative stress [92] (Figure 3).
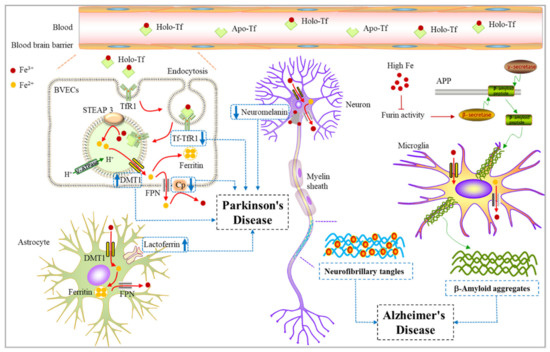
Figure 3.
Relationship between iron and Alzheimer’s disease/Parkinson’s disease. Fe3+ can be absorbed and utilized by astrocytes, neurons, oligodendrocytes, and microglia in the brain. When the iron-related proteins change in neurons, the iron homeostasis is disturbed, leading to neurodegenerative diseases. Upregulation of some iron-storage proteins such as lactoferrin and transferrin increased expression of DMT1 in dopamine neurons. Both ceruloplasmin dysfunction and a decreased level of neuromelanin can cause an accelerated onset of Parkinson’s disease. Alzheimer’s disease is characterized by the formation of Aβ plaques and NFTs in the brain. The increase of intracellular iron level promotes β-secretase and γ-secretase activities to enhance the amyloid production pathway, and finally causes Aβ aggregation around microglia. In addition, iron also binds to tau, affecting its phosphorylation and inducing hyperphosphorylated tau tangles in neurons. Aβ aggregation and neurofibrillary tangles together lead to the occurrence of AD. Apo-Tf, Apo-transferrin; Holo-Tf, Holo-transferrin; BVECs, brain vascular endothelial cells; DMT1, Divalent metal-ion transporter 1; NFTs, Neurofibrillary tangles.
There is a close connection between iron homeostasis and the pathology of AD. It has been reported that the translation of amyloid precursor protein APP is directly regulated by cellular iron levels [93]. Most of the APP is cleaved by a nonamyloid production pathway. APP is first cleaved by α-secretase to produce sAPPα, which is then cleaved by γ-secretase to release the N- terminal fragment p3, leaving the intracellular domain of APP on the membrane. Under pathological conditions, APP passes through the neurodegenerative pathway of amyloid. APP can be cut by β-secretase and then cut by γ-secretase to produce Aβ [94]. The formation of Aβ and its accumulation in the brain can be weakened by stimulating α-secretase. Proteolytic activation of inactive forms of α -secretase and β -secretase is regulated by furin. The transcription of furin is regulated by intracellular iron concentration. Excessive iron leads to the reduction of furin concentration, thus increasing the activity of β-secretase and enhancing the amyloid production pathway. In contrast, iron deficiency increases the activity of furin, which enhances α-secretase and stimulates the nonamyloid production pathway [95]. Iron may regulate APP processing through IRPS, interacting with the hypothetical IRE in the 5′- untranslated region of APP mRNA [93]. The translation of APP may therefore be up-regulated under the condition of iron excess, increasing the amount of APP available to enter the amyloid production pathway, thereby resulting in the aggregation of Aβ peptide [93]. In addition to causing the accumulation of Aβ peptide, iron also binds to tau, affecting its phosphorylation and inducing hyperphosphorylated tau aggregation [96]. Accumulating hyperphosphorylated forms of tau have been demonstrated to be possible primary drivers of AD and play a key role in promoting neurotoxicity and neuronal loss [96]. In summary, iron can affect the progression of Alzheimer’s disease by regulating amyloid (Aβ), amyloid precursor protein (APP), and hyperphosphorylated tau (Figure 3).
The congenital iron overload disease, hereditary hemochromatosis (HH), is caused by mutation of the HFE gene [97]. The association between HH and AD has become increasingly apparent in recent years. HFE is expressed by reactive astrocytes as well as neurons in the brains of AD patients. The induction of HFE in the AD brain by stress factors, such as cells serum deprivation, menadione and beta-amyloid, were determined using BV-2 cells. The labile iron pool was consistently decreased when HFE expression increased. These data provide insight into the induction of HFE in AD and indicate that HFE expression may be a protective function to limit cellular iron exposure during cell stress [98]. Gene variants involved in iron homeostasis such as the HFE gene (His63Asp and Cys282Tyr variants) have been associated with a higher risk for AD. In single nucleotide polymorphisms (SNPs), the HFE 282Y allele yielded a three-fold risk reduction in the whole cohort of patients [99]. On the other hand, some studies have shown that C282Y and H63D HFE variants increase the risk for and severity of AD, especially in synergy with polymorphisms in the Tf gene [100]. An increased frequency of the transferrin C2 subtype was also noted in AD patients compared with age-matched controls [101]. The C2 variant, combined with the presence of the HFE mutation, increases the risk of AD fivefold [102]. To date, the relationship between HFE mutations and Alzheimer’s disease needs further investigation.
4.2. Iron Metabolism and Parkinson’s Disease
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder worldwide, and its pathological feature is the degeneration of dopaminergic neurons in the dense part of the substantia nigra [103]. Iron can participate in the formation of oxygen free radicals and the induction of lipid peroxidation in the system due to its unique chemical properties, which are closely related to the PD process [104]. The results of the histochemical analysis showed that the iron deposition level in the substantia nigra of patients with PD was increased compared with that in the control group [105]. Quantitative analysis of brain iron content in PD patients using Mossbauer spectroscopy, atomic absorption, and atomic emission spectroscopy as well as colorimetric analysis showed that excessive iron shifted the Fe3+/Fe2+ ratio from 2:1 in the substantia nigra of normal people to 1:2 in the substantia nigra of PD patients. This shift to the more toxic form of iron may lead to the generation of hydrogen peroxide-derived reactive hydroxyl radicals through the Fenton reaction and promote the pathological process of Parkinson’s disease. At the same time, elevated iron was also observed in PD animal models induced by 6- hydroxydopamine (6-OHDA) and MPTP. However, the use of iron chelator or genetic manipulation of iron-related genes could prevent neuronal death in these models [106,107]. Furthermore, feeding the neonates of mice with a high-iron diet for 24 months resulted in a significant decrease in the number of TH+ dopaminergic neurons [108]. These studies have indicated that elevated iron may play an important role in the pathogenesis of Parkinson’s disease.
The cause of the accumulation of total iron in the substantia nigra of patients with PD is not well known, but several possible factors have been proposed, such as blood–brain barrier dysfunction [109,110]; upregulation of some iron-storage proteins such as lactoferrin [111] and transferrin [112,113]; Increased expression of DMT1 in dopamine neurons [114]; ceruloplasmin dysfunction and so on (Figure 3).
The blood–brain barrier (BBB) generally protects the brain from plasma iron influx. One potential source of increased iron is from peripheral influx through a disturbed or open BBB in the substantia nigra. In a previous study, researchers used radiolabeled verapamil hydrochloride and positron emission tomography (PET) in PD patients and age-matched healthy controls. Verapamil is a specific substrate for the P-glycoprotein (Pgp) multidrug resistance system in the cell membrane. Pgp functions as an efflux pump, and verapamil does not cross the BBB. The results showed that the uptake of verapamil in the midbrain of PD patients was higher, while the control group had no uptake. The reason is that the BBB’s efflux pump system (Pgp) cannot work properly in certain brain areas of PD patients, so that serum iron can enter the brain [110]. Therefore, blood–brain barrier dysfunction may be one of the causes for the accumulation of iron in the brains of patients with PD.
Some iron-storage proteins, for instance, lactoferrin, are closely related to PD. Studies have shown that human lactoferrin produces a neuroprotective effect and probably stimulates neuroregeneration under conditions of MPTP toxicity in the animal model of PD [115]. In addition, the transferrin/transferrin receptor transport pathway is also connected to neuronal iron uptake. For example, after MPTP was injected into the monkey brain to induce PD, the expression of transferrin receptor (TfR) in dopaminergic neurons and the substantia nigra of PD rats damaged by 6-OHDA was decreased [112]. Moreover, transferrin has been shown to decrease in the substantia nigra of PD by 35% [116]. Therefore, the analysis of these proteins in PD patients may provide important clues to clarify the mechanism of increased iron content in the brain of the patients.
Elevated iron concentrations in the substantia nigra might result from the manipulation of genes relevant to iron transport and binding. One example is that the haplotype (C alleles of 1254T and IVS4 + 44C/A polymorphisms) occurred at greater frequencies in PD subjects compared with that of control, suggesting that CC haplotype in DMT1 gene is a possible risk factor for PD in this Han Chinese population [117]. In addition, mice with mutant DMT1 with gene loss of function were partially resistant to 6-OHDA and MPTP-induced toxicity, further supporting the pathogenic role of DMT1 in iron-related neurodegeneration [114,118].
Both results in animal models [114] and patients [119,120] with PD have shown that ceruloplasmin dysfunction may be related to the disease. The decrease in ceruloplasmin iron oxidase activity and increase in copper concentration in the cerebrospinal fluid of PD may lead to increased mobilization of reactive iron and oxidative stress in the brain of the patient. Several missense mutations in genes encoding ceruloplasmin have been reported in PD patients, which may affect the mobilization of active iron [121], further indicating that ceruloplasmin iron oxidase may affect the pathogenesis of PD. In the substantia nigra, iron is stored in neuromelanin. Under normal circumstances, 50% of neuromelanin (NM) is saturated with iron. Neuromelanin can act protectively by chelating redox-active iron in the cytosol of neurons [122]. Previous work in patients with PD has shown that the iron content in neuromelanin was significantly reduced, increasing the content of redox-active iron (unstable Fe2+) in the substantia nigra [123].
The protein aggregation is mainly composed of highly ubiquitinated α-synuclein in the form of Lewy bodies, which is a pathological sign of PD. Lewy bodies are often found in the substantia nigra and are also present in neurodegenerative diseases, such as dementia with Lewy bodies [124]. Previous studies have shown that iron can stimulate intracellular α -synuclein and ubiquitin aggregation in PD patients [125]. There are toxic feedback loops of iron, reactive oxygen species, and α -synuclein in PD patients. ROS and iron will increase each other’s levels, which will put the cells under oxidative stress and lead to the aggregation of α-synuclein. α-synuclein can in turn increase ROS and iron by inducing mitochondrial dysfunction and iron reductase activity. These effects are transmitted to neighboring neurons in a toxic feedback loop, which leads to PD pathology [126].
Heme oxygenase-1 (HO-1) is an inducible enzyme known for its anti-inflammatory, antioxidant, and neuroprotective effects. However, increased expression of HO-1 during aging and age-related neurodegenerative diseases has been associated with neurotoxic ferric iron deposits. It is highlighted that microglial HO-1 overexpression contributes to neurotoxic iron accumulation, creating deleterious effects in aged mice exposed to an inflammatory insult [127]. Plasma HO-1 levels may be a promising biomarker of early PD. A recent study found that plasma HO-1 levels were significantly elevated in PD patients, predominantly those with early-stage PD, compared with controls [128]. Besides, elevated HO-1 correlates with increased brain iron deposition measured by quantitative susceptibility mapping and decreased hemoglobin in patients with Parkinson’s disease [129].
Briefly, iron can play an important role in the regulation of the activity of neurons in a variety of ways. Understanding the mechanism of iron in the regulation of PD is expected to provide certain clues for the subsequent treatment of Parkinson’s disease.
5. Other Iron-Related Neurological Diseases
Some less common neurodegenerative diseases, mainly include a series of neurodegeneration with brain iron accumulation (NBIA), such as Friedreich’s ataxia (FRDA), Aceruloplasminemia, Neuroferritinopathy, and Pantothenate kinase-associated neurodegeneration (PKAN).
Friedreich’s ataxia (FRDA) is an autosomal recessive neurodegenerative disorder that principally affects the heart and the nervous system [130]. The pathogenic mechanism of Friedreich’s ataxia at the gene level is relatively clear. The GAA trinucleotide repeat sequence amplified in the first intron of the frataxin gene is the most common mutation in the frataxin gene, inducing a substantial reduction in the concentration of the mitochondrial protein frataxin [131]. Conceptually, FRDA is a disorder of iron distribution rather than simply an overload. Iron accumulates in the mitochondria in FRDA and animal models based on frataxin deficiency [15].
Aceruloplasminemia is caused by a mutation in the gene coding for ceruloplasmin protein [132]. Ceruloplasmin (Cp), also known as copper oxidase, is a blue-looking copper (Cu) glycoprotein. It has six compact domains that can bind to six Cu atoms. Cp carries 40–70% of Cu in plasma and plays important roles in Cu transport, iron (Fe) regulation, free radical scavenging, and antioxidant processes [133]. Among the numerous NBIA, Aceruloplasminemia has the highest level of iron accumulation and it has the final impact on the liver, pancreas, retina, and many brain regions, including basal ganglia and cortex [134].
Neuroferritinopathy is a rare autosomal dominant hereditary disease that is caused by a mutation in the L-ferritin gene. It can increase the level of free redox-active iron, and thus make neuron cells undergo oxidative stress [135]. The most common clinical manifestations are dyskinesia, behavioral abnormalities, and cognitive deficits., Iron deposition in the basal ganglia, abnormal ferritin and iron accumulation in the globus pallidus and substantia nigra, and low serum ferritin concentration were found in patients with Neuroferritinopathy [136].
Pantothenate kinase-associated neurodegeneration (PKAN) is caused by mutations in the gene encoding pantothenate kinase 2 (PANK2) which is a major genetic defect in NBIA [16]. PKAN is an autosomal recessive genetic disease characterized by dystonia and retinopathy pigmentosa in children and speech or neuropsychiatric defects in adults. In PKAN patients, the accumulation of iron in the globus pallidus is 3–4 times that of normal subjects [137].
6. Treatment Strategies Using Iron Chelators
Metal chelators can strongly bind metal ions to become stable and large molecular weight compounds, thus preventing metal ions from their action. It can therefore be used for detoxification. Iron chelation therapy may prevent iron-induced ROS, oxidative stress, and α-synuclein and Aβ aggregation, implying that iron chelation is a feasible neuroprotective approach for neurodegenerative disease and other nervous systems disease associated with abnormal iron metabolism [138].
Considering that iron deficiency can also cause harmful effects, unlimited removal of iron is undesirable. Potential therapeutic agents should promote the homeostasis of iron, rather than its complete removal. Iron chelators have some characteristics, such as being highly soluble and easily crossing the blood-brain barrier. In addition, they must have a high degree of specificity and selectivity for the chelation of iron ions [139]. It must also be noted that potential chelators can not only function by removing metal ions but also protect tissues by affecting other cellular mechanisms. For example, desferrioxamine has an additional neuroprotective effect by inducing hypoxia-induced transcription factor 1 DNA binding and erythropoietin transcription [140]. The characteristics and targeted diseases of several common chelating agents are described below in Table 1.

Table 1.
Properties of common chelating agents, characteristics and targeted diseases.
As mentioned above, with increasing evidence that iron homeostasis disorder could be one of the mechanisms of pathological deterioration in PD, the recovery of iron homeostasis in the brain may become a feasible target for a new therapeutic design. For example, in rat PD models, desferrioxamine can be used to protect dopaminergic neurons from death efficiently [141]. Considering that desferrioxamine is unable to travel across the BBB due to its size and hydrophilic nature, new research declared that compound Clioquinol improved motor and non-motor deficits in an MPTP-induced monkey model of PD through the AKT/mTOR pathway [144]. Many other-chelating agents have also been found, including deironiprone, which can protect dopaminergic neurons from neurodegeneration [151].
In addition to being used for the treatment of PD, deferoxamine is also the first metal chelator studied in AD patients. Studies have found that compared, with placebo and no treatment, treatment with this compound significantly reduces the clinical progression of dementia [142]. This manner avoids using a traditional iron-chelating agent, which is hydrophilic and difficult to pass through the BBB. In addition, deferoxamine also has a high affinity for other metal ions (aluminum, copper, and zinc). In other words, it has low selectivity for metal ions [152]. An advanced compound, PBT2, mainly binds to excess copper and zinc and possibly iron in the brain, thereby reducing the number of amyloid plaques and relocating these metal ions to the depleted cell and neuron compartments [145].
At present, there have been many experiments on the administration of deferiprone to Friedreich ataxia patients. One of the studies found that, compared with the control group, the iron content in the dentate nucleus of the deferiprone treatment group had been reduced [153]. In addition, the nervous system-related functions have also been significantly improved, such as manipulative dexterity, speech fluency, and ataxia gait, especially for young patients. Another study revealed when deferiprone and antioxidant idebenone were given to FRDA patients aged 8–25 years old, the combination had a stable effect on the normal function of the nervous system [154]. In short, suitable doses might be beneficial in younger patients with less severe disease, although more cases are needed for validation.
Many natural polyphenols, due to their ability to chelate metal ions, play an important role in the treatment of neurological diseases. Simultaneously, these polyphenols themselves have antioxidation and anti-inflammatory effects that can effectively alleviate the oxidative stress caused by metal ions [146]. For instance, curcumin chelates Cu and Fe transition ions, which play roles in AD pathology [147]. Epigallocatechin-3-gallate (EGCG) is the most abundant catechin found in the green tea plant. EGCG has the potential to combine with Aβ to protect nerves from damage because of its distinct antiamyloidogenic reactivity toward metal-Aβ species with a structure-based mechanism [146]. Besides, quercetin is a promising therapeutic candidate due to its bifunctionality as an antioxidant and chelating molecular [148]. Therefore, it is believed that natural polyphenols have great potential in the treatment of neurodegenerative diseases, implying that the development of more efficient natural polyphenols is critical in the future.
More recently, many novel chelating agents for the treatment of neurodegenerative diseases are emerging. A substance called pro-chelators must be induced by H2O2 to chelate metal ions, which can effectively reduce the oxidative stress caused by metals. Compared with traditional chelating agents, they can more effectively chelate metals and maintain intracellular metal homeostasis [155]. Some non-toxic lipophilic brain-permeable iron chelators provide potential therapeutic benefits for progressive neurodegenerative diseases, such as VK-28, HLA-20, and M30 [149]. Another promising compound, 6-methoxysalicylaldehyde nicotinoyl hydrazone (SNH6), showed low cytotoxicity, potent iron-chelation efficacy, significant inhibition of copper-mediated Aβ aggregation, alleviation of oxidative stress, as well as the effective donation of NAD+ to NAD-dependent metabolic processes (PARP and sirtuin activity). It significantly increased the median lifespan of C. elegans. Based on these benefits to the organisms, SNH6 has the potential to act as a multifunctional therapeutic agent for AD treatment [150].
In summary, iron is believed to be a novel target for pharmacological interventions in these disorders (Table 1). Reducing iron toward normal levels or hampering the increases in the iron of the brain is a promising therapeutic strategy for all iron-related neurodegenerative disorders.
7. Conclusions
Iron is a necessary trace metal element for organisms and is involved in many important physiological processes. However, iron is also a double-edged sword, which will produce toxic effects when overloaded. Therefore, the maintenance of iron homeostasis is particularly vital for the normal physiological function of the organism. Excess iron in the body can produce large amounts of ROS through the Fenton reaction, which in turn damages many macromolecular substances, such as lipids, proteins, and DNA. The increased mitochondrial iron level results in the accumulation of ROS, leading to mitochondrial dysfunction and eventually cell senescence. The accumulation of iron in the brain affects the normal function of the brain and leads to the occurrence of many age-related neurodegenerative diseases. However, many iron chelators can be applied to chelate excess iron to reduce iron levels for the treatment of diseases.
Nevertheless, we should carefully investigate whether iron overload is the cause or the result of aging. If iron overload is the cause of aging, iron chelators may be effective in the treatment and may delay aging. On the other hand, if iron overload is a consequence of aging, clarifying why iron accumulates with age will indicate the treatment of neurodegenerative diseases related to iron overload. In either case, the role of iron in cell senescence remains largely uncertain. Therefore, research on the mechanisms underlying this effect of iron metabolism on aging and aging-related neurodegenerative diseases still needs to be explored further.
Author Contributions
Conceptualization, Y.T. (Yao Tian) and M.Y.; formal analysis, Y.T (Yuanliangzi Tian) and X.F.; data curation, Z.Y. and S.W.; resources, S.W.; project administration, Y.Z.; writing—original draft preparation, Y.T. (Yao Tian); writing—review and editing, Y.T. (Yuanliangzi Tian), D.Y. and M.Y.; supervision, M.Y.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31771338.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank all lab members for invaluable comments on the manuscript. We apologize to those whose work pertinent to the issues discussed was not cited due to limitations of the format or to inadvertent omissions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, S.; Uliana, A.; Taylor, M.K.; Chakarawet, K.; Bandaru, S.R.S.; Gul, S.; Xu, J.; Ackerman, C.M.; Chatterjee, R.; Furukawa, H.; et al. Iron detection and remediation with a functionalized porous polymer applied to environmental water samples. Chem. Sci. 2019, 10, 6651–6660. [Google Scholar] [CrossRef] [PubMed]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tan, Y.; Ouyang, S.; He, J.; Liu, L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 2022, 808, 145968. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Buffenstein, R.; Edrey, Y.H.; Yang, T.; Mele, J. The oxidative stress theory of aging: Embattled or invincible? Insights from non-traditional model organisms. Age 2008, 30, 99–109. [Google Scholar] [CrossRef]
- Levi, S.; Rovida, E. The role of iron in mitochondrial function. Biochim. Biophys. Acta 2009, 1790, 629–636. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef]
- Chen, H.; Lukas, T.J.; Du, N.; Suyeoka, G.; Neufeld, A.H. Dysfunction of the retinal pigment epithelium with age: Increased iron decreases phagocytosis and lysosomal activity. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A. Iron on the brain. Nat. Genet. 2001, 28, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Youdim, M.B.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, M.; Ben-Shachar, D.; Riederer, P.; Youdim, M.B. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J. Neurochem. 1994, 63, 793–807. [Google Scholar] [CrossRef]
- Chiang, S.; Huang, M.L.H.; Park, K.C.; Richardson, D.R. Antioxidant defense mechanisms and its dysfunctional regulation in the mitochondrial disease, Friedreich’s ataxia. Free Radic. Biol. Med. 2020, 159, 177–188. [Google Scholar] [CrossRef]
- Svetel, M.; Dragašević, N.; Petrović, I.; Novaković, I.; Tomić, A.; Kresojević, N.; Stanković, I.; Kostić, V. NBIA Syndromes: A Step Forward from the Previous Knowledge. Neurol. India 2021, 69, 1380–1388. [Google Scholar] [CrossRef]
- Fan, X.; McLaughlin, C.; Robinson, C.; Ravasini, J.; Schelch, K.; Johnson, T.; van Zandwijk, N.; Reid, G.; George, A.M. Zeolites ameliorate asbestos toxicity in a transgenic model of malignant mesothelioma. FASEB Bioadv. 2019, 1, 550–560. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, B. Iron homeostasis in insects: Insights from Drosophila studies. IUBMB Life 2013, 65, 863–872. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.B.; Wei, X.E.; Li, Y.H.; Dai, Y.M. An investigation of age-related iron deposition using susceptibility weighted imaging. PLoS ONE 2012, 7, e50706. [Google Scholar] [CrossRef]
- Zeidan, R.S.; Han, S.M.; Leeuwenburgh, C.; Xiao, R. Iron homeostasis and organismal aging. Ageing Res. Rev. 2021, 72, 101510. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Sobh, A.; Loguinov, A.; Zhou, J.; Jenkitkasemwong, S.; Zeidan, R.; El Ahmadie, N.; Tagmount, A.; Knutson, M.; Fraenkel, P.G.; Vulpe, C.D. Genetic screens reveal CCDC115 as a modulator of erythroid iron and heme trafficking. Am. J. Hematol. 2020, 95, 1085–1098. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Mumbauer, S.; Pascual, J.; Kolotuev, I.; Hamaratoglu, F. Ferritin heavy chain protects the developing wing from reactive oxygen species and ferroptosis. PLoS Genet. 2019, 15, e1008396. [Google Scholar] [CrossRef]
- Baldi, A.; Lombardi, D.; Russo, P.; Palescandolo, E.; De Luca, A.; Santini, D.; Baldi, F.; Rossiello, L.; Dell’Anna, M.L.; Mastrofrancesco, A.; et al. Ferritin contributes to melanoma progression by modulating cell growth and sensitivity to oxidative stress. Clin. Cancer Res. 2005, 11, 3175–3183. [Google Scholar] [CrossRef][Green Version]
- Qiao, B.; Sugianto, P.; Fung, E.; Del-Castillo-Rueda, A.; Moran-Jimenez, M.J.; Ganz, T.; Nemeth, E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012, 15, 918–924. [Google Scholar] [CrossRef]
- Rishi, G.; Subramaniam, V.N. Signaling pathways regulating hepcidin. Vitam. Horm. 2019, 110, 47–70. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.Z. Cellular Iron Metabolism and Regulation. Adv. Exp. Med. Biol. 2019, 1173, 21–32. [Google Scholar]
- Torti, S.V.; Torti, F.M. Iron: The cancer connection. Mol. Asp. Med. 2020, 75, 100860. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Bullock, K.; Price, A.; Inderias, L.; Osorio, C. Ferrosenescence: The iron age of neurodegeneration? Mech. Ageing Dev. 2018, 174, 63–75. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Ogura, J.; Grippo, P.J.; Torres, C.; Sato, T.; Wachtel, M.; Ramachandran, S.; Babu, E.; Sivaprakasam, S.; Rajasekaran, D.; et al. Chronic exposure to excess iron promotes EMT and cancer via p53 loss in pancreatic cancer. Asian J. Pharm. Sci. 2020, 15, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Choudhary, S.K.; Liu, J.; Finkel, T. The role of mitochondria in cellular senescence. FASEB J. 2021, 35, e21991. [Google Scholar] [CrossRef]
- Hughes, C.E.; Coody, T.K.; Jeong, M.Y.; Berg, J.A.; Winge, D.R.; Hughes, A.L. Cysteine Toxicity Drives Age-Related Mitochondrial Decline by Altering Iron Homeostasis. Cell 2020, 180, 296–310.e18. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Chen, L.; Jiang, J. Iron-mediated lysosomal-mitochondrial crosstalk: A new direction in the treatment of aging and aging-related diseases. Acta Biochim. Biophys. Sin. 2020, 52, 1293–1295. [Google Scholar] [CrossRef]
- Braymer, J.J.; Lill, R. Iron-sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 2017, 292, 12754–12763. [Google Scholar] [CrossRef]
- Chiang, S.; Braidy, N.; Maleki, S.; Lal, S.; Richardson, D.R.; Huang, M.L. Mechanisms of impaired mitochondrial homeostasis and NAD (+) metabolism in a model of mitochondrial heart disease exhibiting redox active iron accumulation. Redox Biol. 2021, 46, 102038. [Google Scholar] [CrossRef]
- Mollet, I.G.; Patel, D.; Govani, F.S.; Giess, A.; Paschalaki, K.; Periyasamy, M.; Lidington, E.C.; Mason, J.C.; Jones, M.D.; Game, L.; et al. Low Dose Iron Treatments Induce a DNA Damage Response in Human Endothelial Cells within Minutes. PLoS ONE 2016, 11, e0147990. [Google Scholar] [CrossRef] [PubMed]
- Bolinches-Amorós, A.; Mollá, B.; Pla-Martín, D.; Palau, F.; González-Cabo, P. Mitochondrial dysfunction induced by frataxin deficiency is associated with cellular senescence and abnormal calcium metabolism. Front. Cell. Neurosci. 2014, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.A.; Botella, J.A.; Metzendorf, C.; Lind, M.I.; Schneuwly, S. Mitoferrin modulates iron toxicity in a Drosophila model of Friedreich’s ataxia. Free Radic. Biol. Med. 2015, 85, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, R.; Liu, L.; Zhou, Y.; Chen, Z. Lactoferrin ameliorates pathological cardiac hypertrophy related to mitochondrial quality control in aged mice. Food Funct. 2021, 12, 7514–7526. [Google Scholar] [CrossRef] [PubMed]
- Issitt, T.; Bosseboeuf, E.; De Winter, N.; Dufton, N.; Gestri, G.; Senatore, V.; Chikh, A.; Randi, A.M.; Raimondi, C. Neuropilin-1 Controls Endothelial Homeostasis by Regulating Mitochondrial Function and Iron-Dependent Oxidative Stress. iScience 2019, 11, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.Y.; Xu, J.; Servais, S.; Hofer, T.; Marzetti, E.; Wohlgemuth, S.E.; Knutson, M.D.; Chung, H.Y.; Leeuwenburgh, C. Mitochondrial iron accumulation with age and functional consequences. Aging Cell 2008, 7, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Ven, T.N.; Crane, M.M.; Brunner, M.L.C.; Pun, A.K.; Helget, K.L.; Brower, K.; Chen, D.E.; Doan, H.; Dillard-Telm, J.D.; et al. Loss of vacuolar acidity results in iron-sulfur cluster defects and divergent homeostatic responses during aging in Saccharomyces cerevisiae. Geroscience 2020, 42, 749–764. [Google Scholar] [CrossRef]
- Veatch, J.R.; McMurray, M.A.; Nelson, Z.W.; Gottschling, D.E. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 2009, 137, 1247–1258. [Google Scholar] [CrossRef]
- Rouault, T.A.; Tong, W.H. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat. Rev. Mol. Cell Biol. 2005, 6, 345–351. [Google Scholar] [CrossRef]
- Atamna, H.; Liu, J.; Ames, B.N. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: Revelance to aging. J. Biol. Chem. 2001, 276, 48410–48416. [Google Scholar] [CrossRef]
- Picca, A.; Saini, S.K.; Mankowski, R.T.; Kamenov, G.; Anton, S.D.; Manini, T.M.; Buford, T.W.; Wohlgemuth, S.E.; Xiao, R.; Calvani, R.; et al. Altered Expression of Mitoferrin and Frataxin, Larger Labile Iron Pool and Greater Mitochondrial DNA Damage in the Skeletal Muscle of Older Adults. Cells 2020, 9, 2579. [Google Scholar] [CrossRef] [PubMed]
- Molz, P.; de Freitas, B.S.; Uberti, V.H.; da Costa, K.M.; Kist, L.W.; Bogo, M.R.; Schröder, N. Effects of lipoic acid supplementation on age- and iron-induced memory impairment, mitochondrial DNA damage and antioxidant responses. Eur. J. Nutr. 2021, 60, 3679–3690. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Michaelides, C.; Ekonomou, A.; Geraki, K.; Parkes, H.G.; Suessmilch, M.; Herlihy, A.H.; Crum, W.R.; So, P.W. Dissociation between iron accumulation and ferritin upregulation in the aged substantia nigra: Attenuation by dietary restriction. Aging 2016, 8, 2488–2508. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Michaelides, C.; Walker, T.A.; Ekonomou, A.; Suessmilch, M.; Sriskanthanathan, A.; Abraha, S.; Parkes, A.; Parkes, H.G.; Geraki, K.; et al. Regional Distributions of Iron, Copper and Zinc and Their Relationships with Glia in a Normal Aging Mouse Model. Front. Aging Neurosci. 2019, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Menzies, S.L.; St Martin, S.M.; Mufson, E.J. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J. Neurosci. Res. 1990, 27, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Snyder, B.S.; Arosio, P.; Loeffler, D.A.; LeWitt, P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer’s diseased brains. J. Neurochem. 1995, 65, 717–724. [Google Scholar] [CrossRef]
- Zecca, L.; Gallorini, M.; Schünemann, V.; Trautwein, A.X.; Gerlach, M.; Riederer, P.; Vezzoni, P.; Tampellini, D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: Consequences for iron storage and neurodegenerative processes. J. Neurochem. 2001, 76, 1766–1773. [Google Scholar] [CrossRef]
- Lee, D.W.; Andersen, J.K. Iron elevations in the aging Parkinsonian brain: A consequence of impaired iron homeostasis? J. Neurochem. 2010, 112, 332–339. [Google Scholar] [CrossRef]
- Levenson, C.W. Trace metal regulation of neuronal apoptosis: From genes to behavior. Physiol. Behav. 2005, 86, 399–406. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Youdim, M.B.H. Targeting dysregulation of brain iron homeostasis in ageing. Nutr. Aging 2012, 1, 27–39. [Google Scholar] [CrossRef]
- Han, K.; Jin, X.; Guo, X.; Cao, G.; Tian, S.; Song, Y.; Zuo, Y.; Yu, P.; Gao, G.; Chang, Y.Z. Nrf2 knockout altered brain iron deposition and mitigated age-related motor dysfunction in aging mice. Free Radic. Biol. Med. 2021, 162, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Uberti, V.H.; de Freitas, B.S.; Molz, P.; Bromberg, E.; Schröder, N. Iron Overload Impairs Autophagy: Effects of Rapamycin in Ameliorating Iron-Related Memory Deficits. Mol. Neurobiol. 2020, 57, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, E.; Medina-Leendertz, S.; Díaz, S. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp. Gerontol. 2002, 37, 629–638. [Google Scholar] [CrossRef]
- Massie, H.R.; Aiello, V.R.; Williams, T.R. Inhibition of iron absorption prolongs the life span of Drosophila. Mech. Ageing Dev. 1993, 67, 227–237. [Google Scholar] [CrossRef]
- Kennedy, T.P.; Rao, N.V.; Noah, W.; Michael, J.R.; Jafri, M.H., Jr.; Gurtner, G.H.; Hoidal, J.R. Ibuprofen prevents oxidant lung injury and in vitro lipid peroxidation by chelating iron. J. Clin. Investig. 1990, 86, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.E.; Pham, H.M.; Nguyen, B.V.; Tahmasian, Y.; Ramsden, S.; Coskun, V.; Schriner, S.E.; Jafari, M. Green tea polyphenols require the mitochondrial iron transporter, mitoferrin, for lifespan extension in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2016, 93, 210–221. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, S.; Tan, G.; Ye, W.; Liu, D.; Qian, X.; Ding, Z.; Zhong, Y.; Zhang, J.; Jiang, D.; et al. Reduction of mitoferrin results in abnormal development and extended lifespan in Caenorhabditis elegans. PLoS ONE 2012, 7, e29666. [Google Scholar] [CrossRef][Green Version]
- Schiavi, A.; Maglioni, S.; Palikaras, K.; Shaik, A.; Strappazzon, F.; Brinkmann, V.; Torgovnick, A.; Castelein, N.; De Henau, S.; Braeckman, B.P.; et al. Iron-Starvation-Induced Mitophagy Mediates Lifespan Extension upon Mitochondrial Stress in C. elegans. Curr. Biol. 2015, 25, 1810–1822. [Google Scholar] [CrossRef]
- Klang, I.M.; Schilling, B.; Sorensen, D.J.; Sahu, A.K.; Kapahi, P.; Andersen, J.K.; Swoboda, P.; Killilea, D.W.; Gibson, B.W.; Lithgow, G.J. Iron promotes protein insolubility and aging in C. elegans. Aging 2014, 6, 975–991. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J., IV; Christine Pietsch, E.; Buss, J.L.; Wang, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160. [Google Scholar] [CrossRef]
- Chin, D.; Huebbe, P.; Frank, J.; Rimbach, G.; Pallauf, K. Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2014, 2, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.R.; Parnell, L.D.; Ordovas, J.M.; Lai, C.Q. Curcumin and aging. Biofactors 2013, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Tsuchiyama, S.K.; Nguyen, Q.T.; Plyusnina, E.N.; Terrill, S.R.; Sahibzada, S.; Patel, B.; Faulkner, A.R.; Shaposhnikov, M.V.; Tian, R.; et al. Enhanced longevity by ibuprofen, conserved in multiple species, occurs in yeast through inhibition of tryptophan import. PLoS Genet. 2014, 10, e1004860. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Hatef, A.; Imran ul-Haq, M.; Nair, N.; Unniappan, S.; Kizhakkedathu, J.N. Clinically approved iron chelators influence zebrafish mortality, hatching morphology and cardiac function. PLoS ONE 2014, 9, e109880. [Google Scholar] [CrossRef]
- Almeida, T.; Marques, M.; Mojzita, D.; Amorim, M.A.; Silva, R.D.; Almeida, B.; Rodrigues, P.; Ludovico, P.; Hohmann, S.; Moradas-Ferreira, P.; et al. Isc1p plays a key role in hydrogen peroxide resistance and chronological lifespan through modulation of iron levels and apoptosis. Mol. Biol. Cell 2008, 19, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Liu, X.G.; Zhao, W.; Cui, H.; Ruan, J.; Yuan, Y.; Tu, Z. MET18 Deficiency Increases the Sensitivity of Yeast to Oxidative Stress and Shortens Replicative Lifespan by Inhibiting Catalase Activity. BioMed Res. Int. 2017, 2017, 7587395. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Mittal, N.; Deswal, S.; Roy, N. Calorie restriction up-regulates iron and copper transport genes in Saccharomyces cerevisiae. Mol. Biosyst. 2011, 7, 394–402. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, G.; Casey, K.; Curry, S.; Oliver, M.; Han, S.M.; Leeuwenburgh, C.; Xiao, R. A novel role of the mitochondrial iron-sulfur cluster assembly protein ISCU-1/ISCU in longevity and stress response. Geroscience 2021, 43, 691–707. [Google Scholar] [CrossRef]
- Prischi, F.; Konarev, P.V.; Iannuzzi, C.; Pastore, C.; Adinolfi, S.; Martin, S.R.; Svergun, D.I.; Pastore, A. Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat. Commun. 2010, 1, 95. [Google Scholar] [CrossRef]
- Britti, E.; Delaspre, F.; Feldman, A.; Osborne, M.; Greif, H.; Tamarit, J.; Ros, J. Frataxin-deficient neurons and mice models of Friedreich ataxia are improved by TAT-MTScs-FXN treatment. J. Cell. Mol. Med. 2018, 22, 834–848. [Google Scholar] [CrossRef]
- Zarse, K.; Schulz, T.J.; Birringer, M.; Ristow, M. Impaired respiration is positively correlated with decreased life span in Caenorhabditis elegans models of Friedreich Ataxia. FASEB J. 2007, 21, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Runko, A.P.; Griswold, A.J.; Min, K.T. Overexpression of frataxin in the mitochondria increases resistance to oxidative stress and extends lifespan in Drosophila. FEBS Lett. 2008, 582, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.L.; James, S.A.; Salim, A.; Sumardy, F.; Speed, T.P.; Conrad, M.; Richardson, D.R.; Bush, A.I.; McColl, G. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. Elife 2020, 9, e56580. [Google Scholar] [CrossRef] [PubMed]
- Mangan, D. Iron: An underrated factor in aging. Aging 2021, 13, 23407–23415. [Google Scholar] [CrossRef] [PubMed]
- Go, S.; Kang, M.; Kwon, S.P.; Jung, M.; Jeon, O.H.; Kim, B.S. The Senolytic Drug JQ1 Removes Senescent Cells via Ferroptosis. Tissue Eng. Regen. Med. 2021, 18, 841–850. [Google Scholar] [CrossRef]
- Beydoun, R.; Hamood, M.A.; Gomez Zubieta, D.M.; Kondapalli, K.C. Na+/H+ Exchanger 9 Regulates Iron Mobilization at the Blood-Brain Barrier in Response to Iron Starvation. J. Biol. Chem. 2017, 292, 4293–4301. [Google Scholar] [CrossRef]
- Austin, R.N.; Freeman, J.L.; Guilarte, T.R. Neurochemistry of lead and manganese. Metallomics 2016, 8, 561–562. [Google Scholar] [CrossRef][Green Version]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef]
- Khattar, N.; Triebswetter, C.; Kiely, M.; Ferrucci, L.; Resnick, S.M.; Spencer, R.G.; Bouhrara, M. Investigation of the association between cerebral iron content and myelin content in normative aging using quantitative magnetic resonance neuroimaging. Neuroimage 2021, 239, 118267. [Google Scholar] [CrossRef]
- Faux, N.G.; Rembach, A.; Wiley, J.; Ellis, K.A.; Ames, D.; Fowler, C.J.; Martins, R.N.; Pertile, K.K.; Rumble, R.L.; Trounson, B.; et al. An anemia of Alzheimer’s disease. Mol. Psychiatry 2014, 19, 1227–1234. [Google Scholar] [CrossRef]
- Nobili, A.; Krashia, P.; D’Amelio, M. Cisd2: A promising new target in Alzheimer’s disease†. J. Pathol. 2020, 251, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Perry, G.; Harris, P.L.; Liu, Y.; Schubert, K.A.; Smith, M.A. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: A central role for bound transition metals. J. Neurochem. 2000, 74, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chang, X.; Lang, M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12442. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Song, C.; Du, Y.; Gaur, U.; Yang, M. Pharmacological Treatment of Alzheimer’s Disease: Insights from Drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 4621. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kwon, K.C.; Hwang, D.J.; Koo, J.H.; Um, H.S.; Song, H.S.; Kim, J.S.; Jang, Y.; Cho, J.Y. Treadmill Exercise Alleviates Brain Iron Dyshomeostasis Accelerating Neuronal Amyloid-β Production, Neuronal Cell Death, and Cognitive Impairment in Transgenic Mice Model of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 3208–3223. [Google Scholar] [CrossRef] [PubMed]
- Spotorno, N.; Acosta-Cabronero, J.; Stomrud, E.; Lampinen, B.; Strandberg, O.T.; van Westen, D.; Hansson, O. Relationship between cortical iron and tau aggregation in Alzheimer’s disease. Brain 2020, 143, 1341–1349. [Google Scholar] [CrossRef]
- Nairz, M.; Metzendorf, C.; Vujic-Spasic, M.; Mitterstiller, A.M.; Schroll, A.; Haschka, D.; Hoffmann, A.; Von Raffay, L.; Sparla, R.; Huck, C.W.; et al. Cell-specific expression of Hfe determines the outcome of Salmonella enterica serovar Typhimurium infection in mice. Haematologica 2021, 106, 3149–3161. [Google Scholar] [CrossRef]
- Lee, S.Y.; Connor, J.R. Regulation of Hfe by stress factors in BV-2 cells. Neurobiol. Aging 2005, 26, 803–812. [Google Scholar] [CrossRef]
- Tisato, V.; Zuliani, G.; Vigliano, M.; Longo, G.; Franchini, E.; Secchiero, P.; Zauli, G.; Paraboschi, E.M.; Vikram Singh, A.; Serino, M.L.; et al. Gene-gene interactions among coding genes of iron-homeostasis proteins and APOE-alleles in cognitive impairment diseases. PLoS ONE 2018, 13, e0193867. [Google Scholar] [CrossRef]
- Lehmann, D.J.; Schuur, M.; Warden, D.R.; Hammond, N.; Belbin, O.; Kölsch, H.; Lehmann, M.G.; Wilcock, G.K.; Brown, K.; Kehoe, P.G.; et al. Transferrin and HFE genes interact in Alzheimer’s disease risk: The Epistasis Project. Neurobiol. Aging 2012, 33, e1–e13. [Google Scholar] [CrossRef]
- Namekata, K.; Imagawa, M.; Terashi, A.; Ohta, S.; Oyama, F.; Ihara, Y. Association of transferrin C2 allele with late-onset Alzheimer’s disease. Hum. Genet. 1997, 101, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Robson, K.J.; Lehmann, D.J.; Wimhurst, V.L.; Livesey, K.J.; Combrinck, M.; Merryweather-Clarke, A.T.; Warden, D.R.; Smith, A.D. Synergy between the C2 allele of transferrin and the C282Y allele of the haemochromatosis gene (HFE) as risk factors for developing Alzheimer’s disease. J. Med. Genet. 2004, 41, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.; Domingues, H.S.; Salgado, A.J.; Teixeira, F.G. From regenerative strategies to pharmacological approaches: Can we fine-tune treatment for Parkinson’s disease? Neural Regen. Res. 2022, 17, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Waworuntu, R.V.; Berg, B.M.; Dilger, R.N. Dietary Alpha-Lipoic Acid Alters Piglet Neurodevelopment. Front. Pediatr. 2016, 4, 44. [Google Scholar] [CrossRef]
- Lhermitte, J.; Kraus, W.M.; McAlpine, D. Original Papers: On the occurrence of abnormal deposits of iron in the brain in parkinsonism with special reference to its localisation. J. Neurol. Psychopathol. 1924, 5, 195–208. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, B.; Tao, K.; Yang, H.; Wang, Y.; Zhou, T.; Yang, Y.; Yuan, L.; Liu, X.; Duan, Y. Iron accumulation and microglia activation contribute to substantia nigra hyperechogenicity in the 6-OHDA-induced rat model of Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 36, 76–82. [Google Scholar] [CrossRef]
- Kaur, D.; Yantiri, F.; Rajagopalan, S.; Kumar, J.; Mo, J.Q.; Boonplueang, R.; Viswanath, V.; Jacobs, R.; Yang, L.; Beal, M.F.; et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson’s disease. Neuron 2003, 37, 899–909. [Google Scholar] [CrossRef]
- Kaur, D.; Peng, J.; Chinta, S.J.; Rajagopalan, S.; Di Monte, D.A.; Cherny, R.A.; Andersen, J.K. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol. Aging 2007, 28, 907–913. [Google Scholar] [CrossRef]
- Faucheux, B.A.; Bonnet, A.M.; Agid, Y.; Hirsch, E.C. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet 1999, 353, 981–982. [Google Scholar] [CrossRef]
- Kortekaas, R.; Leenders, K.L.; van Oostrom, J.C.; Vaalburg, W.; Bart, J.; Willemsen, A.T.; Hendrikse, N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef]
- Faucheux, B.A.; Nillesse, N.; Damier, P.; Spik, G.; Mouatt-Prigent, A.; Pierce, A.; Leveugle, B.; Kubis, N.; Hauw, J.J.; Agid, Y.; et al. Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 1995, 92, 9603–9607. [Google Scholar] [CrossRef]
- Borie, C.; Gasparini, F.; Verpillat, P.; Bonnet, A.M.; Agid, Y.; Hetet, G.; Brice, A.; Dürr, A.; Grandchamp, B. Association study between iron-related genes polymorphisms and Parkinson’s disease. J. Neurol. 2002, 249, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.J.; Bras, J.M.; Santana, I.; Januario, C.; Santiago, B.; Morgadinho, A.S.; Ribeiro, M.H.; Hardy, J.; Singleton, A.; Oliveira, C. Association of HFE common mutations with Parkinson’s disease, Alzheimer’s disease and mild cognitive impairment in a Portuguese cohort. BMC Neurol. 2006, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Mena, N.; Hunot, S.; Prigent, A.; Alvarez-Fischer, D.; Arredondo, M.; Duyckaerts, C.; Sazdovitch, V.; Zhao, L.; Garrick, L.M.; et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2008, 105, 18578–18583. [Google Scholar] [CrossRef] [PubMed]
- Kopaeva, M.Y.; Cherepov, A.B.; Nesterenko, M.V.; Zarayskaya, I.Y. Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure. Biology 2021, 10, 24. [Google Scholar] [CrossRef]
- Ayton, S.; Lei, P.; McLean, C.; Bush, A.I.; Finkelstein, D.I. Transferrin protects against Parkinsonian neurotoxicity and is deficient in Parkinson’s substantia nigra. Signal Transduct. Target. Ther. 2016, 1, 16015. [Google Scholar] [CrossRef]
- He, Q.; Du, T.; Yu, X.; Xie, A.; Song, N.; Kang, Q.; Yu, J.; Tan, L.; Xie, J.; Jiang, H. DMT1 polymorphism and risk of Parkinson’s disease. Neurosci. Lett. 2011, 501, 128–131. [Google Scholar] [CrossRef]
- Jiang, H.; Song, N.; Xu, H.; Zhang, S.; Wang, J.; Xie, J. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res. 2010, 20, 345–356. [Google Scholar] [CrossRef]
- Martínez-Hernández, R.; Montes, S.; Higuera-Calleja, J.; Yescas, P.; Boll, M.C.; Diaz-Ruiz, A.; Rios, C. Plasma ceruloplasmin ferroxidase activity correlates with the nigral sonographic area in Parkinson’s disease patients: A pilot study. Neurochem. Res. 2011, 36, 2111–2115. [Google Scholar] [CrossRef]
- Olivieri, S.; Conti, A.; Iannaccone, S.; Cannistraci, C.V.; Campanella, A.; Barbariga, M.; Codazzi, F.; Pelizzoni, I.; Magnani, G.; Pesca, M.; et al. Ceruloplasmin oxidation, a feature of Parkinson’s disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J. Neurosci. 2011, 31, 18568–18577. [Google Scholar] [CrossRef]
- Hochstrasser, H.; Bauer, P.; Walter, U.; Behnke, S.; Spiegel, J.; Csoti, I.; Zeiler, B.; Bornemann, A.; Pahnke, J.; Becker, G.; et al. Ceruloplasmin gene variations and substantia nigra hyperechogenicity in Parkinson disease. Neurology 2004, 63, 1912–1917. [Google Scholar] [CrossRef]
- Sian-Hülsmann, J.; Mandel, S.; Youdim, M.B.; Riederer, P. The relevance of iron in the pathogenesis of Parkinson’s disease. J. Neurochem. 2011, 118, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, B.A.; Martin, M.E.; Beaumont, C.; Hauw, J.J.; Agid, Y.; Hirsch, E.C. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J. Neurochem. 2003, 86, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease-lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Riederer, P.; Monoranu, C.; Strobel, S.; Iordache, T.; Sian-Hülsmann, J. Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson’s disease. J. Neural Transm. 2021, 128, 1577–1598. [Google Scholar] [CrossRef] [PubMed]
- Jansen van Rensburg, Z.; Abrahams, S.; Bardien, S.; Kenyon, C. Toxic Feedback Loop Involving Iron, Reactive Oxygen Species, α-Synuclein and Neuromelanin in Parkinson’s Disease and Intervention with Turmeric. Mol. Neurobiol. 2021, 58, 5920–5936. [Google Scholar] [CrossRef]
- Fernández-Mendívil, C.; Luengo, E.; Trigo-Alonso, P.; García-Magro, N.; Negredo, P.; López, M.G. Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism. Redox Biol. 2021, 38, 101789. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, J.; Ma, J.; Wang, Z.; Shi, X.; Li, M.; Huang, S.; Hu, S.; Zhao, Z.; Li, D. Increased Plasma Heme Oxygenase-1 Levels in Patients with Early-Stage Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 621508. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, C.; Song, W.; Cui, X.; Pan, M.; Wang, Q.; Feng, Y.; Xu, Y. Elevated Heme Oxygenase-1 Correlates with Increased Brain Iron Deposition Measured by Quantitative Susceptibility Mapping and Decreased Hemoglobin in Patients with Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 656626. [Google Scholar] [CrossRef]
- Monda, E.; Lioncino, M.; Rubino, M.; Passantino, S.; Verrillo, F.; Caiazza, M.; Cirillo, A.; Fusco, A.; Di Fraia, F.; Fimiani, F.; et al. Diagnosis and Management of Cardiovascular Involvement in Friedreich Ataxia. Heart Fail. Clin. 2022, 18, 31–37. [Google Scholar] [CrossRef]
- Yang, W.; Thompson, B.; Kwa, F.A.A. Molecular approaches for the treatment and prevention of Friedreich’s ataxia. Drug Discov. Today 2021, 27, 866–880. [Google Scholar] [CrossRef]
- Nittis, T.; Gitlin, J.D. The copper-iron connection: Hereditary aceruloplasminemia. Semin. Hematol. 2002, 39, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Zhang, C.; Zhou, S.; Ji, G. Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology. Diabetes Metab. Syndr. Obes. 2022, 15, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Ikeda, S.; Yamamoto, K.; Morita, S.; Yoshida, K.; Nomoto, S.; Kato, M.; Yanagisawa, N. Hereditary ceruloplasmin deficiency with hemosiderosis: A clinicopathological study of a Japanese family. Ann. Neurol. 1995, 37, 646–656. [Google Scholar] [CrossRef]
- Nair, T.; Ram, C.V.S. Normotensive aging: Finally, an ‘iron in the fire’. J. Hypertens. 2021, 39, 1102–1103. [Google Scholar] [CrossRef]
- Crompton, D.E.; Chinnery, P.F.; Fey, C.; Curtis, A.R.; Morris, C.M.; Kierstan, J.; Burt, A.; Young, F.; Coulthard, A.; Curtis, A.; et al. Neuroferritinopathy: A window on the role of iron in neurodegeneration. Blood Cells Mol. Dis. 2002, 29, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Dusek, P.; Tovar Martinez, E.M.; Madai, V.I.; Jech, R.; Sobesky, J.; Paul, F.; Niendorf, T.; Wuerfel, J.; Schneider, S.A. 7-Tesla Magnetic Resonance Imaging for Brain Iron Quantification in Homozygous and Heterozygous PANK2 Mutation Carriers. Mov. Disord. Clin. Pract. 2014, 1, 329–335. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Hagemeier, J.; Geurts, J.J.; Zivadinov, R. Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev. Neurother. 2012, 12, 1467–1480. [Google Scholar] [CrossRef]
- Prass, K.; Ruscher, K.; Karsch, M.; Isaev, N.; Megow, D.; Priller, J.; Scharff, A.; Dirnagl, U.; Meisel, A. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J. Cereb. Blood Flow Metab. 2002, 22, 520–525. [Google Scholar] [CrossRef]
- Jiang, H.; Luan, Z.; Wang, J.; Xie, J. Neuroprotective effects of iron chelator Desferal on dopaminergic neurons in the substantia nigra of rats with iron-overload. Neurochem. Int. 2006, 49, 605–609. [Google Scholar] [CrossRef]
- Crapper McLachlan, D.R.; Dalton, A.J.; Kruck, T.P.; Bell, M.Y.; Smith, W.L.; Kalow, W.; Andrews, D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef]
- Lin, G.; Zhu, F.; Kanaan, N.M.; Asano, R.; Shirafuji, N.; Sasaki, H.; Yamaguchi, T.; Enomoto, S.; Endo, Y.; Ueno, A.; et al. Clioquinol Decreases Levels of Phosphorylated, Truncated, and Oligomerized Tau Protein. Int. J. Mol. Sci. 2021, 22, 12063. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Huang, C.; Luo, Q.; Xia, Y.; Liu, W.; Zeng, W.; Cheng, A.; Shi, R.; Zhengli, C. Clioquinol improves motor and non-motor deficits in MPTP-induced monkey model of Parkinson’s disease through AKT/mTOR pathway. Aging 2020, 12, 9515–9533. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.L.; Roseman, G.P.; Sopasis, G.J.; Millhauser, G.L.; Harris, H.H.; Pickering, I.J.; George, G.N. Copper (II) Binding to PBT2 Differs from That of Other 8-Hydroxyquinoline Chelators: Implications for the Treatment of Neurodegenerative Protein Misfolding Diseases. Inorg. Chem. 2020, 59, 17519–17534. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; González, Y.; Doens, D.; Stephens, D.E.; Santamaría, R.; Murillo, E.; Gutiérrez, M.; Fernández, P.L.; Rao, K.S.; Larionov, O.V.; et al. Assessment of Novel Curcumin Derivatives as Potent Inhibitors of Inflammation and Amyloid-β Aggregation in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, s59–s68. [Google Scholar] [CrossRef]
- Elmegeed, G.A.; Ahmed, H.H.; Hashash, M.A.; Abd-Elhalim, M.M.; El-kady, D.S. Synthesis of novel steroidal curcumin derivatives as anti-Alzheimer’s disease candidates: Evidences-based on in vivo study. Steroids 2015, 101, 78–89. [Google Scholar] [CrossRef]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef]
- Zheng, H.; Gal, S.; Weiner, L.M.; Bar-Am, O.; Warshawsky, A.; Fridkin, M.; Youdim, M.B. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: In vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J. Neurochem. 2005, 95, 68–78. [Google Scholar] [CrossRef]
- Wu, Z.; Palanimuthu, D.; Braidy, N.; Salikin, N.H.; Egan, S.; Huang, M.L.H.; Richardson, D.R. Novel multifunctional iron chelators of the aroyl nicotinoyl hydrazone class that markedly enhance cellular NAD+/NADH ratios. Br. J. Pharmacol. 2020, 177, 1967–1987. [Google Scholar] [CrossRef]
- Dexter, D.T.; Statton, S.A.; Whitmore, C.; Freinbichler, W.; Weinberger, P.; Tipton, K.F.; Della Corte, L.; Ward, R.J.; Crichton, R.R. Clinically available iron chelators induce neuroprotection in the 6-OHDA model of Parkinson’s disease after peripheral administration. J. Neural Transm. 2011, 118, 223–231. [Google Scholar] [CrossRef]
- Kenche, V.B.; Barnham, K.J. Alzheimer’s disease & metals: Therapeutic opportunities. Br. J. Pharmacol. 2011, 163, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B. Therapeutic developments in Friedreich ataxia. J. Child Neurol. 2012, 27, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Sánchez, D.; Aracil, A.; Montero, R.; Mas, A.; Jiménez, L.; O’Callaghan, M.; Tondo, M.; Capdevila, A.; Blanch, J.; Artuch, R.; et al. Combined therapy with idebenone and deferiprone in patients with Friedreich’s ataxia. Cerebellum 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leed, M.G.; Wolkow, N.; Pham, D.M.; Daniel, C.L.; Dunaief, J.L.; Franz, K.J. Prochelators triggered by hydrogen peroxide provide hexadentate iron coordination to impede oxidative stress. J. Inorg. Biochem. 2011, 105, 1161–1172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).