Abstract
Oxidative dissolution of stibnite (Sb2S3), one of the most prevalent geochemical processes for antimony (Sb) release, can be promoted by Sb-oxidizing microbes, which were studied under alkaline and neutral conditions but rarely under acidic conditions. This work is dedicated to unraveling the enhancement mechanism of stibnite dissolution by typical acidophile Acidithiobacillus ferrooxidans under extremely acidic conditions. The results of solution behavior showed that the dissolution of Sb2S3 was significantly enhanced by A. ferrooxidans, with lower pH and higher redox potential values and higher [Sb(III)] and [Sb(V)] than the sterile control. The surface morphology results showed that the cells adsorbed onto the mineral surface and formed biofilms. Much more filamentous secondary minerals were formed for the case with A. ferrooxidans. Further mineral phase compositions and Sb/S speciation transformation analyses showed that more secondary products Sb2O3/SbO2−, Sb2O5/SbO3−, SO42−, as well as intermediates, such as S0, S2O32− were formed for the biotic case, indicating that the dissolution of Sb2S3 and the Sb/S speciation transformation was promoted by A. ferrooxidans. These results were further clarified by the comparative transcriptome analysis. This work demonstrated that through the interaction with Sb2S3, A. ferrooxidans promotes S/Sb oxidation, so as to enhance S/Sb transformation and thus the dissolution of Sb2S3.
1. Introduction
Antimony (Sb) is widely used as a metal additive in brake pads, alloys, semiconductors and flame retardants [1,2]. The extensive demand for antimony has promoted the massive mining of antimony ore, leaving behind a large number of abandoned mines and waste tailings, the main sources for the serious Sb pollution around Sb mines areas [1,3,4]. It is known that stibnite (Sb2S3) is the main form of Sb in the abandoned mines and waste tailings as well as Sb ores, and the oxidative dissolution of Sb2S3 may be the most predominant cause for serious Sb pollution, the mechanism, however, remains with little known as of yet [5]. It is thus, of great significance to further study and unravel the oxidative dissolution process of stibnite.
Under abiotic conditions, Sb2S3 can be oxidized slowly by dissolved oxygen possibly by two typical stages, i.e., the dissolution of Sb2S3 to form aqueous Sb(OH)3 and H2S (Equation (1)), and the oxidations of Sb(OH)3 and H2S by dissolved oxygen to form Sb(OH)5/Sb(OH)6− and sulfate, respectively (Equations (2) and (3)) [6,7]. It was reported that Sb2S3 oxidation can be significantly promoted under the action of Sb-oxidizing bacteria in nature environments [4,8]. For example, Li et al. isolated six Sb-oxidizing bacteria from Xikuangshan, Hunan, China, the largest Sb ores area in the world, and found that the strain with the highest Sb-oxidation activity could aerobically oxidize 50 mmol/L Sb(III) in 3 days [9]. Nguyen et al. isolated two Sb-oxidizing strains and found they can aerobically oxidize 500 mmol/L Sb(III) within 24 h, and one strain can even anaerobically oxidize Sb(III) with nitrate as the electron acceptor [10]. Xiang et al. demonstrated that the Sb-oxidizing bacteria AS-1 significantly enhanced the oxidations of Sb and S (Equations (4) and (5)), resulting in enhancement of the mobilization of Sb [4]. Up to date, most of the relevant studies were under alkaline, neutral and weak acidic conditions [4,5,11], but rarely under extremely acidic conditions (pH < 3) [1,12].
Sb2S3 (s) + 6H2O (l) ⇋ 2Sb(OH)3 (aq) + 3H2S (aq)
2Sb(OH)3 + O2 + 2H2O → 2Sb(OH)5
H2S + 2O2 → H2SO4
Of note, is that extremely acidic environments commonly occur for Sb2S3 when it coexists with other sulfide minerals typically pyrite and arsenopyrite and they are oxidized by acidophiles [1,13]. Torma et al. reported that Sb2S3 can be oxidized by Acidithiobacillus ferrooxidans, the most typical acidophile with a variety of sulfur oxidation pathways (Equations (6)–(8)) [12]. Nguyen et al., however, found that Sb in the contaminated sediment was difficult to solubilize by A. ferrooxidans and the extraction efficiency of Sb was the lowest in comparison to those of Cr, Cu, Mn, Ni, and Zn [14]. The reason for that may be the speciation transformation of Sb occurring during the dissolution process of Sb2S3, i.e., the soluble Sb was transformed into the insoluble Sb, which is unclear as of yet.
Therefore, in this work, we focused on the dissolution process of Sb2S3 mediated by A. ferrooxidans under extremely acidic conditions, with a systematic investigation of the Sb and S speciation transformation by Sb and S X-ray photoelectron spectroscopy (XPS) and X-ray absorption near-edge structure (XANES) spectroscopy, besides the determination of the solution parameters, surface morphology and phase composition of the solid residues. We found that under the action of A. ferrooxidans, the content of aqueous Sb(OH)3 increased significantly, and much of the Sb-oxides (Sb2O3/SbO2−, Sb2O5/SbO3−) and secondary sulfur products (S0, S2O32−, SO32−, SO42−) appeared, indicating that by promoting the Sb/S oxidation, A. ferrooxidans enhanced the oxidative dissolution process of Sb2S3. These findings could be of value for deeply understanding Sb occurrence and fate during Sb2S3 dissolution and providing insights into prevention and control of Sb pollution in acidic Sb mine areas and tailings.
2. Results
2.1. Physicochemical Parameters
The physicochemical parameters of the solution for the case with A. ferrooxidans and sterile control were in terms of pH, redox potential (ORP), [SO42−], [Sb(III)], [Sb(V)] and [SbT] (the concentration of total dissolved Sb), which are shown in Figure 1a,b. The pH values for both cases gradually decreased, and the pH for the case with A. ferrooxidans was significantly lower than that in the sterile control (Figure 1a). The ORP values for the sterile control gradually decreased from 335 mV to 274 mV and then increased to 325 mV, while for the case with A. ferrooxidans, the ORP values changed slightly on days 0–13, increased sharply by ~100 mV to ~460 mV from day 13 to day 18 and then slightly decreased (Figure 1a). The [SO42−] gradually increased to ~4.0 g/L for both cases and then remained basically steady, but it took four days more to reach the steady stage for the sterile control compared to A. ferrooxidans (Figure 1b). The [SbT] gradually increased during the whole experiment for both cases, while it was higher for the case with A. ferrooxidans than in the sterile control, and the former was approximately two times higher than the latter at the late stage of the experiment (Figure 1b). The concentrations of different forms of dissolved antimony, i.e., [Sb(III)] and [Sb(V)], show a similar trend to [SbT] for both cases. The [Sb(III)] and [Sb(V)] had significant differences on days 0–30 for both cases, while there was only a small [Sb(V)] on days 0–12 for the sterile control (Figure 1b).
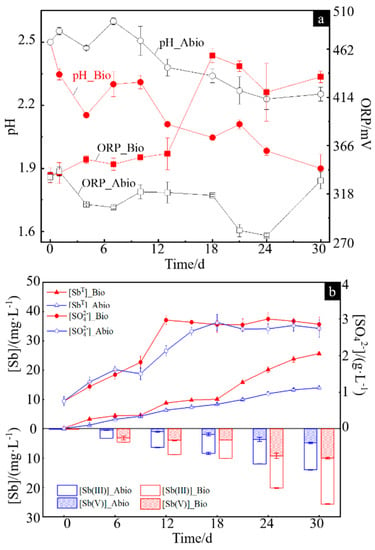
Figure 1.
Changes in the pH and ORP values (a) and the concentrations of Sb(III), Sb(V), SbT and SO42− (b) in the solution during dissolution of stibnite in the presence or absence of A. ferrooxidans.
2.2. Surface Morphology of Solid Residues
The surface morphology of solid residues for the case with A. ferrooxidans and the sterile control are shown in Figure 2a–f, respectively. During the dissolution of Sb2S3 in the presence of A. ferrooxidans, the bacteria would absorb on the surface of the mineral and form many corrosion pits (Figure 2a). At the same time, there were many filamentous secondary minerals distributed in the culture system, and the EDS results showed that the main elements of the secondary minerals were Sb, S and O (Figure 2b,c). Meanwhile, the attached bacteria on the mineral surface wrapped the solid to generate biofilms, which were bright blue in the fluorescence microscope (FM) image (Figure 2d). Of note, different from the surface of the mineral with the adsorbed bacterial cells (Figure 2a,d), the secondary minerals formed for the case with A. ferrooxidans contained no bacterial cells, because no significant N and P elements were detected in the secondary minerals (Figure 2c) and no blue was observed after DAPI staining by FM observation (not shown). In contrast, no significant changes occurred on the surface of Sb2S3, and the main composition of minerals remained basically steady for the sterile control (Figure 2e,f).

Figure 2.
SEM-EDS and FM images of the solid for the group with A. ferrooxidans (a–d) and the sterile group (e,f) on day 30. Images (a,b) represent surface morphology of the solid for the group in present of A. ferrooxidans, where the dash arrow and circle in image (a) shows the attached bacterial cells and corrosion pits, respectively, and dash oval shows the filamentous secondary minerals. Image (c) is the elemental composition of the selected area in image (b). Image (d) represents the biofilm on the solid surface by fluorescence microscopic analysis after 4′,6-diamidino-2-phenylindole (DAPI) staining with DNA, where the square in the lower left corner shows the non-stained image of the same solid particle under the optical microscopy. Images (e,f) show the surface morphology of the solid for the group in the sterile experiment and the elemental composition of the selected area, respectively.
2.3. Phase and Composition of Solid Residues
The XRD patterns of solid residues for the cases with A. ferrooxidans and the sterile control are presented in Figure 3a. The results show that the phase types of the solid residues remained unchanged for both cases in comparison with pristine stibnite, comprising Sb2S3, quartz, Sb2O3 and calcite, which is consistent with Wu et al. [15]. However, the diffraction signal at 27° associated with Sb2O3 became stronger for the case with A. ferrooxidans, but there was no difference for the case of the sterile control, indicating that more Sb2O3 was generated [16].
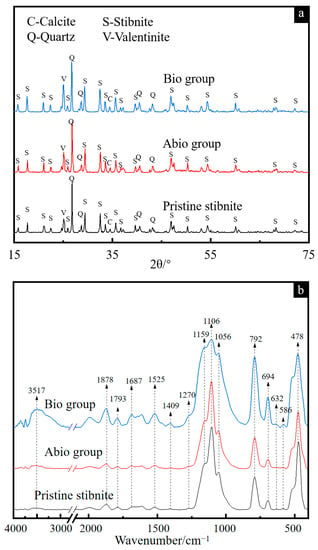
Figure 3.
XRD patterns (a) and FT-IR spectra (b) of pristine stibnite and the solid for the group with A. ferrooxidans and the sterile group on day 30.
The FT-IR spectra (Figure 3b) show that the bands at 1056, 1106 and 1159 cm−1 assigned to the C-O and C-O-C vibrations of polysaccharides became apparently broader for the case with A. ferrooxidans than for the sterile control, while the intensities of the bands at 1878, 1793, 1687, 1525 and 1409 cm−1 assigned to the C=O and N-H vibrations of proteins were apparently higher for the case with A. ferrooxidans [17,18,19,20,21,22], indicating that the bacterial cells were adsorbed on the mineral surface, which is consistent with the FM results (Figure 2d). Notably, strong bands appeared in the range from 3000 cm−1 to 4000 cm−1 associated with O-H, Sb-S and Sb-O vibrations [23], indicating the enhancement of Sb2S3 dissolution by A. ferrooxidans and the secondary products of antimony oxide(s).
The Raman spectra of the reference samples show significant differences in the intensity and positions of pristine Sb2S3 mineral and other Sb and/or S-containing compounds (Figure S1), where the bands at 154, 200, 630, 720, and 995 cm−1 could be signed as the characteristic peaks of S0, Sb2O5, SbO3−, Sb2O3, and SO42−, respectively [24,25,26,27]. By comparing the Raman spectra of solid residues for the case with A. ferrooxidans and the sterile control (Figure 4) with the reference spectra, strong and broad bands at 154 and 200 associated with S0 and Sb2O5 appeared, respectively, indicating the oxidation of Sb2S3 and the formation of S0 and Sb2O5. The new bands at 216 and 720 cm−1 assigned to Sb2O3 [28,29] were strongest for the case with A. ferrooxidans at day 30, indicating the formation of Sb2O3 due to the dissolution of Sb2S3 by A. ferrooxidans. Notably, a new band at 630 cm−1 assigned to sodium antimonate (NaSbO3) [30] appeared for the case with A. ferrooxidans rather than the sterile control, indicating of the bio-oxidation of Sb(III) by A. ferrooxidans, the formation of Sb2O5-Sb(V) species and the promotion of Sb2S3 dissolution by A. ferrooxidans. In addition, a new broad band at 995 cm−1 assigned to SO42− species appeared at day 30 for the case with A. ferrooxidans [31], indicating that the S2− was oxidized to SO42−.
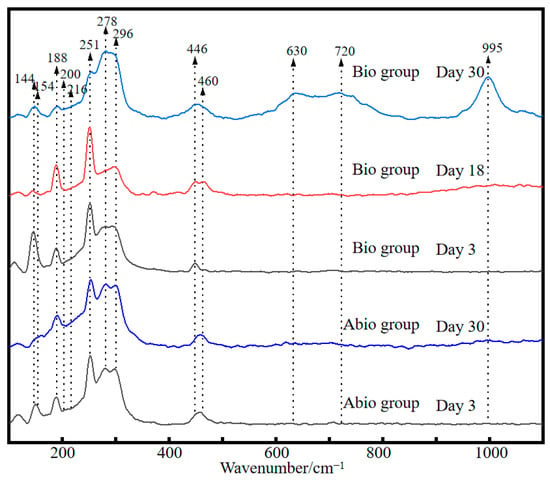
Figure 4.
Raman spectra of pristine stibnite and the solid for the group with A. ferrooxidans and the sterile group on day 30.
2.4. S and Sb Speciation Transformation
The S 2p(3/2) XPS spectra and the fitted results of the solid residues for the case with A. ferrooxidans at days 3, 18 and 30 and the sterile control at days 3 and 30 are shown in Figure 5a and Table S1, respectively. The results in Figure 5a shows that the surface sulfur species comprised S2− (161.35 eV), S− (eV), S0 (164.20 eV), SO32− (166.50 eV), and SO42− (168.50 eV) [32] for the case with A. ferrooxidans. The fitted results (Table S1) further show that during bio-oxidation, the content of S2− species gradually decreased from 94.8% to 50.0%, and the content of SO42− species gradually increased from 5.2% to 34.3%. Meanwhile, 11.4% of S−, 2.9% of S0 and 1.4% of SO32− were detected, indicating that surface S2− of Sb2S3 was oxidized to SO42− via S−, S0 and SO32− by A. ferrooxidans. In contrast, only S2− and SO42− species were detected in the sterile control, and the content of SO42− species was apparently lower than that in the Bio group, indicating that A. ferrooxidans could notably promote the oxidization of sulfur in stibnite.
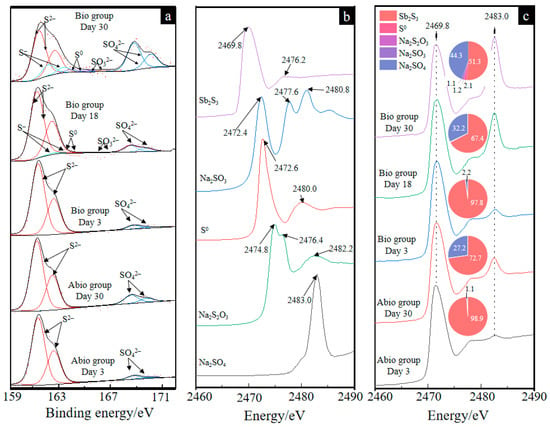
Figure 5.
The S 2p(3/2) XPS spectra of solid residues (a) and the XANES spectra of the reference sample (b) and solid residues for the group with A. ferrooxidans on days 3, 18 and 30 and the sterile group on days 3 and 30 (c). The pie charts in figure (c) represent the percentage composition of S species of each XANES spectrum.
The S K-edge XANES spectra of the reference samples (Figure 5b) show different peak intensities and positions, which can be used to differentiate the S speciation composition of the unknown sample. It can be seen from Figure 5c that for both the case of A. ferrooxidans and the sterile control, the intensity of the peak at 2483.0 eV gradually increased, indicating the formation of SO42−, which is consistent with the S 2p(3/2) XPS results. According to a previous study [26], the linear combination fitting of the unknown spectra with the reference spectra is suitable to analyze the content of S speciation compositions because of high sensitivity, and the fitted results are shown in Figure 5c. The fitted results of the S K-edge XANES spectra show that Sb2S3, NaSO3, Na2S2O3, S0 and Na2SO4 are detected for both the cases with A. ferrooxidans and in the sterile control, and the content of S2− and SO42− species had similar trends to the XPS results, further demonstrating that in the presence of A. ferrooxidans, the surface sulfur was oxidized and transformed faster during the bio-oxidation of Sb2S3. Notably, stibnite, as an acid insoluble mineral, is mainly dissolved by thiosulfate, and the formation of thiosulfate is also found.
According to the Sb 3d(3/2) and 3d(5/2) XPS spectra (Figure 6a), the surface Sb species comprised Sb2S3 (529.5 eV; 539.2 eV), Sb2O3 (532.0 eV; 539.6 eV), and Sb2O5 (532.1 eV; 540.2 eV) and ranged from an approximate peak O 1s (532.6 eV) [33]. The fitted results (Table S2) further show that for the case with A. ferrooxidans, the content of Sb2S3 species gradually decreased from 75.1% to 35.9%, and the content of Sb2O3 and Sb2O5 species gradually increased to 22.1% and 25.4%, respectively. In contrast, for the sterile control, 14.2% Sb2O3 and 3.4% Sb2O5 were detected.
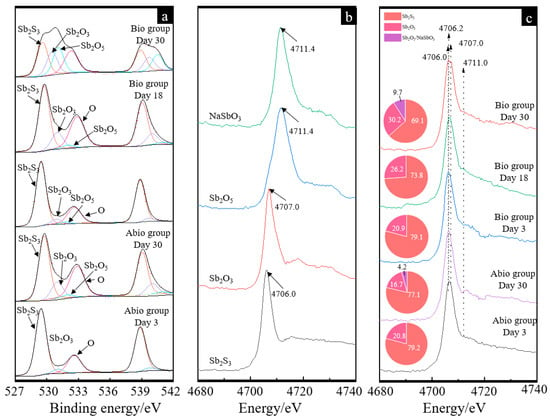
Figure 6.
Antimony XPS patterns (a), XANES standard and experimental spectra (b,c) of pristine stibnite and the solid for the group with A. ferrooxidans and the sterile group on day 30. The curves in image (a) and image (c) represent the antimony XPS patterns for the sterile control case on days 3 and 30 and for the case with A. ferrooxidans on days 3, 18, and 30, respectively. The pie charts in figure (c) represent the percentage composition of Sb species of each XANES spectrum.
The Sb L1-edge XANES spectra of the different referenced samples show different peak positions (Figure 6b), where the max adsorption bands for Sb2S3, Sb2O3 and Sb2O5/NaSbO3 were 4.706, 4.707 and 4.711 keV, respectively. For the XANES spectra of the case with A. ferrooxidans and the sterile control, apparent adsorption bands at 4.706, 4.707 and 4.711 keV were detected, indicating the formation of Sb2O3 and Sb2O5/NaSbO3. The fitted results (Figure 6c) further show that the Sb species for the case with A. ferrooxidans were mainly composed of 76.8–73.8% Sb2S3 and 20.9–26.2% Sb2O3 on days 3 and 18, while it became 59.1 Sb2S3, 30.2% Sb2O3 and 9.7% Sb2O5, indicating the oxidation of Sb2O3 to Sb2O5. For the sterile control, the Sb species Sb2O3 and Sb2O5 were also detected on day 30.
2.5. Comparative Transcriptome Analysis
The transcriptomes of bacterial cells grown on stibnite and S0 were sequenced and analyzed, which is of value to clarify the enhancement mechanism of the formation of S and Sb-containing intermediates during stibnite dissolution by A. ferrooxidans at the transcriptome level. Figure 7 shows the statistical diagram of the number of the differential expression genes between the cells grown on stibnite and S0, indicating that the expression of most genes for the bacterial cells grown on stibnite were significantly inhibited in comparison with that on S0, which is due to the high toxicity of dissolved Sb(III) and Sb(V) to the bacterial cells during the dissolution of stibnite [11,23]. Of note, there are some genes significantly up-regulated for the cells grown on stibnite than S0 (as presented in Table S3), i.e., AFE_1575 (encoding type I restriction enzyme, R subunit), AFE_1577 (encoding type I restriction enzyme M protein), AFE_2393 (encoding transposase), AFE_3223 (encoding NAD(P)H dehydrogenase, quinone), AFE_1654 (encoding formate dehydrogenase (FdhD) protein), AFE_1652 (encoding oxidoreductase alpha subunit), AFE_1651 (encoding 3-hydroxyisobutyrate dehydrogenase family protein), AFE_1589 (encoding DNA-damage-inducible protein), AFE_2312 (encoding Major facilitator superfamily (MFS) transporter), AFE_1636 (encoding hypothetical protein). These up-regulated genes are mainly annotated as the function of metabolic process, cellular process, binding, catalytic activity, transporter activity, molecular carrier activity and cellular anatomical entity in the GO (gene ontology) level 2, indicating these processes are mostly related to stibnite dissolution by A. ferrooxidans.
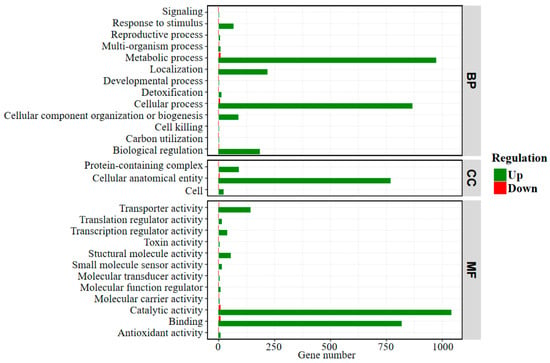
Figure 7.
Statistical diagram of the number of differentially expressed genes in GO (gene ontology) Level 2 between the bacterial cells grown on stibnite and S0. The GO annotation includes three major functional classifications, i.e., BP: biological process; CC: cellular component; MF: molecular function.
3. Discussion
3.1. The Dissolution and Sb/S Intermediates Formation
Previous laboratory experiments have shown that some bacteria in neutral and alkaline environments can accelerate Sb oxidation, reduction and methylation [1,34]. In the present study, we experimentally proved that A. ferrooxidans can grow on Sb2S3 and significantly promote the release of Sb and S of Sb2S3 and the subsequent oxidation processes in an extremely acidic environment.
The dissolution process of Sb2S3 is a dissolution equilibrium process, and the dissolution of minerals in the sterile control group is very slow. However, after the addition of A. ferrooxidans, the concentrations of H+, soluble Sb(III) and Sb(V) in the solution increased significantly (Figure 1). During the dissolution of Sb2S3, massive amounts of Sb- and S-containing intermediates were released, where the oxidation of S0, and ultimately reduced sulfur intermediates to sulfuric acid by A. ferrooxidans is one main factor affecting the change in pH [21,35] and the increase in cell density (Figure S2), resulting in a decrease in the pH of the solution (Figure 1a). On the other hand, the attached bacterial cells, evidenced by the FM observation (Figure 2d) and the FT-IR spectroscopy (Figure 3b), can fall off into the culture with the maturation and abscission of biofilms [36], which also results in an increase in the bacterial density of the solution. According to Multani et al., high ORP in the environment promoted Sb(III) oxidation to Sb(V), which could be the main reason for the higher [Sb(V)] for the case with A. ferrooxidans than the sterile control (Figure 1b) [3].
The morphology of the intermediates formed in the presence and absence of A. ferrooxidans is significantly different, where many filamentous secondary minerals are distributed in the culture system in the presence of A. ferrooxidans, while little secondary mineral is formed on the mineral surface in the absence of A. ferrooxidans. These intermediates are mainly comprised of S0, S2O32−, SO42−, Sb2O3/SbO2−, Sb2O5/SbO3− (Figure 5 and Figure 6). According to a previous study, the S2O32− was formed due to the oxidation of Sb2S3 by the thiosulfate pathway, and then oxidized to other S species according to Equations (6)–(8) [12]. Both the solid phases Sb(III)-Sb2O3 and Sb(V)-Sb2O5 and the dissolved phases Sb(III)-SbO2− and Sb(V)-SbO3− were detected, which is probably because the occurrence of Sb2O3/SbO2− or Sb2O5/SbO3− is a dynamic balance process, depending on the pH and ORP of the solution [1]. Of note, during bio-oxidation, the decrease of the content of S2− species and the increase of the content of SO42− and Sb2O5 species were significantly higher than the sterile control experiment (Figure 5 and Figure 6), indicating that A. ferrooxidans significantly promoted the dissolution of Sb2S3 by enhancing Sb/S transformation.
3.2. The Differential Expression Genes Related to Sb2S3 Bio-Oxidation
Though most of the genes are down-regulated for the bacterial cells grown on stibnite, the bacterial sulfur oxidation activity is still existing evidenced by the expression of the relevant genes, contributing to the rapid formation of reduced sulfur species and the significant increase of [SO42−] and decrease of pH in the solution.
The genes with significant up-regulation for the bacterial cells grown on stibnite in comparison with that on S0 are mostly related to stibnite dissolution by A. ferrooxidans. According to previous studies, AFE_1652 and AFE_1654 belong to the cluster that involves the oxidation of formate, which is a basic biochemical process of A. ferrooxidans, indicates that the oxidation of formate is probably related to the bio-oxidation stibnite [37,38]. The gene AFE_3223 is responsible for the biosynthesis of ubiquinone and other terpenoid-quinones, which are electron carriers in electron transfer pathways. According to previous studies, there are two electron transfer pathways proposed in A. ferrooxidans for the oxidation of ferrous, by which the one is the “downhill electron pathway” through c-cytochrome Cyc1 to aa3 cytochrome oxidase, and the other is “uphill electron pathway” through c-cytochrome CycA1--> bc1 complex-->ubiquinone pool--> NAD(P) [38,39,40]. The latter pathway has been proved to be related to the Arsenic (III) biotransformation via A. ferrooxidans [41], the up-regulation of AFE_3223 indicates the probable contribution of this pathway to the electron transfer in the biotransformation of Sb (III) in the presence of trace iron. In addition, AFE_2312 is very similar to xylose and galactose proton symporters, which is proposed to be related to the MFS transporter superfamily contributing to the carbohydrate transporter of the outer membrane [38,42], thus the up-regulation of this gene indicated that the extracellular substances probably took a key role in the stibnite dissolution process and contributes to the biofilm formation of A. ferrooxidans on the stibnite surface.
3.3. Enhancement Mechanism of Stibnite Dissolution Mediated by A. ferrooxidans
It can be derived from the above discussion that A. ferrooxidans promotes the dissolution of Sb2S3 most probably by the three following coherent aspects: (i) A. ferrooxidans adsorbed on the surface of Sb2S3 with the up-regulation of genes encoding the MFS transporter, resulting in the formation of biofilms and many corroded pits on the mineral surface with obvious changes in the mineral surface structure and chemical speciation, so as to strengthen the cell-mineral interaction and the dissolution process of minerals; (ii) A. ferrooxidans enhanced S oxidation due to the bacterial sulfur oxidation activities, resulting in enhancement of S transformation and cell growth, producing more sulfuric acid to reduce the pH and further enhance the oxidation of Sb(OH)3 with Sb transformation forming a large number of Sb2O3/SbO2−, Sb2O5/SbO3−, and thus accelerating the dissolution of Sb2S3; (iii) the presence of trace iron enhances the uphill electron transfer to NAD(P), accelerating the transformation of Sb(III) by A. ferrooxidans. Moreover, the pertinent enhancement mechanism can be shown schematically in Figure 8. All these findings are of significance to understanding the biogeochemistry of Sb at acidic mining areas and tailings.
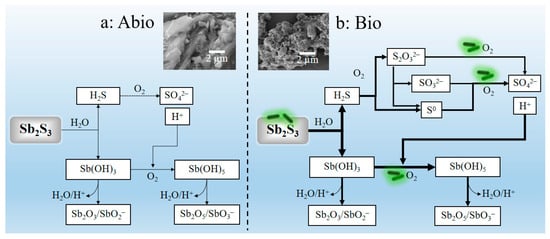
Figure 8.
The acidic dissolution mechanisms of Sb2S3 under abiotic condition (a) and biotic condition in the presence of A. ferrooxidans (b), accompanied with comparison of the dissolution effect (SEM graphs). Where the heavy lines in figure (b) were used to demonstrate the enhancement effects of the A. ferrooxidans in comparison with the dissolution effects in the abiotic case in figure (a).
4. Materials and Methods
4.1. Mineral Sample
The original stibnite sample used in this study was from Xikuangshan, Hunan, China. The mineral composition was determined by X-ray fluorescence (XRF, RIGAKU, ZSX Priums, Tokyo, Japan). The X-ray diffraction (XRD) patterns (Figure S3) show that the original Sb2S3 mainly includes Sb2S3, Sb2O3, SiO2 and CaCO3 phases, and the XRF results (Table S4) show that its composition mainly includes Sb, S and Si. The original Sb2S3 mineral was dried, ground, passed through −200 mesh screens and retained by −400 mesh screens to obtain particles with sizes of 38 to 75 μm.
4.2. Bacterial Strain and Culture Condition
The bacterial strain A. ferrooxidans ATCC 23,270 (Accession number of 16S rRNA gene in GenBank: NR_041888) used in this study was provided by the Key Laboratory of Biometallurgy of Ministry of Education of China, Changsha, China. The basal medium for cultivation of A. ferrooxidans comprised the following ingredients (in g/L): (NH4)2SO4, 3.0; MgSO4, 0.5; K2HPO4, 0.5; KCl, 0.1; Ca(NO3)2, 0.01, with an initial pH of 2.5, which is adjusted by 1 M H2SO4.
4.3. Dissolution Experiment
Prior to the biodissolution experiment, the strain was adapted to the energy substrate Sb2S3 by cultivation for several generations with the addition of 10 g/L Sb2S3 to the basal medium. The biodissolution experiment of Sb2S3 by A. ferrooxidans was carried out in 250 mL Erlen–Meyer flasks containing 100 mL basal medium and 1 g stibnite (referred to as the Bio group). In contrast, the experiment without A. ferrooxidans was taken as the sterile control group (referred to as the Abio group). The initial cell density was 6 × 107 cells/mL. Cultivation of the Bio and Abio groups was performed in an incubator shaker (ZQZY-C8) at 30 °C and 180 r/min.
4.4. Analyses Methods
For the culture medium, the pH and redox potential (ORP) values, the cell densities, and the concentrations of SO42− and Sb were determined at 3–5 day intervals during cultivation. The pH of the solution was measured with a pH meter (PHS-3C) by standing the culture for 3–5 min and placing the pH electrode in the solution at 30 °C. Similarly, the ORP was measured with a platinum (Pt) electrode using a calomel electrode (Ag/AgCl) as the reference electrode. The cell density was directly counted by a light microscope (Olympus, Center Valley, PA, USA) with a blood corpuscle counter (XB-K-25). For the [SO42−], [Sb(III)] and [Sb(V)], 1 mL of the solution was collected, centrifuged and preserved at −80 °C until analysis. The [SO42−] in the solutions was analyzed by using inductively coupled plasma-atomic emission spectroscopy (ICP–OES) (IRIS Intrepid II XSP, Thermo Fisher, Waltham, MA, USA), and the [Sb(III)] was measured by hydride generation (PS Analytical Ltd., Kent, UK) -inductively coupled plasma-atomic emission spectrometry (ICP-AES) (Beijing Haiguang, Beijing, China). Hydride was produced when 2% KBH4 (prepared in 0.5% KOH) and 5% HCl were mixed in reducing reagents (5% ascorbic acid + 5% thiourea), and 6 mol/L HCl was added to the solution for the determination of [SbT] [43]. The [Sb(V)] was obtained by subtracting the [Sb(III)] from the [SbT]. The above data were analyzed statistically by Excel 2015 and SPSS 20.0 software and are presented in terms of the mean value with the standard deviation (SD) as the error bar from triplicate cultures (n = 3).
For the solid residues, the morphology and composition were analyzed by scanning electron microscopy (SEM) coupled with an energy dispersive spectroscopy (EDS) facility (Oxford AZtecLive Ultim Max 20, Abingdon, UK). Briefly, the samples were first prefixed with 25% formaldehyde overnight, dehydrated using a graded ethanol series, coated with gold and introduced into the SEM chamber for observation. The adsorption of bacterial cells on the mineral surface was observed under a fluorescence microscope (FM) (Nexcope NE900, Ningbo, China) with an intelligent mercury lamp power box (NFP-1N, Ningbo, China) by staining with 4′,6-diamidino-2-phenylindole (DAPI).
The mineral phase and composition were measured by XRD, FT-IR and Raman spectroscopy. Before analysis, the solid samples taken out during cultivation were immediately frozen with liquid N2, and then dried under vacuum conditions. The Fourier transform infrared (FT-IR) spectra were collected in the range of 4000–500 cm−1 by an FT-IR spectrometer (Nexus 670, Nicolet, Madison, WI, USA). Raman spectra were recorded at room temperature in the range of 200–4000 cm−1 by a Raman spectrometer (Thermo Fisher, Sunnyvale, CA, USA). The Sb and S speciation was characterized by Sb and S XPS and XANES spectroscopy. Briefly, XPS spectra were collected by an X-ray photoelectron spectrometer (Thermo Fisher Scientific, East Grinstead, UK) with a voltage and current on X-rays of 12 kV and 6 mA, respectively, and all photoelectron binding energies (BEs) were referenced to the C1 s adventitious contamination peak set at 284.5 eV BE. The Sb L-edge and S K-edge XANES spectra were collected at beamline 4B7A at the Beijing Synchrotron Radiation Facility (BSRF), Beijing, China. The S K-edge XANES spectra were recorded in fluorescence mode from 11,820 eV to 11,980 eV at a step of 0.2 eV and a dwell time of 2 s at each energy level. The Sb L1-edge XANES spectra were recorded in total electron yield (TEY) mode from 4660 eV to 4760 eV at a step of 0.2 eV. Athena software was used for the normalization of XANES spectra and linear combination fitting (LCF) analysis [44].
For the comparative transcriptome analysis, the bacterial cells were grown on the energy substrates S0, or stibnite, and were collected at the mid-post logarithmic growth phase. The cell samples for the transcriptome analysis were triplicate (n = 3) for each case. The transcriptome was sequenced with an Illumina Hiseq 2500 platform (Illumina, San Diego, CA, USA) by Magigene Co. Ltd., Guangzhou, China. Before sequencing, the total RNA was extracted by Trizol, and then the RNA library was constructed by NEBNextő Ultra II Directional RNA Library Prep Kit for Illumina after removing the ribosomal RNA. The differential expression of genes (DEGs) of A. ferrooxidans grown on S0 and stibnite were analyzed according to previous studies [45,46,47].
5. Conclusions
The dissolution of Sb2S3 can be enhanced in presence of A. ferrooxidans with an obvious decrease in pH and increase in cell density, soluble Sb/S concentration and ORP values, formation of biofilm on the mineral surface with many corroded pits and producing filamentous secondary minerals distributing in the culture system. Comprehensive FT-IR, XRD, XPS, XANES and Raman spectroscopic analyses showed that the main S and Sb speciation in the culture system is S0, S2O32−, SO42− and Sb(OH)3, Sb(OH)5, Sb2O3/SbO2−, Sb2O5/SbO3−. It can be inferred that A. ferrooxidans accelerated the oxidation of S getting more energy to enhance the cell growth and thus, strengthen the cell-mineral interaction and S transformation; produce more sulfuric acid to promote the oxidation of Sb(OH)3 and thus, strengthen Sb transformation. The up-regulated genes related to uphill electron transfer to NAD(P) also contribute to accelerating the transformation of Sb (III) by A. ferrooxidans. All the above promote the dissolution of Sb2S3. All these findings are of significance to understanding the biogeochemistry of Sb at acidic mining areas and tailings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23073580/s1.
Author Contributions
Conceptualization, J.-L.X., H.-C.L. and Z.-Y.N.; methodology, J.-L.X., H.-C.L. and Z.-Y.N.; validation, Y.-H.Z.; investigation, C.W. and L.C.; data curation, C.W.; writing—original draft preparation, C.W. and H.-C.L.; writing—review and editing, J.-L.X. and H.-C.L.; supervision, J.-L.X.; project administration, J.-L.X.; funding acquisition, J.-L.X. and W.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 41830318 and 51861135305).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chan, T.; Jing, C. Mechanistic study for stibnite oxidative dissolution and sequestration on pyrite. Environ. Pollut. 2020, 262, 114309. [Google Scholar] [CrossRef] [PubMed]
- Multani, R.S.; Feldmann, T.; Demopoulos, G.P. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy 2016, 164, 141–153. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, C.Y.; Liu, D.; Ma, L.Y.; Qiu, X.; Wang, H.M.; Lu, X.L. Antimony transformation and mobilization from stibnite by an antimonite oxidizing bacterium Bosea sp. AS-1. J. Environ. Sci. 2022, 111, 273–281. [Google Scholar] [CrossRef]
- Loni, P.C.; Wu, M.X.J.; Wang, W.Q.; Wang, H.M.; Ma, L.Y.; Liu, C.Y.; Song, Y.Y.; Tuovinen, O.H. Mechanism of microbial dissolution and oxidation of antimony in stibnite under ambient conditions. J. Hazard. Mater. 2020, 385, 121561. [Google Scholar] [CrossRef] [PubMed]
- Biver, M.; Shotyk, W. Stibnite (Sb2S3) oxidative dissolution kinetics from pH 1 to 11. Geochim. Cosmochim. Acta 2012, 79, 127–139. [Google Scholar] [CrossRef]
- Hu, X.Y.; Guo, X.J.; He, M.C.; Li, S.S. pH-dependent release characteristics of antimony and arsenic from typical antimony-bearing ores. J. Environ. Sci. 2016, 44, 171–179. [Google Scholar] [CrossRef]
- Qin, Z.M.; Zhao, S.T.; Shi, T.R.; Zhang, F.R.; Pei, Z.R.; Wang, Y.H.; Liang, Y.R. Accumulation, regional distribution, and environmental effects of Sb in the largest Hg–Sb mine area in Qinling Orogen, China. Sci. Total Environ. 2022, 804, 150218. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Zhang, S.Z.; Qin, D.; Wang, G.J. Phylogenetic and genome analyses of antimony-oxidizing bacteria isolated from antimony mined soil. Int. Biodeter. Biodegr. 2013, 76, 76–80. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Choi, W.; Yu, J.; Lee, T. Microbial oxidation of antimonite and arsenite by bacteria isolated from antimony-contaminated soils. Int. J. Hydrogen Energy 2017, 42, 27832–27842. [Google Scholar] [CrossRef]
- He, M.C.; Wang, N.N.; Long, X.J.; Zhang, C.J.; Ma, C.L.; Zhong, Q.Y.; Wang, A.H.; Wang, Y.; Pervaiz, A.; Shan, J. Antimony speciation in the environment: Recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. 2019, 75, 14–39. [Google Scholar] [CrossRef] [PubMed]
- Torma, A.E.; Gabra, G.G. Oxidation of stibnite by Thiobacillus ferrooxidans. Antonie Leeuwenhoek 1977, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. The Experimental Studies on HQ0211 Bacteria Oxidation of Refractory Gold Complex. Master’s Thesis, Northeastern University, Shenyang, China, 2014. (In Chinese). [Google Scholar]
- Nguyen, V.K.; Tran, T.; Han, H.-J.; Lee, S.-H.; Lee, J.-U. Possibility of bacterial leaching of antimony, chromium, copper, manganese, nickel, and zinc from contaminated sediment. J. Geochem. Explor. 2015, 156, 153–161. [Google Scholar] [CrossRef][Green Version]
- Wu, F.F.; Sun, F.H.; Wu, S.; Yan, Y.B.; Xing, B.S. Removal of antimony(iii) from aqueous solution by freshwater cyanobacteria Microcystis biomass. Chem. Eng. J. 2012, 183, 172–179. [Google Scholar] [CrossRef]
- Karale-Unde, N.J.; Nikumbh, A.K.; Khanvilkar, M.B.; Nagawade, P.A.; Pawar, R.A.; Nighot, D.V.; Misal, S.B.; Gugale, G.S. Synthesis, structural and electrical conduction of some dual doped semiconductor oxides nanoparticles for photocatalytic degradation of Victoria blue-B and brilliant yellow under solar light irradiation. J. Mater. Sci. Mater. Electron. 2021, 32, 4998–5034. [Google Scholar] [CrossRef]
- Auernik, K.S.; Maezato, Y.; Blum, P.H.; Kelly, R.M. The genome sequence of the metal-mobilizing, extremely thermoacidophilic archaeon Metallosphaera sedula provides insights into bioleaching-associated metabolism. Appl. Environ. Microbiol. 2008, 74, 682–692. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yang, Y.; Xia, J.L.; Ding, J.N.; Zhao, X.J.; Nie, Z.Y. Growth and surface properties of new thermoacidophilic archaea strain Acidianus manzaensis YN-25 grown on different substrates. Trans. Nonferrous Met. Soc. China 2008, 18, 1374–1378. [Google Scholar] [CrossRef]
- Kletzin, A.; Urich, T.; Müller, F.; Bandeiras, T.M.; Gomes, C.M. Dissimilatory oxidation and reduction of elemental sulfur in thermophilic archaea. J. Bioenerg. Biomembr. 2004, 36, 77–91. [Google Scholar] [CrossRef]
- Liang, C.L.; Xia, J.L.; Zhao, X.J.; Yang, Y.; Gong, S.Q.; Nie, Z.Y.; Ma, C.Y.; Zheng, L.; Zhao, Y.D.; Qiu, G.Z. Effect of activated carbon on chalcopyrite bioleaching with extreme thermophile Acidianus manzaensis. Hydrometallurgy 2010, 105, 179–185. [Google Scholar] [CrossRef]
- Peng, A.A.; Liu, H.C.; Nie, Z.Y.; Xia, J.L. Effect of surfactant tween-80 on sulfur oxidation and expression of sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2012, 22, 3147–3155. [Google Scholar] [CrossRef]
- Zhu, W.; Xia, J.L.; Yang, Y.; Nie, Z.Y.; Zheng, L.; Ma, C.Y.; Zhang, R.Y.; Peng, A.A.; Tang, L.; Qiu, G. Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite. Bioresour. Technol. 2011, 102, 3877–3882. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Boyanov, M.I.; Kemner, K.M.; O’Loughlin, E.J.; Kwon, M.J. Distribution and speciation of Sb and toxic metal(loid)s near an antimony refinery and their effects on indigenous microorganisms. J. Hazard. Mater. 2021, 403, 123625. [Google Scholar] [CrossRef] [PubMed]
- Ammari, A.; Trari, M.; Zebbar, N. Transport properties in Sb-doped SnO2 thin films: Effect of UV illumination and temperature dependence. Mater. Sci. Semicond. Process. 2019, 89, 97–104. [Google Scholar] [CrossRef]
- Drewett, N.E.; Aldous, I.M.; Zou, J.; Hardwick, L.J. In situ Raman spectroscopic analysis of the lithiation and sodiation of antimony microparticles. Electrochim. Acta 2017, 247, 296–305. [Google Scholar] [CrossRef]
- Xia, J.L.; Yang, Y.; He, H.; Zhao, X.J.; Liang, C.L.; Zheng, L.; Ma, C.Y.; Zhao, Y.D.; Nie, Z.Y.; Qiu, G.Z. Surface analysis of sulfur speciation on pyrite bioleached by extreme thermophile Acidianus manzaensis using Raman and XANES spectroscopy. Hydrometallurgy 2010, 100, 129–135. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.A.; Ma, Y.; Wang, X.; Zhao, B.; Ruan, W.D. Study of charge transfer effect in surface-enhanced Raman scattering (sers) by using antimony-doped tin oxide (ATO) nanoparticles as substrates with tunable optical band gaps and free charge carrier densities. Spectrochim. Acta A 2022, 264, 120288. [Google Scholar] [CrossRef]
- Roper, A.J.; Williams, P.A.; Filella, M. Secondary antimony minerals: Phases that control the dispersion of antimony in the supergene zone. Geochemistry 2012, 72, 9–14. [Google Scholar] [CrossRef]
- Yalcin, D.; Ozcalik, O.; Altiok, E.; Bayraktar, O. Characterization and recovery of tartaric acid from wastes of wine and grape juice industries. J. Therm. Anal. Calorim. 2008, 94, 767–771. [Google Scholar] [CrossRef]
- Bittarello, E.; Cámara, F.; Ciriotti, M.E.; Marengo, A. Ottensite, brizziite and mopungite from Pereta mine (Tuscany, Italy): New occurrences and crystal structure refinement of mopungite. Miner. Petrol. 2015, 109, 431–442. [Google Scholar] [CrossRef]
- Williams, P.A.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on new minerals, nomenclature and classification (CNMNC). Mineral. Mag. 2014, 78, 1241–1248. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J.; Prarr, A.R. Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochim. Cosmochim. Acta 1995, 59, 1773–1786. [Google Scholar] [CrossRef]
- Kanuchova, M.; Kozakova, L.; Bakalar, T.; Skvarla, J. Characterization of antimony leaching from polyethylene terephthalate bottles by x-ray photoelectron spectroscopy. J. Anal. Chem. 2020, 75, 1304–1309. [Google Scholar] [CrossRef]
- Kulp, T.R.; Miller, L.G.; Braiotta, F.; Webb, S.M.; Kocar, B.D.; Blum, J.S.; Oremland, R.S. Microbiological reduction of Sb(V) in anoxic freshwater sediments. Environ. Sci. Technol. 2014, 48, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.L.; Liu, H.C.; Nie, Z.Y.; Fan, X.L.; Zhang, D.R.; Zheng, X.F.; Liu, L.Z.; Pan, X.; Zhou, Y.H. Taking insights into phenomics of microbe-mineral interaction in bioleaching and acid mine drainage: Concepts and methodology. Sci. Total Environ. 2020, 729, 139005. [Google Scholar] [CrossRef]
- Liu, H.C.; Xia, J.L.; Nie, Z.Y.; Ma, C.Y.; Zheng, L.; Hong, C.H.; Zhao, Y.D.; Wen, W. Bioleaching of chalcopyrite by Acidianus manzaensis under different constant pH. Miner. Eng. 2016, 98, 80–89. [Google Scholar] [CrossRef]
- Skala, J.; Purnelle, B.; Crouzet, M.; Aigle, M.; Goffeau, A. The open reading frame YCR101 located on chromosome III froms accharomyces cerevisiae is a putative protein kinase. Yeast 1991, 7, 651–655. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; Blake, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef]
- Holmes, D.S.; Bonnefoy, V. Genetic and bioinformatic insights into iron and sulfur oxidation mechanisms of bioleaching organisms. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 281–307. [Google Scholar]
- Lu, S.; Gischkat, S.; Reiche, M.; Akob, D.M.; Hallberg, K.B.; Küsel, K. Ecophysiology of Fe-cycling bacteria in acidic sediments. Appl. Environ. Microbiol. 2010, 76, 8174–8183. [Google Scholar] [CrossRef]
- Wang, J.W.; Liu, T.; Sun, W.L.; Chen, Q. Bioavailable metal(loid)s and physicochemical features co-mediating microbial communities at combined metal(loid) pollution sites. Chemosphere 2020, 260, 127619. [Google Scholar] [CrossRef]
- Barreto, M.; Jedlicki, E.; Holmes, D.S. Identification of a gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 2902–2909. [Google Scholar] [CrossRef]
- Shao, Y.L.; Sun, Q.; Wang, L.K.; Zhan, W.Y.; Zhang, H.W.; Zhong, H. Migration and transformation of Sb are affected by Mn(III/IV) associated with lepidocrocite originating from Fe(II) oxidation. J. Environ. Sci. 2022, 115, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef]
- Xia, J.L.; Zhang, R.Y.; Zhang, Q.; Wu, S.; Zhang, C.G.; Nie, Z.Y.; Qiu, G.Z. Differential expression of genes encoding sulfur metabolism-related periplasmic proteins of Acidithiobacillus ferrooxidans ATCC 23270. Trans. Nonferrous Met. Soc. China 2010, 20, 2366–2370. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).