Potential Role of Neutrophil Extracellular Traps in Cardio-Oncology
Abstract
:1. Introduction of Cardio-Oncology
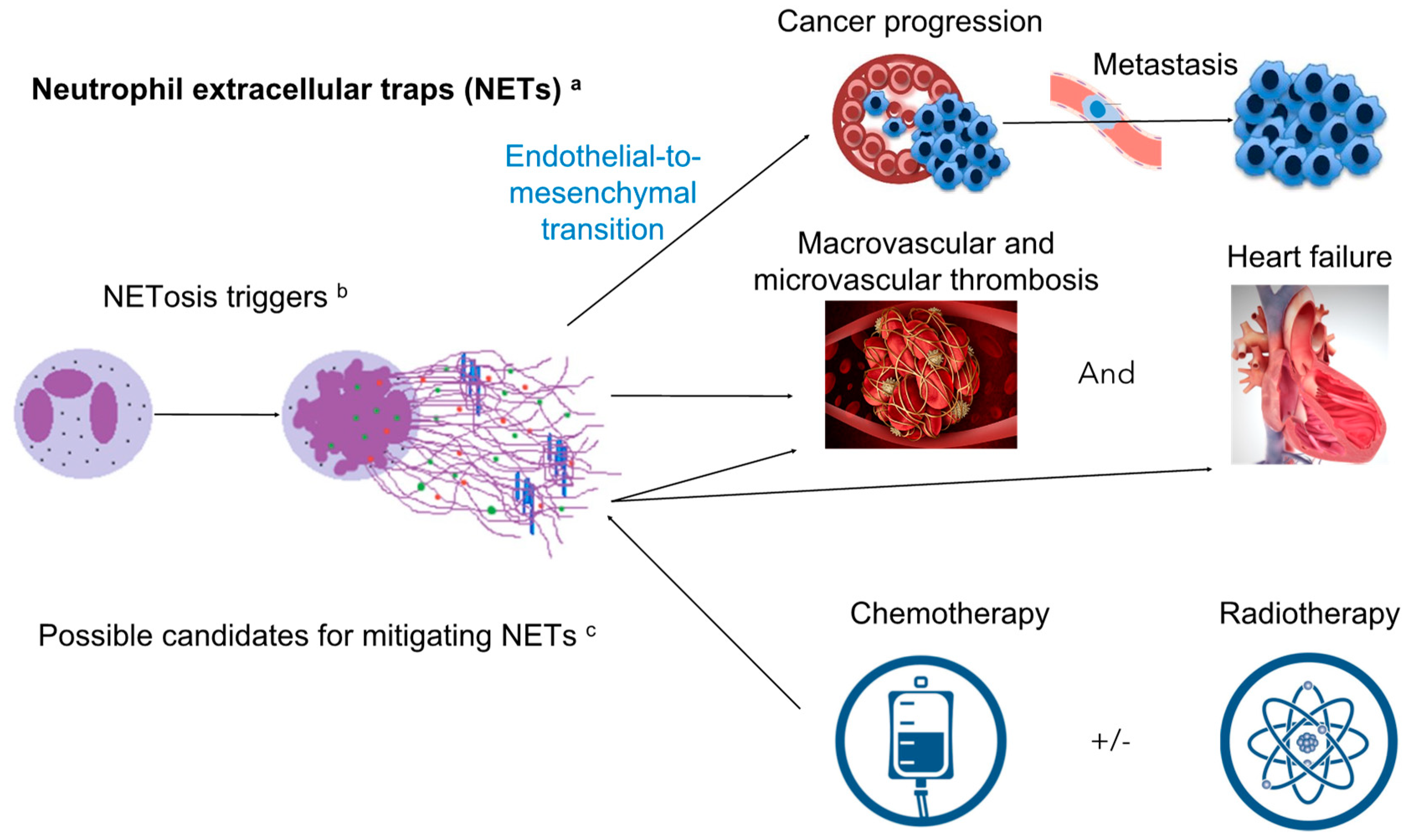
2. Neutrophil Extracellular Traps (NETs): Introduction, Mechanism of Toxicity Focusing on Cardiovascular Toxicity and Contribution to the Cardiovascular Events
2.1. Introduction of NETs: Formation, Definition, and Components
2.2. Mechanism of Toxicity Focusing on Cardiovascular Toxicity and Contribution to Cardiovascular Events
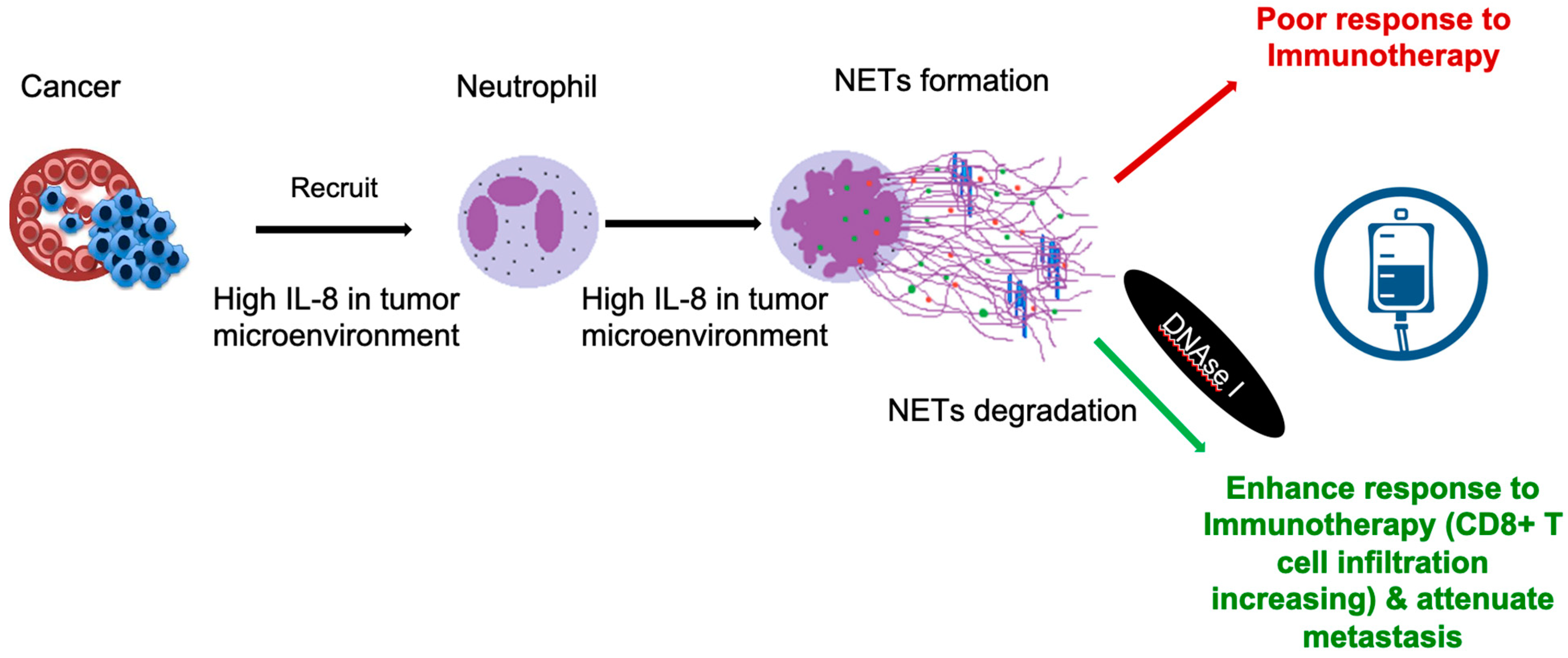
3. NETs in Cancer Itself
4. Effects of Cancer Treatments on Neutrophil Amount, Accumulation in Tissue, Activation, and Possible Effects of NET Formation
4.1. Type I CTRCD Agents
4.2. Type II CTRCD Agents
4.3. Radiotherapy
4.4. Immunotherapies
5. Possible Treatment Modalities
6. Unsolved Questions
7. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.S. Rapid advances in immunotherapy to treat cancer. Clin. Pharmacol. Ther. 2018, 103, 540–544. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Ewer, M.S.; Lippman, S.M. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J. Clin. Oncol. 2005, 23, 2900–2902. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wu, C.-K.; Tsai, M.-L.; Lee, C.-M.; Huang, W.-C.; Chou, H.-H.; Huang, J.-L.; Chi, N.-H.; Yen, H.-W.; Tzeng, B.-H.; et al. 2019 focused update of the guidelines of the taiwan society of cardiology for the diagnosis and treatment of heart failure. Acta Cardiol. Sin. 2019, 35, 244–283. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- De Azambuja, E.; Procter, M.J.; Van Veldhuisen, D.J.D.; Agbor-Tarh, D.D.; Metzger-Filho, O.; Steinseifer, J.; Untch, M.; Smith, I.E.; Gianni, L.; Baselga, J.; et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the herceptin adjuvant trial (BIG 1-01). J. Clin. Oncol. 2014, 32, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Windecker, S.; Lancellotti, P.; Bax, J.J.; Griffin, B.P.; Cahlon, O.; Johnston, D.R. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC scientific expert panel. J. Am. Coll. Cardiol. 2019, 74, 905–927. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Offersen, B.V.; Nielsen, H.M.; Vaage-Nilsen, M.; Yusuf, S.W. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin. Cardiol. 2017, 40, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Nie, X.; Ji, C.; Lin, X.; Liu, L.; Chen, X.; Yao, H.; Wu, S. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J. Am. Heart Assoc. 2017, 6, e005633. [Google Scholar] [CrossRef]
- Boekel, N.B.; Jacobse, J.N.; Schaapveld, M.; Hooning, M.J.; Gietema, J.; Duane, F.K.; Taylor, C.W.; Darby, S.C.; Hauptmann, M.; Seynaeve, C.M.; et al. Cardiovascular disease incidence after internal mammary chain irradiation and anthracycline-based chemotherapy for breast cancer. Br. J. Cancer 2018, 119, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Boekel, N.B.; Duane, F.K.; Jacobse, J.N.; Hauptmann, M.; Schaapveld, M.; Sonke, G.S.; Gietema, J.; Hooning, M.J.; Seynaeve, C.M.; Maas, A.H.; et al. Heart failure after treatment for breast cancer. Eur. J. Heart Fail. 2020, 22, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.; McGale, P.; Brønnum, D.S.; Correa, C.; Cutter, D.; Duane, F.K.; Gigante, B.; Jensen, M.-B.; Lorenzen, E.L.; Rahimi, K.; et al. Cardiac structure injury after radiotherapy for breast cancer: Cross-sectional study with individual patient data. J. Clin. Oncol. 2018, 36, 2288–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, R.M.F.L. Effects of radiotherapy in coronary artery disease. Curr. Atheroscler. Rep. 2019, 21, 50. [Google Scholar] [CrossRef]
- Mansouri, I.; Allodji, R.; Hill, C.; El-Fayech, C.; Pein, F.; Diallo, S.; Schwartz, B.; Vu-Bezin, G.; Veres, C.; Souchard, V.; et al. The role of irradiated heart and left ventricular volumes in heart failure occurrence after childhood cancer. Eur. J. Heart Fail. 2018, 21, 509–518. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Plummer, C.; Steingart, R.M.; Jurczak, W.; Iakobishvili, Z.; Lyon, A.R.; Plastaras, J.P.; Minotti, G. Treatment specific toxicities: Hormones, antihormones, radiation therapy. Semin. Oncol. 2019, 46, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Lambin, P.; De Ruysscher, D.; Widder, J.; Bos, M.; Verheij, M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother. Oncol. 2013, 107, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Lacchetti, C.; Lenihan, D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline summary. J. Oncol. Pract. 2017, 13, 270–275. [Google Scholar] [CrossRef]
- Plana, J.C.; Thavendiranathan, P.; Bucciarelli-Ducci, C.; Lancellotti, P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc. Imaging 2018, 11, 1173–1186. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Bates, J.; Howell, R.M.; Liu, Q.; Yasui, Y.; Mulrooney, D.A.; Dhakal, S.; Smith, S.A.; Leisenring, W.; Indelicato, D.J.; Gibson, T.M.; et al. Therapy-related cardiac risk in childhood cancer survivors: An analysis of the childhood cancer survivor study. J. Clin. Oncol. 2019, 37, 1090–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, M.L.; Niu, J.; Zhang, N.; Giordano, S.H.; Chavez-macgregor, m. cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC Cardiovasc. Imaging 2018, 11, 1084–1093. [Google Scholar] [CrossRef]
- Bijl, J.M.; Roos, M.; van Leeuwen-Segarceanu, E.M.; Vos, J.M.; Bos, W.J.W.; Biesma, D.H.; Post, M.C. Assessment of valvular disorders in survivors of Hodgkin’s lymphoma treated by mediastinal radiotherapy ± chemotherapy. Am. J. Cardiol. 2016, 117, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-H.; Handschumacher, M.D.; Assuncao, B.M.B.L.; Sebag, I.A.; Halpern, E.F.; Scherrer-Crosbie, M. Contraction timing patterns in patients treated for breast cancer before and after anthracyclines therapy. J. Am. Soc. Echocardiogr. 2017, 30, 454–460. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Hancock, S.L.; Lee, B.K.; Mariscal, C.S.; Schnittger, I. Asymptomatic cardiac disease following mediastinal irradiation. J. Am. Coll. Cardiol. 2003, 42, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Van Kessel, K.P.M.; Ebestebroer, J.; Van Strijp, J.A.G. Neutrophil-mediated phagocytosis of staphylococcus aureus. Front. Immunol. 2014, 5, 467. [Google Scholar] [CrossRef] [Green Version]
- Spano, A.; Barni, S.; Sciola, L. PMA withdrawal in PMA-treated monocytic THP-1 cells and subsequent retinoic acid stimulation, modulate induction of apoptosis and appearance of dendritic cells. Cell Prolif. 2013, 46, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Lazzaretto, B.; Fadeel, B. Intra- and extracellular degradation of neutrophil extracellular traps by macrophages and dendritic cells. J. Immunol. 2019, 203, 2276–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.-K. Reassessing the mechanisms of acute coronary syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef]
- Heinecke, J.W.; Li, W.; Francis, G.A.; Goldstein, J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J. Clin. Investig. 1993, 91, 2866–2872. [Google Scholar] [CrossRef]
- Weinrauch, Y.; Drujan, D.; Shapiro, S.D.; Weiss, J.; Zychlinsky, A. Neutrophil elastase targets virulence factors of enterobacteria. Nature 2002, 417, 91–94. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J. Neutrophil extracellular traps: A new player in cancer metastasis and therapeutic target. J. Exp. Clin. Cancer Res. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases: Recent experimental and clinical insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef]
- Suzuki, M.; Ikari, J.; Anazawa, R.; Tanaka, N.; Katsumata, Y.; Shimada, A.; Suzuki, E.; Tatsumi, K. PAD4 Deficiency improves bleomycin-induced neutrophil extracellular traps and fibrosis in mouse lung. Am. J. Respir. Cell Mol. Biol. 2020, 63, 806–818. [Google Scholar] [CrossRef]
- Megens, R.T.A.; Vijayan, S.; Lievens, D.; Döring, Y.; van Zandvoort, M.A.M.J.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012, 107, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.K.; Hsu, P.-C.; Wei, J.Y.; Lopez-Candales, A.; Chen, J.Z.; Su, L.J.; Makhoul, I. Biomarkers of inflammation, hypercoagulability and endothelial injury predict early asymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. Am. J. Cancer Res. 2020, 10, 2933–2945. [Google Scholar] [PubMed]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, R.; Yu, M.; Sausen, G.; Bichsel, C.A.; Corbo, C.; Folco, E.J.; Lee, G.Y.; Liu, Y.; Tesmenitsky, Y.; Shvartz, E.; et al. Targeted delivery of protein arginine deiminase-4 inhibitors to limit arterial intimal NETosis and preserve endothelial integrity. Cardiovasc. Res. 2021, 117, 2652–2663. [Google Scholar] [CrossRef] [PubMed]
- Vulesevic, B.; Lavoie, S.S.; Neagoe, P.-E.; Dumas, E.; Räkel, A.; White, M.; Sirois, M.G. CRP induces NETosis in heart failure patients with or without diabetes. ImmunoHorizons 2019, 3, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Langseth, M.S.; Andersen, G.Ø.; Husebye, T.; Arnesen, H.; Zucknick, M.; Solheim, S.; Eritsland, J.; Seljeflot, I.; Opstad, T.B.; Helseth, R. Neutrophil extracellular trap components and myocardial recovery in post-ischemic acute heart failure. PLoS ONE 2020, 15, e0241333. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Xu, J.-W. NETosis as a pathogenic factor for heart failure. Oxidative Med. Cell. Longev. 2021, 2021, 1–24. [Google Scholar] [CrossRef]
- Mozzini, C.; Pagani, M. Cardiovascular diseases: Consider netosis. Curr. Probl. Cardiol. 2021, 100929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, T.; Chen, Z.; Wang, H.; Yin, Y.; Wang, L.; Wang, Y.; Xu, B.; Xu, W. HMGB1-Promoted Neutrophil Extracellular Traps Contribute to Cardiac Diastolic Dysfunction in Mice. J. Am. Heart Assoc. 2022, 11, e023800. [Google Scholar] [CrossRef]
- Streiff, M.B.; Abutalib, S.A.; Farge, D.; Murphy, M.; Connors, J.M.; Piazza, G. Update on guidelines for the management of cancer-associated thrombosis. Oncologist 2020, 26, e24–e40. [Google Scholar] [CrossRef]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.; Panageas, K.S.; DeAngelis, L. Risk of arterial thromboembolism in patients with cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef]
- Lyman, G.H. Venous thromboembolism in the patient with cancer: Focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011, 117, 1334–1349. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, W.; Shen, F.; Du, W.; Xu, Z.; Liu, Z. The emerging role of neutrophil extracellular traps in arterial, venous and cancer-associated thrombosis. Front. Cardiovasc. Med. 2021, 8, 786387. [Google Scholar] [CrossRef]
- Efrimescu, C.I.; Buggy, P.M.; Buggy, D.J. Neutrophil extracellular trapping role in cancer, metastases, and cancer-related thrombosis: A narrative review of the current evidence base. Curr. Oncol. Rep. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Li, X.; Zhuo, W. Neutrophil extracellular traps in tumor metastasis: Pathological functions and clinical applications. Cancers 2021, 13, 2832. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Nam, J.-K.; Kim, J.-H.; Choi, S.-H.; Lee, Y.-J. Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage. Exp. Mol. Med. 2020, 52, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, E.; Rother, N.; Garsen, M.; Hofstra, J.M.; Satchell, S.C.; Hoffmann, M.; Loeven, M.A.; Knaapen, H.K.; van der Heijden, O.W.; Berden, J.H.; et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arter. Thromb. Vasc. Biol. 2017, 37, 1371–1379. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.; Fritz, A.J.; Zaidi, S.K.; van Wijnen, A.J.; Nickerson, J.A.; Imbalzano, A.N.; Lian, J.B.; Stein, J.L.; Stein, G.S. Epithelial-to-mesenchymal transition and cancer stem cells contribute to breast cancer heterogeneity. J. Cell. Physiol. 2018, 233, 9136–9144. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2021, 22, 173–187. [Google Scholar] [CrossRef]
- Martins-Cardoso, K.; Almeida, V.H.; Bagri, K.M.; Rossi, M.I.D.; Mermelstein, C.S.; König, S.; Monteiro, R.Q. Neutrophil Extracellular Traps (NETs) Promote pro-metastatic phenotype in human breast cancer cells through epithelial-mesenchymal transition. Cancers 2020, 12, 1542. [Google Scholar] [CrossRef]
- Demers, M.; Wong, S.L.; Martinod, K.; Gallant, M.; Cabral, J.E.; Wang, Y.; Wagner, D.D. Priming of neutrophils toward NETosis promotes tumor growth. OncoImmunology 2016, 5, e1134073. [Google Scholar] [CrossRef] [Green Version]
- Faget, J.; Groeneveld, S.; Boivin, G.; Sankar, M.; Zangger, N.; Garcia, M.; Guex, N.; Zlobec, I.; Steiner, L.; Piersigilli, A.; et al. Neutrophils and snail orchestrate the establishment of a pro-tumor microenvironment in lung cancer. Cell Rep. 2017, 21, 3190–3204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snoderly, H.T.; Boone, B.A.; Bennewitz, M.F. Neutrophil extracellular traps in breast cancer and beyond: Current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019, 21, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Várady, C.B.; Oliveira, A.C.; Monteiro, R.Q.; Gomes, T. Recombinant human DNase I for the treatment of cancer-associated thrombosis: A pre-clinical study. Thromb. Res. 2021, 203, 131–137. [Google Scholar] [CrossRef]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil extracellular traps: Villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; D’Ovidio, A.; Tran, H.; Palaniyar, N. Anthracyclines suppress both NADPH oxidase- dependent and -independent NETosis in human neutrophils. Cancers 2019, 11, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todorova, V.K.; Wei, J.Y.; Makhoul, I. Subclinical doxorubicin-induced cardiotoxicity update: Role of neutrophils and endothelium. Am. J. Cancer. Res. 2021, 11, 4070–4091. [Google Scholar]
- Todorova, V.K.; Pennisi, A.; Makhoul, I. Elevated levels of circulating neutrophil extracellular traps and prothrombotic state after one dose of chemotherapy to predict doxorubicin cardiotoxicity in breast cancer. J. Clin. Oncol. 2018, 36, e12579. [Google Scholar] [CrossRef]
- Mendez, N.; Alarcón, P.; Millán, C.; Burgos, R.A.; Morera, F.J.; Ojeda, J. Vincristine, carboplatin and cisplatin increase oxidative burst induced by PAF in canine neutrophils. Vet. Immunol. Immunopathol. 2020, 221, 110011. [Google Scholar] [CrossRef] [PubMed]
- Basyreva, L.Y.; Voinova, E.V.; Gusev, A.A.; Mikhalchik, E.V.; Kuskov, A.N.; Goryachaya, A.V.; Gusev, S.A.; Shtilman, M.I.; Velonia, K.; Tsatsakis, A.M. Fluorouracil neutrophil extracellular traps formation inhibited by polymer nanoparticle shielding. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110382. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Jung, C.-J.; Huang, Y.-M.; Chiao, L.; Chang, Y.-L.; Hsieh, S.-C.; Lin, C.-H.; Kuo, Y.-M. The first reported case of trastuzumab induced interstitial lung disease associated with anti-neutrophil cytoplasmic antibody vasculitis-A case report and a prospective cohort study on the usefulness of neutrophil derived biomarkers in monitoring vasculitis disease activity during follow-up. Breast 2021, 61, 35–42. [Google Scholar] [CrossRef]
- Telerman, A.; Granot, G.; Leibovitch, C.; Yarchovsky-Dolberg, O.; Shacham-Abulafia, A.; Partouche, S.; Yeshurun, M.; Ellis, M.H.; Raanani, P.; Wolach, O. Neutrophil extracellular traps are increased in chronic myeloid leukemia and are differentially affected by tyrosine kinase inhibitors. Cancers 2021, 14, 119. [Google Scholar] [CrossRef]
- Takeshima, T.; Pop, L.M.; Laine, A.; Iyengar, P.; Vitetta, E.S.; Hannan, R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. Proc. Natl. Acad. Sci. USA 2016, 113, 11300–11305. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Hao, Y.; Du, R.; Hu, D.; Xie, J.; Zhang, J.; Deng, G.; Liang, N.; Tian, T.; Käsmann, L.; et al. Radiotherapy programs neutrophils to an antitumor phenotype by inducing mesenchymal-epithelial transition. Transl. Lung Cancer Res. 2021, 10, 1424–1443. [Google Scholar] [CrossRef]
- Shinde-Jadhav, S.; Mansure, J.J.; Rayes, R.F.; Marcq, G.; Ayoub, M.; Skowronski, R.; Kool, R.; Bourdeau, F.; Brimo, F.; Spicer, J.; et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Muravlyova, L.; Maratkyzy, M.; Kabildina, N.; Mkhitaryan, K.; Ponamareva, O.; Bakirova, R.; Molotov-Luchanskiy, V.; Sirota, V. Quantitative characteristics of neutrophil extracellular traps in tumor tissues in colorectal cancer. Open Access Maced. J. Med. Sci. 2020, 8, 1152–1156. [Google Scholar] [CrossRef]
- Moon, E.K.; Wang, L.-C.; Dolfi, D.V.; Wilson, C.B.; Ranganathan, R.; Sun, J.; Kapoor, V.; Scholler, J.; Puré, E.; Milone, M.C.; et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 2014, 20, 4262–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangachari, D.; Costa, D. From hope to reality: Durable overall survival with immune checkpoint inhibitors for advanced lung cancer. J. Clin. Oncol. 2019, 37, 2511–2513. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.A.; Melenhorst, J.J.; Lacey, S.F.; Ambrose, D.E.; Gonzalez, V.; Levine, B.L.; June, C.H.; Schuster, S.J. PD-1 blockade modulates chimeric antigen receptor (CAR)–modified T cells: Refueling the CAR. Blood 2017, 129, 1039–1041. [Google Scholar] [CrossRef] [Green Version]
- Martín-Ruiz, A.; Fiuza-Luces, C.; Martínez-Martínez, E.; Arias, C.F.; Gutiérrez, L.; Ramírez, M.; Martin-Acosta, P.; Coronado, M.J.; Lucia, A.; Provencio, M. Effects of anti-PD-1 immunotherapy on tumor regression: Insights from a patient-derived xenograft model. Sci. Rep. 2020, 10, 7078. [Google Scholar] [CrossRef]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular events among adults treated with Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Salem, J.-E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef] [Green Version]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Beckermann, K.E.; Wang, D.Y. Immune checkpoint inhibitor therapy in patients with autoimmune disease. Oncology 2018, 32, 190–194. [Google Scholar] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Khunger, A.; Vachhani, P.; Colvin, T.A.; Hattoum, A.; Spangenthal, E.; Curtis, A.B.; Dy, G.K.; Ernstoff, M.S.; Puzanov, I. Cardiac toxicity associated with immune checkpoint inhibitors: Case series and review of the literature. Case Rep. Oncol. 2019, 12, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.A.; Zlotoff, D.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Larco, J.A.; Abbasi, M.; Madhani, S.I.; Mereuta, M.O.; Liu, Y.; Dai, D.; Kadirvel, R.; Savastano, L.; Kallmes, D.F.; Brinjikji, W. Correlation of neutrophil to lymphocyte ratio with expression of neutrophil extracellular traps within stroke emboli. Interv. Neuroradiol. 2021. [Google Scholar] [CrossRef]
- Blanch-Ruiz, M.A.; Ortega-Luna, R.; Gómez-García, G.; Martínez-Cuesta, M. Ángeles; Álvarez, Ángeles Role of neutrophil extracellular traps in COVID-19 progression: An insight for effective treatment. Biomedicines 2021, 10, 31. [Google Scholar] [CrossRef]
- Naik, G.S.; Buchbinder, E.I.; Cohen, J.V.; Manos, M.P.; Johnson, A.E.; Bowling, P.; Aizer, A.A.; Schoenfeld, J.D.; Lawrence, D.P.; Haq, R.; et al. Long-term overall survival and predictors in Anti-PD-1-naive melanoma patients with brain metastases treated with immune checkpoint inhibitors in the real-world setting: A multicohort study. J. Immunother. 2021, 44, 307–318. [Google Scholar] [CrossRef]
- Moey, M.Y.; Tomdio, A.N.; McCallen, J.D.; Vaughan, L.M.; O’Brien, K.; Naqash, A.R.; Cherry, C.; Walker, P.R.; Carabello, B.A. Characterization of Immune checkpoint inhibitor-related cardiotoxicity in lung cancer patients from a rural setting. JACC CardioOncol. 2020, 2, 491–502. [Google Scholar] [CrossRef]
- Lynce, F.; Barac, A.; Geng, X.; Dang, C.; Yu, A.F.; Smith, K.L.; Gallagher, C.; Pohlmann, P.R.; Nunes, R.; Herbolsheimer, P.; et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: The SAFE-HEaRt study. Breast Cancer Res. Treat. 2019, 175, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronchetti, L.; Boubaker, N.S.; Barba, M.; Vici, P.; Gurtner, A.; Piaggio, G. Neutrophil extracellular traps in cancer: Not only catching microbes. J. Exp. Clin. Cancer Res. 2021, 40, 23. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Villalba, M.; Olivera, I.; Cirella, A.; Eguren-Santamaria, I.; Berraondo, P.; Schalper, K.A.; de Andrea, C.E.; et al. IL8, neutrophils, and NETs in a collusion against cancer immunity and immunotherapy. Clin. Cancer Res. 2021, 27, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, A.; Seong, K.M. Chronic radiation exposure aggravates atherosclerosis by stimulating neutrophil infiltration. Int. J. Radiat. Biol. 2021, 10, 1–12. [Google Scholar] [CrossRef]
- Majewski, P.; Majchrzak-Gorecka, M.; Grygier, B.; Skrzeczynska-Moncznik, J.; Osiecka, O.; Cichy, J. Inhibitors of serine proteases in regulating the production and function of neutrophil extracellular traps. Front. Immunol. 2016, 7, 261. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Chen, H.; Davids, J.; Bryant, M.; Lucas, A. Serpins for diagnosis and therapy in cancer. Cardiovasc. Hematol. Disord. Drug. Targets 2013, 13, 123–132. [Google Scholar] [CrossRef]
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014, 156, 1002–1016. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Hou, S.; Liang, Q.; He, W.; Li, R.; Wang, H.; Zhu, Y.; Zhang, B.; Chen, L.; Dai, X.; et al. Localized degradation of neutrophil extracellular traps by photoregulated enzyme delivery for cancer immunotherapy and metastasis suppression. ACS Nano 2022, 16, 2585–2597. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Onuma, A.; He, J.; Wang, H.; Xia, Y.; Lal, R.; Cheng, X.; Kasumova, G.; Hu, Z.; et al. Neutrophils extracellular traps inhibition improves pd-1 blockade immunotherapy in colorectal cancer. Cancers 2021, 13, 5333. [Google Scholar] [CrossRef]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Büller, H.R.; Van Es, N. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef] [Green Version]
- Josefs, T.; Barrett, T.J.; Brown, E.J.; Quezada, A.; Wu, X.; Voisin, M.; Amengual, J.; Fisher, E.A. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alique, M.; Luna, C.; Carracedo, J.; Ramírez, R. LDL biochemical modifications: A link between atherosclerosis and aging. Food Nutr. Res. 2015, 59, 29240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obama, T.; Itabe, H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 8312. [Google Scholar] [CrossRef] [PubMed]
| Prediction of CTRCD | 1. Compare Individual NETs with current standard -GLS and hsTn 2. Combination with NETs, GLS and hsTn better than GLS and hsTn? |
| Prediction of thrombosis Events including VTE, ACS and stroke | 1. Compare circulating components of NETs with Khorana score 2. New score system with NETs to predict thrombosis events |
| NETs with mLDL & Atherosclerosis after Cancer Treatment | 1. The causality between NETs and mLDL formation after cancer treatment 2.NETs burden after cancer treatment with atherosclerosis development |
| NETs Clearance with Cancer Recurrence, Metastasis and CTRCT | 1. NETs clearance to prevent cancer recurrence, metastasis, and CTRCT 2. The best solution/mechanism for NETs clearance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.-H.; Contreras, G.P.; Yeh, T.-Y. Potential Role of Neutrophil Extracellular Traps in Cardio-Oncology. Int. J. Mol. Sci. 2022, 23, 3573. https://doi.org/10.3390/ijms23073573
Cheng K-H, Contreras GP, Yeh T-Y. Potential Role of Neutrophil Extracellular Traps in Cardio-Oncology. International Journal of Molecular Sciences. 2022; 23(7):3573. https://doi.org/10.3390/ijms23073573
Chicago/Turabian StyleCheng, Kai-Hung, Gregory P. Contreras, and Ting-Yu Yeh. 2022. "Potential Role of Neutrophil Extracellular Traps in Cardio-Oncology" International Journal of Molecular Sciences 23, no. 7: 3573. https://doi.org/10.3390/ijms23073573
APA StyleCheng, K.-H., Contreras, G. P., & Yeh, T.-Y. (2022). Potential Role of Neutrophil Extracellular Traps in Cardio-Oncology. International Journal of Molecular Sciences, 23(7), 3573. https://doi.org/10.3390/ijms23073573






