State of the Art in the Current Management and Future Directions of Targeted Therapy for Differentiated Thyroid Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Conventional Management of DTC
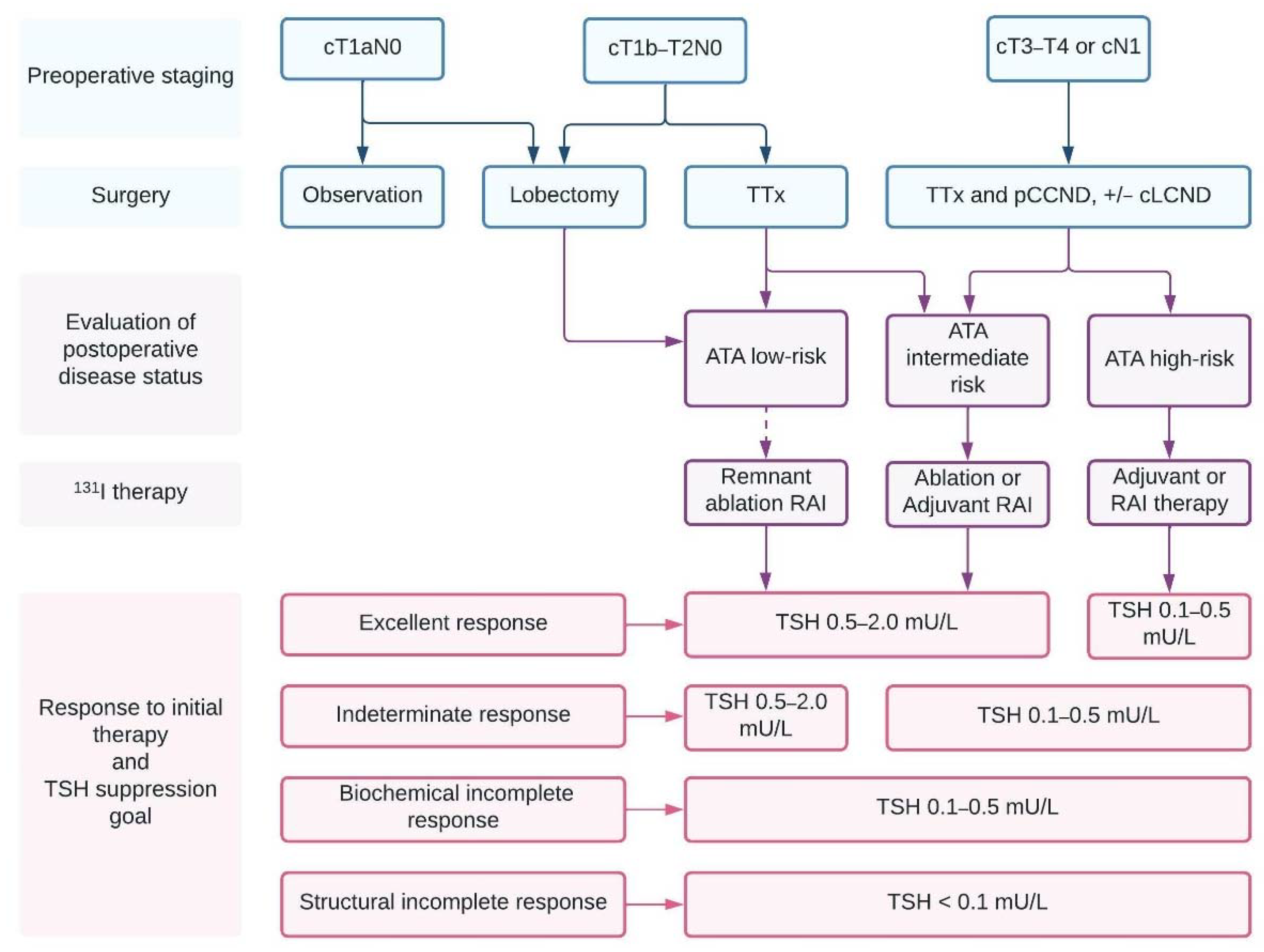
3.1. Surgical Treatment
3.2. Extent of Lymphadenectomy
3.3. Postoperative RAI Therapy
3.4. TSH Suppression following Initial Therapy
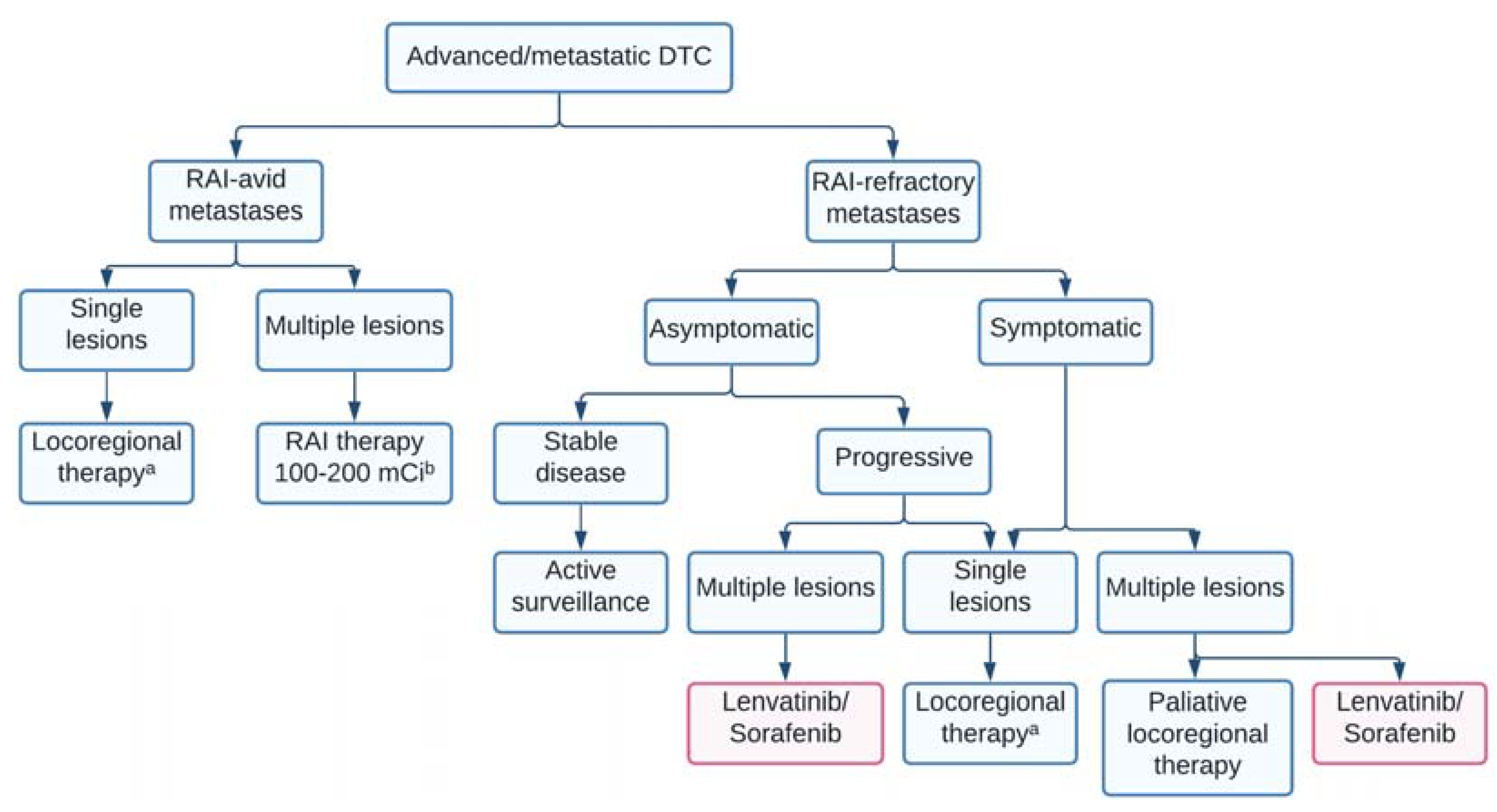
4. Treatment of RAI-Avid Metastatic Disease
5. Treatment of Structural Neck Recurrence
6. Radioiodine-Refractory DTC
6.1. Definition and Current Management
6.2. First-Line Tyrosine Kinase Inhibitors in Advanced, Metastatic RAIR-DTC
6.3. Salvage Targeted Therapy after First-Line Tyrosine Kinase Inhibitors Failure
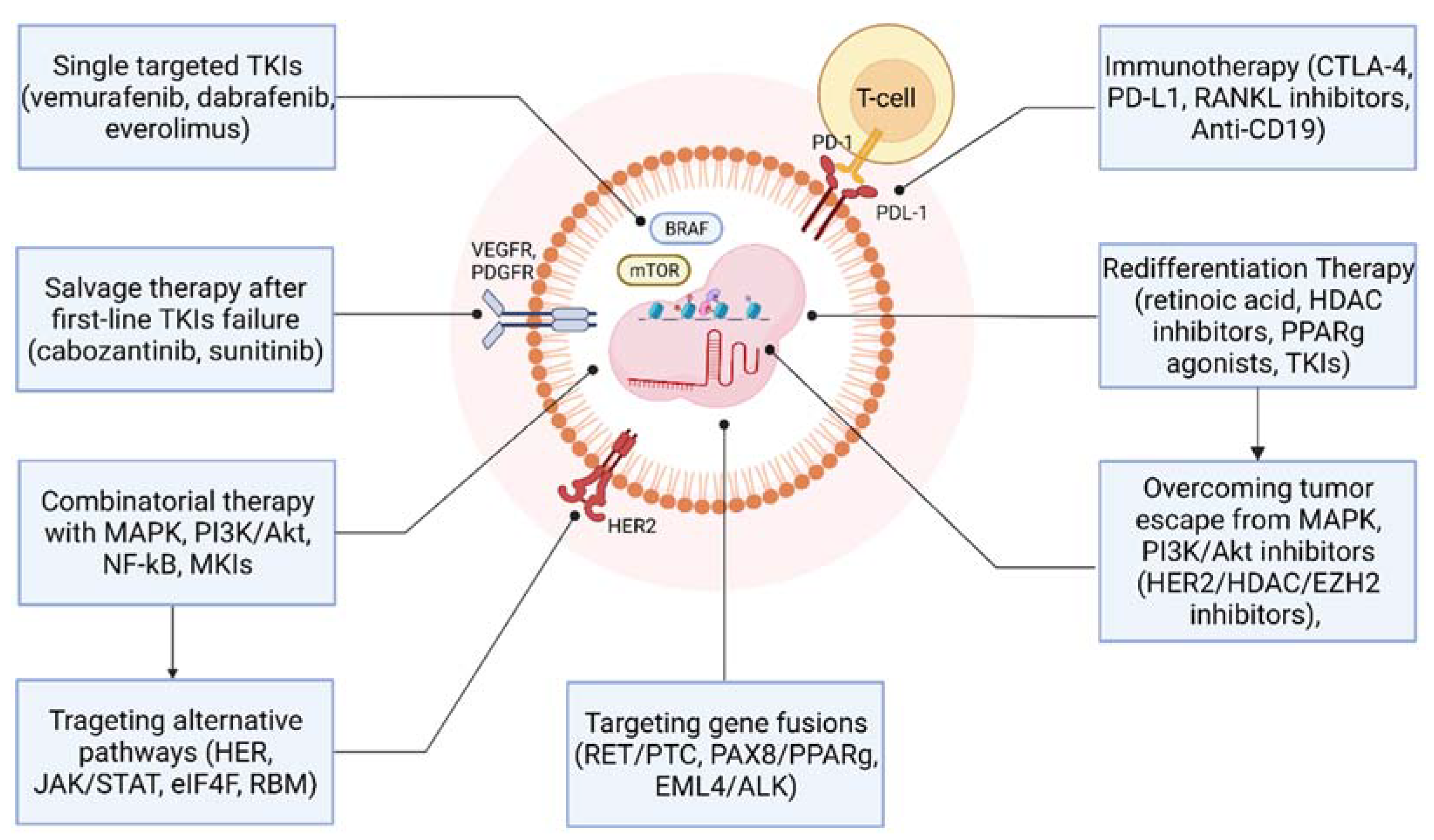
7. Combinatorial Therapy with MAPK, PI3K/Akt/mTOR, NF-κB, and MTKs Inhibitors
8. Targeting Alternative Pathways to Overcome the Resistance to MAPK and PI3K Pathways Inhibitors
9. Redifferentiation Therapy
9.1. Redifferentiation Therapy Achievements Acquired during Recent Decades
9.2. Modulation of MAPK and PI3K/AKT/mTOR Pathways in Restoring DTC Radio-Sensitivity: Preclinical and Clinical Evidence
9.3. Perspectives in Overcoming Tumour Escape from MAPK and PI3K Inhibitors, and RAI Resensitizing Effect
10. Immunotherapy of Advanced TC
11. Targeting Gene Fusions in Differentiated Thyroid Cancer
12. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H. Long-term survival rates of cancer patients achieved by the end of the 20th century: A period analysis. Lancet 2002, 360, 1131–1135. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Saftencu, M.; Braicu, C.; Cojocneanu, R.; Buse, M.; Irimie, A.; Piciu, D.; Berindan-Neagoe, I. Gene Expression Patterns Unveil New Insights in Papillary Thyroid Cancer. Medicina 2019, 55, 500. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019, 8, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- MAZZAFERRI, E.L. An Overview of the Management of Papillary and Follicular Thyroid Carcinoma. Thyroid 1999, 9, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Kim, K.H.; Kang, D.W.; Kim, S.H.; Seong, I.O.; Kang, D.Y. Mutations of the BRAF Gene in Papillary Thyroid Carcinoma in a Korean Population. Yonsei Med. J. 2004, 45, 818. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.; Trovisco, V.; Rocha, A.S.; Lima, J.; Castro, P.; Preto, A.; Máximo, V.; Botelho, T.; Seruca, R.; Sobrinho-Simões, M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 2003, 22, 4578–4580. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Kimura, E.T.; Gandhi, M.; Biddinger, P.W.; Knauf, J.A.; Basolo, F.; Zhu, Z.; Giannini, R.; Salvatore, G.; Fusco, A.; et al. BRAF Mutations in Thyroid Tumors Are Restricted to Papillary Carcinomas and Anaplastic or Poorly Differentiated Carcinomas Arising from Papillary Carcinomas. J. Clin. Endocrinol. Metab. 2003, 88, 5399–5404. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L.; Mannavola, D.; Cirello, V.; Vannucchi, G.; Muzza, M.; Vicentini, L.; Beck-Peccoz, P. BRAF mutations in an Italian cohort of thyroid cancers. Clin. Endocrinol. 2004, 61, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Trovisco, V.; Vieira de Castro, I.; Soares, P.; Máximo, V.; Silva, P.; Magalhães, J.; Abrosimov, A.; Guiu, X.M.; Sobrinho-Simões, M. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J. Pathol. 2004, 202, 247–251. [Google Scholar] [CrossRef]
- Cappola, A.R.; Mandel, S.J. Molecular Testing in Thyroid Cancer. JAMA 2013, 309, 1529. [Google Scholar] [CrossRef]
- Biondi, B.; Filetti, S.; Schlumberger, M. Thyroid-hormone therapy and thyroid cancer: A reassessment. Nat. Clin. Pract. Endocrinol. Metab. 2005, 1, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.T.; Nikiforova, M.N.; Zhu, Z.; Knauf, J.A.; Nikiforov, Y.E.; Fagin, J.A. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003, 63, 1454–1457. [Google Scholar] [PubMed]
- Kim, M.; Jeon, M.J.; Oh, H.-S.; Park, S.; Kim, T.Y.; Shong, Y.K.; Kim, W.B.; Kim, K.; Kim, W.G.; Song, D.E. BRAF and RAS Mutational Status in Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features and Invasive Subtype of Encapsulated Follicular Variant of Papillary Thyroid Carcinoma in Korea. Thyroid 2018, 28, 504–510. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma. JAMA Oncol. 2016, 2, 1023. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Gaweł, D.; Godlewska, M. Novel Inhibitor-Based Therapies for Thyroid Cancer—An Update. Int. J. Mol. Sci. 2021, 22, 11829. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Koenig, R.; Rosen, C.; Auchus, R.; Goldfine, A. Williams Textbook of Endocrinology, 14th ed.; Elsevier: Amsterdam, The Nederlands, 2019; ISBN 9780323711548. [Google Scholar]
- Haddad, R.I.; Nasr, C.; Bischoff, L.; Busaidy, N.L.; Byrd, D.; Callender, G.; Dickson, P.; Duh, Q.-Y.; Ehya, H.; Goldner, W.; et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Barney, B.M.; Hitchcock, Y.J.; Sharma, P.; Shrieve, D.C.; Tward, J.D. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck 2011, 33, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Haigh, P.I.; Urbach, D.R.; Rotstein, L.E. Extent of Thyroidectomy Is Not a Major Determinant of Survival in Low- or High-Risk Papillary Thyroid Cancer. Ann. Surg. Oncol. 2005, 12, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, F.; Shaha, A.; Fish, S.; Tuttle, R.M. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin. Endocrinol. 2011, 75, 112–119. [Google Scholar] [CrossRef]
- Randolph, G.W.; Shin, J.J.; Grillo, H.C.; Mathisen, D.; Katlic, M.R.; Kamani, D.; Zurakowski, D. The surgical management of goiter: Part II. Surgical treatment and results. Laryngoscope 2011, 121, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, A. Clinical Trials of Active Surveillance of Papillary Microcarcinoma of the Thyroid. World J. Surg. 2016, 40, 516–522. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Higashiyama, T.; Kobayashi, K.; Miya, A. Patient Age Is Significantly Related to the Progression of Papillary Microcarcinoma of the Thyroid Under Observation. Thyroid 2014, 24, 27–34. [Google Scholar] [CrossRef]
- Sugitani, I.; Toda, K.; Yamada, K.; Yamamoto, N.; Ikenaga, M.; Fujimoto, Y. Three Distinctly Different Kinds of Papillary Thyroid Microcarcinoma should be Recognized: Our Treatment Strategies and Outcomes. World J. Surg. 2010, 34, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.T.; Doherty, G.M. Central Neck Dissection for Papillary Thyroid Cancer. Cancer Control 2011, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.H.-H.; Ng, S.-H.; Lau, L.L.H.; Cowling, B.J.; Wong, K.P.; Wan, K.Y. A Systematic Review and Meta-Analysis of Prophylactic Central Neck Dissection on Short-Term Locoregional Recurrence in Papillary Thyroid Carcinoma after Total Thyroidectomy. Thyroid 2013, 23, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Viola, D.; Materazzi, G.; Valerio, L.; Molinaro, E.; Agate, L.; Faviana, P.; Seccia, V.; Sensi, E.; Romei, C.; Piaggi, P.; et al. Prophylactic Central Compartment Lymph Node Dissection in Papillary Thyroid Carcinoma: Clinical Implications Derived From the First Prospective Randomized Controlled Single Institution Study. J. Clin. Endocrinol. Metab. 2015, 100, 1316–1324. [Google Scholar] [CrossRef]
- Wang, T.S.; Evans, D.B.; Fareau, G.G.; Carroll, T.; Yen, T.W. Effect of Prophylactic Central Compartment Neck Dissection on Serum Thyroglobulin and Recommendations for Adjuvant Radioactive Iodine in Patients with Differentiated Thyroid Cancer. Ann. Surg. Oncol. 2012, 19, 4217–4222. [Google Scholar] [CrossRef]
- Wang, T.S.; Cheung, K.; Farrokhyar, F.; Roman, S.A.; Sosa, J.A. A Meta-analysis of the Effect of Prophylactic Central Compartment Neck Dissection on Locoregional Recurrence Rates in Patients with Papillary Thyroid Cancer. Ann. Surg. Oncol. 2013, 20, 3477–3483. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Führer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131 I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular. Thyroid 2019, 29, 461–470. [Google Scholar] [CrossRef]
- Schlumberger, M.; Catargi, B.; Borget, I.; Deandreis, D.; Zerdoud, S.; Bridji, B.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Strategies of Radioiodine Ablation in Patients with Low-Risk Thyroid Cancer. N. Engl. J. Med. 2012, 366, 1663–1673. [Google Scholar] [CrossRef]
- Mallick, U.; Harmer, C.; Yap, B.; Wadsley, J.; Clarke, S.; Moss, L.; Nicol, A.; Clark, P.M.; Farnell, K.; McCready, R.; et al. Ablation with Low-Dose Radioiodine and Thyrotropin Alfa in Thyroid Cancer. N. Engl. J. Med. 2012, 366, 1674–1685. [Google Scholar] [CrossRef]
- Pacini, F.; Ladenson, P.W.; Schlumberger, M.; Driedger, A.; Luster, M.; Kloos, R.T.; Sherman, S.; Haugen, B.; Corone, C.; Molinaro, E.; et al. Radioiodine Ablation of Thyroid Remnants after Preparation with Recombinant Human Thyrotropin in Differentiated Thyroid Carcinoma: Results of an International, Randomized, Controlled Study. J. Clin. Endocrinol. Metab. 2006, 91, 926–932. [Google Scholar] [CrossRef]
- Jonklaas, J.; Sarlis, N.J.; Litofsky, D.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Cooper, D.S.; Haugen, B.R.; Ladenson, P.W.; Magner, J.; et al. Outcomes of Patients with Differentiated Thyroid Carcinoma Following Initial Therapy. Thyroid 2006, 16, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Fujimoto, Y. Does Postoperative Thyrotropin Suppression Therapy Truly Decrease Recurrence in Papillary Thyroid Carcinoma? A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2010, 95, 4576–4583. [Google Scholar] [CrossRef]
- COOPER, D.S.; SPECKER, B.; HO, M.; SPERLING, M.; LADENSON, P.W.; ROSS, D.S.; AIN, K.B.; BIGOS, S.T.; BRIERLEY, J.D.; HAUGEN, B.R.; et al. Thyrotropin Suppression and Disease Progression in Patients with Differentiated Thyroid Cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998, 8, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Pujol, P.; Daures, J.P.; Nsakala, N.; Baldet, L.; Bringer, J.; Jaffiol, C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 1996, 81, 4318–4323. [Google Scholar]
- Hovens, G.C.; Stokkel, M.P.; Kievit, J.; Corssmit, E.P.; Pereira, A.M.; Romijn, J.A.; Smit, J.W.A. Associations of Serum Thyrotropin Concentrations with Recurrence and Death in Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2007, 92, 2610–2615. [Google Scholar] [CrossRef]
- Ruegemer, J.J.; Hay, I.D.; Bergstralh, E.J.; Ryan, J.J.; Offord, K.P.; Gorman, C.A. Distant Metastases in Differentiated Thyroid Carcinoma: A Multivariate Analysis of Prognostic Variables. J. Clin. Endocrinol. Metab. 1988, 67, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, G.M.; Yatin, M.; Marcinek, R.; Ain, K.B. Restoration of Iodide Uptake in Dedifferentiated Thyroid Carcinoma: Relationship to Human Na+/I− Symporter Gene Methylation Status1. J. Clin. Endocrinol. Metab. 1999, 84, 2449–2457. [Google Scholar] [CrossRef][Green Version]
- Tuttle, R.M.; Brokhin, M.; Omry, G.; Martorella, A.J.; Larson, S.M.; Grewal, R.K.; Fleisher, M.; Robbins, R.J. Recombinant Human TSH–Assisted Radioactive Iodine Remnant Ablation Achieves Short-Term Clinical Recurrence Rates Similar to Those of Traditional Thyroid Hormone Withdrawal. J. Nucl. Med. 2008, 49, 764–770. [Google Scholar] [CrossRef][Green Version]
- Scharpf, J.; Tuttle, M.; Wong, R.; Ridge, D.; Smith, R.; Hartl, D.; Levine, R.; Randolph, G. Comprehensive management of recurrent thyroid cancer: An American Head and Neck Society consensus statement: AHNS consensus statement. Head Neck 2016, 38, 1862–1869. [Google Scholar] [CrossRef]
- Tufano, R.P.; Clayman, G.; Heller, K.S.; Inabnet, W.B.; Kebebew, E.; Shaha, A.; Steward, D.L.; Tuttle, R.M. Management of Recurrent/Persistent Nodal Disease in Patients with Differentiated Thyroid Cancer: A Critical Review of the Risks and Benefits of Surgical Intervention Versus Active Surveillance. Thyroid 2015, 25, 15–27. [Google Scholar] [CrossRef]
- Ahn, B.-C. Sodium Iodide Symporter for Nuclear Molecular Imaging and Gene Therapy: From Bedside to Bench and Back. Theranostics 2012, 2, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef]
- Capdevila, J.; Galofré, J.C.; Grande, E.; Zafón Llopis, C.; Ramón y Cajal Asensio, T.; Navarro González, E.; Jiménez-Fonseca, P.; Santamaría Sandi, J.; Gómez Sáez, J.M.; Riesco Eizaguirre, G. Consensus on the management of advanced radioactive iodine-refractory differentiated thyroid cancer on behalf of the Spanish Society of Endocrinology Thyroid Cancer Working Group (GTSEEN) and Spanish Rare Cancer Working Group (GETHI). Clin. Transl. Oncol. 2017, 19, 279–287. [Google Scholar] [CrossRef]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard, G.E.; Gilbert, J.A.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81, 1–122. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.F.; Wahl, R.L.; Lodge, M.A.; Javadi, M.S.; Cho, S.Y.; Chien, D.T.; Ewertz, M.E.; Esaias, C.E.; Ladenson, P.W.; Sgouros, G. 124 I PET-Based 3D-RD Dosimetry for a Pediatric Thyroid Cancer Patient: Real-Time Treatment Planning and Methodologic Comparison. J. Nucl. Med. 2009, 50, 1844–1847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robbins, R.J.; Wan, Q.; Grewal, R.K.; Reibke, R.; Gonen, M.; Strauss, H.W.; Tuttle, R.M.; Drucker, W.; Larson, S.M. Real-Time Prognosis for Metastatic Thyroid Carcinoma Based on 2-[18F]Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography Scanning. J. Clin. Endocrinol. Metab. 2006, 91, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Van Nostrand, D.; Cheng, L.; Liu, M.; Chen, L. Radioiodine refractory differentiated thyroid cancer. Crit. Rev. Oncol. Hematol. 2018, 125, 111–120. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Gupta-Abramson, V.; Troxel, A.B.; Nellore, A.; Puttaswamy, K.; Redlinger, M.; Ransone, K.; Mandel, S.J.; Flaherty, K.T.; Loevner, L.A.; O’Dwyer, P.J.; et al. Phase II Trial of Sorafenib in Advanced Thyroid Cancer. J. Clin. Oncol. 2008, 26, 4714–4719. [Google Scholar] [CrossRef]
- Kloos, R.T.; Ringel, M.D.; Knopp, M.V.; Hall, N.C.; King, M.; Stevens, R.; Liang, J.; Wakely, P.E.; Vasko, V.V.; Saji, M.; et al. Phase II Trial of Sorafenib in Metastatic Thyroid Cancer. J. Clin. Oncol. 2009, 27, 1675–1684. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Schlumberger, M.; Jarzab, B.; Martins, R.G.; Pacini, F.; Robinson, B.; McCaffrey, J.C.; Shah, M.H.; Bodenner, D.L.; Topliss, D.; et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer 2015, 121, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.L.; Mankoff, D.A.; Goulart, B.H.; Eaton, K.D.; Capell, P.T.; Kell, E.M.; Bauman, J.E.; Martins, R.G. Phase II Study of Daily Sunitinib in FDG-PET–Positive, Iodine-Refractory Differentiated Thyroid Cancer and Metastatic Medullary Carcinoma of the Thyroid with Functional Imaging Correlation. Clin. Cancer Res. 2010, 16, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Ravaud, A.; de la Fouchardière, C.; Caron, P.; Doussau, A.; Do Cao, C.; Asselineau, J.; Rodien, P.; Pouessel, D.; Nicolli-Sire, P.; Klein, M.; et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: Mature data from the THYSU study. Eur. J. Cancer 2017, 76, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Leboulleux, S.; Bastholt, L.; Krause, T.; de la Fouchardiere, C.; Tennvall, J.; Awada, A.; Gómez, J.M.; Bonichon, F.; Leenhardt, L.; Soufflet, C.; et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 2 trial. Lancet Oncol. 2012, 13, 897–905. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Rosen, L.S.; Vokes, E.E.; Kies, M.S.; Forastiere, A.A.; Worden, F.P.; Kane, M.A.; Sherman, E.; Kim, S.; Bycott, P.; et al. Axitinib Is an Active Treatment for All Histologic Subtypes of Advanced Thyroid Cancer: Results From a Phase II Study. J. Clin. Oncol. 2008, 26, 4708–4713. [Google Scholar] [CrossRef]
- Locati, L.D.; Licitra, L.; Agate, L.; Ou, S.-H.I.; Boucher, A.; Jarzab, B.; Qin, S.; Kane, M.A.; Wirth, L.J.; Chen, C.; et al. Treatment of advanced thyroid cancer with axitinib: Phase 2 study with pharmacokinetic/pharmacodynamic and quality-of-life assessments. Cancer 2014, 120, 2694–2703. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; de Souza, J.A.; Geyer, S.; Wirth, L.J.; Menefee, M.E.; Liu, S.V.; Shah, K.; Wright, J.; Shah, M.H. Cabozantinib As Salvage Therapy for Patients With Tyrosine Kinase Inhibitor-Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase II International Thyroid Oncology Group Trial. J. Clin. Oncol. 2017, 35, 3315–3321. [Google Scholar] [CrossRef]
- Brose, M.S.; Shenoy, S.; Bhat, N.; Harlacker, A.K.; Yurtal, R.K.; Posey, Z.A.; Torrente, D.M.; Grande, C.; Squillante, C.M.; Troxel, A.B.; et al. A Phase 2 Trial of Cabozantinib for the Treatment of Radioiodine-Refractory Differentiated Thyroid Carcinoma in the First-Line Setting. Int. J. Radiat. Oncol. 2018, 100, 1311. [Google Scholar] [CrossRef]
- Brose, M.S.; Robinson, B.; Sherman, S.I.; Krajewska, J.; Lin, C.-C.; Vaisman, F.; Hoff, A.O.; Hitre, E.; Bowles, D.W.; Hernando, J.; et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 2021, 22, 1126–1138. [Google Scholar] [CrossRef]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Sideras, K.; Morris, J.C.; McIver, B.; et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol. 2010, 11, 962–972. [Google Scholar] [CrossRef]
- de la Fouchardière, C.; Godbert, Y.; Dalban, C.; Illouz, F.; Wassermann, J.; Do Cao, C.; Bardet, S.; Zerdoud, S.; Chougnet, C.N.; Zalzali, M.; et al. Intermittent versus continuous administration of pazopanib in progressive radioiodine refractory thyroid carcinoma: Final results of the randomised, multicenter, open-label phase II trial PAZOTHYR. Eur. J. Cancer 2021, 157, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I.; Wirth, L.J.; Droz, J.-P.; Hofmann, M.; Bastholt, L.; Martins, R.G.; Licitra, L.; Eschenberg, M.J.; Sun, Y.-N.; Juan, T.; et al. Motesanib Diphosphate in Progressive Differentiated Thyroid Cancer. N. Engl. J. Med. 2008, 359, 31–42. [Google Scholar] [CrossRef]
- Brose, M.S.; Cabanillas, M.E.; Cohen, E.E.W.; Wirth, L.J.; Riehl, T.; Yue, H.; Sherman, S.I.; Sherman, E.J. Vemurafenib in patients with BRAFV600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1272–1282. [Google Scholar] [CrossRef]
- Shah, M.H.; Wei, L.; Wirth, L.J.; Daniels, G.A.; De Souza, J.A.; Timmers, C.D.; Sexton, J.L.; Beshara, M.; Nichols, D.; Snyder, N.; et al. Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J. Clin. Oncol. 2017, 35, 6022. [Google Scholar] [CrossRef]
- Falchook, G.S.; Millward, M.; Hong, D.; Naing, A.; Piha-Paul, S.; Waguespack, S.G.; Cabanillas, M.E.; Sherman, S.I.; Ma, B.; Curtis, M.; et al. BRAF Inhibitor Dabrafenib in Patients with Metastatic BRAF -Mutant Thyroid Cancer. Thyroid 2015, 25, 71–77. [Google Scholar] [CrossRef]
- Hanna, G.J.; Busaidy, N.L.; Chau, N.G.; Wirth, L.J.; Barletta, J.A.; Calles, A.; Haddad, R.I.; Kraft, S.; Cabanillas, M.E.; Rabinowits, G.; et al. Genomic Correlates of Response to Everolimus in Aggressive Radioiodine-refractory Thyroid Cancer: A Phase II Study. Clin. Cancer Res. 2018, 24, 1546–1553. [Google Scholar] [CrossRef]
- Schneider, T.C.; de Wit, D.; Links, T.P.; van Erp, N.P.; van der Hoeven, J.J.; Gelderblom, H.; Roozen, C.F.M.; Bos, M.; Corver, W.E.; van Wezel, T.; et al. Everolimus in patients with advanced follicular-derived thyroid cancer; results of a phase II clinical trial. J. Clin. Endocrinol. Metab. 2016, 102, 698–707. [Google Scholar] [CrossRef]
- Negri, F.; Porta, C. Donafenib in Chinese patients with advanced hepatocellular carcinoma (HCC): Really a new standard of care, or should we change paradigm for drug development in HCC? Oncol. Rev. 2021, 15, 564. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Yang, H.; Ding, Y.; Cheng, Y.-Z.; Shi, F.; Tan, J.; Deng, Z.-Y.; Chen, Z.-D.; Wang, R.-F.; Ji, Q.-H.; et al. Donafenib in Progressive Locally Advanced or Metastatic Radioactive Iodine-Refractory Differentiated Thyroid Cancer: Results of a Randomized, Multicenter Phase II Trial. Thyroid 2021, 31, 607–615. [Google Scholar] [CrossRef]
- Robinson, B.; Schlumberger, M.; Wirth, L.J.; Dutcus, C.E.; Song, J.; Taylor, M.H.; Kim, S.-B.; Krzyzanowska, M.K.; Capdevila, J.; Sherman, S.I.; et al. Characterization of Tumor Size Changes Over Time From the Phase 3 Study of Lenvatinib in Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Berg, E.; Adam, P.; Weber, G.-L.; Pfestroff, A.; Luster, M.; Kutsch, J.M.; Lapa, C.; Sandner, B.; Rayes, N.; et al. Real world efficacy and safety of multi-tyrosine kinase inhibitors in radioiodine refractory thyroid cancer. Thyroid 2021, 31, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-J.; Chang, Y.-H.; Chen, Y.-H.; Wu, C.-W.; Wang, P.-W.; Hsiao, P.-J. Low Dose of Lenvatinib Treatment for Patients of Radioiodine-Refractory Differentiated Thyroid Carcinoma—A Real-World Experience. Cancer Manag. Res. 2021, 13, 7139–7148. [Google Scholar] [CrossRef] [PubMed]
- Rendl, G.; Sipos, B.; Becherer, A.; Sorko, S.; Trummer, C.; Raderer, M.; Hitzl, W.; Ardelt, M.; Gallowitsch, H.J.; Pirich, C. Real-World Data for Lenvatinib in Radioiodine-Refractory Differentiated Thyroid Cancer (RELEVANT): A Retrospective Multicentric Analysis of Clinical Practice in Austria. Int. J. Endocrinol. 2020, 2020, 8834148. [Google Scholar] [CrossRef]
- Treistman, N.; Nobre, G.M.; Tramontin, M.Y.; da Silva, G.M.W.; Herchenhorn, D.; de Lima Araujo, L.H.; de Andrade, F.A.; Corbo, R.; Bulzico, D.; Vaisman, F. Prognostic factors in patients with advanced differentiated thyroid cancer treated with multikinase inhibitors—A single Brazilian center experience. Arch. Endocrinol. Metab. 2021, 65, 411–420. [Google Scholar] [CrossRef]
- Iwasaki, H.; Toda, S.; Murayama, D.; Kato, S.; Matsui, A. Relationship between adverse events associated with lenvatinib treatment for thyroid cancer and patient prognosis. Mol. Clin. Oncol. 2020, 14, 28. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Brose, M.S.; Ramies, D.A.; Lee, Y.; Miles, D.; Sherman, S.I. Antitumor activity of cabozantinib (XL184) in a cohort of patients (pts) with differentiated thyroid cancer (DTC). J. Clin. Oncol. 2012, 30, 5547. [Google Scholar] [CrossRef]
- Dadu, R.; Devine, C.; Hernandez, M.; Waguespack, S.G.; Busaidy, N.L.; Hu, M.I.; Jimenez, C.; Habra, M.A.; Sellin, R.V.; Ying, A.K.; et al. Role of Salvage Targeted Therapy in Differentiated Thyroid Cancer Patients Who Failed First-Line Sorafenib. J. Clin. Endocrinol. Metab. 2014, 99, 2086–2094. [Google Scholar] [CrossRef]
- Jin, N.; Jiang, T.; Rosen, D.M.; Nelkin, B.D.; Ball, D.W. Synergistic Action of a RAF Inhibitor and a Dual PI3K/mTOR Inhibitor in Thyroid Cancer. Clin. Cancer Res. 2011, 17, 6482–6489. [Google Scholar] [CrossRef]
- Yi, H.; Ye, X.; Long, B.; Ye, T.; Zhang, L.; Yan, F.; Yang, Y.; Li, L. Inhibition of the AKT/mTOR Pathway Augments the Anticancer Effects of Sorafenib in Thyroid Cancer. Cancer Biother. Radiopharm. 2017, 32, 176–183. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Xing, M. The Akt Inhibitor MK2206 Synergizes, but Perifosine Antagonizes, the BRAF V600E Inhibitor PLX4032 and the MEK1/2 Inhibitor AZD6244 in the Inhibition of Thyroid Cancer Cells. J. Clin. Endocrinol. Metab. 2012, 97, E173–E182. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.J.; Dunn, L.A.; Ho, A.L.; Baxi, S.S.; Ghossein, R.A.; Fury, M.G.; Haque, S.; Sima, C.S.; Cullen, G.; Fagin, J.A.; et al. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer 2017, 123, 4114–4121. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Kumar, S.; Kushchayeva, Y.; Gaskins, K.; Boufraqech, M.; Wei, D.; Gara, S.K.; Zhang, L.; Zhang, Y.; Shen, M.; et al. A Combinatorial Strategy for Targeting BRAF V600E -Mutant Cancers with BRAF V600E Inhibitor (PLX4720) and Tyrosine Kinase Inhibitor (Ponatinib). Clin. Cancer Res. 2020, 26, 2022–2036. [Google Scholar] [CrossRef] [PubMed]
- Tsumagari, K.; Abd Elmageed, Z.Y.; Sholl, A.B.; Green, E.A.; Sobti, S.; Khan, A.R.; Kandil, A.; Murad, F.; Friedlander, P.; Boulares, A.H.; et al. Bortezomib sensitizes thyroid cancer to BRAF inhibitor in vitro and in vivo. Endocr. Relat. Cancer 2018, 25, 99–109. [Google Scholar] [CrossRef]
- She, Q.-B.; Halilovic, E.; Ye, Q.; Zhen, W.; Shirasawa, S.; Sasazuki, T.; Solit, D.B.; Rosen, N. 4E-BP1 Is a Key Effector of the Oncogenic Activation of the AKT and ERK Signaling Pathways that Integrates Their Function in Tumors. Cancer Cell 2010, 18, 39–51. [Google Scholar] [CrossRef]
- Faber, A.C.; Li, D.; Song, Y.; Liang, M.-C.; Yeap, B.Y.; Bronson, R.T.; Lifshits, E.; Chen, Z.; Maira, S.-M.; Garcia-Echeverria, C.; et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc. Natl. Acad. Sci. USA 2009, 106, 19503–19508. [Google Scholar] [CrossRef]
- Liu, D.; Xing, J.; Trink, B.; Xing, M. BRAF mutation-selective inhibition of thyroid cancer cells by the novel MEK inhibitor RDEA119 and genetic-potentiated synergism with the mTOR inhibitor temsirolimus. Int. J. Cancer 2010, 127, 2965–2973. [Google Scholar] [CrossRef]
- Montero-Conde, C.; Ruiz-Llorente, S.; Dominguez, J.M.; Knauf, J.A.; Viale, A.; Sherman, E.J.; Ryder, M.; Ghossein, R.A.; Rosen, N.; Fagin, J.A. Relief of Feedback Inhibition of HER3 Transcription by RAF and MEK Inhibitors Attenuates Their Antitumor Effects in BRAF -Mutant Thyroid Carcinomas. Cancer Discov. 2013, 3, 520–533. [Google Scholar] [CrossRef]
- Ma, J.; Lyu, H.; Huang, J.; Liu, B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol. Cancer 2014, 13, 105. [Google Scholar] [CrossRef]
- Boussemart, L.; Malka-Mahieu, H.; Girault, I.; Allard, D.; Hemmingsson, O.; Tomasic, G.; Thomas, M.; Basmadjian, C.; Ribeiro, N.; Thuaud, F.; et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 2014, 513, 105–109. [Google Scholar] [CrossRef]
- Poulikakos, P.I.; Persaud, Y.; Janakiraman, M.; Kong, X.; Ng, C.; Moriceau, G.; Shi, H.; Atefi, M.; Titz, B.; Gabay, M.T.; et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011, 480, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Menzies, A.M.; Zimmer, L.; Eroglu, Z.; Ye, F.; Zhao, S.; Rizos, H.; Sucker, A.; Scolyer, R.A.; Gutzmer, R.; et al. Acquired BRAF inhibitor resistance: A multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur. J. Cancer 2015, 51, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Antonello, Z.A.; Hsu, N.; Bhasin, M.; Roti, G.; Joshi, M.; Van Hummelen, P.; Ye, E.; Lo, A.S.; Karumanchi, S.A.; Bryke, C.R.; et al. Vemurafenib-resistance via de novo RBM genes mutations and chromosome 5 aberrations is overcome by combined therapy with palbociclib in thyroid carcinoma with BRAFV600E. Oncotarget 2017, 8, 84743–84760. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; Corbo, R.; Buescu, A.; Carvalho, D.P.; Vaisman, M. Retinoic acid in patients with radioiodine non-responsive thyroid carcinoma. J. Endocrinol. Investig. 2004, 27, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. Action of thiazolidinediones on differentiation, proliferation and apoptosis of normal and transformed thyrocytes in culture. Endocr. Relat. Cancer 2005, 12, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Lindsay, S.; Clark, O.H.; Woeber, K.A.; Hawkins, R.; Greenspan, F.S. Results of Rosiglitazone Therapy in Patients with Thyroglobulin-Positive and Radioiodine-Negative Advanced Differentiated Thyroid Cancer. Thyroid 2009, 19, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Zheng, Y.; Tyner, J.W.; Chng, W.J.; Chien, W.W.; Gery, S.; Leong, G.; Braunstein, G.D.; Koeffler, H.P. Belinostat and panobinostat (HDACI): In vitro and in vivo studies in thyroid cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1507–1514. [Google Scholar] [CrossRef]
- Dunn, L.A.; Sherman, E.J.; Baxi, S.S.; Tchekmedyian, V.; Grewal, R.K.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Sabra, M.M.; et al. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers. J. Clin. Endocrinol. Metab. 2019, 104, 1417–1428. [Google Scholar] [CrossRef]
- Chow, L.Q.; Santana-Davila, R.; Pantel, A.; Roth, M.; Anderson, L.N.; Failor, A.; Doot, R.; Mankoff, D. A phase I study of pazopanib in combination with escalating doses of 131I in patients with well-differentiated thyroid carcinoma borderline refractory to radioiodine. PLoS ONE 2017, 12, e0178325. [Google Scholar] [CrossRef]
- Park, J.-W.; Zarnegar, R.; Kanauchi, H.; Wong, M.G.; Hyun, W.C.; Ginzinger, D.G.; Lobo, M.; Cotter, P.; Duh, Q.-Y.; Clark, O.H. Troglitazone, the Peroxisome Proliferator-Activated Receptor-γ Agonist, Induces Antiproliferation and Redifferentiation in Human Thyroid Cancer Cell Lines. Thyroid 2005, 15, 222–231. [Google Scholar] [CrossRef]
- Ho, A.L.; Grewal, R.K.; Leboeuf, R.; Sherman, E.J.; Pfister, D.G.; Deandreis, D.; Pentlow, K.S.; Zanzonico, P.B.; Haque, S.; Gavane, S.; et al. Selumetinib-Enhanced Radioiodine Uptake in Advanced Thyroid Cancer. N. Engl. J. Med. 2013, 368, 623–632. [Google Scholar] [CrossRef]
- Banerji, U.; Camidge, D.R.; Verheul, H.M.W.; Agarwal, R.; Sarker, D.; Kaye, S.B.; Desar, I.M.E.; Timmer-Bonte, J.N.H.; Eckhardt, S.G.; Lewis, K.D.; et al. The First-in-Human Study of the Hydrogen Sulfate (Hyd-Sulfate) Capsule of the MEK1/2 Inhibitor AZD6244 (ARRY-142886): A Phase I Open-Label Multicenter Trial in Patients with Advanced Cancer. Clin. Cancer Res. 2010, 16, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Dedecjus, M.; Truttle, R.M.; Tennaval, J.; So, K.; Fagin, J. ASTRA: A phase III, randomized, placebo-controlled study evaluating complete remission rate (CRR) with short-course selumetinib plus adjuvant radioactive iodine (RAI) in patients (pts) with differentiated thyroid cancer (DTC). In Proceedings of the 88th Annual Meeting of the American Thyroid Association, Washington, DC, USA, 3–7 October 2018. [Google Scholar]
- Rothenberg, S.M.; McFadden, D.G.; Palmer, E.L.; Daniels, G.H.; Wirth, L.J. Redifferentiation of Iodine-Refractory BRAF V600E-Mutant Metastatic Papillary Thyroid Cancer with Dabrafenib. Clin. Cancer Res. 2015, 21, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P. Current and future immunotherapies for thyroid cancer. Expert Rev. Anticancer Ther. 2018, 18, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Fesnak, A.; Lin, C.; Siegel, D.L.; Maus, M.V. CAR-T Cell Therapies From the Transfusion Medicine Perspective. Transfus. Med. Rev. 2016, 30, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, T.M.; Liang, H.; Moore, M.D.; Al-Jamed, I.; Gray, K.D.; Limberg, J.; Stefanova, D.; Buicko, J.L.; Finnerty, B.; Beninato, T.; et al. Dual inhibition of BRAF and MEK increases expression of sodium iodide symporter in patient-derived papillary thyroid cancer cells in vitro. Surgery 2020, 167, 56–63. [Google Scholar] [CrossRef]
- Jafri, S.; Yaqub, A. Redifferentiation of BRAF V600E-Mutated Radioiodine Refractory Metastatic Papillary Thyroid Cancer After Treatment With Dabrafenib and Trametinib. Cureus 2021, 13, e17488. [Google Scholar] [CrossRef]
- Jaber, T.; Waguespack, S.G.; Cabanillas, M.E.; Elbanan, M.; Vu, T.; Dadu, R.; Sherman, S.I.; Amit, M.; Santos, E.B.; Zafereo, M.; et al. Targeted Therapy in Advanced Thyroid Cancer to Resensitize Tumors to Radioactive Iodine. J. Clin. Endocrinol. Metab. 2018, 103, 3698–3705. [Google Scholar] [CrossRef]
- Hayes, D.N.; Lucas, A.S.; Tanvetyanon, T.; Krzyzanowska, M.K.; Chung, C.H.; Murphy, B.A.; Gilbert, J.; Mehra, R.; Moore, D.T.; Sheikh, A.; et al. Phase II Efficacy and Pharmacogenomic Study of Selumetinib (AZD6244; ARRY-142886) in Iodine-131 Refractory Papillary Thyroid Carcinoma with or without Follicular Elements. Clin. Cancer Res. 2012, 18, 2056–2065. [Google Scholar] [CrossRef]
- Iravani, A.; Solomon, B.; Pattison, D.A.; Jackson, P.; Ravi Kumar, A.; Kong, G.; Hofman, M.S.; Akhurst, T.; Hicks, R.J. Mitogen-Activated Protein Kinase Pathway Inhibition for Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer: An Evolving Protocol. Thyroid 2019, 29, 1634–1645. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Zhang, X.; Ringel, M.D.; Jhiang, S.M. Modulation of sodium iodide symporter expression and function by LY294002, Akti-1/2 and Rapamycin in thyroid cells. Endocr. Relat. Cancer 2012, 19, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Kogai, T.; Sajid-Crockett, S.; Newmarch, L.S.; Liu, Y.-Y.; Brent, G.A. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J. Endocrinol. 2008, 199, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, T.S.; Heinhuis, B.; Gerrits, D.; Netea, M.G.; Joosten, L.A.B.; Hermus, A.R.M.M.; Oyen, W.J.G.; Schweppe, R.E.; Haugen, B.R.; Boerman, O.C.; et al. mTOR Inhibition Promotes TTF1-Dependent Redifferentiation and Restores Iodine Uptake in Thyroid Carcinoma Cell Lines. J. Clin. Endocrinol. Metab. 2014, 99, E1368–E1375. [Google Scholar] [CrossRef][Green Version]
- de Souza, E.C.L.; Padrón, Á.S.; Braga, W.M.O.; de Andrade, B.M.; Vaisman, M.; Nasciutti, L.E.; Ferreira, A.C.F.; de Carvalho, D.P. MTOR downregulates iodide uptake in thyrocytes. J. Endocrinol. 2010, 206, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, Y.; Liu, M.; Ruan, M.; Chen, L. HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E. Oncotarget 2017, 8, 19843–19854. [Google Scholar] [CrossRef]
- Fu, H.; Cheng, L.; Sa, R.; Jin, Y.; Chen, L. Combined tazemetostat and MAPKi enhances differentiation of papillary thyroid cancer cells harbouring BRAF V600E by synergistically decreasing global trimethylation of H3K27. J. Cell. Mol. Med. 2020, 24, 3336–3345. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, J.; Yan, J.; Zhang, Y.; Yin, Z. Targeting EZH2 as a novel therapeutic strategy for sorafenib-resistant thyroid carcinoma. J. Cell. Mol. Med. 2019, 23, 4770–4778. [Google Scholar] [CrossRef]
- Tchekmedyian, V.; Dunn, L.; Sherman, E.J.; Baxi, S.S.; Grewal, R.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Sabra, M.; et al. Enhancing Radioiodine Incorporation in BRAF-Mutant, Radioiodine-Refractory Thyroid Cancers with Vemurafenib and the Anti-ErbB3 Monoclonal Antibody CDX-3379: Results of a Pilot Clinical Trial. Thyroid 2022. Online Ahead of Editing. [Google Scholar] [CrossRef]
- Bastman, J.J.; Serracino, H.S.; Zhu, Y.; Koenig, M.R.; Mateescu, V.; Sams, S.B.; Davies, K.D.; Raeburn, C.D.; McIntyre, R.C.; Haugen, B.R.; et al. Tumor-Infiltrating T Cells and the PD-1 Checkpoint Pathway in Advanced Differentiated and Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 2863–2873. [Google Scholar] [CrossRef]
- Chowdhury, S.; Veyhl, J.; Jessa, F.; Polyakova, O.; Alenzi, A.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 2016, 7, 32318–32328. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.-C.; Prawira, A.; de Braud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and antitumor activity of the anti–PD-1 antibody pembrolizumab in patients with advanced, PD-L1–positive papillary or follicular thyroid cancer. BMC Cancer 2019, 19, 196. [Google Scholar] [CrossRef]
- Zimmer, L.; Livingstone, E.; Krackhardt, A.; Schultz, E.S.; Göppner, D.; Assaf, C.; Trebing, D.; Stelter, K.; Windemuth-Kieselbach, C.; Ugurel, S.; et al. Encorafenib, binimetinib plus pembrolizumab triplet therapy in patients with advanced BRAFV600 mutant melanoma: Safety and tolerability results from the phase I IMMU-TARGET trial. Eur. J. Cancer 2021, 158, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.; French, J.; Worden, F.P.; Konda, B.; Sherman, E.J.; Dadu, R.; Gianoukakis, A.G.; Wolfe, E.G.; Foster, N.R.; Bowles, D.W.; et al. Lenvatinib plus pembrolizumab combination therapy in patients with radioiodine-refractory (RAIR), progressive differentiated thyroid cancer (DTC): Results of a multicenter phase II international thyroid oncology group trial. J. Clin. Oncol. 2020, 38, 6512. [Google Scholar] [CrossRef]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Naoum, G.E.; Morkos, M.; Kim, B.; Arafat, W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol. Cancer 2018, 17, 51. [Google Scholar] [CrossRef]
- Rinaldi, L.; Vetrano, E.; Rinaldi, B.; Galiero, R.; Caturano, A.; Salvatore, T.; Sasso, F.C. HCC and Molecular Targeting Therapies: Back to the Future. Biomedicines 2021, 9, 1345. [Google Scholar] [CrossRef]
- Min, I.M.; Shevlin, E.; Vedvyas, Y.; Zaman, M.; Wyrwas, B.; Scognamiglio, T.; Moore, M.D.; Wang, W.; Park, S.; Park, S.; et al. CAR T Therapy Targeting ICAM-1 Eliminates Advanced Human Thyroid Tumors. Clin. Cancer Res. 2017, 23, 7569–7583. [Google Scholar] [CrossRef]
- Carneiro, R.M.; Carneiro, B.A.; Agulnik, M.; Kopp, P.A.; Giles, F.J. Targeted therapies in advanced differentiated thyroid cancer. Cancer Treat. Rev. 2015, 41, 690–698. [Google Scholar] [CrossRef]
- Staubitz, J.I.; Schad, A.; Springer, E.; Rajalingam, K.; Lang, H.; Roth, W.; Hartmann, N.; Musholt, T.J. Novel rearrangements involving the RET gene in papillary thyroid carcinoma. Cancer Genet. 2019, 230, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hsu, C.; Lai, C.; Hang, J. NTRK -rearranged papillary thyroid carcinoma demonstrates frequent subtle nuclear features and indeterminate cytologic diagnoses. Cancer Cytopathol. 2022, 130, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-H.; Wirth, L.J.; Farahani, A.A.; Nosé, V.; Faquin, W.C.; Dias-Santagata, D.; Sadow, P.M. Clinicopathologic features of kinase fusion-related thyroid carcinomas: An integrative analysis with molecular characterization. Mod. Pathol. 2020, 33, 2458–2472. [Google Scholar] [CrossRef]
- Zhu, Z.; Ciampi, R.; Nikiforova, M.N.; Gandhi, M.; Nikiforov, Y.E. Prevalence of RET/PTC Rearrangements in Thyroid Papillary Carcinomas: Effects of the Detection Methods and Genetic Heterogeneity. J. Clin. Endocrinol. Metab. 2006, 91, 3603–3610. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; De Falco, V.; Rao, D.M.; Bellelli, R.; Muthu, M.; Basolo, F.; Fusco, A.; Gutkind, J.S.; Santoro, M. The β-Catenin Axis Integrates Multiple Signals Downstream from RET/Papillary Thyroid Carcinoma Leading to Cell Proliferation. Cancer Res. 2009, 69, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Berthelet, J.; Renaud, E.; Rosigkeit, S.; Distler, U.; Stawiski, E.; Wang, J.; Modrusan, Z.; Fiedler, M.; Bienz, M.; et al. Proteogenomics analysis unveils a TFG-RET gene fusion and druggable targets in papillary thyroid carcinomas. Nat. Commun. 2020, 11, 2056. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Carydis, B.; Ezzat, S.; Bedard, Y.C.; Asa, S.L. Analysis of ret/PTC Gene Rearrangements Refines the Fine Needle Aspiration Diagnosis of Thyroid Cancer. J. Clin. Endocrinol. Metab. 2001, 86, 2187–2190. [Google Scholar] [CrossRef]
- Castro, P.; Rebocho, A.P.; Soares, R.J.; Magalhães, J.; Roque, L.; Trovisco, V.; Vieira de Castro, I.; Cardoso-de-Oliveira, M.; Fonseca, E.; Soares, P.; et al. PAX8-PPAR γ Rearrangement Is Frequently Detected in the Follicular Variant of Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 213–220. [Google Scholar] [CrossRef]
- Kroll, T.G.; Sarraf, P.; Pecciarini, L.; Chen, C.-J.; Mueller, E.; Spiegelman, B.M.; Fletcher, J.A. PAX8-PPAR γ 1 Fusion in Oncogene Human Thyroid Carcinoma. Science 2000, 289, 1357–1360. [Google Scholar] [CrossRef]
- Dwight, T.; Thoppe, S.R.; Foukakis, T.; Lui, W.O.; Wallin, G.; Höög, A.; Frisk, T.; Larsson, C.; Zedenius, J. Involvement of the PAX8/Peroxisome Proliferator-Activated Receptor γ Rearrangement in Follicular Thyroid Tumors. J. Clin. Endocrinol. Metab. 2003, 88, 4440–4445. [Google Scholar] [CrossRef]
- Giordano, T.J.; Haugen, B.R.; Sherman, S.I.; Shah, M.H.; Caoili, E.M.; Koenig, R.J. Pioglitazone Therapy of PAX8-PPARγ Fusion Protein Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 1277–1281. [Google Scholar] [CrossRef]
- Sabir, S.; Yeoh, S.; Jackson, G.; Bayliss, R. EML4-ALK Variants: Biological and Molecular Properties, and the Implications for Patients. Cancers 2017, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Aydemirli, M.D.; van Eendenburg, J.D.H.; van Wezel, T.; Oosting, J.; Corver, W.E.; Kapiteijn, E.; Morreau, H. Targeting EML4-ALK gene fusion variant 3 in thyroid cancer. Endocr. Relat. Cancer 2021, 28, 377–389. [Google Scholar] [CrossRef] [PubMed]

| Name of the Drug | Targets | Phase II/III | RCT | PFS (Months) | RR (%) |
|---|---|---|---|---|---|
| Multitargeted Kinase Inhibitors | |||||
| Sorafenib | VEGFR, PDGFR, c-KIT, RET, RAF | III | DECISION [59] | 10.8 | 12 |
| II | Gupta et al., 2008 [61] | 79 | 23 | ||
| II | Kloos et al., 2009 [62] | 15 | 15 | ||
| Lenvatinib | VEGFR, PDGFR, c-KIT, RET, FGFR | III | SELECT [60] | 18.3 | 64.8 |
| II | Cabanillas et al., 2015 [63] | 12.6 | 50 | ||
| Sunitinib | VEGFR, PDGFR, c-KIT, RET, FLT3, GCSFR | II | Carr et al., 2010 [64] | 12.8 | 31 |
| II | Ravaud et al., 2017 [65] | 22 | 13.1 | ||
| Vandetanib | VEGFR, EGFR, RET | II | Leboulleux et al., 2012 [66] | 11 | 8.3 |
| Axitinib | VEGFR, PDGFR, c-KIT, RET | II | Cohen et al., 2008 [67] | 18 | 30 |
| II | Locati et al., 2014 [68] | 16 | 35 | ||
| Cabozantinib | VEGFR, RET, c-MET, FLT3, TEK | II | Cabanillas et al., 2017 [69] | 12.7 | 40 |
| II | Brose et al., 2018 [70] | NA | 54 | ||
| III | Brose et al., 2021 (COSMIC-311) [71] | 5.7 | 15 | ||
| Pazopanib | VEGFR, PDGFR, c-KIT | II | Bible et al., 2010 [72] | 11.7 | 49 |
| II | De la Fouchardiere et al., 2021 (PAZOTHYR) [73] | 9.2 | 35.6 | ||
| Motesanib | VEGFR, PDGFR, c-KIT, RET | II | Sherman et al., 2008 [74] | 9.3 | 14 |
| Single Targeted Kinase Inhibitors | |||||
| Vemurafenib | BRAFV600E | II | Brose et al., 2016 [75] | 15 | 38.5 |
| Dabrafenib | BRAFV600E | II | Shah et al., 2017 [76] | 11.4 | 50 |
| I | Falchook et al., 2015 [77] | 1.3 | 29 | ||
| Everolimus | mTOR | II | Hanna et al., 2018 [78] | 12.9 | 6 |
| II | Schneider et al., 2016 [79] | 9 | 0 | ||
| Targets | MAPK Pathway | Multiple Tyrosine Kinases | ||
|---|---|---|---|---|
| RAF/BRAFV600E | MEK-1/-2 | |||
| PI3K/AKT/mTOR pathway | PI3K | RAF 265 + Dactolisib (BEZ 235) (Jin et al., 2011) [90] | Sorafenib + Dactolisib (Yi et al., 2017) [91] | |
| mTOR | Refametinib + Temsirolimus (Liu et al., 2012) [92] | Sorafenib + Temsirolimus (Sherman et al., 2017) [93] | ||
| AKT | MK 2206 + Vemurafenib (Liu et al., 2012) [92] | MK 2206 + Selumetinib (Liu et al., 2012) [92] | ||
| Multiple tyrosine kinases | PLX4720 + Ponatinib (Ghosh et al., 2020) [94] | |||
| NF-κB | Vemurafenib + Bortezomib (Tsumagari et al., 2018) [95] | |||
| Drug (target) | Study Type | Patients (Number) | RAI Uptake Threshold | PR | SD | PFS (Month) | Study |
|---|---|---|---|---|---|---|---|
| Monotherapy | |||||||
| Selumetinib (MEK-1/-2) | Prospective | 20 | 8/20 | 5/8 | 3/8 | Ho et al., 2013 [112] | |
| II | 3 | 1/32 | 21/32 | 8 | Hayes et al., 2012 [121] | ||
| III | 157 | 60/157 | Ho et al., 2018 [114] | ||||
| Dabrafenib (BRAF) | I Prospective | 10 | 6/10 | 2/6 | 4/6 | Rothenberg et al., 2015 [115] | |
| Vemurafenib (BRAF) | Pilot Prospective | 10 | 6/10 | 2/4 | 2/4 | 6 | Dunn et al., 2019 [109] |
| Retrospective | 6 | 4/6 | 3/4 | 1/4 | Iravani et al., 2019 [122] | ||
| Pazopanib (MTKI) | I | 6 | 0/6 * | 0/6 | 5/6 | 6.7 | Chow et al., 2017 [110] |
| Combined Therapy | |||||||
| Dabrafenib/Verumafenib + Trametinib/Cobimetinib (MEK) | Retrospective | 6 | 4/6 | 3/4 | 1/4 | Jaber et al., 2018 [120] | |
| Dabrafenib + Trametinib | II | 53 | - | 9/24 | 10/27 | 15.1 | Shah et al., 2017 [76] |
| Combination of MAPK Pathway Inhibitors with: | Preclinical Studies Targeting Redifferentiation Therapy of RAIR-DTC | References |
|---|---|---|
| HER2 inhibitors | Dabrafenib (BRAF) + lapatinib (HER2) | Cheng et al., 2017 [128] |
| Selumetinib (MEK-1/-2) + lapatinib (HER2) | Cheng et al., 2017 [128] | |
| HDAC inhibitor | Dabrafenib/selumetinib + panabinostat (HDAC) | Fu et al., 2020 [129] |
| EZH2 inhibitor | Dabrafenib/selumetinib + Tazemetostat (EZH2) | Fu et al., 2020 [129] |
| Selumetinib + Tazemetostat (EZH2) | Wang et al., 2019 [130] |
| Trial | Trial Phase | Patients/Diagnosis | Drug or Drugs Combination | Drug Targets | Status |
|---|---|---|---|---|---|
| NCT04061980 | II | BRAF V600-positive metastatic and RAIR-DTC | encorafenib + binimetinib ± nivolumab | BRAF, MEK with or without PD-1 | Recruiting |
| NCT02973997 | II | lenvatinib-naïve with progressive RAIR-DTC | lenvatinib + pembrolizumab | VEGFR and PD-1 | Active, not recruiting |
| NCT02614495 | II | advanced MTC and RAIR-DTC | sulfatinib | VEGFR, FGFR-1, and CSF1R | Recruitment completed |
| NCT03914300 | II | advanced MTC and RAIR-DTC with cancer progression after one VEGFR-treatment | cabozantinib + nivolumab + ipilimumab | VEGFR, PD-1, and CTLA-4 | Recruitment suspended |
| NCT04560127 | II | RAIR-DTC | camrelizumab + apatinib | PD-1, VEGFR2/KDR | Recruiting |
| NCT04544111 | II | RAIR-DTC | spartalizumab, + trametinib/dabrafenib | PD-1, MEK-1 and MEK-2, BRAF | Recruiting |
| NCT03732495 | II | bone metastatic RAIR-DTC | lenvatinib + denosumab | VEGFR and RANKL | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silaghi, H.; Lozovanu, V.; Georgescu, C.E.; Pop, C.; Nasui, B.A.; Cătoi, A.F.; Silaghi, C.A. State of the Art in the Current Management and Future Directions of Targeted Therapy for Differentiated Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 3470. https://doi.org/10.3390/ijms23073470
Silaghi H, Lozovanu V, Georgescu CE, Pop C, Nasui BA, Cătoi AF, Silaghi CA. State of the Art in the Current Management and Future Directions of Targeted Therapy for Differentiated Thyroid Cancer. International Journal of Molecular Sciences. 2022; 23(7):3470. https://doi.org/10.3390/ijms23073470
Chicago/Turabian StyleSilaghi, Horatiu, Vera Lozovanu, Carmen Emanuela Georgescu, Cristina Pop, Bogdana Adriana Nasui, Adriana Florinela Cătoi, and Cristina Alina Silaghi. 2022. "State of the Art in the Current Management and Future Directions of Targeted Therapy for Differentiated Thyroid Cancer" International Journal of Molecular Sciences 23, no. 7: 3470. https://doi.org/10.3390/ijms23073470
APA StyleSilaghi, H., Lozovanu, V., Georgescu, C. E., Pop, C., Nasui, B. A., Cătoi, A. F., & Silaghi, C. A. (2022). State of the Art in the Current Management and Future Directions of Targeted Therapy for Differentiated Thyroid Cancer. International Journal of Molecular Sciences, 23(7), 3470. https://doi.org/10.3390/ijms23073470







