40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs?
Abstract
1. Introduction
Herpesviruses, Taxonomy, and Pathogenicity
- Order: Herpesvirales
- Family: Herpesviridae
- Subfamily: Alphaherpesvirinae
- ➢
- Genus: SimplexvirusSpecies: Human alphaherpesvirus 1 (known as herpes simplex virus type 1; HSV-1)Human alphaherpesvirus 2 (known as herpes simplex virus type 2; HSV-2)
- ➢
- Genus: VaricellovirusSpecies: Human alphaherpesvirus 3 (known as varicella-zoster virus; VZV)
- Subfamily: Betaherpesvirinae
- ➢
- Genus: CytomegalovirusSpecies: Human betaherpesvirus 5 (known as cytomegalovirus; CMV)Genus: Roseolovirus
- ➢
- Species: Human betaherpesvirus 6AHuman betaherpesvirus 6BHuman betaherpesvirus 7
- Subfamily: Gammaherpesvirinae
- ➢
- Genus: LymphocryptovirusSpecies: Human gammaherpesvirus 4 (known as Epstein–Barr virus; EBV)
- ➢
- Genus: RhadinovirusSpecies: Human gammaherpesvirus 8 (known as Kaposi’s sarcoma-associated herpesvirus; KSHV) [10].
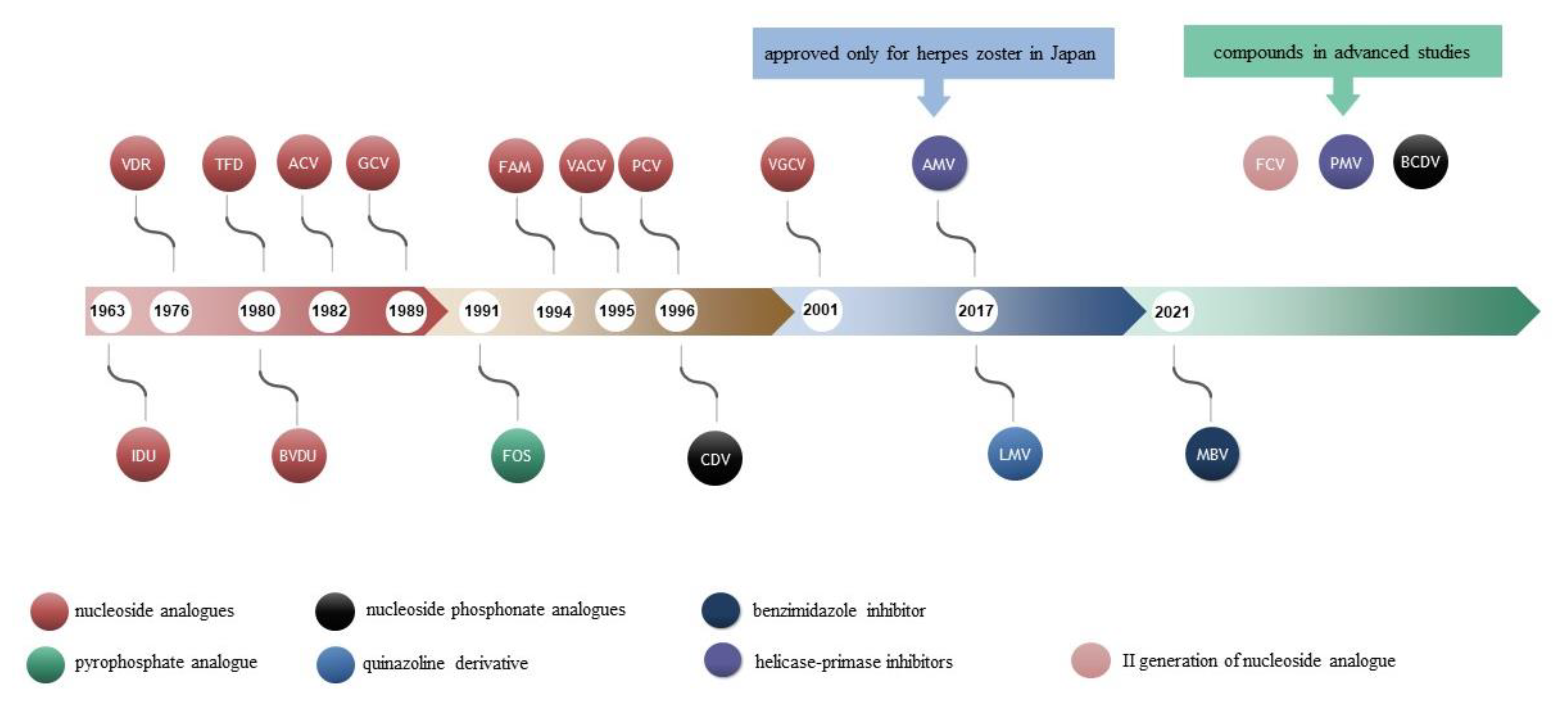
2. Antiviral Drugs
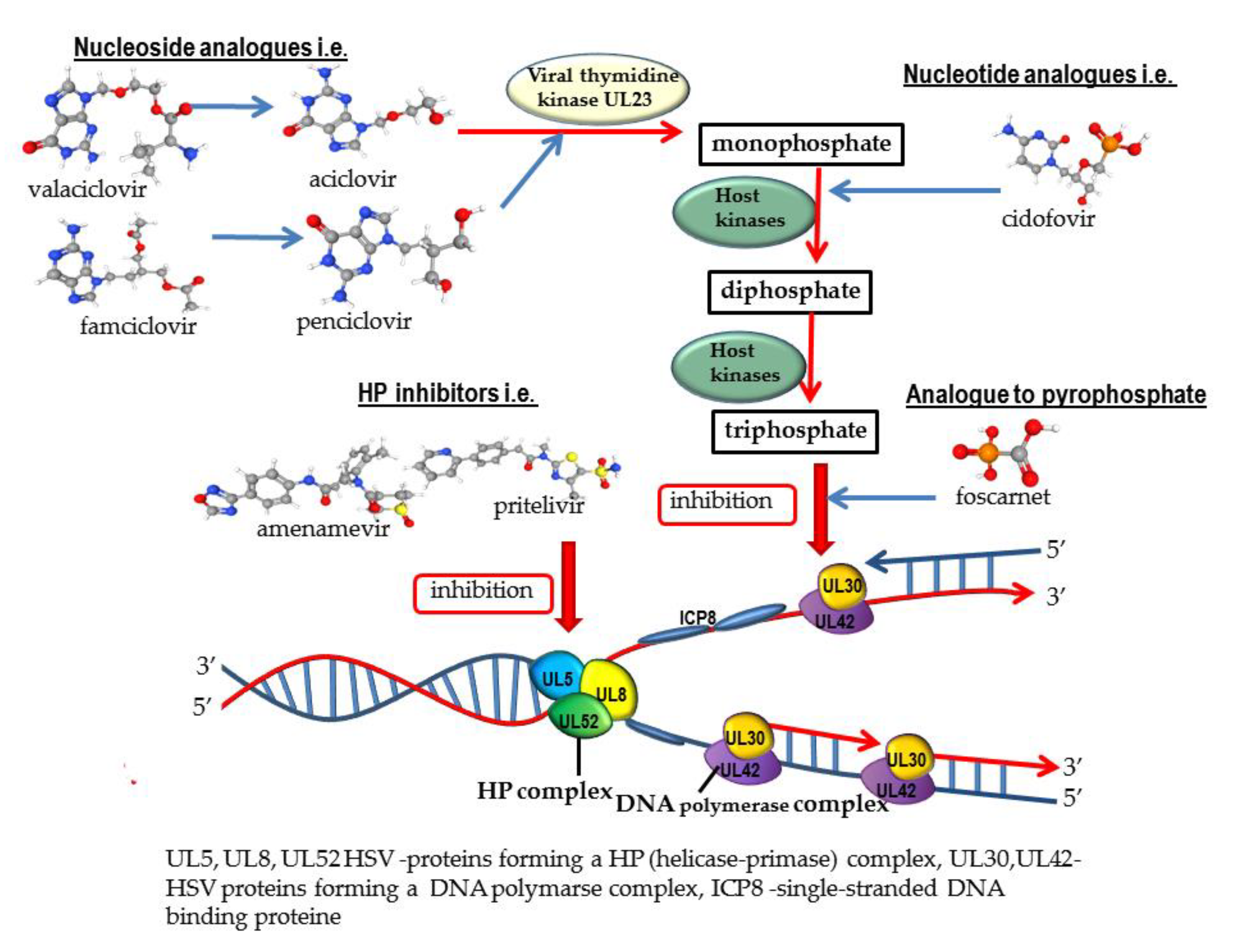
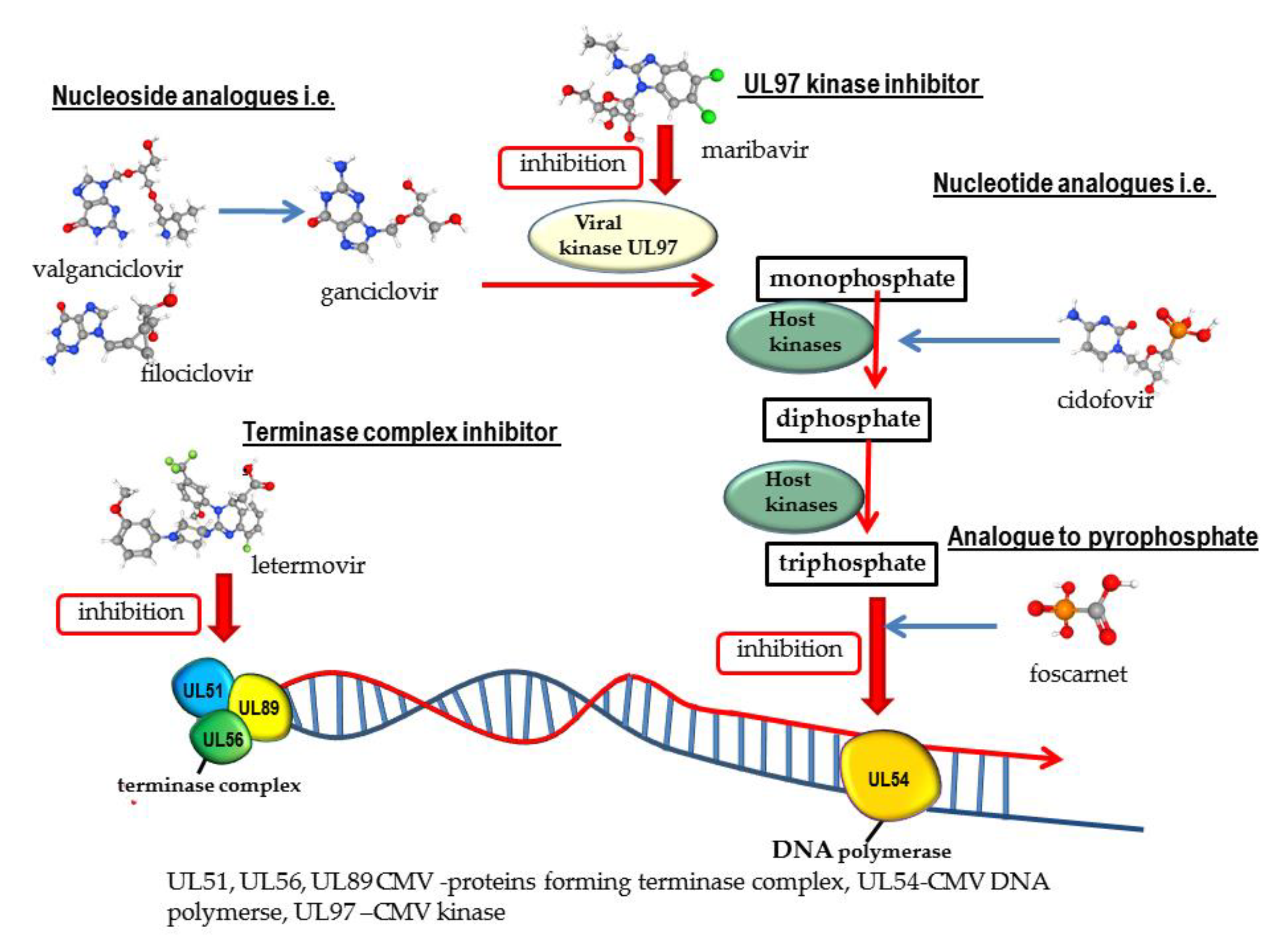
2.1. Nucleoside Analogues
2.2. Nucleotide Analogues
2.3. Analogue to Pyrophosphate
2.4. Quinazoline Derivative
2.5. Benzimidazole Inhibitor
3. Compounds with Potential Use in the Treatment of Herpesvirus Infections
3.1. Helicase-Primase Inhibitors (HPIs)
- ➢
- 2-amino-thiazolylphenyl derivatives (BILS 179 BS),
- ➢
- thiazole urea (BAY 57-1293; pritelivir), and
- ➢
- oxadiazolylphenyl type (ASP2151, amenamevir) [12].
3.2. Methylenecyclopropane Analogue
3.3. Other Selected Substances in Preclinical Studies
4. Vaccines
4.1. VZV
4.2. CMV
4.3. HSV
5. Conclusions
- Viral DNA polymerase inhibitors such as:
- -
- nucleoside analogues (ACV, GCV, PCV, VACV, VGCV, and BVDU FCV);
- -
- nucleotide analogues (CDV); and
- -
- analogue to pyrophosphate (FOS).
- Helicase-primase inhibitors (AMV in Japan).
- CMV UL97 kinase inhibitor (MBV).
- CMV terminase complex inhibitor (LMV).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACV | acyclovir |
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| AMV | Amenamevir |
| ATP | adenosine triphosphate |
| BCDV | brincidofovir |
| BVDU | Brivudin |
| CDV | cidofovir |
| CMV | cytomegalovirus |
| CNS | central nervous system |
| dNTPs | deoxyribonucleotides triphosphate |
| dsDNA | double-stranded DNA |
| EBV | Epstein–Barr virus |
| EC50 | half-maximal effective concentration |
| FCV | famciclovir |
| FCV | filociclovir |
| FDA | Food and Drug Administration |
| FOS | foscarnet |
| gB | glycoprotein B |
| GCV | ganciclovir |
| gD | glycoprotein D |
| HBV | hepatitis B virus |
| HHV6 | human herpesvirus |
| HP | helicase-primase complex |
| HPIs | helicase-primase inhibitors |
| HPV | human papillomavirus |
| HSC | hematopoietic stem cell |
| HSV-1 | herpes simplex virus type 1 |
| HSV-2 | herpes simplex virus type 2 |
| IDU | iododeoxyuridine |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| LMV | letermovir |
| MBV | maribavir |
| MBZM-N-IBT1 | [(2-methyl benzimidazole-1-yl) methyl]-2-oxo-indolin-3-ylidene] amino] thiourea |
| MDV | Marek’s disease virus |
| PCV | penciclovir |
| PRV | pseudorabies virus |
| PTV | pritelivir |
| SOT | solid organ transplant |
| ssDNA | single-stranded DNA |
| TFD | trifluridine |
| TK | thymidine kinase |
| VACV | valacyclovir |
| VDR | vidarabine |
| VGCV | valganciclovir |
| VZIG | varicella-zoster immune globulin |
| VZV | varicella-zoster virus |
References
- Saxena, S.K.; Saxena, S.; Saxena, R.; Swamy, A.; Gupta, A.; Nair, M. Emerging trends, challenges and prospects in antiviral therapeutics and drug development for infectious diseases. Electron. J. Biol. 2010, 6, 26–31. [Google Scholar]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.M.; Seabra, A.B. Bactericidal and virucidal activities of biogenic metal-based nanoparticles: Advances and perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Symons, J.A.; Deval, J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antiviral. Res. 2018, 155, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Immunomodulatory strategies in herpes simplex virus encephalitis. Clin. Microbiol. Rev. 2020, 33, e00105-19. [Google Scholar] [CrossRef]
- Majewska, A.; Lasek, W.; Janyst, M.; Młynarczyk, G. In vitro inhibition of HHV-1 replication by inosine pranobex and interferon-α. Acta Pol. Pharm. 2016, 73, 637–644. [Google Scholar]
- Gonçalves, B.C.; Lopes Barbosa, M.G.; Silva Olak, A.P.; BelebechaTerezo, N.; Nishi, L.; Watanabe, M.A.; Marinello, P.; Zendrini Rechenchoski, D.; Dejato Rocha, S.P.; Faccin-Galhardi, L.C. Antiviral therapies: Advances and perspectives. Fundam. Clin. Pharmacol. 2021, 35, 305–320. [Google Scholar] [CrossRef]
- Holland, E.J.; Fingeret, M.; Mah, F.S. Use of topical steroids in conjunctivitis: A review of the evidence. Cornea 2019, 38, 1062–1067. [Google Scholar] [CrossRef]
- Woźniakowski, G.; Samorek-Salamonowicz, E. Animal herpesviruses and their zoonotic potential for cross-species infection. Ann. Agric. Environ. Med. 2015, 22, 191–194. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses (ICTV). Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 31 January 2022).
- Frick, D.N.; Lam, A.M. Understanding helicases as a means of virus control. Curr. Pharm. Des. 2006, 12, 1315–1338. [Google Scholar] [CrossRef]
- Shiraki, K.; Yasumoto, S.; Toyama, N.; Fukuda, H. Amenamevir, a helicase-primase inhibitor, for the optimal treatment of herpes zoster. Viruses 2021, 13, 1547. [Google Scholar] [CrossRef] [PubMed]
- Bermek, O.; Williams, R.S. The three-component helicase/primase complex of herpes simplex virus-1. Open. Biol. 2021, 11, 210011. [Google Scholar] [CrossRef] [PubMed]
- Van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral active compounds derived from natural sources against herpes simplex viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk-Bonikowska, B.; Majewska, A.; Malejczyk, M.; Mlynarczyk, G.; Majewski, S. Antiviral medication in sexually transmitted diseases. Part, I.: HSV, HPV. Mini Rev. Med. Chem. 2013, 13, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.; Kimberlin, D.W.; Prober, C.G. Pathogenesis and disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Eds.; Cambridge University Press: Cambridge, MA, USA, 2007; Chapter 32; ISBN 978-0-521-82714-0. [Google Scholar]
- Da Rocha, W.M.; da Silva, K.C.; Cavalcanti, S.M.B. The role of oncogenic DNA viruses in penile cancer development. Crit. Rev. Oncog. 2019, 24, 385–402. [Google Scholar] [CrossRef]
- Majewska, A.; Mlynarczyk-Bonikowska, B.; Malejczyk, M.; Majewski, S.; Mlynarczyk, G. Possibilities of prevention and treatment of human cytomegalovirus infections including new drugs and compounds with potential application. Adv. Microbiol. 2019, 58, 291–299. [Google Scholar] [CrossRef]
- Pei, Y.; Wong, J.H.; Robertson, E.S. Herpesvirus epigenetic reprogramming and oncogenesis. Annu. Rev. Virol. 2020, 7, 309–331. [Google Scholar] [CrossRef]
- Cleary, J.M.; Rosen, L.S.; Yoshida, K.; Rasco, D.; Shapiro, G.I.; Sun, W. A phase 1 study of the pharmacokinetics of nucleoside analog trifluridine and thymidine phosphorylase inhibitor tipiracil (components of TAS-102) vs trifluridine alone. Investig. New Drugs. 2017, 35, 189–197. [Google Scholar] [CrossRef]
- Elion, G.B.; Furman, P.A.; Fyfe, J.A.; de Miranda, P.; Beauchamp, L.; Schaeffer, H.J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. USA 1977, 74, 5716–5720. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- King, D.H. History, pharmacokinetics, and pharmacology of acyclovir. J. Am. Acad. Dermatol. 1988, 18, 176–179. [Google Scholar] [CrossRef]
- Krawczyk, E.; Kniotek, M.; Nowaczyk, M.; Dzieciatkowski, T.; Przybylski, M.; Majewska, A.; Luczak, M. N-acetylphenylisoserinates of Lactarius sesquiterpenoid alcohols-cytotoxic, antiviral, antiproliferative and immunotropic activities in vitro. Planta Med. 2006, 72, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Kuczer, M.; Czarniewska, E.; Majewska, A.; Różanowska, M.; Rosiński, G.; Lisowski, M. Novel analogs of alloferon: Synthesis, conformational studies, pro-apoptotic and antiviral activity. Bioorg. Chem. 2016, 66, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K. Antiviral Drugs Against Alphaherpesvirus. In Human Herpesviruses. Advances in Experimental Medicine and Biology; Kawaguchi, Y., Mori, Y., Kimura, H., Eds.; Springer: Singapore, 2018; Volume 1045, pp. 103–122. ISBN 978-981-10-7230-7. [Google Scholar]
- Li, G.; Yue, T.; Zhang, P.; Gu, W.; Gao, L.-W.; Tan, L. Drug discovery of nucleos(t)ide antiviral agents: Dedicated to prof. dr. Erik De Clercq on occasion of his 80th birthday. Molecules 2021, 26, 923. [Google Scholar] [CrossRef] [PubMed]
- Birkmann, A.; Zimmermann, H. HSV antivirals—Current and future treatment options. Curr. Opin. Virol. 2016, 18, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Einsele, H.; Ljungman, P.; Boeckh, M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020, 13, 1619–1629. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3415, Foscarnet. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Foscarnet (accessed on 5 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 60613, Cidofovir. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cidofovir (accessed on 5 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398742, Valacyclovir. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Valacyclovir (accessed on 5 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398748, Penciclovir. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Penciclovir (accessed on 5 February 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 135398740, Ganciclovir” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ganciclovir (accessed on 5 February 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 135413535, Valganciclovir” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Valganciclovir (accessed on 5 February 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 135409256, Filociclovir” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Filociclovir (accessed on 5 February 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 471161, Maribavir” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Maribavir (accessed on 5 February 2022).
- Allen, U.D.; Robinson, J.L.; Canadian Paediatric Society; Infectious Diseases and Immunization Committee. Prevention and management of neonatal herpes simplex virus infections. Paediatr. Child Health 2014, 19, 201–212. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Newman, L.M.; Gottlieb, S.L. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health 2017, 5, e300–e309. [Google Scholar] [CrossRef]
- Poole, C.L.; Kimberlin, D.W. Antiviral approaches for the treatment of Herpes Simplex Virus infections in newborn infants. Annu. Rev. Virol. 2018, 5, 407–425. [Google Scholar] [CrossRef]
- Melvin, A.J.; Mohan, K.M.; Vora, S.B.; Selke, S.; Sullivan, E.; Wald, A. Neonatal Herpes Simplex Virus infection: Epidemiology and outcomes in the modern era. J. Pediatric Infect. Dis. Soc. 2021, 11, piab105. [Google Scholar] [CrossRef]
- Sailer, C.A.; Spruance, S.L.; Hul, C.M. Acyclovir and hydrocortisone cream for the early treatment of recurrent cold sores. Virus Adapt. Treat. 2011, 3, 1–6. [Google Scholar] [CrossRef][Green Version]
- Hull, C.M.; Harmenberg, J.; Arlander, E.; Aoki, F.; Bring, J.; Darpö, B.; Levin, M.J.; Tyring, S.; Spruance, S.L.; ME-609 Study Group. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: A randomized, double-blind, placebo-controlled, patient-initiated clinical trial. J. Am. Acad. Dermatol. 2011, 64, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current drugs to treat infections with herpes simplex viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Snoeck, R. Advances and perspectives in the management of Varicella-Zoster Virus infections. Molecules 2021, 26, 1132. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, S.; Yokota, T.; Iwabuchi, T.; Baba, M.; Konno, K.; Ogata, M.; De Clercq, R. Comparative efficacy of antiherpes drugs against various strains of Varicella-Zoster virus. J. Infect. Dis. 1983, 147, 576–584. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Fifty years in search of selective antiviral drugs. J. Med. Chem. 2019, 62, 7322–7339. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A.; Bohn-Wippert, K.; Kaspar, M.; Krumbholz, A.; Karrasch, M.; Zell, R. Database on natural polymorphisms and resistance-related non-synonymous mutations in thymidine kinase and DNA polymerase genes of herpes simplex virus types 1 and 2. J. Antimicrob. Chemother. 2016, 71, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E. Antivirals against Herpesviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 577–595. [Google Scholar]
- Lee, D.H.; Zuckerman, R.A. Herpes simplex virus infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13526. [Google Scholar] [CrossRef]
- Perera, M.R.; Wills, M.R.; Sinclair, J.H. HCMV antivirals and strategies to target the latent reservoir. Viruses 2021, 13, 817. [Google Scholar] [CrossRef]
- Frange, F.; Leruez-Ville, M. Maribavir, brincidofovir and letermovir: Efficacy and safety of new antiviral drugs for treating cytomegalovirus infections. Médecine et Maladies Infectieuses 2018, 48, 495–502. [Google Scholar] [CrossRef]
- Ongrádi, J.; Ablashi, D.V.; Yoshikawa, T.; Stercz, B.; Ogata, M. Roseolovirus-associated encephalitis in immunocompetent and immunocompromised individuals. J. Neurovirol. 2017, 23, 1–19. [Google Scholar] [CrossRef]
- Lehto, J.T.; Halme, M.; Tukiainen, P.; Harjula, A.; Sipponen, J.; Lautenschlager, I. Human herpesvirus-6 and -7 after lung and heart-lung transplantation. J. Heart Lung Transplant. 2007, 26, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, Y.; Jin, Y.; Sun, H.; Wang, W.; Zhan, L. Difference between acyclovir and ganciclovir in the treatment of children with Epstein–Barr Virus-associated infectious mononucleosis. Evid. Based Complement. Alternat. Med. 2021, 2021, 8996934. [Google Scholar] [CrossRef] [PubMed]
- Galar, A.; Valerio, M.; Catalán, P.; García-González, X.; Burillo, A.; Fernández-Cruz, A.; Zataráin, E.; Sousa-Casasnovas, I.; Anaya, F.; Rodríguez-Ferrero, M.L.; et al. Valganciclovir-ganciclovir use and systematic therapeutic drug monitoring. An invitation to antivirals stewardship. Antibiotics 2021, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A.; The Transplantation Society International CMV Consensus Group. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Winston, D.J.; Razonable, R.R.; Lyon, G.M.; Silveira, F.P.; Wagener, M.M.; Stevens-Ayers, T.; Edmison, B.; Boeckh, M.; Limaye, A.P. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors: A randomized clinical trial. JAMA 2020, 323, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Styczyński, J. Prophylaxis vs preemptive therapy in prevention of CMV infection: New insight on prophylactic strategy after allogeneic hematopoietic cell transplantation. Acta Haematol. Pol. 2020, 51, 17–23. [Google Scholar] [CrossRef]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Yager, J.E.; Magaret, A.S.; Kuntz, S.R.; Selke, S.; Huang, M.L.; Corey, L.; Casper, C.; Wald, A. Valganciclovir for the suppression of Epstein-Barr Virus replication. J. Infect. Dis. 2017, 216, 198–202. [Google Scholar] [CrossRef]
- Casper, C.; Krantz, E.M.; Corey, L.; Kuntz, S.R.; Wang, J.; Selke, S.; Hamilton, S.; Huang, M.L.; Wald, A. Valganciclovir for suppression of human herpesvirus-8 replication: A randomized, double-blind, placebo-controlled, crossover trial. J. Infect. Dis. 2008, 198, 23–30. [Google Scholar] [CrossRef]
- Labrunie, T.; Ducastelle, S.; Domenech, C.; Ader, F.; Morfin, F.; Frobert, E. UL23, UL30, and UL5 characterization of HSV1 clinical strains isolated from hematology department patients. Antivir. Res. 2019, 168, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Anton-Vazquez, V.; Mehra, V.; Mbisa, J.L.; Bradshaw, D.; Basu, T.N.; Daly, M.L.; Mufti, G.J.; Pagliuca, A.; Potter, V.; Zuckerman, M. Challenges of aciclovir-resistant HSV infection in allogeneic bone marrow transplant recipients. J. Clin. Virol. 2020, 128, 104421. [Google Scholar] [CrossRef] [PubMed]
- Frobert, E.; Burrel, S.; Ducastelle-Lepretre, S.; Billaud, G.; Ader, F.; Casalegno, J.S.; Nave, V.; Boutolleau, D.; Michallet, M.; Lina, B.; et al. Resistance of herpes simplex viruses to acyclovir: An update from a ten-year survey in France. Antivir. Res. 2014, 111, 36–41. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, V.W.; Romeiro, N.C.; Paixão, I.C.N.P.; Abreu, P.A. Mechanism of resistance to acyclovir in thymidine kinase mutants from Herpes simplex virus type 1: A computational approach. J. Biomol. Struct. Dyn. 2020, 38, 2116–2127. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Antiviral drug resistance in herpesviruses other than cytomegalovirus. Rev. Med. Virol. 2014, 24, 186–218. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: Diagnosis and management. Curr. Opin. Infect. Dis. 2016, 29, 654–662. [Google Scholar] [CrossRef]
- Poole, C.L.; James, S.H. Antiviral therapies for Herpesviruses: Current agents and new directions. Clin. Ther. 2018, 40, 1282–1298. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; Liu, M.; et al. Alpha-herpesvirus thymidine kinase genes mediate viral virulence and are potential therapeutic targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Burrel, S.; Deback, C.; Agut, H.; Boutolleau, D. Genotypic characterization of UL23 thymidine kinase and UL30 DNA polymerase of clinical isolates of herpes simplex virus: Natural polymorphism and mutations associated with resistance to antivirals. Antimicrob. Agents Chemother. 2010, 54, 4833–4842. [Google Scholar] [CrossRef]
- Schmidt, S.; Bohn-Wippert, K.; Schlattmann, P.; Zell, R.; Sauerbrei, A. Sequence analysis of herpes simplex virus 1 thymidine kinase and DNA polymerase genes from over 300 clinical isolates from 1973 to 2014 finds novel mutations that may be relevant for development of antiviral resistance. Antimicrob. Agents Chemother. 2015, 59, 4938–4945. [Google Scholar] [CrossRef] [PubMed]
- Hussin, A.; Md Nor, N.S.; Ibrahim, N. Phenotypic and genotypic characterization of induced acyclovir-resistant clinical isolates of herpes simplex virus type 1. Antivir. Res. 2013, 100, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Karamitros, T.; Harrison, I.; Piorkowska, R.; Katzourakis, A.; Magiorkinis, G.; Mbisa, J.L. De novo assembly of Human Herpes Virus Type 1 (HHV-1) genome, mining of non-canonical structures and detection of novel drug-resistance mutations using short- and long-read next generation sequencing technologies. PLoS ONE 2016, 11, e0157600. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, N.; Jamalidoust, M.; Pouladfar, G.; Heydari Marandi, N.; Ziyaeyan, A.; Amanati, A.; Ziyaeyan, M. Susceptibility evaluation of clinically isolated HSV-1 strains to acyclovir: A phenotypic and genotypic study. Jundishapur J. Microbiol. 2021, 14, e117928. [Google Scholar] [CrossRef]
- Burrel, S.; Aime, C.; Hermet, L.; Ait-Arkoub, Z.; Agut, H.; Boutolleau, D. Surveillance of herpes simplex virus resistance to antivirals: A 4-year survey. Antivir. Res. 2013, 100, 365–372. [Google Scholar] [CrossRef]
- Usage of Antivirals and the Occurrence of Antiviral Resistance in Norway 2020. Available online: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2021/ravn-rapport-2021.pdf (accessed on 31 January 2022).
- De Clercq, E. A 40-year journey in search of selective antiviral chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 1–24. [Google Scholar] [CrossRef]
- Loeffelholz, M.; Young, S.A.; Pinksy, B.A. Clinical Virology Manual, 5th ed.; John Wiley & Sons: Washington, DC, USA, 2016; Available online: https://www.perlego.com/book/1343048/clinical-virology-manual-pdf (accessed on 31 January 2022).
- Fajfr, M.; Pliskova, L.; Bolehovská, R.; Uhlířová, Z.; Vrbacký, F. Herpes simplex virus resistant to acyclovir: A single-centre experience from the Czech Republic. J. Glob. Antimicrob. Resist. 2019, 19, 269–273. [Google Scholar] [CrossRef]
- Hoffmann, A.; Döring, K.; Seeger, N.T.; Bühler, M.; Schacke, M.; Krumbholz, A.; Sauerbrei, A. Genetic polymorphism of thymidine kinase (TK) and DNA polymerase (pol) of clinical varicella-zoster virus (VZV) isolates collected over three decades. J. Clin. Virol. 2017, 95, 61–65. [Google Scholar] [CrossRef]
- Perrier, M.; Désiré, N.; Deback, C.; Agut, H.; Boutolleau, D.; Burrel, S. Complementary assays for monitoring susceptibility of varicella-zoster virus resistance to antivirals. J. Virol. Methods 2016, 233, 10–14. [Google Scholar] [CrossRef]
- Leung, P.Y.M.; Tran, T.; Testro, A.; Paizis, K.; Kwong, J.; Whitlam, J.B. Ganciclovir-resistant post-transplant cytomegalovirus infection due to combined deletion mutation at codons 595-596 of the UL97 gene. Transpl. Infect. Dis. 2019, 21, e13168. [Google Scholar] [CrossRef]
- Benzi, F.; Vanni, I.; Cassina, G.; Ugolotti, E.; Di Marco, E.; Cirillo, C.; Cristina, E.; Morreale, G.; Melioli, G.; Malnati, M.; et al. Detection of ganciclovir resistance mutations by pyrosequencing in HCMV-infected pediatric patients. J. Clin. Virol. 2012, 54, 48–55. [Google Scholar] [CrossRef]
- Vejražková, E.; Hubáček, P.; Kutová, R.; Plíšková, L.; Košťál, M.; Štěpánová, V.; Zavřelová, A.; Radocha, J.; Malá, E.; Žák, P. Ganciclovir treatment failure in adult allogeneic hematopoietic stem cell transplant recipients with cytomegalovirus infection-a single centre experience. Epidemiol. Mikrobiol. Imunol. 2015, 64, 160–168. [Google Scholar] [PubMed]
- Guermouche, H.; Burrel, S.; Mercier-Darty, M.; Kofman, T.; Rogier, O.; Pawlotsky, J.M.; Boutolleau, D.; Rodriguez, C. Characterization of the dynamics of human cytomegalovirus resistance to antiviral drugs by ultra-deep sequencing. Antivir. Res. 2020, 173, 104647. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cardona, J.J.; Whited, L.K.; Chemaly, R.F. Brincidofovir: Understanding its unique profile and potential role against adenovirus and other viral infections. Future Microbiol. 2020, 15, 389–400. [Google Scholar] [CrossRef]
- Marty, F.M.; Winston, D.J.; Chemaly, R.F.; Mullane, K.M.; Shore, T.B.; Papanicolaou, G.A.; Chittick, G.; Brundage, T.M.; Wilson, C.; Morrison, M.E.; et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2019, 25, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Chan-Tack, K.; Harrington, P.; Bensman, T.; Choi, S.Y.; Donaldson, E.; O’Rear, J.; McMillan, D.; Myers, L.; Seaton, M.; Ghantous, G.; et al. Benefit-risk assessment for brincidofovir for the treatment of smallpox: U.S. Food and Drug Administration’s Evaluation. Antivir. Res. 2021, 195, 105182. [Google Scholar] [CrossRef] [PubMed]
- Tippin, T.K.; Morrison, M.E.; Brundage, T.M.; Momméja-Marin, H. Brincidofovir is not a substrate for the human organic anion transporter 1: A mechanistic explanation for the lack of nephrotoxicity observed in clinical studies. Ther. Drug Monit. 2016, 38, 777–786. [Google Scholar] [CrossRef]
- Topalis, D.; Gillemot, S.; Snoeck, R.; Andrei, G. Distribution and effects of amino acid changes in drug-resistant α and β herpesviruses DNA polymerase. Nucleic. Acids. Res. 2016, 44, 9530–9554. [Google Scholar] [CrossRef]
- Razonable, R.R. Drug-resistant cytomegalovirus: Clinical implications of specific mutations. Curr. Opin. Organ Transplant. 2018, 23, 388–394. [Google Scholar] [CrossRef]
- Erice, A. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 1999, 12, 286–297. [Google Scholar] [CrossRef]
- Chou, S.; Ercolani, R.J.; Lanier, E.R. Novel Cytomegalovirus UL54 DNA polymerase gene mutations selected in vitro that confer brincidofovir resistance. Antimicrob. Agents Chemother. 2016, 60, 3845–3848. [Google Scholar] [CrossRef]
- Garikapati, S.; Nguyen, M. Foscarnet. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556108/ (accessed on 31 January 2022).
- Zarrouk, K.; Zhu, X.; Pham, V.D.; Goyette, N.; Piret, J.; Shi, R.; Boivin, G. Impact of Amino Acid Substitutions in Region II and Helix K of Herpes Simplex Virus 1 and Human Cytomegalovirus DNA Polymerases on Resistance to Foscarnet. Antimicrob. Agents Chemother. 2021, 65, e0039021. [Google Scholar] [CrossRef] [PubMed]
- Tchesnokov, E.P.; Gilbert, C.; Boivin, G.; Götte, M. Role of Helix P of the Human Cytomegalovirus DNA Polymerase in Resistance and Hypersusceptibility to the Antiviral Drug Foscarnet. J. Virol. 2006, 80, 1440–1450. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance. Antivir. Res. 2019, 163, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Winstead, R.J.; Kumar, D.; Brown, A.; Yakubu, I.; Song, C.; Thacker, L.; Gupta, G. Letermovir prophylaxis in solid organ transplant—Assessing CMV breakthrough and tacrolimus drug interaction. Transpl. Infect. Dis. 2021, 23, e13570. [Google Scholar] [CrossRef] [PubMed]
- Shigle, T.L.; Handy, V.W.; Chemaly, R.F. Letermovir and its role in the prevention of cytomegalovirus infection in seropositive patients receiving an allogeneic hematopoietic cell transplant. Ther. Adv. Hematol. 2020, 11, 2040620720937150. [Google Scholar] [CrossRef] [PubMed]
- MK-8228 (Letermovir) in the Prevention of Human Cytomegalovirus (CMV) Infection and Disease in Adult Japanese Kidney Transplant Recipients (MK-8228-042). Available online: https://clinicaltrials.gov/ct2/show/NCT04129398?term=letermovir&draw=2&rank=5 (accessed on 31 January 2022).
- Letermovir Versus Valganciclovir to Prevent Human Cytomegalovirus Disease in Kidney Transplant Recipients (MK-8228-002). Available online: https://clinicaltrials.gov/ct2/show/NCT03443869?term=letermovir&draw=3&rank=12 (accessed on 31 January 2022).
- Letermovir for CMV Prevention After Lung Transplantation. Available online: https://clinicaltrials.gov/ct2/show/NCT05041426?term=letermovir&draw=2&rank=1 (accessed on 31 January 2022).
- Letermovir Use in Heart Transplant Recipients. Available online: https://clinicaltrials.gov/ct2/show/NCT04904614?term=letermovir&draw=2&rank=4 (accessed on 31 January 2022).
- El Helou, G.; Razonable, R.R. Letermovir for the prevention of cytomegalovirus infection and disease in transplant recipients: An evidence-based review. Infect. Drug Resist. 2019, 12, 1481–1491. [Google Scholar] [CrossRef]
- Veit, T.; Munker, D.; Barton, J.; Milger, K.; Kauke, T.; Meiser, B.; Michel, S.; Zoller, M.; Nitschko, H.; Keppler, O.T.; et al. Letermovir in lung transplant recipients with cytomegalovirus infection: A retrospective observational study. Am. J. Transplant. 2021, 21, 3449–3455. [Google Scholar] [CrossRef]
- Chou, S. Rapid In Vitro Evolution of Human Cytomegalovirus UL56 Mutations That Confer Letermovir Resistance. Antimicrob. Agents Chemother. 2015, 59, 6588–6593. [Google Scholar] [CrossRef]
- Jo, H.; Kwon, D.E.; Han, S.H.; Min, S.Y.; Hong, Y.-M.; Lim, B.J.; Lee, K.H.; Jo, J.-H. De Novo Genotypic Heterogeneity in the UL56 Region in Cytomegalovirus-Infected Tissues: Implications for Primary Letermovir Resistance. J. Infect. Dis. 2019, 221, 1480–1487. [Google Scholar] [CrossRef]
- Turner, N.; Strand, A.; Grewal, D.S.; Cox, G.; Arif, S.; Baker, A.W.; Maziarz, E.K.; Saullo, J.H.; Wolfe, C.R. Use of Letermovir as Salvage Therapy for Drug-Resistant Cytomegalovirus Retinitis. Antimicrob. Agents Chemother. 2019, 63, e02337-18. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Michel, M.; Stamminger, T.; Michel, D. Fast breakthrough of resistant cytomegalovirus during secondary letermovir prophylaxis in a hematopoietic stem cell transplant recipient. BMC Infect. Dis. 2019, 19, 388. [Google Scholar] [CrossRef] [PubMed]
- Chou, S. Comparison of Cytomegalovirus Terminase Gene Mutations Selected after Exposure to Three Distinct Inhibitor Compounds. Antimicrob. Agents Chemother. 2017, 61, e01325-17. [Google Scholar] [CrossRef]
- Hakki, M. Moving Past Ganciclovir and Foscarnet: Advances in CMV Therapy. Curr. Hematol. Malign Rep. 2020, 15, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Ercolani, R.J.; Derakhchan, K. Antiviral activity of maribavir in combination with other drugs active against human cytomegalovirus. Antivir. Res. 2018, 157, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Novel Drug Approvals for 2021. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 (accessed on 31 January 2022).
- Chou, S.; Song, K.; Wu, J.; Bo, T.; Crumpacker, C. Drug resistance mutations and associated phenotypes detected in clinical trials of maribavir for treatment of cytomegalovirus infection. J. Infect. Dis. 2020, 29, jiaa462. [Google Scholar] [CrossRef]
- Bravo, M.S.; Plault, N.; Palomino, S.S.; Gutierrez, M.M.M.; Avilés, F.F.; Lledo, M.S.; Fernández, N.S.; Rovira, M.; Alain, S.; Maeso, M.M. Phenotype and Genotype Study of Novel C480F Maribavir-Ganciclovir Cross-Resistance Mutation Detected in Hematopoietic Stem Cell and Solid Organ Transplant Recipients. J. Infect. Dis. 2021, 224, 1024–1028. [Google Scholar] [CrossRef]
- Chou, S.; Wu, J.; Song, K.; Bo, T. Novel UL97 drug resistance mutations identified at baseline in a clinical trial of maribavir for resistant or refractory cytomegalovirus infection. Antivir. Res. 2019, 172, 104616. [Google Scholar] [CrossRef]
- Chou, S. Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance. Antivir. Res. 2020, 176, 104711. [Google Scholar] [CrossRef]
- Papanicolaou, G.A.; Silveira, F.P.; Langston, A.A.; Pereira, M.R.; Avery, R.K.; Uknis, M.; Wijatyk, A.; Wu, J.; Boeckh, M.; Marty, F.M.; et al. Maribavir for Refractory or Resistant Cytomegalovirus Infections in Hematopoietic-cell or Solid-organ Transplant Recipients: A Randomized, Dose-ranging, Double-blind, Phase 2 Study. Clin. Infect. Dis. 2018, 68, 1255–1264. [Google Scholar] [CrossRef]
- Komazin, G.; Ptak, R.G.; Emmer, B.T.; Townsend, L.B.; Drach, J.C. Resistance of Human Cytomegalovirus to the Benzimidazole l -Ribonucleoside Maribavir Maps to UL27. J. Virol. 2003, 77, 11499–11506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bogner, E.; Egorova, A.; Makarov, V. Small Molecules—Prospective Novel HCMV Inhibitors. Viruses 2021, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Dropulic, L.K.; Cohen, J.I. Update on New Antivirals Under Development for the Treatment of Double-Stranded DNA Virus Infections. Clin. Pharmacol. Ther. 2010, 88, 610–619. [Google Scholar] [CrossRef]
- Weller, S.K.; Kuchta, R.D. The DNA helicase-primase complex as a target for herpes viral infection. Expert Opin. Ther. Targets. 2013, 17, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Chono, K.; Katsumata, K.; Kontani, T.; Kobayashi, M.; Sudo, K.; Yokota, T.; Konno, K.; Shimizu, Y.; Suzuki, H. ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2. J. Antimicrob. Chemother. 2010, 65, 1733–1741. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11397521, Amenamevir. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amenamevir (accessed on 3 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 491941, Pritelivir. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pritelivir (accessed on 3 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398513, Acyclovir. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acyclovir (accessed on 3 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3324, Famciclovir. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Famciclovir (accessed on 3 February 2022).
- Gege, C.; Bravo, F.J.; Uhlig, N.; Hagmaier, T.; Schmachtenberg, R.; Elis, J.; Burger-Kentischer, A.; Finkelmeier, D.; Hamprecht, K.; Grunwald, T.; et al. A helicase-primase drug candidate with sufficient target tissue exposure affects latent neural herpes simplex virus infections. Sci. Transl. Med. 2021, 13, eabf8668. [Google Scholar] [CrossRef]
- Wald, A.; Corey, L.; Timmler, B.; Magaret, A.; Warren, T.; Tyring, S.; Johnston, C.; Kriesel, J.; Fife, K.; Galitz, L.; et al. Helicase–Primase Inhibitor Pritelivir for HSV-2 Infection. N. Engl. J. Med. 2014, 370, 201–210. [Google Scholar] [CrossRef]
- Wald, A.; Timmler, B.; Magaret, A.; Warren, T.; Tyring, S.; Johnston, C.; Fife, K.; Selke, S.; Huang, M.-L.; Stobernack, H.-P.; et al. Effect of Pritelivir Compared with Valacyclovir on Genital HSV-2 Shedding in Patients With Frequent Recurrences. JAMA 2016, 316, 2495–2503. [Google Scholar] [CrossRef]
- Cannon, L.; Tholouli, E.; Ward, C.; Farooq, H.; Kingston, M. Use of pritelivir in refractory aciclovir-resistant herpes simplex virus type 2. Int. J. STD AIDS 2021, 32, 978–980. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Swan, D.A.; Magaret, A.; Corey, L.; Wald, A.; Ossig, J.; Ruebsamen-Schaeff, H.; Stoelben, S.; Timmler, B.; Zimmermann, H.; et al. Mathematical modeling of herpes simplex virus-2 suppression with pritelivir predicts trial outcomes. Sci. Transl. Med. 2016, 8, 324ra15. [Google Scholar] [CrossRef]
- Trial on Efficacy and Safety of Pritelivirtablets for Treatment of Acyclovir-Resistant Mucocutaneous HSV (Herpes Simplex Virus) Infections in Immunocompromised Subjects (PRIOH-1). Available online: https://clinicaltrials.gov/ct2/show/NCT03073967?term=pritelivir&draw=2&rank=2 (accessed on 31 January 2022).
- Edlefsen, P.T.; Birkmann, A.; Huang, M.-L.; Magaret, C.A.; Kee, J.J.; Diem, K.; Goldner, T.; Timmler, B.; Stoelben, S.; Ruebsamen-Schaeff, H.; et al. No Evidence of Pritelivir Resistance Among Herpes Simplex Virus Type 2 Isolates After 4 Weeks of Daily Therapy. J. Infect. Dis. 2016, 214, 258–264. [Google Scholar] [CrossRef]
- Sato, Y.; Suenaga, T.; Kobayashi, M.; Miyazaki, N.; Suzuki, T.; Ishioka, K.; Suzutani, T. Characteristics of Helicase-Primase Inhibitor Amenamevir-Resistant Herpes Simplex Virus. Antimicrob. Agents Chemother. 2021, 65, e00494-21. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Field, H.J. Herpes Simplex Virus Helicase—Primase Inhibitors: Recent Findings from the Study of Drug Resistance Mutations. Antivir. Chem. Chemother. 2008, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Crute, J.J.; Grygon, C.A.; Hargrave, K.D.; Simoneau, B.; Faucher, A.-M.; Bolger, G.; Kibler, P.; Liuzzi, M.; Cordingley, M.G. Herpes simplex virus helicase-primase inhibitors are active in animal models of human disease. Nat. Med. 2002, 8, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, K.; Chono, K.; Suzuki, H. Antiviral efficacy of the helicase-primase inhibitor amenamevir in murine models of severe herpesvirus infection. Biochem. Pharmacol. 2018, 158, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Tyring, S.; Wald, A.; Zadeikis, N.; Dhadda, S.; Takenouchi, K.; Rorig, R. ASP2151 for the Treatment of Genital Herpes: A Randomized, Double-Blind, Placebo- and Valacyclovir-Controlled, Dose-Finding Study. J. Infect. Dis. 2012, 205, 1100–1110. [Google Scholar] [CrossRef]
- Chono, K.; Katsumata, K.; Kontani, T.; Shiraki, K.; Suzuki, H. Characterization of virus strains resistant to the herpes virus helicase–primase inhibitor ASP2151 (Amenamevir). Biochem. Pharmacol. 2012, 84, 459–467. [Google Scholar] [CrossRef]
- Shoji, N.; Tanese, K.; Sasaki, A.; Horiuchi, T.; Utsuno, Y.; Fukuda, K.; Hoshino, Y.; Noda, S.; Minami, H.; Asakura, W.; et al. Pharmaceuticals and Medical Device Agency approval summary: Amenamevir for the treatment of herpes zoster. J. Dermatol. 2020, 47, 683–688. [Google Scholar] [CrossRef]
- Biswas, S.; Kleymann, G.; Swift, M.; Tiley, L.S.; Lyall, J.; Aguirre-Hernández, J.; Field, H.J. A single drug-resistance mutation in HSV-1 UL52 primase points to a difference between two helicase–primase inhibitors in their mode of interaction with the antiviral target. J. Antimicrob. Chemother. 2008, 61, 1044–1047. [Google Scholar] [CrossRef]
- Pacreau, M.-L.; Bomme, O.; Burrel, S.; Boutolleau, D. High conservation of varicella-zoster virus helicase-primase complex, the target of the new antiviral drug amenamevir. Antivir. Res. 2021, 195, 105189. [Google Scholar] [CrossRef]
- Zhou, S.; Zemlicka, J.; Kern, E.R.; Drach, J.C. Fluoroanalogues of Anti-Cytomegalovirus Agent Cyclopropavir: Synthesis and Antiviral Activity of (E)- and (Z)-9-{[2,2-Bis(Hydroxymethyl)-3-Fluorocyclopropylidene]Methyl}-Adenines and Guanines. Nucleosides Nucleotides Nucleic Acids 2007, 26, 231–243. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Zemlicka, J.; Gentry, B.G.; Bowlin, T.L.; Coen, D.M. Potency and Stereoselectivity of Cyclopropavir Triphosphate Action on Human Cytomegalovirus DNA Polymerase. Antimicrob. Agents Chemother. 2016, 60, 4176–4182. [Google Scholar] [CrossRef] [PubMed]
- Hussein, I.T.; Brooks, J.; Bowlin, T.L. The discovery and development of filociclovir for the prevention and treatment of human cytomegalovirus-related disease. Antivir. Res. 2020, 176, 104710. [Google Scholar] [CrossRef]
- Chen, S.J.; Wang, S.C.; Chen, Y.C. Antiviral Agents as Therapeutic Strategies Against Cytomegalovirus Infections. Viruses 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.G.; Hurwitz, S.J.; Hart, M.; Beck, A.; Anderson, E.J.; Deye, G.; Osborn, B.; Cai, S.Y.; Focht, C.; Amegashie, C.; et al. Phase Ib Trial to Evaluate the Safety and Pharmacokinetics of Multiple Ascending Doses of Filociclovir (MBX-400, Cyclopropavir) in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 63, e00717-19. [Google Scholar] [CrossRef] [PubMed]
- Toscani, A.; Denaro, R.; Pacheco, S.F.C.; Biolatti, M.; Anselmi, S.; Dell’Oste, V.; Castagnolo, D. Synthesis and Biological Evaluation of Amidinourea Derivatives against Herpes Simplex Viruses. Molecules 2021, 26, 4927. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; De, S.; Moharana, A.K.; Nayak, T.K.; Saswat, T.; Datey, A.; Mamidi, P.; Mishra, P.; Subudhi, B.B.; Chattopadhyay, S. Inhibition of herpes simplex virus-1 infection by MBZM-N-IBT: In silico and in vitro studies. Virol. J. 2021, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Luganini, A.; Sibille, G.; Mognetti, B.; Sainas, S.; Pippione, A.C.; Giorgis, M.; Boschi, D.; Lolli, M.L.; Gribaudo, G. Effective deploying of a novel DHODH inhibitor against herpes simplex type 1 and type 2 replication. Antivir. Res. 2021, 189, 105057. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. Stones: A Valuable By-Product as an Ellagitannin Source with High Antioxidant Potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef]
- Lin, L.-T.; Chen, T.-Y.; Chung, C.-Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.-C.; Wang, G.-H.; Lin, C.-C.; Richardson, C.D. Hydrolyzable Tannins (Chebulagic Acid and Punicalagin) Target Viral Glycoprotein-Glycosaminoglycan Interactions to Inhibit Herpes Simplex Virus 1 Entry and Cell-to-Cell Spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Jacquet, R.; Quideau, S.; Galabov, A. Ellagitannins as synergists of ACV on the replication of ACV-resistant strains of HSV 1 and 2. Antivir. Res. 2014, 110, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. Morb. Mortal. Wkly. Rep. 2013, 62, 574–576. [Google Scholar]
- Aschner, C.B.; Herold, B.C. Alphaherpesvirus Vaccines. Curr. Issues Mol. Biol. 2021, 41, 469–508. [Google Scholar] [CrossRef] [PubMed]
- Gabutti, G.; Bolognesi, N.; Sandri, F.; Florescu, C.; Stefanati, A. Varicella zoster virus vaccines: An update. ImmunoTargets Ther. 2019, 8, 15–28. [Google Scholar] [CrossRef]
- Shah, R.A.; Limmer, A.L.; Nwannunu, C.E.; Patel, R.R.; Mui, U.N.; Tyring, S. Shingrix for herpes zoster: A review. Skin. Ther. Lett. 2019, 24, 5–7. [Google Scholar]
- Gerna, G.; Lilleri, D. Human Cytomegalovirus Congenital (cCMV) Infection Following Primary and Nonprimary Maternal Infection: Perspectives of Prevention through Vaccine Development. Vaccines 2020, 8, 194. [Google Scholar] [CrossRef]
- Nelson, C.S.; Baraniak, I.; Lilleri, D.; Reeves, M.B.; Griffiths, P.D.; Permar, S.R. Immune Correlates of Protection Against Human Cytomegalovirus Acquisition, Replication, and Disease. J. Infect. Dis. 2020, 221, S45–S59. [Google Scholar] [CrossRef]
- Manandhar, T.; Hò, G.-G.T.; Pump, W.C.; Blasczyk, R.; Bade-Doeding, C. Battle between Host Immune Cellular Responses and HCMV Immune Evasion. Int. J. Mol. Sci. 2019, 20, 3626. [Google Scholar] [CrossRef]
- Park, A.; Ra, E.A.; Lee, T.A.; Choi, H.J.; Lee, E.; Kang, S.; Seo, J.-Y.; Lee, S.; Park, B. HCMV-encoded US7 and US8 act as antagonists of innate immunity by distinctively targeting TLR-signaling pathways. Nat. Commun. 2019, 10, 4670. [Google Scholar] [CrossRef]
- Xia, L.; Su, R.; An, Z.; Fu, T.-M.; Luo, W. Human cytomegalovirus vaccine development: Immune responses to look into vaccine strategy. Hum. Vaccines Immunother. 2017, 14, 292–303. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Wang, D.; Oualim, A.; Diamond, D.J.; Kotton, C.N.; Mossman, S.; Carfi, A.; Anderson, D.; Dormitzer, P.R. The status of vaccine development against the human cytomegalovirus. J. Infect. Dis. 2020, 221, S113–S122. [Google Scholar] [CrossRef] [PubMed]
- Scarpini, S.; Morigi, F.; Betti, L.; Dondi, A.; Biagi, C.; Lanari, M. Development of a Vaccine against Human Cytomegalovirus: Advances, Barriers, and Implications for the Clinical Practice. Vaccines 2021, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.P.; Lewis, N.; Conlon, A.; Christiansen, M.P.; Al-Ibrahim, M.; Rupp, R.; Fu, T.-M.; Bautista, O.; Tang, H.; Wang, D.; et al. Phase 1 Clinical Trial of a Conditionally Replication-Defective Human Cytomegalovirus (CMV) Vaccine in CMV-Seronegative Subjects. J. Infect. Dis. 2019, 220, 411–419. [Google Scholar] [CrossRef] [PubMed]
- V160 2-Dose and 3-Dose Regimens in Healthy Cytomegalovirus (CMV) Seronegative Females (V160-002). Available online: https://clinicaltrials.gov/ct2/show/NCT03486834 (accessed on 29 November 2021).
- Bernstein, D.I.; Munoz, F.M.; Callahan, S.T.; Rupp, R.; Wootton, S.H.; Edwards, K.M.; Turley, C.B.; Stanberry, L.R.; Patel, S.M.; Mcneal, M.M.; et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine 2015, 34, 313–319. [Google Scholar] [CrossRef]
- Baraniak, I.; Gomes, A.C.; Sodi, I.; Langstone, T.; Rothwell, E.; Atkinson, C.; Pichon, S.; Piras-Douce, F.; Griffiths, P.D.; Reeves, M.B. Seronegative patients vaccinated with cytomegalovirus gB-MF59 vaccine have evidence of neutralising antibody responses against gB early post-transplantation. EBioMedicine 2019, 50, 45–54. [Google Scholar] [CrossRef]
- La Rosa, C.; Longmate, J.; Lingaraju, C.R.; Zhou, Q.; Kaltcheva, T.; Hardwick, N.; Aldoss, I.; Nakamura, R.; Diamond, D.J. Rapid Acquisition of Cytomegalovirus-Specific T Cells with a Differentiated Phenotype, in Nonviremic Hematopoietic Stem Transplant Recipients Vaccinated with CMVPepVax. Biol. Blood Marrow Transplant. 2018, 25, 771–784. [Google Scholar] [CrossRef]
- Vaccine Therapy in Reducing the Frequency of Cytomegalovirus Events in Patients with Hematologic Malignancies Undergoing Donor Stem Cell Transplant. Available online: https://clinicaltrials.gov/ct2/show/NCT02396134 (accessed on 29 November 2021).
- Kirchmeier, M.; Fluckiger, A.-C.; Soare, C.; Bozic, J.; Ontsouka, B.; Ahmed, T.; Diress, A.; Pereira, L.; Schödel, F.; Plotkin, S.; et al. Enveloped Virus-Like Particle Expression of Human Cytomegalovirus Glycoprotein B Antigen Induces Antibodies with Potent and Broad Neutralizing Activity. Clin. Vaccine Immunol. 2014, 21, 174–180. [Google Scholar] [CrossRef]
- Study to Evaluate Safety, Tolerability, and Immunogenicity of Candidate Human Cytomegalovirus Vaccine in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT02826798 (accessed on 29 November 2021).
- Schleiss, M.R.; Berka, U.; Watson, E.; Aistleithner, M.; Kiefmann, B.; Mangeat, B.; Swanson, E.C.; Gillis, P.A.; Hernandez-Alvarado, N.; Fernández-Alarcón, C.; et al. Additive Protection against Congenital Cytomegalovirus Conferred by Combined Glycoprotein B/pp65 Vaccination Using a Lymphocytic Choriomeningitis Virus Vector. Clin. Vaccine Immunol. 2017, 24, e00300-16. [Google Scholar] [CrossRef]
- A Study of CMV Vaccine (HB-101) in Kidney Transplant Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT03629080 (accessed on 29 November 2021).
- Aldoss, I.; La Rosa, C.; Baden, L.R.; Longmate, J.; Ariza-Heredia, E.J.; Rida, W.N.; Lingaraju, C.R.; Zhou, Q.; Martinez, J.; Kaltcheva, T.; et al. Poxvirus Vectored Cytomegalovirus Vaccine to Prevent Cytomegalovirus Viremia in Transplant Recipients. Ann. Intern. Med. 2020, 172, 306. [Google Scholar] [CrossRef]
- Ljungman, P.; Bermudez, A.; Logan, A.C.; Kharfan-Dabaja, M.A.; Chevallier, P.; Martino, R.; Wulf, G.; Selleslag, D.; Kakihana, K.; Langston, A.; et al. A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients. eClinicalMedicine 2021, 33, 100787. [Google Scholar] [CrossRef]
- Mori, T.; Kanda, Y.; Takenaka, K.; Okamoto, S.; Kato, J.; Kanda, J.; Yoshimoto, G.; Gondo, H.; Doi, S.; Inaba, M.; et al. Safety of ASP0113, a cytomegalovirus DNA vaccine, in recipients undergoing allogeneic hematopoietic cell transplantation: An open-label phase 2 trial. Int. J. Hematol. 2016, 105, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Safety, Reactogenicity, and Immunogenicity of Cytomegalovirus Vaccines mRNA-1647 and mRNA-1443 in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT03382405 (accessed on 29 November 2021).
- John, S.; Yuzhakov, O.; Woods, A.; Deterling, J.; Hassett, K.; Shaw, C.A.; Ciaramella, G. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 2018, 36, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Chemaitelly, H.; Abu-Raddad, L.J. Epidemiological impact of novel preventive and therapeutic HSV-2 vaccination in the United States: Mathematical modeling analyses. Vaccines 2020, 8, 366. [Google Scholar] [CrossRef]
- Freeman, E.E.; White, R.G.; Bakker, R.; Orroth, K.K.; Weiss, H.A.; Buvé, A.; Hayes, R.J.; Glynn, J.R. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine 2009, 27, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Herpevac trial for women. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Aschner, C.B.; Loh, L.N.; Galen, B.; Delwel, I.; Jangra, R.K.; Garforth, S.J.; Chandran, K.; Almo, S.; Jacobs, W.R.; Ware, C.F.; et al. HVEM signaling promotes protective antibody-dependent cellular cytotoxicity (ADCC) vaccine responses to herpes simplex viruses. Sci. Immunol. 2020, 5, eaax2454. [Google Scholar] [CrossRef]
- Truong, N.R.; Smith, J.B.; Sandgren, K.J.; Cunningham, A.L. Mechanisms of immune control of mucosal HSV infection: A guide to rational vaccine design. Front. Immunol. 2019, 10, 10373. [Google Scholar] [CrossRef]
- Tognarelli, E.I.; Palomino, T.F.; Corrales, N.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Herpes simplex virus evasion of early host antiviral responses. Front. Cell Infect. Microbiol. 2019, 9, 127. [Google Scholar] [CrossRef]
- Awasthi, S.; Huang, J.; Shaw, C.; Friedman, H.M. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J. Virol. 2014, 88, 8421–8432. [Google Scholar] [CrossRef]
- Egan, K.P.; Hook, L.M.; Naughton, A.; Pardi, N.; Awasthi, S.; Cohen, G.H.; Weissman, D.; Friedman, H.M. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection. PLoS Pathog. 2020, 16, e1008795. [Google Scholar] [CrossRef]
- Egan, K.; Hook, L.M.; LaTourette, P.; Desmond, A.; Awasthi, S.; Friedman, H.M. Vaccines to prevent genital herpes. Transl. Res. 2020, 220, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalova, M.; McKay, J.; Mbaye, A.; Sanford-Crane, H.; Blanco, J.C.G.; Huber, A.; Herold, B.C. Efficacy of the Herpes Simplex Virus 2 (HSV-2) Glycoprotein D/AS04 Vaccine against Genital HSV-2 and HSV-1 Infection and Disease in the Cotton Rat Sigmodon hispidus Model. J. Virol. 2015, 89, 9825–9840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernstein, D.I.; Flechtner, J.B.; McNeil, L.K.; Heineman, T.; Oliphant, T.; Tasker, S.; Wald, A.; Hetherington, S. Therapeutic HSV-2 vaccine decreases recurrent virus shedding and recurrent genital herpes disease. Vaccine 2019, 37, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Ike, A.C.; Onu, C.J.; Ononugbo, C.M.; Reward, E.E.; Muo, S.O. Immune Response to Herpes Simplex Virus Infection and Vaccine Development. Vaccines 2020, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Woo, Y.; Dutton, J.L.; Xu, Y.; Li, B.; Kinrade, S.; Druce, J.; Finlayson, N.; Griffin, P.; Laing, K.J.; et al. Immune responses to a HSV-2 polynucleotide immunotherapy COR-1 in HSV-2 positive subjects: A randomized double blinded phase I/IIa trial. PLoS ONE 2019, 14, e0226320. [Google Scholar] [CrossRef]
- De Bruyn, G.; Vargas-Cortez, M.; Warren, T.; Tyring, S.K.; Fife, K.H.; Lalezari, J.; Brady, R.C.; Shahmanesh, M.; Kinghorn, G.; Beutner, K.R.; et al. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine 2006, 24, 914–920. [Google Scholar] [CrossRef]
- Dropulic, L.K.; Oestreich, M.C.; Pietz, H.L.; Laing, K.J.; Hunsberger, S.; Lumbard, K.; Garabedian, D.; Turk, S.P.; Chen, A.; Hornung, R.L.; et al. A Randomized, Double-Blinded, Placebo-Controlled, Phase 1 Study of a Replication-Defective Herpes Simplex Virus (HSV) Type 2 Vaccine, HSV529, in Adults with or Without HSV Infection. J. Infect. Dis. 2019, 220, 990–1000. [Google Scholar] [CrossRef]
- Halford, W.P.; Geltz, J.; Messer, R.J.; Hasenkrug, K.J. Antibodies Are Required for Complete Vaccine-Induced Protection against Herpes Simplex Virus 2. PLoS ONE 2015, 10, e0145228. [Google Scholar] [CrossRef]
- Stanfield, B.A.; Kousoulas, K.G.; Fernandez, A.; Gershburg, E. Rational Design of Live-Attenuated Vaccines against Herpes Simplex Viruses. Viruses 2021, 13, 1637. [Google Scholar] [CrossRef]
- Safety and Efficacy of 4 Investigational HSV 2 Vaccines in Adults with Recurrent Genital Herpes Caused by HSV 2 (HSV15). Available online: https://clinicaltrials.gov/ct2/show/NCT04222985 (accessed on 29 November 2021).
- HSV529 Vaccine in HSV-2 Seropositive Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT02571166 (accessed on 29 November 2021).
- Joyce, J.; Patel, A.; Murphy, B.; Carr, D.; Gershburg, E.; Bertke, A. Assessment of Two Novel Live-Attenuated Vaccine Candidates for Herpes Simplex Virus 2 (HSV-2) in Guinea Pigs. Vaccines 2021, 9, 258. [Google Scholar] [CrossRef]
- Van Wagoner, N.; Fife, K.; Leone, P.A.; Bernstein, D.I.; Warren, T.; Panther, L.; Novak, R.M.; Beigi, R.; Kriesel, J.; Tyring, S.; et al. Effects of Different Doses of GEN-003, a Therapeutic Vaccine for Genital Herpes Simplex Virus-2, on Viral Shedding and Lesions: Results of a Randomized Placebo-Controlled Trial. J. Infect. Dis. 2018, 218, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Maintenance Dose Study of GEN-003 in Subjects with Genital Herpes Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT03146403 (accessed on 29 November 2021).
- Biological Efficacy Study of HerpV Vaccine With QS-21 to Treat Participants with Recurrent Genital Herpes. Available online: https://clinicaltrials.gov/ct2/show/NCT01687595 (accessed on 29 November 2021).
- Safety and Efficacy Study of Herpes Simplex Virus Type 2 (HSV-2) Therapeutic DNA Vaccine (HSV-2). Available online: https://clinicaltrials.gov/ct2/show/NCT02837575 (accessed on 29 November 2021).
| Drug Name/IUPAC Name | Chemical Formula |
|---|---|
| ACICLOVIR (ACV) 2-amino-9-(2-hydroxyethoxymethyl)-1H-purin-6-one | 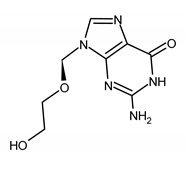 |
| Mutations conferring resistance to ACV | Ref. |
| HSV-1 UL23: G200C/S/D, R261C, R281STOP, L298P, A189V, A93, V204G, Y53C/D/H, Y53stop, D55N, G56S, P57H, K62N/R, A174P, H58R/Y, Q259stop, Q67stop, Y172S, C336Y, D162N/T, L242P, L170P, W88stop, C171stop, R176stop, S181N, R216S, R220C, R221H, G246V, T287M, R41H, Q261R, P84S, M121L, E83K, P84L, Y87H, G ins to 7Gs nt.430-436, stop codon at AA 224, A ins at nt. 438, stop codon at AA 227, E257K, S263stop (del nt. 781–793), L288stop, E39G, L208F, del C548-55399, R51W, and G59P UL30: R700G/M, A719V/T, A605V, S724N/S729N, V813M, G841C, G850I, L773M, Y941H, V573M, L1007M, I1028T, E188K, E222K, M501I, M553L, G901V, del G432-438, S599L, F733C, S775N, T821M, R881C, I619K, V715S, N820NS, R1047L, V621S, and H1228D | [66,67,68,69,72,73,74,75,76,77,78,79,80,81] |
| HSV-2 UL23: M183stop, R220C, R221C/H, D137stop, Q222C, G61W, G201D, E84K/G, S169P, T288M, R51W, R177W, R217H, E39G, Q105P, R271V, add G nt. 433–439, del G nt. 439–440, del C nt. 467, and G59P UL30: Y577H, E678G, D785N, D307N, Q619R, A724T/V, Q732R, N820S, T843A, T844I, G846A, and A915V | [66,68,69,72,77,78,81] |
| VZV ORF36: S179N, W225R, W225stop, E48G/L, G24E/R, K25R, A37stop, I38stop, R54stop, E59, T86, C90stop, L92, H97R, D129N, R130Q, C138R, R143G/K, L154P, A163STOP, V171STOP, V194STOP, Y206stop, C231STOP, T256A/M, L298stop, Q303stop, L332P, N334stop, D338stop, ins. nt. 412-3, and del nt. 641 ORF28: E512K, K662E, R665G, V666L, D668Y, V680A, A684T/N, Q692R, A773V, N779S, I804T, G805C, R806S, M808V, L809S, V855M, and M874I | [68,69,79,82,83] |
| VALACICLOVIR (VACV) 2-[(2-amino-6-oxo-1H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate | 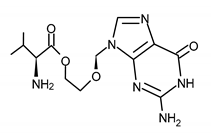 |
| PENCICLOVIR (PCV) 2-amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-1H-purin-6-one | 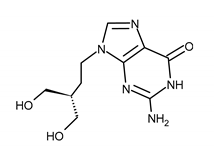 |
| FAMCYCLOVIR (FCV) [2-(acetyloxymethyl)-4-(2-aminopurin-9-yl)butyl] acetate |  |
| GANCICLOVIR (GCV) 2-amino-9-(1,3-dihydroxypropan-2-yloxymethyl)-1H-purin-6-one |  |
| VALGANCICLOVIR (VGCV) [2-[(2-amino-6-oxo-1H-purin-9-yl)methoxy]-3-hydroxypropyl] (2S)-2-amino-3-methylbutanoate | 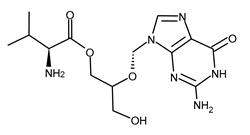 |
| Mutations conferring resistance to GCV | Ref. |
| CMV UL54: D301N, N408D, N410K, F412C, D413E, L501F/I, T503I, K513R/E, P522A, Q578H, V781I, L802M, A809V, G841A, A834, V787L, and del. 524 UL97: del595-596, C592G, H520Q, K599T, M460V/I, V466G, A594V/E/G/T/P, C603W/R, L405P, and L595F | [84,85,86,87] |
| BRIVUDIN (BVDU) [(E)-5-(2-bromovinyl)-2′-deoxyuridine] | 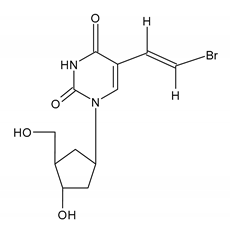 |
| Drug Name/IUPAC Name | Chemical Formula |
|---|---|
| CIDOFOVIR (CDV) [(2S)-1-(4-amino-2-oxopyrimidin-1-yl)-3-hydroxypropan-2-yl]oxymethylphosphonic acid | 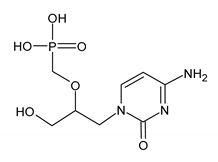 |
| Mutations conferring resistance to CDV | Ref. |
| HSV UL30: R700M, G841C, G850I, L773M, Y941H, V573M, L1007M, I1028T, K960R, and N408K i V812L CMV UL54: K805Q, D542E, D301N, N408D, N410K, F412C/V, D413A/E, L501I, T503I, L516R, I521T, P522A, L545S, D588N, E756K, V812L, T813S, G841A, A987G, K513E/R, S676G/S Y751H, and del 981–982 VCV ORF28:K662E, V666L, D668Y, L767S, N779, G805C, and R808V | [18,69,70,83,92,93,94] |
| BRINCIDOFOVIR (BCDV) [(2S)-1-(4-amino-2-oxopyrimidin-1-yl)-3-hydroxypropan-2-yl]oxymethyl-(3-hexadecoxypropoxy)phosphinic acid | 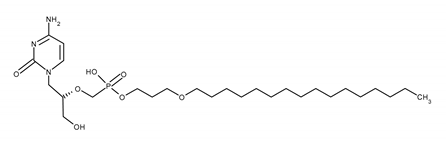 |
| Mutations conferring resistance to BCDV | Ref. |
| CMV U54: N408K, V812L, D413Y, E303D, E303G, D542E, and E303G+ V812L | [52,89,95] |
| FOSCARNET (FOS) phosphonoformic acid | 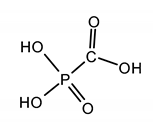 |
| Mutations conferring resistance to FOS | Ref. |
| HSV-1 and HSV-2 UL30: V715G, S724N/S729N, Y941H, I922N, I619K, V715S, and A719T CMV UL54: Q697P, V715M/S, A719T, T700A, M715V, V781I, V787L, L802M, A809V, V812L, T821I, Q578H, N495K, D588E, and E756D/N/Q T838A. V715M, S290R, T813S, A834, G841, del. 981-982, VCV ORF28:E519K, K662E, R665G, V666L, D668Y, L767S, G805C, R806S, M808V, L809S, V855M, Q692R, and N774 | [18,68,69,92,93,94,95] |
| Letermovir (LMV) 2-[(4S)-8-fluoro-2-[4-(3-methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-4H-quinazolin-4-yl]acetic acid | 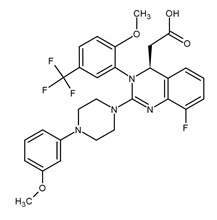 |
| Mutations conferring resistance to LMV | Ref. |
| CMV UL56: C25F, L328V, V231L, (C25F +V231L) V236M, (V236M+L257I+M329T), V236A(V236L+L257I), L241P, L257F, C325Y, C325W, C325F, A365S, R369G, R369M, R369S, T4270, T4189, T4257, T4237, and T4217 UL89: N320H, N329S, D344E and T350M, especially when coexisting with Q204R, E237D, F261L, and M329T in UL56 (UL89 D334E +UL56 E237D) UL51 P91S (UL51 P91S + UL5 R369M) | [52,87,94,99,108] |
| Maribavir (MBV) (2S,3S,4R,5S)-2-[5,6-dichloro-2-(propan-2-ylamino)benzimidazol-1-yl]-5-(hydroxymethyl)oxolane-3,4-diol | 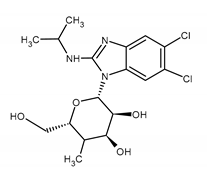 |
| Mutations conferring resistance to MBV | Ref. |
| CMV UL97: T409M, H411Y, F342Y, F342F, C480F, V466G, P521L, V356G, D456N C480R, Y617del., M460V/I, H520Q, A594V, L595S, C603W H411N, H411L, L347R, V353A UL27: L335P, 480F | [99,116,117,118,119,120,121] |
| Drug Name/IUPAC Name | Chemical Formula |
|---|---|
| PRITELIVIR (PTV) N-methyl-N-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-(4-pyridin-2-ylphenyl)acetamide | 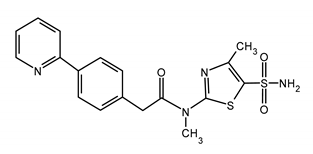 |
| Mutations conferring resistance to PTV | Ref. |
| HSV-1UL5:K356T; UL52: A899T, UL52: A899T, and UL5: K356T | [131,138] |
| AMENAMEVIR (AMV) N-(2,6-dimethylphenyl)-N-[2-[4-(1,2,4-oxadiazol-3-yl)anilino]-2-oxoethyl]-1,1-dioxothiane-4-carboxamide | 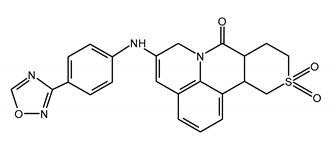 |
| Mutations conferring resistance to AMV | Ref. |
| HSV-1UL5:G352V+M355I; UL52: R367H + S364G; and HSV-2 UL5: K355N + K451R | [12,132,136] |
| VZV OFR55: R66H and OFR6: N939D | [139] |
| FILOCICLOVIR (FCV) 2-amino-9-[(Z)-[2,2-bis(hydroxymethyl)cyclopropylidene]methyl]-1H-purin-6-one | 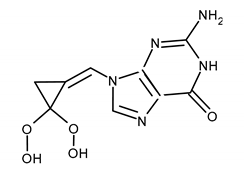 |
| Mutations conferring resistance to FCV | Ref. |
| CMVUL97: M460V, M460I, H520Q, C592G, A594V, and C603W (cross-resistance with ganciclovir in all cases) | [119,143] |
| Name of Vaccine (Developer) | Antigen/Other Vaccine Components | Type of Vaccine | Research Stage | Results | References |
|---|---|---|---|---|---|
| V160 (Merck) | whole virus and AD169 with restored pentamer complex | Live attenuated | Phase 2 completed | Phase 1: well-tolerated and immune response similar as in natural infection. Phase 2: 42.4% preventive efficacy in seronegative women of reproductive age | [168,169] |
| gB/MF59 (Sanofi Pasteur) | gB with MF59 adjuvant | Recombinant subunit | Phase 2 completed | Safe and immunogenic, 43% preventive efficacy in seronegative adolescent girls, and was found to be insufficient. Neutralizing antibody production in a small group of seronegative transplant recipients from seropositive donors | [170,171] |
| CMVPepVax (City of Hope) | pp65 fused to a natural tetanus sequence and PF-03512676 adjuvant | Peptide | Phase 2b completed | Safe, immunogenic, and reduces the risk of recurrent HCMV infections in seropositive HCT recipients | [156,157] |
| VBI-1501 (VBI Vaccines) | gB in eVLPs * and alum adjuvant | Recombinant and VLP | Phase 1 completed | Safe and immunogenic | [174,175] |
| HB-101 (Hookipa) | replication-defective LCMV ** encoding HCMV gB and pp65 | DNA, virus-vectored | Phase 2 ongoing | Phase 1: safe and immunogenic. Phase 2: (seronegative kidney transplant recipients) NA | [176,177] |
| CMV-MVA Triplex (City of Hope) | Modified MVA ***containing HCMVUL83 (pp65), UL123 (IE1), and UL122 (IE2) | DNA, virus-vectored | Phase 2 completed | Well-tolerated and immunogenic Reduces the risk of complications associated with HCMV infection by 50% in seropositive HCT recipients | [178] |
| ASP0113 or VCL-CB01 (Astellas) | gB and pp65 with the adiuvants CRL1005 and benzalkonium chloride | DNA, plasmid-based | Phase2/Phase 3 completed | Well-tolerated; however, does not affect the rate of complications related to HCMV infection in HSCT recipients and renal transplant recipients | [179,180] |
| mRNA-1647 (Moderna) | six mRNAs, of which five encode the pentamer complex and one encodes gB | Self-replicating mRNA | Phase 2 and initiation of phase 3 announced in October 2021 | NA and, according to the interim phase 2, well-tolerated | [181,182] |
| Name of Vaccine (Developer) | Antigen/Other Vaccine Components | Type of Vaccine | Research Stage | Results | References |
|---|---|---|---|---|---|
| HSV529 (SanofiPasteur) | HSV-2 with deletions in UL5 and UL29 | Replication defective virus | Phase 1/2 | Safe and immunogenic only in HSV-1 and 2 seronegative individuals | [197,200,201] |
| HSV-2 ΔgD-2 (X-Vax Technology) | HSV-2 ΔgD | Single -cycle virus | Preclinical, preparing for phase 1 | Immunogenic in mice including HSV-1 seropositive and 100% protective in seronegative mice | [158,199] |
| RVx201 -ΔNLS (RationalVaccines) | HSV-2ΔNLS | Live attenuated | Preclinical, preparing for phase 1 | Reduced recurrence rates in a small group of genital herpes patients studied without FDA approval | [202] |
| RVx1001 -HSV-1 VC2 (Rational Vaccines) | HSV-1 with deletion in gK (AA31-68) | Live attenuated | Preclinical, preparing for phase 1 | Immunogenic in animals and protective against both HSV-2 genital infection (guinea pigs) and HSV-1 ocular infection (mice) | [198,199] |
| Gen-003 (Genocea) | gD2, infected cell polypeptide 4 (ICP4), and matrix-M2 adjuvant | Recombinant subunit | Phase 2 completed | Acceptable safety profile and immunogenic. Significant reduction of viral shedding and lesions rates in patients with genital herpes | [203,204] |
| HerpV (Agenus) | 32 HSV peptides’ complex with HHSP 70 and QS21-adjuvant | Peptide | Phase 2 completed | Significant (17%) reduction in viral shedding in genital herpes patients | [205] |
| VCL-HB01 Vaxfectin (Vical) | gD, UL46/UL46, and Vaxfectin adjuvant | Plasmid-based DNA | Phase 2 completed (NCT02837575) | No difference from placebo in relapse rate in genital herpes patients tested | [194,206] |
| COR-1 (Admedus) | Codon-optimized gD2 and shortened gD2 fused with ubiquitin | DNA | Phase 2 completed | Herpes recurrence rate in the study group lower than baseline but the same as the placebo group | [195] |
| (UPenn, BioNTech) | HSV-2 mRNA coding gC2, gD2, gE2, and LNP * | mRNA | Preclinical, preparing for phase 1 | Immunogenic and protective in mice and guinea pigs | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. https://doi.org/10.3390/ijms23073431
Majewska A, Mlynarczyk-Bonikowska B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? International Journal of Molecular Sciences. 2022; 23(7):3431. https://doi.org/10.3390/ijms23073431
Chicago/Turabian StyleMajewska, Anna, and Beata Mlynarczyk-Bonikowska. 2022. "40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs?" International Journal of Molecular Sciences 23, no. 7: 3431. https://doi.org/10.3390/ijms23073431
APA StyleMajewska, A., & Mlynarczyk-Bonikowska, B. (2022). 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? International Journal of Molecular Sciences, 23(7), 3431. https://doi.org/10.3390/ijms23073431






