Chemical Fingerprinting of Heat Stress Responses in the Leaves of Common Wheat by Fourier Transform Infrared Spectroscopy
Abstract
1. Introduction
2. Results
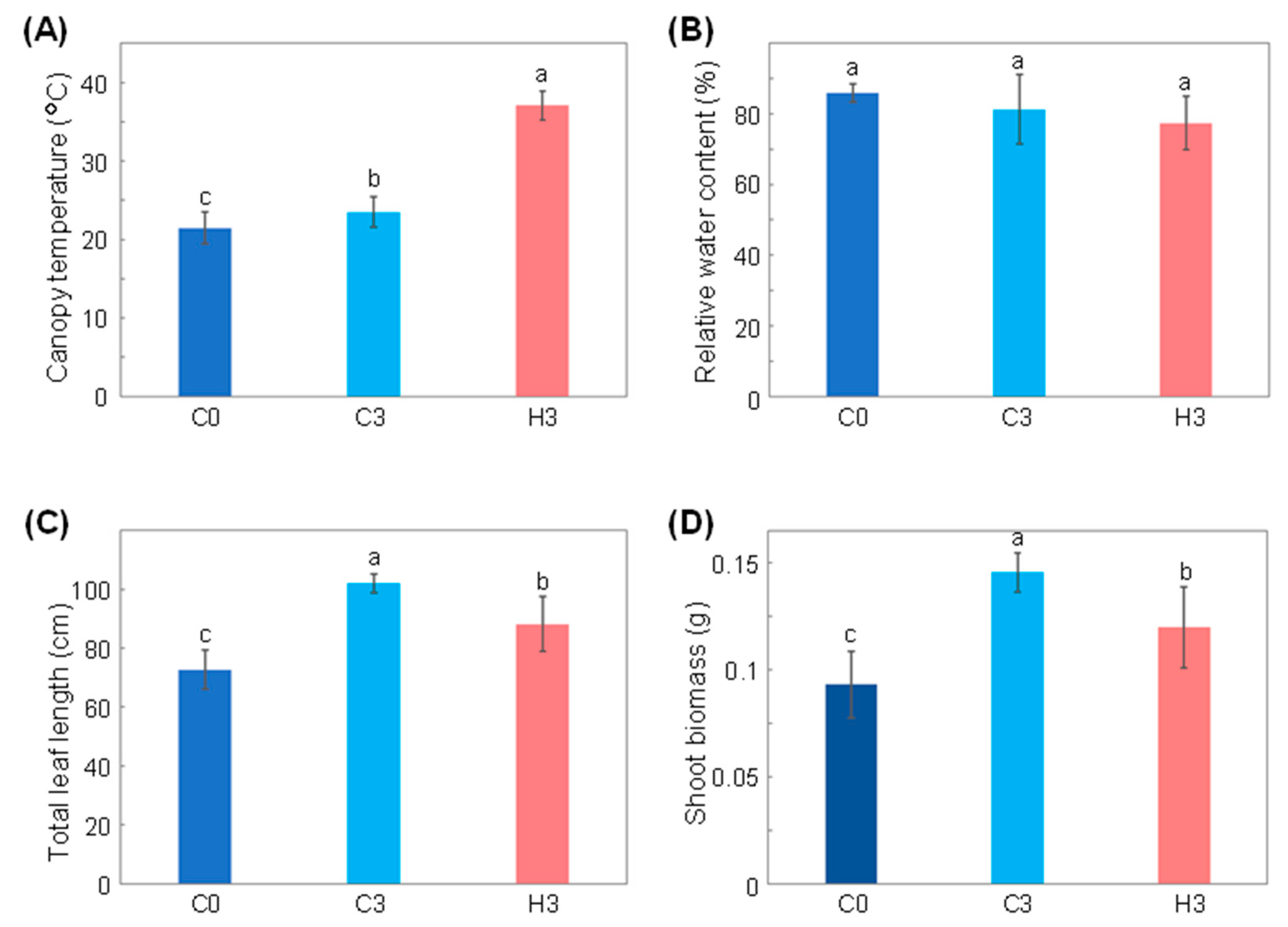
2.1. Growth and Physiological Response of Wheat to Heat Stress
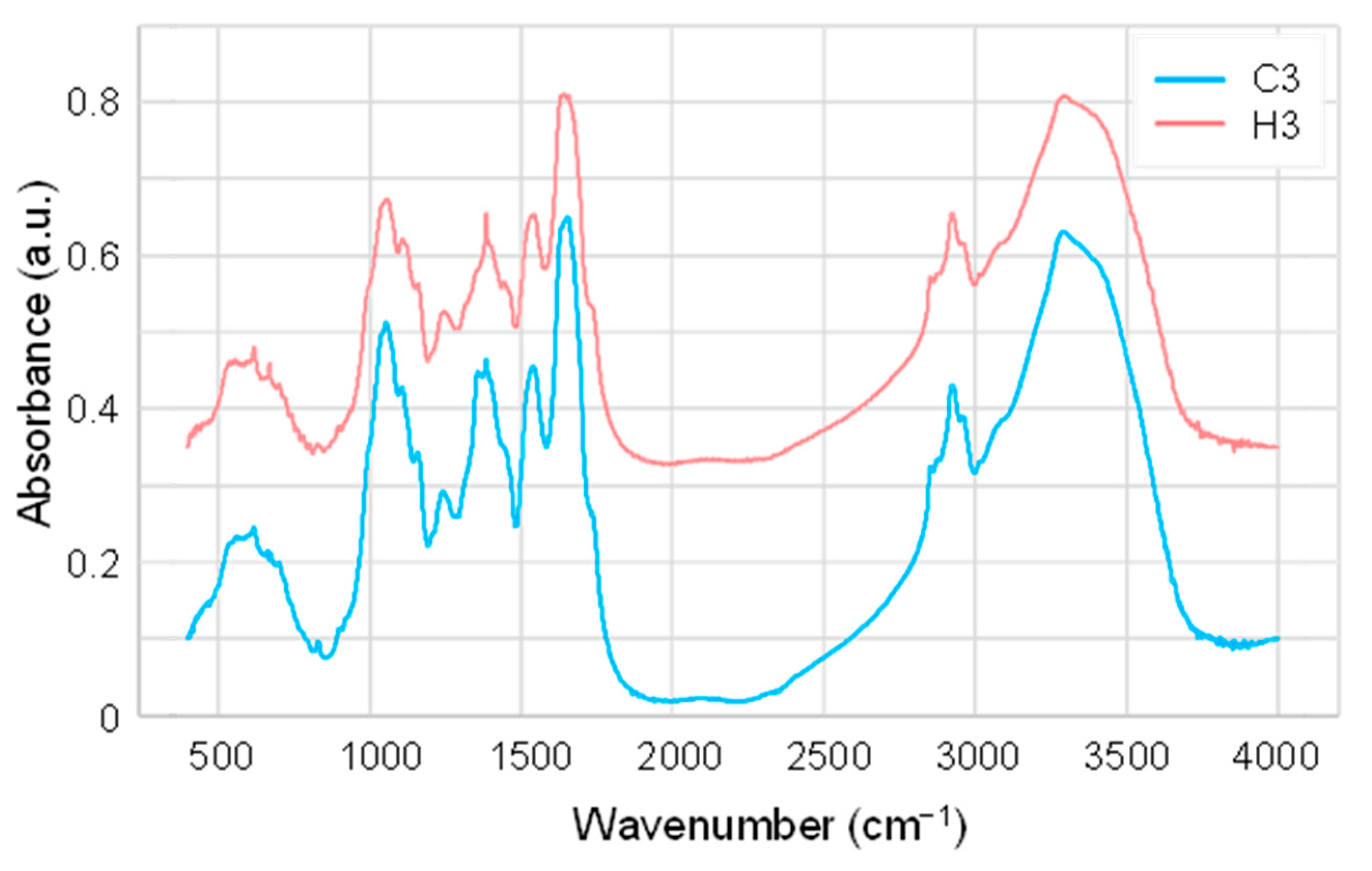
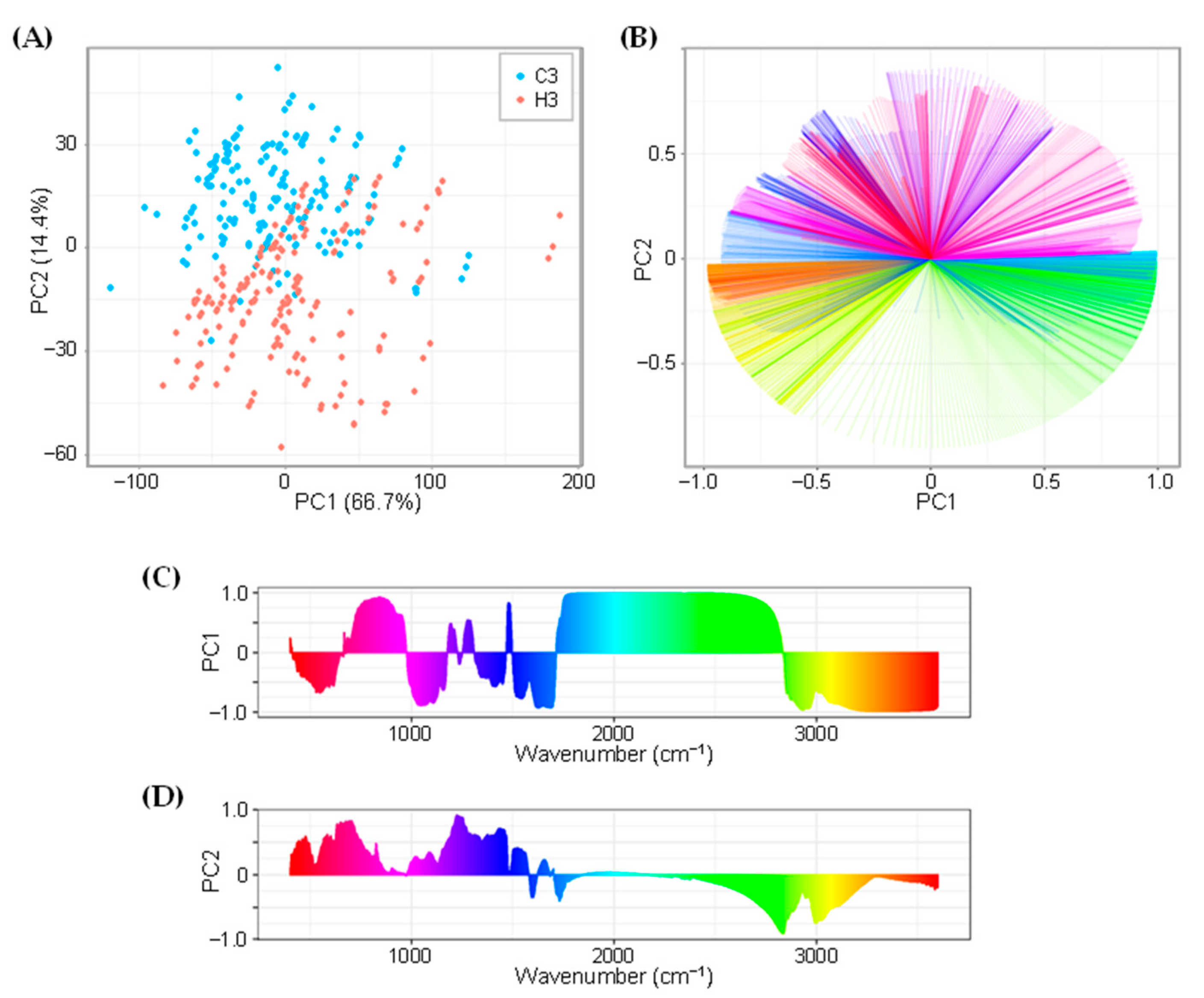
2.2. FTIR and Principal Component Analysis
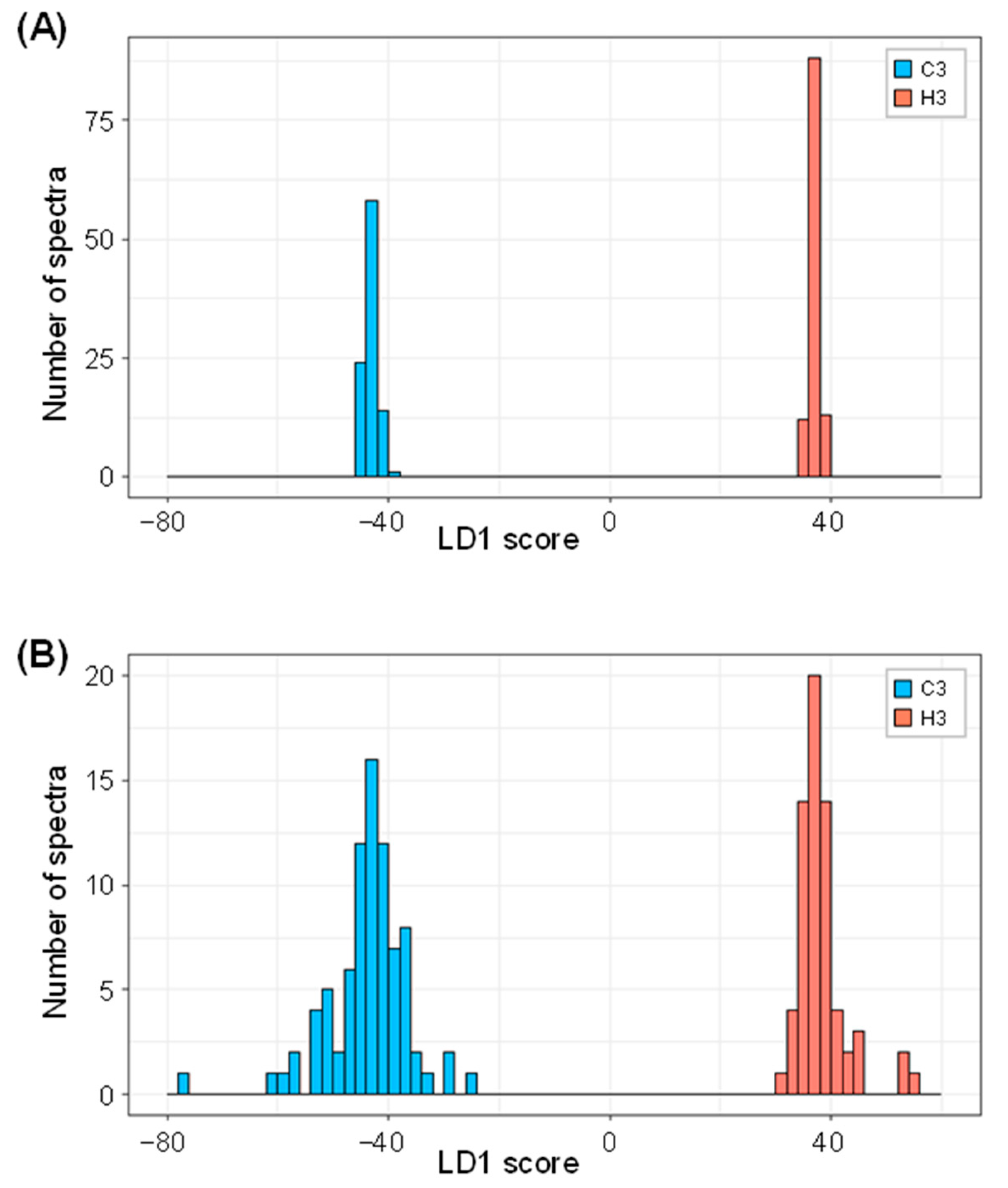
2.3. Linear Discriminant Analysis
2.4. Spectral Biomarkers for Heat Stress Response
3. Discussion
3.1. Sensitivity of FTIR Spectral Response in Heat-Stressed Wheat Leaves
3.2. Chemometrics Using FTIR Spectra
3.3. FTIR-Based Biomarker for Chemical Changes under Heat Stress
3.4. Application of FTIR-Based Metabolome Profiling on Agronomy
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurements of Plant Growth and Physiology
4.3. FTIR Measurement
4.4. Chemometrics of Spectral Data
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Yamasaki, Y.; Toda, Y.; Mega, R.; Akashi, K.; Tsujimoto, H. Stage-specific characterization of physiological response to heat stress in the wheat cultivar norin 61. Int. J. Mol. Sci. 2021, 22, 6942. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.S.M.H.M.; McDonald, G.K.; Gill, G.S. Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat. Field Crops Res. 2014, 160, 54–63. [Google Scholar] [CrossRef]
- Iizumi, T.; Ali-Babiker, I.E.A.; Tsubo, M.; Tahir, I.S.A.; Kurosaki, Y.; Kim, W.; Gorafi, Y.S.A.; Idris, A.A.M.; Tsujimoto, H. Rising temperatures and increasing demand challenge wheat supply in Sudan. Nat. Food 2021, 2, 19–27. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in plant stress physiology. Adv. Biochem. Eng. Biotechnol. 2018, 164, 187–236. [Google Scholar] [CrossRef]
- Djande, C.Y.H.; Pretorius, C.; Tugizimana, F.; Piater, L.A.; Dubery, I.A. Metabolomics: A tool for cultivar phenotyping and investigation of grain crops. Agronomy 2020, 10, 831. [Google Scholar] [CrossRef]
- Thomason, K.; Babar, M.A.; Erickson, J.E.; Mulvaney, M.; Beecher, C.; MacDonald, G. Comparative physiological and metabolomics analysis of wheat (Triticum Aestivum L.) following post-anthesis heat stress. PLoS ONE 2018, 13, e0197919. [Google Scholar] [CrossRef]
- Matsunaga, S.; Yamasaki, Y.; Mega, R.; Toda, Y.; Akashi, K.; Tsujimoto, H. Metabolome profiling of heat priming effects, senescence, and acclimation of bread wheat induced by high temperatures at different growth stages. Int. J. Mol. Sci. 2021, 22, 13139. [Google Scholar] [CrossRef]
- De Leonardis, A.M.; Fragasso, M.; Beleggia, R.; Ficco, D.B.M.; de Vita, P.; Mastrangelo, A.M. Effects of heat stress on metabolite accumulation and composition, and nutritional properties of durum wheat grain. Int. J. Mol. Sci. 2015, 16, 30382–30404. [Google Scholar] [CrossRef]
- McCann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier transform infrared microspectroscopy is a new way to look at plant cell walls. Plant Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Renard, G.M.G.C.; Bureau, S.; Bourvellec, C.L. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef] [PubMed]
- Bouyanfif, A.; Liyanage, S.; Hewitt, J.E.; Vanapalli, S.A.; Moustaid-Moussa, N.; Hequet, E.; Abidi, N. FTIR imaging detects diet and genotype-dependent chemical composition changes in wild type and mutant C. elegans strains. Analyst 2017, 142, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Munz, E.; Rolletschek, H.; Oeltze-Jafra, S.; Fuchs, J.; Guendel, A.; Neuberger, T.; Ortleb, S.; Jakob, P.M.; Borisjuk, L. A functional imaging study of germinating oilseed rape seed. New Phytol. 2017, 216, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Z.; Mamtimin, A.; Liu, J.; Liu, Y.; Ju, C.; Zhang, H.; Gao, Z. A new linear relation for estimating surface broadband emissivity in arid regions based on FTIR and MODIS products. Remote Sens. 2021, 13, 1686. [Google Scholar] [CrossRef]
- Westworth, S.; Ashwath, N.; Cozzolino, D. Application of FTIR-ATR spectroscopy to detect salinity response in beauty leaf tree (Calophyllum inophyllum L). Energy Proc. 2019, 160, 761–768. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Kumar, J.; Nikam, T.D.; Suprasanna, P. FT-IR profiling reveals differential response of roots and leaves to salt stress in a halophyte Sesuvium portulacastrum (L.) L. Biotechnol. Rep. 2019, 23, e00352. [Google Scholar] [CrossRef]
- Allwood, J.W.; Chandra, S.; Xu, Y.; Dunn, W.B.; Correa, E.; Hopkins, L.; Goodacre, R.; Tobin, A.K.; Bowsher, C.G. Profiling of spatial metabolite distributions in wheat leaves under normal and nitrate limiting conditions. Phytochemistry 2015, 115, 99–111. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Shi, Y.; Chen, G.; Li, X. Protein and lipid characterization of wheat roots plasma membrane damaged by Fe and H2O2 using ATR-FTIR method. J. Biophys. Chem. 2013, 4, 28–35. [Google Scholar] [CrossRef][Green Version]
- Georget, D.M.R.; Belton, P.S. Effects of temperature and water content on the secondary structure of wheat gluten studied by FTIR spectroscopy. Biomacromolecules 2006, 7, 469–475. [Google Scholar] [CrossRef]
- Demir, P.; Onde, S.; Severcan, F. Phylogeny of cultivated and wild wheat species using ATR-FTIR spectroscopy. Spectrochimica Acta A 2015, 135, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B. Spectral analysis. In Infrared Spectroscopy: Fundamentals and Applications; Stuart, B., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 2004; pp. 45–70. [Google Scholar]
- Kamnev, A.A.; Tugarova, A.V.; Dyatlova, Y.A.; Tarantilis, P.A.; Grigoryeva, O.P.; Fainleib, A.M.; De Luca, S. Methodological effects in Fourier transform infrared (FTIR) spectroscopy: Implications for structural analyses of biomacromolecular samples. Spectrochimica Acta A 2018, 193, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Kamnev, A.A.; Dyatlova, Y.A.; Kenzhegulov, O.A.; Vladimirova, A.A.; Mamchenkova, P.V.; Tugarova, A.V. Fourier transform infrared (FTIR) spectroscopic analyses of microbiological samples and biogenic selenium nanoparticles of microbial origin: Sample preparation effects. Molecules 2021, 26, 1146. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B. Biological application. In Infrared Spectroscopy: Fundamentals and Applications; Stuart, B., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 2004; pp. 137–165. [Google Scholar]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2016, 52, 456–506. [Google Scholar] [CrossRef]
- Keleş, Y.; Öncel, I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiologiae Plantarum 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Ramani, H.R.; Mandavia, M.K.; Dave, R.A.; Bambharolia, R.P.; Silungwe, H.; Garaniya, N.H. Biochemical and physiological constituents and their correlation in wheat (Triticum aestivum L.) genotypes under high temperature at different development stages. Int. J. Plant Physiol. Biochem. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Johnson, H.E.; Broadhurst, D.; Goodacre, R.; Smith, A.R. Metabolic fingerprinting of salt-stressed tomatoes. Phytochemistry 2003, 62, 919–928. [Google Scholar] [CrossRef]
- Giang, L.T.; Thien, T.L.T.; Yen, D.H. Rapid classification of rice in northern Vietnam by using FTIR spectroscopy combined with chemometrics methods. Viet. J. Chem. 2020, 58, 372–379. [Google Scholar] [CrossRef]
- Rohman, A.; Ghazali, M.A.B.; Windarsih, A.; Irnawati Riyanto, S.; Yusof, F.M.; Mustafa, S. Comprehensive review on application of FTIR spectroscopy coupled with chemometrics for authentication analysis of fats and oils in the food products. Molecules 2020, 25, 5485. [Google Scholar] [CrossRef] [PubMed]
- Cortizas, M.A.; López-Costas, O. Linking structural and compositional changes in archaeological human bone collagen: An FTIR-ATR approach. Sci. Rep. 2020, 10, 17888. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Herzog, R.; Wiesenhofer, F.M.; Vychytil, A.; Ehling-Schulz, M.; Kratochwill, K. Vibrational spectroscopy of peritoneal dialysis effluent for rapid assessment of patient characteristics. Biomolecules 2020, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Ami, D.; Natalello, A.; Mereghetti, P.; Neri, T.; Zanoni, M.; Monti, M.; Doglia, S.M.; Redi, C.A. FTIR spectroscopy supported by PCA-LDA analysis for the study of embryonic stem cell differentiation. Spectroscopy 2010, 24, 89–97. [Google Scholar] [CrossRef][Green Version]
- Walsh, M.J.; Fellous, T.G.; Hammiche, A.; Lin, W.-R.; Fullwood, N.J.; Grude, O.; Bahrami, F.; Nicholson, J.M.; Cotte, M.; Susini, J.; et al. Fourier transform infrared microspectroscopy identifies symmetric PO2− modifications as a marker of the putative stem cell region of human intestinal crypts. Stem Cells 2008, 26, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Bhardwaj, S.; Kumar, R. On the spectroscopic investigation of kohl stains via ATR-FTIR and multivariate analysis: Application in forensic trace evidence. Vib. Spectrosc. 2019, 101, 81–91. [Google Scholar] [CrossRef]
- Stewart, D. Fourier transform infrared microspectroscopy of plant tissues. Appl. Spectrosc. 1996, 50, 357–365. [Google Scholar] [CrossRef]
- Lammers, K.; Arbuckle-Keil, G.; Dighton, J. FTIR study of the changes in carbohydrate chemistry of three New Jersey pine barrens leaf litters during simulated control burning. Soil Biol. Biochem. 2009, 41, 340–347. [Google Scholar] [CrossRef]
- Stuart, B. Biological Applications of Infrared Spectroscopy; Wiley: Chichester, UK, 1997. [Google Scholar]
- Gorgulu, S.T.; Dogan, M.; Severcan, F. The characterization and differentiation of higher plants by Fourier transform infrared spectroscopy. Appl. Specrosc. 2007, 61, 300–308. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alteration. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef]
- Lima, R.B.; dos Santos, T.B.; Vieira, L.G.E.; Ferrarese, M.L.L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; Petkowicz, C.L.O. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, W.; Chang, S.X.; Anyia, A.O. Water-deficit and high temperature affected water use efficiency and arabinoxylan concentration in spring wheat. J. Cereal Sci. 2010, 52, 263–269. [Google Scholar] [CrossRef]
- Lahlali, R.; Jiang, Y.; Kumar, S.; Karunakaran, C.; Liu, X.; Borondics, F.; Hallin, E.; Bueckert, R. ATR-FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Front. Plant Sci. 2014, 5, 747. [Google Scholar] [CrossRef] [PubMed]
- Oleszko, A.; OlsztyNska-Janus, S.; Walski, T.; Grzeszczuk-Kuć, K.; Bujok, J.; GaBecka, K.; Czerski, A.; Witkiewicz, W.; Komorowska, M. Application of FTIR-ATR spectroscopy to determine the extent of lipid peroxidation in plasma during haemodialysis. Biomed. Res. Int. 2015, 2015, 245607. [Google Scholar] [CrossRef]
- Savicka, M.; Škute, N. Effects of high temperature on malondialdehyde content superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 2010, 56, 26–33. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Dighton, J.; Arbuckle, G.A. Characterization of plant carbohydrates and changes in leaf carbohydrate chemistry due to chemical and enzymatic degradation measured by microscopic ATR FTIR spectroscopy. Appl. Spectrosc. 2000, 54, 681–686. [Google Scholar] [CrossRef]
- Baron-Epel, O.; Gharyal, P.K.; Schindler, M. Pectins as mediators of wall porosity in soybean cells. Planta 1988, 175, 389–395. [Google Scholar] [CrossRef]
- Kurian, J.K.; Gariepy, Y.; Orsat, V.; Raghavan, V. Microwave-assisted lime treatment and recovery of lignin from hydrothermally treated sweet sorghum bagasse. Biofuels 2015, 6, 341–355. [Google Scholar] [CrossRef]
- Razzaq, A.; Sadia, B.; Raza, A.; Hameed, M.K.; Saleem, F. Metabolomics: A way forward for crop improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef]
- Shapaval, V.; Møretrø, T.; Suso, H.-P.; Åsli, A.W.; Schmitt, J.; Lillehaug, D.; Martens, H.; Böcker, U.; Kohler, A. A high-throughput microcultivation protocol for FTIR spectroscopic characterization and identification of fungi. J. Biophoton. 2010, 3, 512–521. [Google Scholar] [CrossRef]
- Bağcıoğlu, M.; Kohler, A.; Seifert, S.; Kneipp, J.; Zimmermann, B. Monitoring of plant—Environment interactions by high-throughput FTIR spectroscopy of pollen. Methods Ecol. Evol. 2017, 8, 870–880. [Google Scholar] [CrossRef]
- Mandrone, M.; Chiocchio, I.; Barbanti, L.; Tomasi, P.; Tacchini, M.; Poli, F. Metabolomic study of sorghum (Sorghum bicolor) to interpret plant behavior under variable field conditions in view of smart agriculture applications. J. Agric. Food Chem. 2021, 69, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Galleni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and future of plant stress detection: An overview from remote sensing to position emission tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef] [PubMed]
- Yalkun, A.; Mamtimin, A.; Liu, S.; Yang, F.; He, Q.; Qi, F.; Liu, Y. Coefficients optimization of the GLASS broadband emissivity based on FTIR and MODIS data over the Taklimakan Desert. Sci. Rep. 2019, 9, 18460. [Google Scholar] [CrossRef]
- Assouline, S.; Or, D. The concept of field capacity revisited: Defining intrinsic static and dynamic criteria for soil internal drainage dynamics. Water Resour. Res. 2014, 50, 4787–4802. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 2006, 224, 1080–1090. [Google Scholar] [CrossRef]
- McCann, S.E.; Huang, B. Effects of trinexapac-ethyl foliar application on creeping bentgrass responses to combined drought and heat stress. Crop Sci. 2007, 47, 2121–2128. [Google Scholar] [CrossRef]
- ImageJ Home Page, Version 1.80. Available online: https://imagej.nih.gov/ij/index.html (accessed on 10 October 2021).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 1 October 2020).
- Astatsa. Complex Online Web Statistics Calculator. Available online: https://astatsa.com/ (accessed on 3 January 2022).
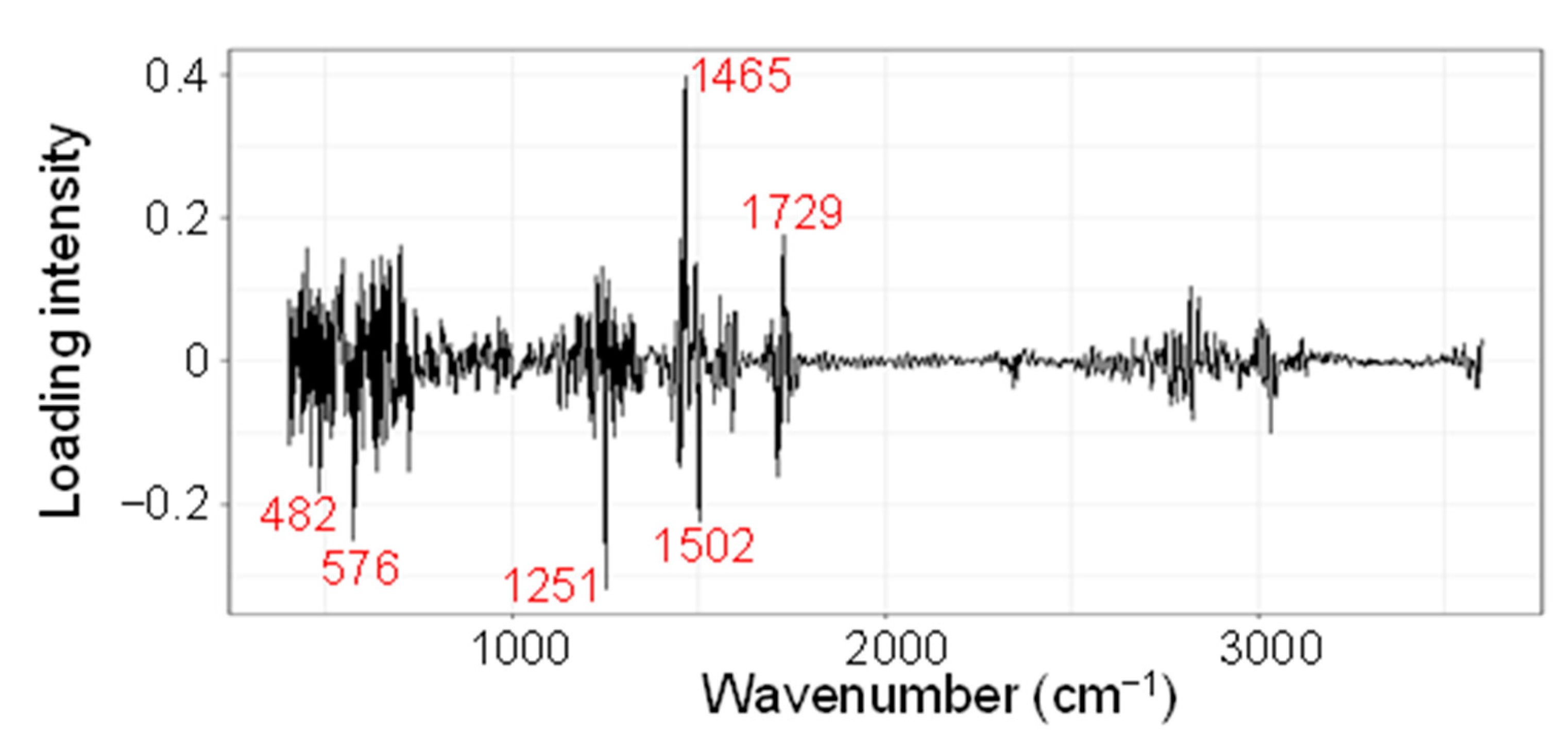
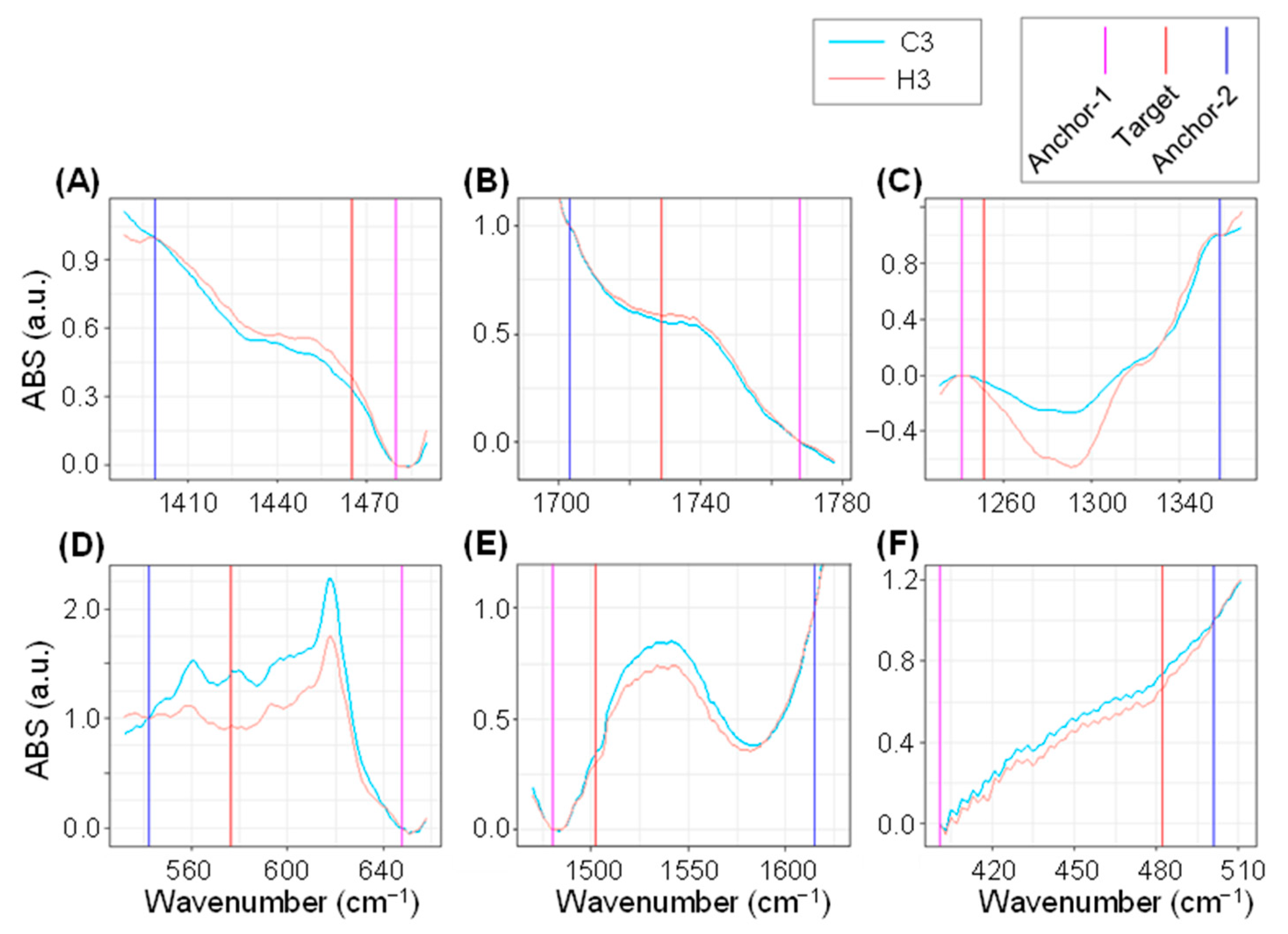
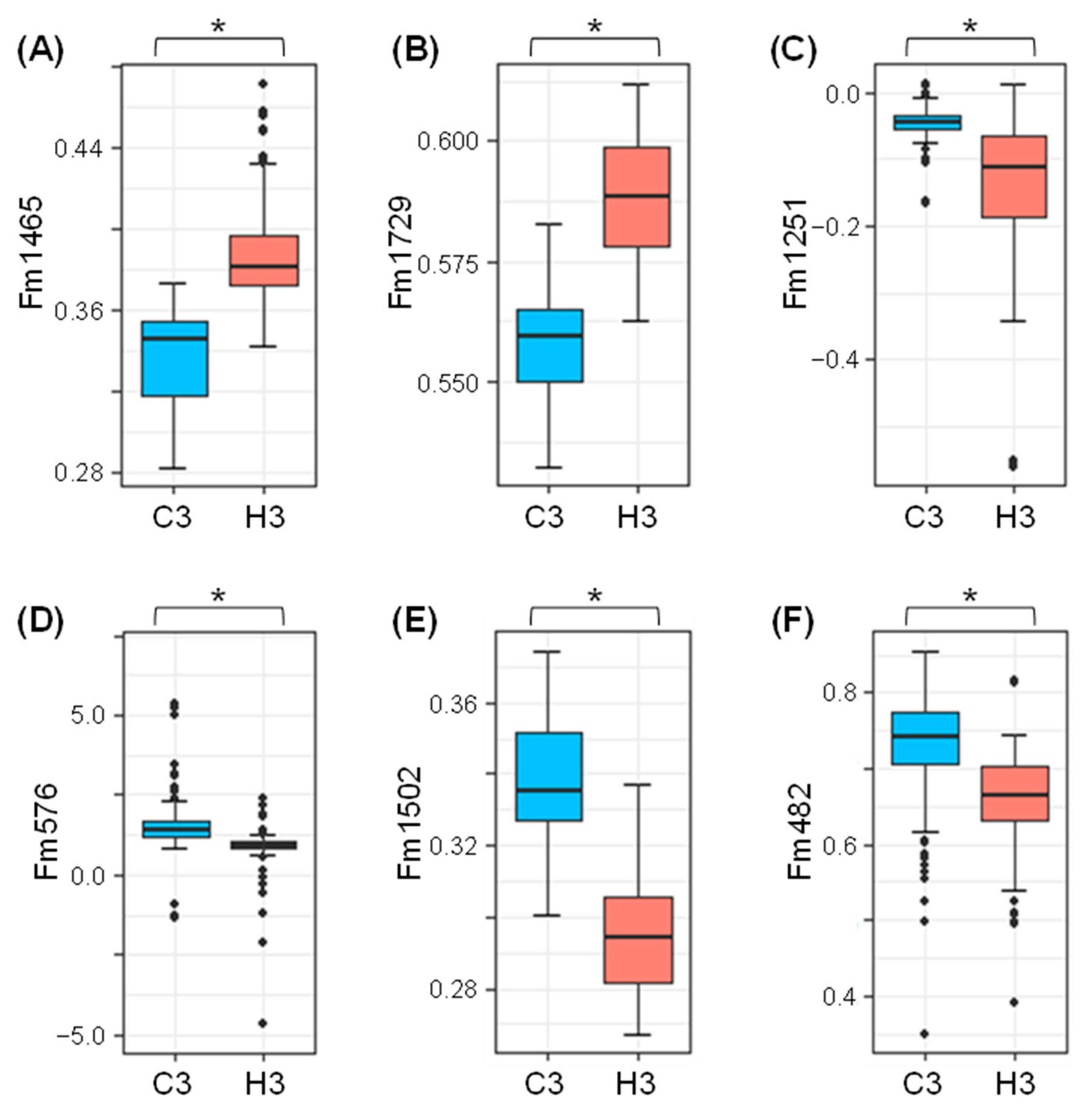
| No. | Wavenumber (cm−1) | Probable Functional Groups |
|---|---|---|
| 1 | 3293 | O–H stretching, N–H stretching. |
| 2 | 2960 | C–H stretching in –CH3 (antisymmetric). |
| 3 | 2925 | C–H stretching in –CH2– (antisymmetric). |
| 4 | 2852 | C–H stretching in –CH2– (symmetric). |
| 5 | 1651 | C=C stretching, C=O stretching (amide), N–H bending (amide I). |
| 6 | 1541 | C=C stretching (aromatic), N–H bending (amide II), C–N stretching. |
| 7 | 1385 | C–H bending (antisymmetric), =C–H in-plain bending. |
| 8 | 1241 | C–O stretching, in-plain C–H bending (aromatic), aliphatic C–O stretching, P=O stretching (aliphatic). |
| 9 | 1158 | C–O stretching, C–N stretching (aliphatic), in-plain C–H bending (aromatic), aliphatic C–O stretching. |
| 10 | 1106 | C–O stretching, C–N stretching (aliphatic), in-plain C–H bending (aromatic), aliphatic C–O stretching. |
| 11 | 1055 | C–O stretching, C–N stretching (aliphatic), in-plain C–H bending (aromatic). |
| 12 | 618 | =C–H out-of-plane bending, =C–H bending, C–S stretching. |
| Marker Name | Wavenumbers (cm−1) | Loading *1 | Median Fm Value | H3/C3 Ratio *2 | p *3 | |||
|---|---|---|---|---|---|---|---|---|
| Target | Anchor-1 | Anchor-2 | C3 | H3 | ||||
| Fm1465 | 1465 | 1480 | 1399 | 0.398 | 0.345 | 0.381 | 1.104 | 2.1 × 10−61 |
| Fm1729 | 1729 | 1768 | 1703 | 0.176 | 0.559 | 0.588 | 1.052 | 3.8 × 10−80 |
| Fm1251 | 1251 | 1241 | 1358 | −0.318 | −0.0428 | −0.112 | 2.607 | 3.1 × 10−26 |
| Fm576 | 576 | 648 | 542 | −0.25 | 1.436 | 0.899 | 0.626 | 1.1 × 10−4 |
| Fm1502 | 1502 | 1480 | 1615 | −0.224 | 0.335 | 0.294 | 0.879 | 4.4 × 10−75 |
| Fm482 | 482 | 401 | 501 | −0.183 | 0.741 | 0.666 | 0.899 | 7.9 × 10−21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, S.O.M.; Saad, A.S.I.; Tadano, S.; Takeda, Y.; Konaka, T.; Yamasaki, Y.; Tahir, I.S.A.; Tsujimoto, H.; Akashi, K. Chemical Fingerprinting of Heat Stress Responses in the Leaves of Common Wheat by Fourier Transform Infrared Spectroscopy. Int. J. Mol. Sci. 2022, 23, 2842. https://doi.org/10.3390/ijms23052842
Osman SOM, Saad ASI, Tadano S, Takeda Y, Konaka T, Yamasaki Y, Tahir ISA, Tsujimoto H, Akashi K. Chemical Fingerprinting of Heat Stress Responses in the Leaves of Common Wheat by Fourier Transform Infrared Spectroscopy. International Journal of Molecular Sciences. 2022; 23(5):2842. https://doi.org/10.3390/ijms23052842
Chicago/Turabian StyleOsman, Salma O. M., Abu Sefyan I. Saad, Shota Tadano, Yoshiki Takeda, Takafumi Konaka, Yuji Yamasaki, Izzat S. A. Tahir, Hisashi Tsujimoto, and Kinya Akashi. 2022. "Chemical Fingerprinting of Heat Stress Responses in the Leaves of Common Wheat by Fourier Transform Infrared Spectroscopy" International Journal of Molecular Sciences 23, no. 5: 2842. https://doi.org/10.3390/ijms23052842
APA StyleOsman, S. O. M., Saad, A. S. I., Tadano, S., Takeda, Y., Konaka, T., Yamasaki, Y., Tahir, I. S. A., Tsujimoto, H., & Akashi, K. (2022). Chemical Fingerprinting of Heat Stress Responses in the Leaves of Common Wheat by Fourier Transform Infrared Spectroscopy. International Journal of Molecular Sciences, 23(5), 2842. https://doi.org/10.3390/ijms23052842







