Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches
Abstract
1. Introduction
2. Results
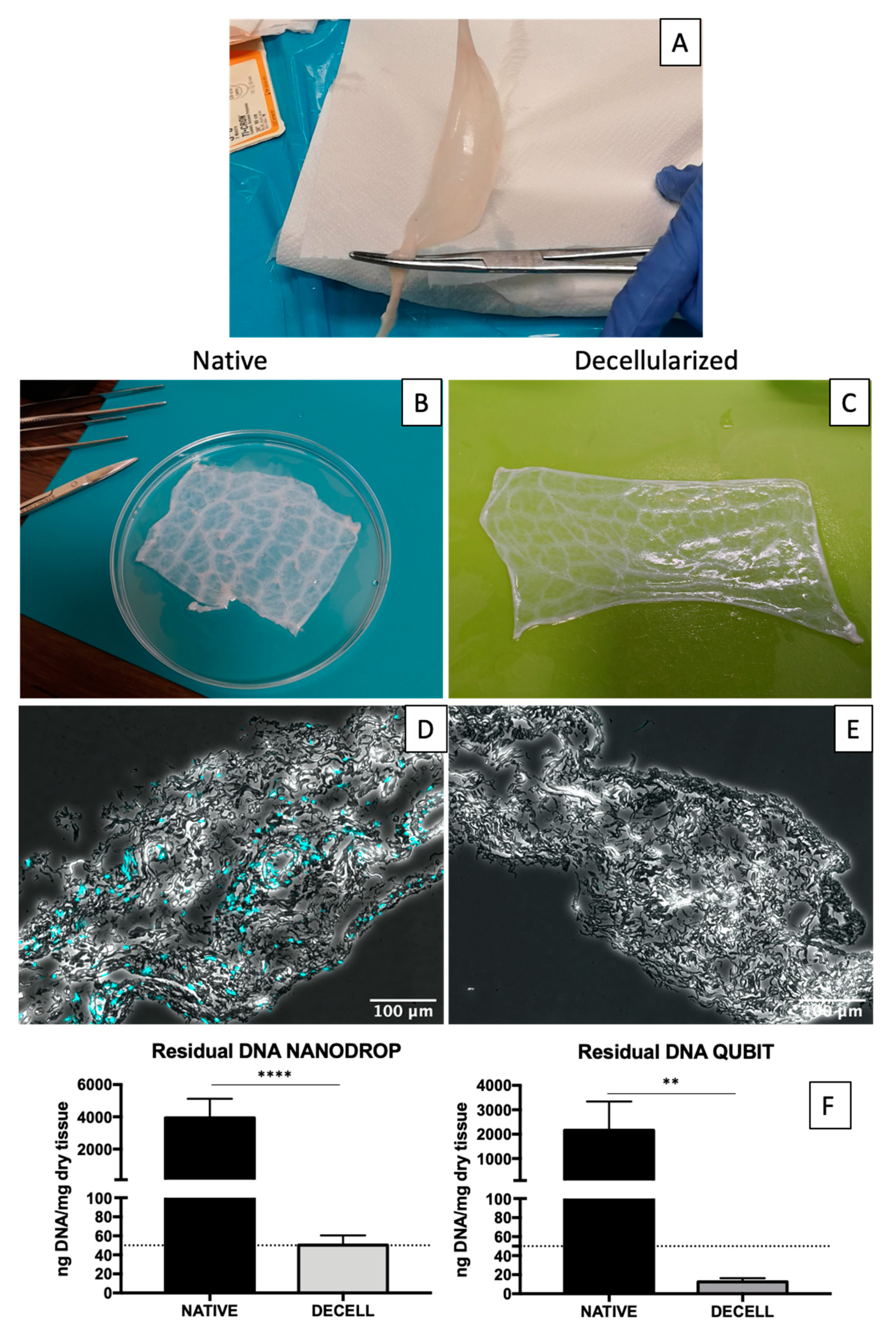
2.1. Decellularization Assessment
2.2. Gross Structure Maintenance
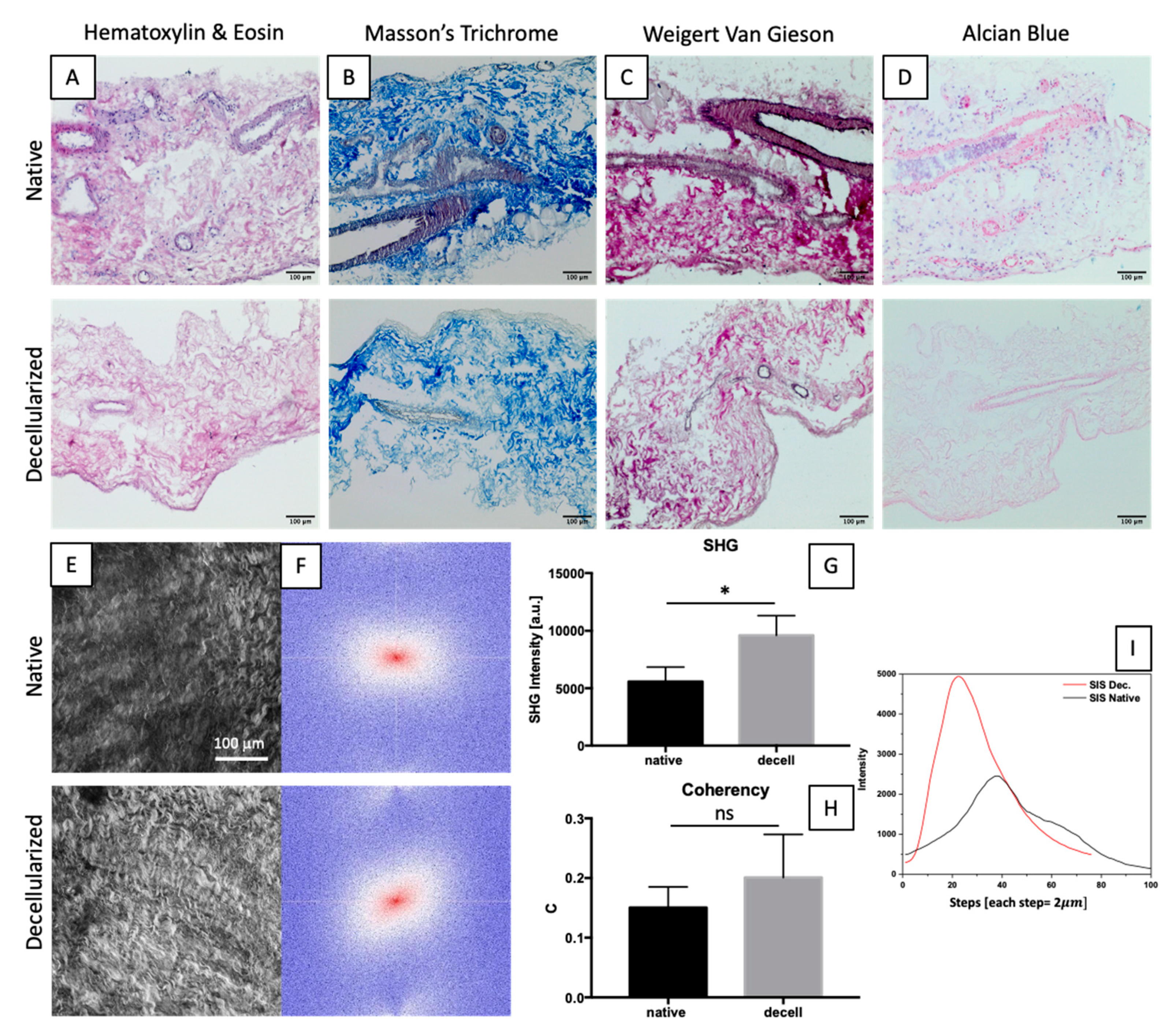
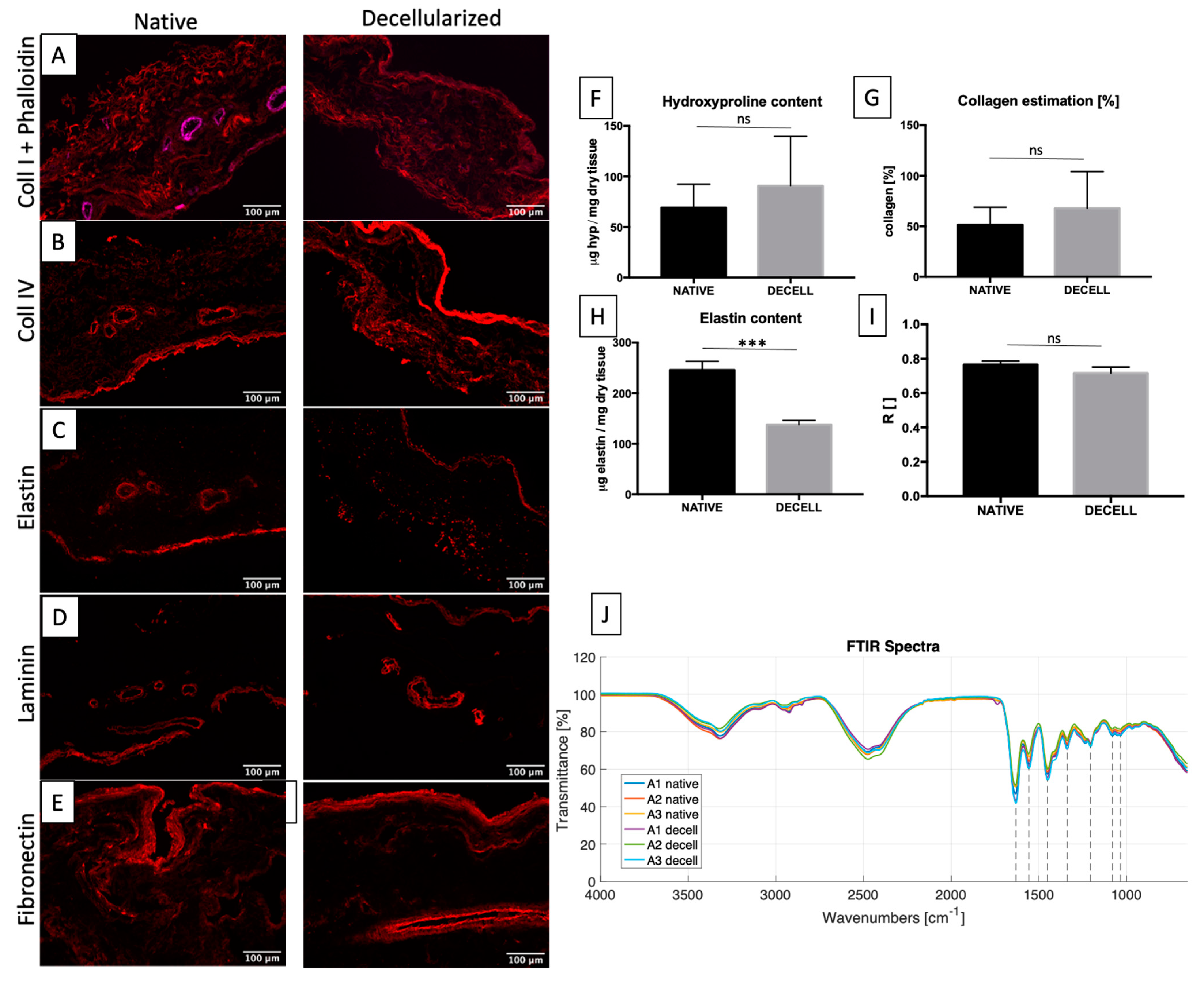
2.3. ECM Maintenance
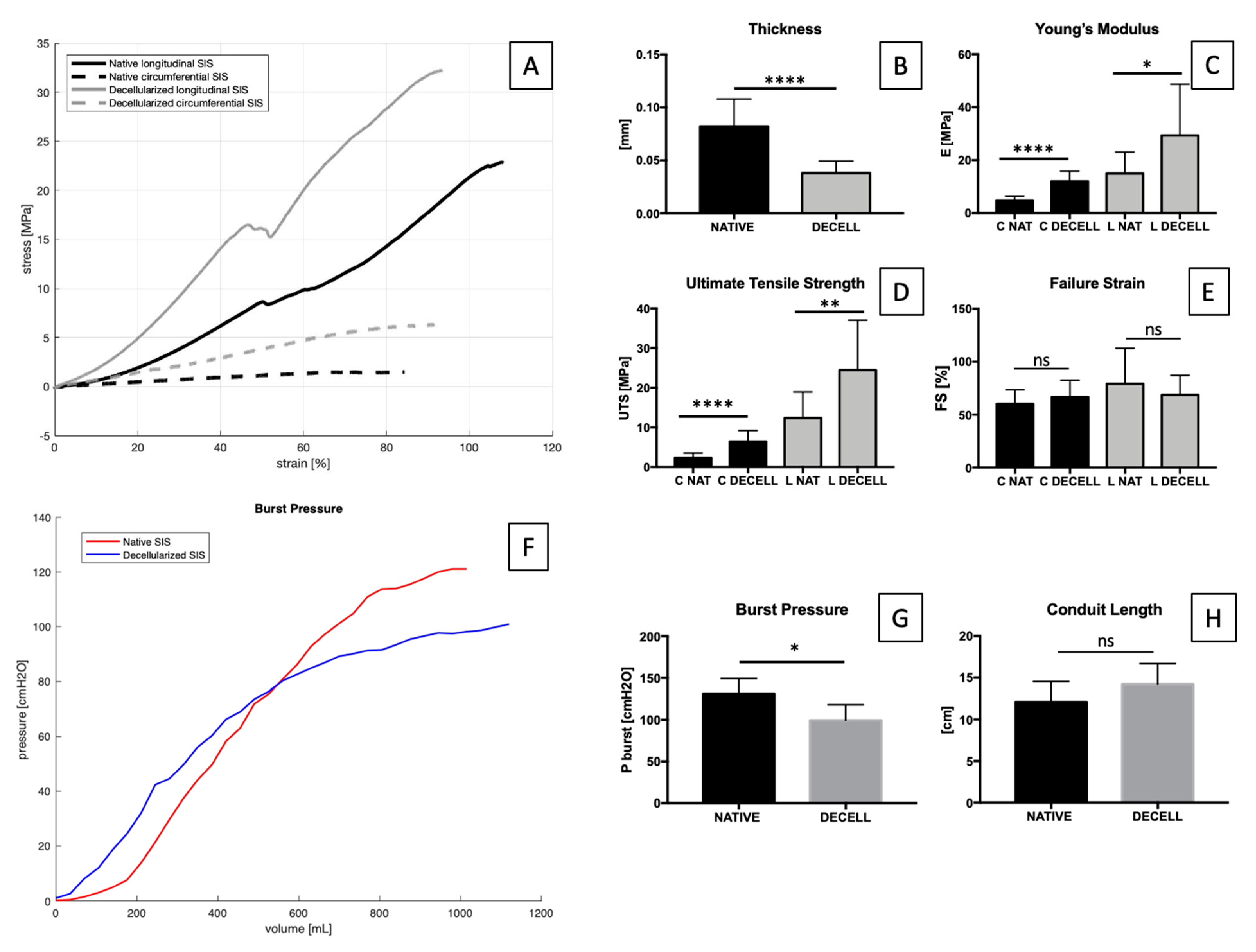
2.4. Tensile and Inflation Tests
2.5. Sterility Assessment
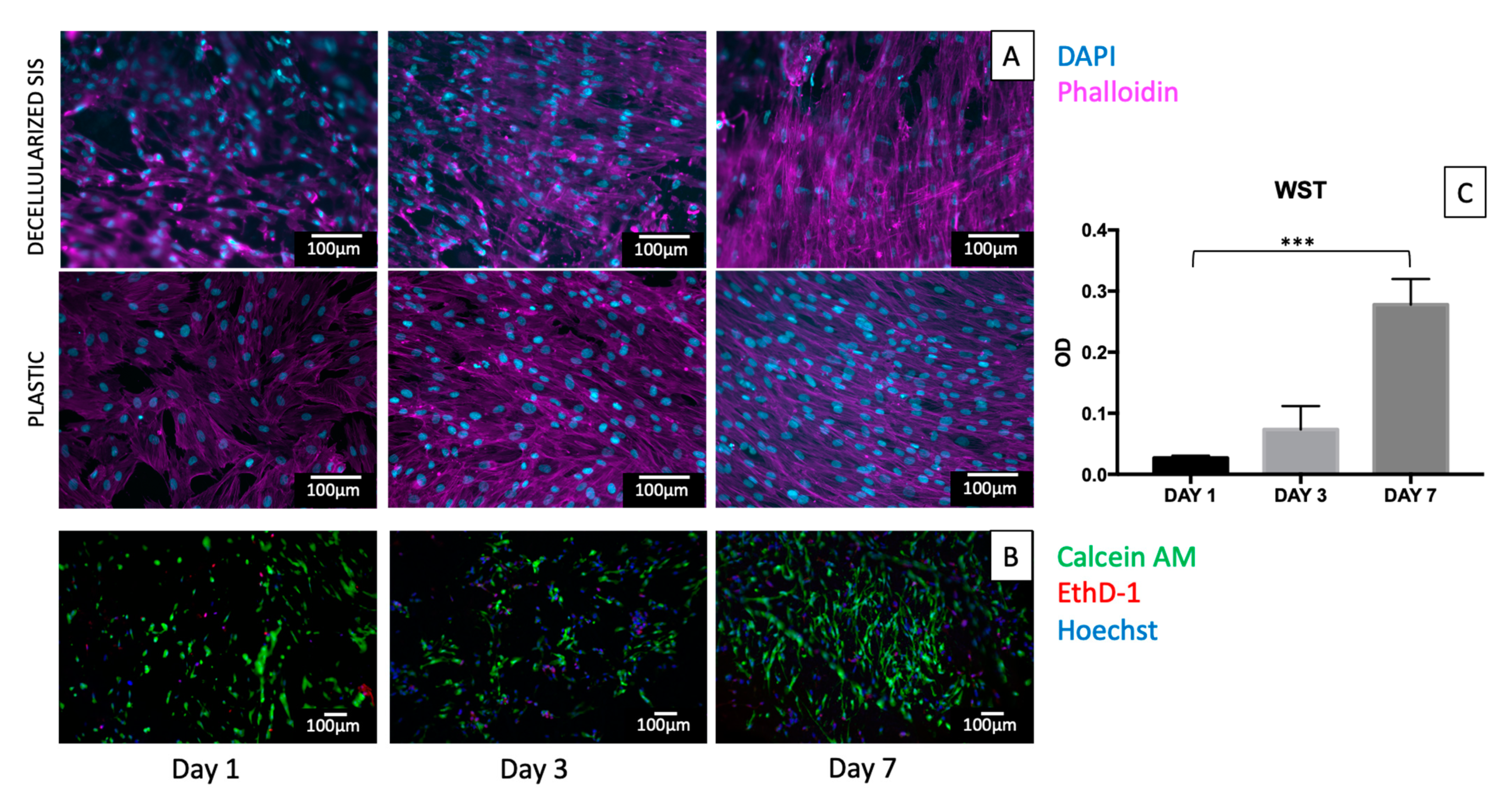
2.6. In Vitro Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Porcine Small Intestinal Submucosa (SIS) Procurement
4.2. SIS Decellularization
4.3. DNA Quantification
4.4. Histological Stainings
4.5. Immunofluorescence
4.6. Two-Photon Microscopy
4.7. Mechanical Tests
4.8. Inflation Tests
4.9. FTIR Analysis
4.10. Protein Analysis
4.10.1. Elastin Quantification
4.10.2. Hydroxyproline Quantification
4.11. SIS Sterilization
4.12. Sterility Assessment
4.13. In Vitro Cytotoxicity Assays
4.14. Live/Dead Assay
4.15. WST Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Pane, K.; Mirabelli, P.; Coppola, L.; Illiano, E.; Salvatore, M.; Franzese, M. New Roadmaps for Non-muscle-invasive Bladder Cancer With Unfavorable Prognosis. Front. Chem. 2020, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, S.T.; Djaladat, H.; Ahmadi, H.; Miranda, G.; Cai, J.; Schuckman, A.K.; Daneshmand, S. Gastrointestinal Complications Following Radical Cystectomy Using Enhanced Recovery Protocol. Eur. Urol. Focus 2018, 4, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009, 55, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, R.S.; Nielsen, M.; Smith, A.; Nix, J.; Schultz, H.; Wallen, E.M. Fast Track Program in Patients Undergoing Radical Cystectomy: Results in 362 Consecutive Patients. J. Am. Coll. Surg. 2010, 210, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; McIntosh, A.G.; Strehlow, R.; Lawrence, V.A.; Parekh, D.J.; Svatek, R.S. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: A systematic review. Eur. Urol. 2013, 64, 588–597. [Google Scholar] [CrossRef]
- Faba, O.R.; Moreno, R.P.; Malca, L.; Martínez, A.P.; Nervo, N.; Breda, A.; Esquinas, C.; Palou, J. Postoperative management of radical cystectomy. Review of the evidence on the prevention and treatment of urological complications. Actas Urológicas Españolas 2018, 42, 143–151. [Google Scholar] [CrossRef]
- Adamowicz, J.; Pokrywczynska, M.; Van Breda, S.V.; Kloskowski, T.; Drewa, T. Concise Review: Tissue Engineering of Urinary Bladder; We Still Have a Long Way to Go? Stem Cells Transl. Med. 2017, 6, 2033–2043. [Google Scholar] [CrossRef]
- Shelbaia, A.; Salem, H.K.; Emran, A.; Raouf, M.A.; Rahman, S.A. Long term complications after radical cystoprostatectomy with orthotopic diversion in male patients: Preliminary experience. African J. Urol. 2013, 19, 89–93. [Google Scholar] [CrossRef][Green Version]
- Drewa, T. The Artificial Conduit for Urinary Diversion in Rats: A Preliminary Study. Transplant. Proc. 2007, 39, 1647–1651. [Google Scholar] [CrossRef]
- Geutjes, P.; Roelofs, L.; Hoogenkamp, H.; Walraven, M.; Kortmann, B.; De Gier, R.; Farag, F.; Tiemessen, D.; Sloff, M.; Oosterwijk, E.; et al. Tissue engineered tubular construct for urinary diversion in a preclinical porcine model. J. Urol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Jayo, M.J.; Ilagan, R.M.; Guthrie, K.I.; Sangha, N.; Genheimer, C.W.; Quinlan, S.F.; Payne, R.; Knight, T.; Rivera, E.; et al. Regeneration of native-like neo-urinary tissue from nonbladder cell sources. Tissue Eng. Part A 2012, 18, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Yang, S.; Song, C.; Li, Y.; Meng, L.; Li, X.; Xiong, Y. Tissue-engineered tubular graft for urinary diversion after radical cystectomy in rabbits. J. Surg. Res. 2013, 182, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sloff, M.; Simaioforidis, V.; Tiemessen, D.M.; Janke, H.P.; Kortmann, B.B.M.; Roelofs, L.A.J.; Geutjes, P.J.; Oosterwijk, E.; Feitz, W.F.J. Tubular Constructs as Artificial Urinary Conduits. J. Urol. 2016, 196, 1279–1286. [Google Scholar] [CrossRef]
- Sartoneva, R.; Haaparanta, A.M.; Lahdes-Vasama, T.; Mannerström, B.; Kellomäki, M.; Salomäki, M.; Sándor, G.; Seppänen, R.; Miettinen, S.; Haimi, S. Characterizing and optimizing poly-L-lactide-co-1-caprolactone membranes for urothelial tissue engineering. J. R. Soc. Interface 2012, 9, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Kloskowski, T.; Jundziłł, A.; Kowalczyk, T.; Nowacki, M.; Bodnar, M.; Marszałek, A.; Pokrywczyńska, M.; Frontczak-Baniewicz, M.; Kowalewski, T.A.; Chłosta, P.; et al. Ureter regeneration-The proper scaffold has to be defined. PLoS ONE 2014, 9, e106023. [Google Scholar] [CrossRef] [PubMed]
- Oberpenning, F.; Meng, J.; Yoo, J.J.; Atala, A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 1999, 17, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Yoon, C.Y.; Yoo, J.J.; Wulf, T.; Atala, A.; Kropp, B. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J. Urol. 2002, 168, 1853–1858. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Chen, G.; Komuro, H.; Ushida, T.; Kaneko, S.; Tateishi, T.; Kaneko, M. Tissue-Engineered Urinary Bladder Wall Using PLGA Mesh-Collagen Hybrid Scaffolds: A Comparison Study of Collagen Sponge and Gel as a Scaffold. J. Pediatr. Surg. 2003, 38, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Freeman, M.R.; Vacanti, J.P.; Shepard, J.; Retik, A.B. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J. Urol. 1993, 150, 608–612. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Micol, L.A.; Arenas da Silva, L.F.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.J.; Frey, P. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef]
- Sayeg, K.; Freitas-Filho, L.G.; Waitzberg, Â.F.L.; Arias, V.E.A.; Laks, M.; Egydio, F.M.; Oliveira, A.S. Integration of collagen matrices into the urethra when implanted as onlay graft. Int. Braz J Urol 2013, 39, 414–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef]
- Aufderklamm, S.; Vaegler, M.; Kelp, A.; Maurer, S.; Gustafsson, L.; Mundhenk, J.; Busch, S.; Daum, L.; Stenzl, A.; Amend, B.; et al. Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: First results in a xenograft minipig model. World J. Urol. 2017, 35, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef]
- Algarrahi, K.; Franck, D.; Ghezzi, C.E.; Cristofaro, V.; Yang, X.; Sullivan, M.P.; Chung, Y.G.; Affas, S.; Jennings, R.; Kaplan, D.L.; et al. Acellular bi-layer silk fibroin scaffolds support functional tissue regeneration in a rat model of onlay esophagoplasty. Biomaterials 2015, 53, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.; Zhou, L.; Sun, Z.; Zheng, J.; Chen, Y.; Dai, Y. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng. Part C Methods 2010, 16, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Kropp, B.P.; Rippy, M.K.; Badylak, S.F.; Adams, M.C.; Keating, M.A.; Rink, R.C.; Thor, K.B. Regenerative urinary bladder augmentation using small intestinal submucosa: Urodynamic and histopathologic assessment in long-term canine bladder augmentations. J. Urol. 1996, 155, 2098–2104. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef]
- Campodonico, F.; Benelli, R.; Michelazzi, A.; Ognio, E.; Toncini, C.; Maffezzini, M. Bladder cell culture on small intestinal submucosa as bioscaffold: Experimental study on engineered urothelial grafts. Eur. Urol. 2004, 46, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Ayyildiz, A.; Akgül, K.T.; Huri, E.; Nuhoğlu, B.; Kiliçoğlu, B.; Ustün, H.; Gürdal, M.; Germiyanoğlu, C. Use of porcine small intestinal submucosa in bladder augmentation in rabbit: Long-term histological outcome. ANZ J. Surg. 2008, 78, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liao, L. Histologic and functional outcomes of small intestine submucosa-regenerated bladder tissue. BMC Urol. 2014, 14, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kropp, B.P.; Eppley, B.L.; Prevel, C.D.; Rippy, M.K.; Harruff, R.C.; Badylak, S.F.; Adams, M.C.; Rink, R.C.; Keating, M.A. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology 1995, 46, 396–400. [Google Scholar] [CrossRef]
- Liu, Y.; Bharadwaj, S.; Lee, S.J.; Atala, A.; Zhang, Y. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials 2009, 30, 3865–3873. [Google Scholar] [CrossRef]
- Liao, W.B.; Song, C.; Li, Y.W.; Yang, S.X.; Meng, L.C.; Li, X.H. Tissue-engineered conduit using bladder acellular matrix and bladder epithelial cells for urinary diversion in rabbits. Chin. Med. J. 2013, 126, 335–339. [Google Scholar]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef]
- Johnson, S.C.; Smith, Z.L.; Sack, B.S.; Steinberg, G.D. Tissue Engineering and Conduit Substitution. Urol. Clin. N. Am. 2018, 45, 133–141. [Google Scholar] [CrossRef]
- Singh, A.; Bivalacqua, T.J.; Sopko, N. Urinary Tissue Engineering: Challenges and Opportunities. Sex. Med. Rev. 2018, 6, 35–44. [Google Scholar] [CrossRef]
- Cao, G.; Huang, Y.; Li, K.; Fan, Y.; Xie, H.; Li, X. Small intestinal submucosa: Superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J. Mater. Chem. B 2019, 7, 5038–5055. [Google Scholar] [CrossRef]
- Pavcnik, D.; Obermiller, J.; Uchida, B.T.; Van Alstine, W.; Edwards, J.M.; Landry, G.J.; Kaufman, J.A.; Keller, F.S.; Rösch, J. Angiographic evaluation of carotid artery grafting with prefabricated small-diameter, small-intestinal submucosa grafts in sheep. Cardiovasc. Intervent. Radiol. 2009, 32, 106–113. [Google Scholar] [CrossRef]
- Record, R.D.; Hillegonds, D.; Simmons, C.; Tullius, R.; Rickey, F.A.; Elmore, D.; Badylak, S.F. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials 2001, 22, 2653–2659. [Google Scholar] [CrossRef]
- Hoeppner, J.; Crnogorac, V.; Marjanovic, G.; Jüttner, E.; Karcz, W.; Weiser, H.F.; Hopt, U.T. Small intestinal submucosa as a bioscaffold for tissue regeneration in defects of the colonic wall. J. Gastrointest. Surg. 2009, 13, 113–119. [Google Scholar] [CrossRef]
- Lee, M.; Chang, P.C.Y.; Dunn, J.C.Y. Evaluation of Small Intestinal Submucosa as Scaffolds for Intestinal Tissue Engineering. J. Surg. Res. 2008, 147, 168–171. [Google Scholar] [CrossRef]
- Karaoglu, S.; Fisher, M.B.; Woo, S.L.Y.; Fu, Y.C.; Liang, R.; Abramowitch, S.D. Use of a bioscaffold to improve healing of a patellar tendon defect after graft harvest for ACL reconstruction: A study in rabbits. J. Orthop. Res. 2008, 26, 255–263. [Google Scholar] [CrossRef]
- Le Roux, P.J. Endoscopic urethroplasty with unseeded small intestinal submucosa collagen matrix grafts: A pilot study. J. Urol. 2005, 173, 140–143. [Google Scholar] [CrossRef]
- Fiala, R.; Vidlar, A.; Vrtal, R.; Belej, K.; Student, V. Porcine Small Intestinal Submucosa Graft for Repair of Anterior Urethral Strictures. Eur. Urol. 2007, 51, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Shindel, A.W.; Brandes, S.B. Small intestinal submucosa for patch grafting after plaque incision in the treatment of Peyronie’s disease. Int. Braz J Urol 2008, 34, 191–197. [Google Scholar] [CrossRef]
- Donkov, I.I.; Bashir, A.; Elenkov, C.H.G.; Panchev, P.K. Dorsal onlay augmentation urethroplasty with small intestinal submucosa: Modified Barbagli technique for strictures of the bulbar urethra. Int. J. Urol. 2006, 13, 1415–1417. [Google Scholar] [CrossRef]
- Franklin, M.E.; Treviño, J.M.; Portillo, G.; Vela, I.; Glass, J.L.; González, J.J. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: Long-term follow-up. Surg. Endosc. Other Interv. Tech. 2008, 22, 1941–1946. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole organ decellularization processes. Biomaterials 2012, 32, 3233–3243. [Google Scholar] [CrossRef]
- Iop, L.; Paolin, A.; Aguiari, P.; Trojan, D.; Cogliati, E.; Gerosa, G. Decellularized Cryopreserved Allografts as Off-the-Shelf Allogeneic Alternative for Heart Valve Replacement: In Vitro Assessment Before Clinical Translation. J. Cardiovasc. Transl. Res. 2017, 10, 93–103. [Google Scholar] [CrossRef]
- Iop, L.; Bonetti, A.; Naso, F.; Rizzo, S.; Cagnin, S.; Bianco, R.; Dal Lin, C.; Martini, P.; Poser, H.; Franci, P.; et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS ONE 2014, 9, e99593. [Google Scholar] [CrossRef]
- European Chemicals Agency. Inclusion of Substances of Very High Concerns in the Candidate List; European Chemicals Agency: Helsinki, Finland, 2012; pp. 1–9. [Google Scholar]
- Luo, J.C.; Chen, W.; Chen, X.H.; Qin, T.W.; Huang, Y.C.; Xie, H.Q.; Li, X.Q.; Qian, Z.Y.; Yang, Z.M. A multi-step method for preparation of porcine small intestinal submucosa (SIS). Biomaterials 2011, 32, 706–713. [Google Scholar] [CrossRef]
- Gallagher, W. FTIR Analysis of Protein Structure. Course Man. Chem. 2009, 455, 1–8. [Google Scholar]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Lazarev, Y.A.; Grishkovsky, B.A.; Khromova, T.B. Amide I band of IR spectrum and structure of collagen and related polypeptides. Biopolymers 1985, 24, 1449–1478. [Google Scholar] [CrossRef]
- Sacks, M.S.; Claire Gloeckner, D. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J. Biomed. Mater. Res. 1999, 46, 1–10. [Google Scholar] [CrossRef]
- Lanir, Y. Constitutive equations for fibrous connective tissues. J. Biomech. 1983, 16, 1–12. [Google Scholar] [CrossRef]
- Council of Europe. 2.6.1. Sterility. Eur. Pharmacopoeia 2005, 5, 145–149. [Google Scholar]
- Yuh, B.E.; Nazmy, M.; Ruel, N.H.; Jankowski, J.T.; Menchaca, A.R.; Torrey, R.R.; Linehan, J.A.; Lau, C.S.; Chan, K.G.; Wilson, T.G. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur. Urol. 2012, 62, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering approaches for bladder regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Morlacco, A.; Moro, F.D. Bladder Substitution: The Role of Tissue Engineering and Biomaterials. Processes 2021, 9, 1643. [Google Scholar] [CrossRef]
- Kropp, B.P. Small-intestinal submucosa for bladder augmentation: A review of preclinical studies. World J. Urol. 1998, 16, 262–267. [Google Scholar] [CrossRef]
- Glynn, J.J.; Polsin, E.G.; Hinds, M.T. Crosslinking decreases the hemocompatibility of decellularized, porcine small intestinal submucosa. Acta Biomater. 2015, 14, 96–103. [Google Scholar] [CrossRef]
- Badylak, S.F. Small Intestinal Submucosa (SIS): A Biomaterial Conducive to Smart Tissue Remodeling. In Tissue Engineering; Birkhäuser: Boston, MA, USA, 1993; pp. 179–189. [Google Scholar]
- Zheng, M.H.; Chen, J.; Kirilak, Y.; Willers, C.; Xu, J.; Wood, D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: Possible implications in human implantation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 61–67. [Google Scholar] [CrossRef]
- Jundziłł, A.; Kwieciński, P.; Balcerczyk, D.; Kloskowski, T.; Grzanka, D.; Antosik, P.; Meger, K.; Pokrywczyńska, M.; Drewa, T. A tissue-engineered urinary conduit in a porcine urinary diversion model. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Lu, S.H.; Sacks, M.S.; Chung, S.Y.; Gloeckner, D.C.; Pruchnic, R.; Huard, J.; De Groat, W.C.; Chancellor, M.B. Biaxial mechanical properties of muscle-derived cell seeded small intestinal submucosa for bladder wall reconstitution. Biomaterials 2005, 26, 443–449. [Google Scholar] [CrossRef]
- Chung, Y.G.; Algarrahi, K.; Franck, D.; Tu, D.D.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R.; Mauney, J.R. The use of bi-layer silk fibroin scaffolds and small intestinal submucosa matrices to support bladder tissue regeneration in a rat model of spinal cord injury. Biomaterials 2014, 35, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Bury, M.I.; Fuller, N.J.; Meisner, J.W.; Hofer, M.D.; Webber, M.J.; Chow, L.W.; Prasad, S.; Thaker, H.; Yue, X.; Menon, V.S.; et al. The promotion of functional urinary bladder regeneration using anti-inflammatory nanofibers. Biomaterials 2014, 35, 9311–9321. [Google Scholar] [CrossRef] [PubMed]
- Pokrywczynska, M.; Jundzill, A.; Adamowicz, J.; Kowalczyk, T.; Warda, K.; Rasmus, M.; Buchholz, L.; Krzyzanowska, S.; Nakielski, P.; Chmielewski, T.; et al. Is the poly (L- lactide- co- caprolactone) nanofibrous membrane suitable for urinary bladder regeneration? PLoS ONE 2014, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Frimberger, D.; Cheng, E.Y.; Lin, H.K.; Kropp, B.P. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006, 98, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, H.K.; Frimberger, D.; Epstein, R.B.; Kropp, B.P. Growth of bone marrow stromal cells on small intestinal submucosa: An alternative cell source for tissue engineered bladder. BJU Int. 2005, 96, 1120–1125. [Google Scholar] [CrossRef]
- Caione, P.; Capozza, N.; Zavaglia, D.; Palombaro, G.; Boldrini, R. In vivo bladder regeneration using small intestinal submucosa: Experimental study. Pediatr. Surg. Int. 2006, 22, 593–599. [Google Scholar] [CrossRef]
- Ashley, R.A.; Roth, C.C.; Palmer, B.W.; Kibar, Y.; Routh, J.C.; Fung, K.M.; Frimberger, D.; Lin, H.K.; Kropp, B.P. Regional variations in small intestinal submucosa evoke differences in inflammation with subsequent impact on tissue regeneration in the rat bladder augmentation model. BJU Int. 2010, 105, 1462–1468. [Google Scholar] [CrossRef]
- Roth, C.C.; Mondalek, F.G.; Kibar, Y.; Ashley, R.A.; Bell, C.H.; Califano, J.A.; Madihally, S.V.; Frimberger, D.; Lin, H.K.; Kropp, B.P. Bladder regeneration in a canine model using hyaluronic acid-poly(lactic-co-glycolic-acid) nanoparticle modified porcine small intestinal submucosa. BJU Int. 2011, 108, 148–155. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bury, M.I.; Marks, A.J.; Fuller, N.J.; Meisner, J.W.; Tapaskar, N.; Halliday, L.C.; Matoka, D.J.; Cheng, E.Y. A Nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells 2011, 29, 241–250. [Google Scholar] [CrossRef]
- Mauney, J.R., Jr.; Lovett, G.M.C.; Gong, M.L.; Divizio, E.M.; Kaplan, D.; Adam, R.M.; Estrada, C.R., Jr. Evaluation of gel spun silk-based biomaterials in a murine model of Bladder Augmentation. Biomaterials 2013, 32, 808–818. [Google Scholar] [CrossRef]
- Seth, A.; Chung, Y.G.; Gil, E.S.; Tu, D.; Franck, D.; Di Vizio, D.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R.; Mauney, J.R. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials 2013, 34, 4758–4765. [Google Scholar] [CrossRef] [PubMed]
- Caione, P.; Boldrini, R.; Salerno, A.; Nappo, S.G. Bladder augmentation using acellular collagen biomatrix: A pilot experience in exstrophic patients. Pediatr. Surg. Int. 2012, 28, 421–428. [Google Scholar] [CrossRef]
- Schaefer, M.; Kaiser, A.; Stehr, M.; Beyer, H.J. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J. Pediatr. Urol. 2013, 9, 878–883. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Deisseroth, A.; Rubartelli, A. Damage associated molecular pattern molecules. Clin. Immunol. 2007, 124, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.C.; Dede Eren, A.; Spierings, J.; de Boer, J.; Ito, K.; Foolen, J. Solid-phase silica-based extraction leads to underestimation of residual DNA in decellularized tissues. Xenotransplantation 2021, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Gottardi, M.; Corbo, V.; Fassan, M.; Mafficini, A.; Malpeli, G.; Lawlor, R.T.; Scarpa, A. DNA Qualification Workflow for Next Generation Sequencing of Histopathological Samples. PLoS ONE 2013, 8, e62692. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yamaguchi, H.; Einaga, N.; Esumi, M. Pitfalls of DNA quantification using dnabinding fluorescent dyes and suggested solutions. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Freund, J.M.; Badylak, S.F. Quantification of DNA in Biologic Scaffold Materials. J. Surg. Res. 2009, 152, 135–139. [Google Scholar] [CrossRef]
- van Rijswijk, J.W.; Talacua, H.; Mulder, K.; van Hout, G.P.J.; Bouten, C.V.C.; Gründeman, P.F.; Kluin, J. Failure of decellularized porcine small intestinal submucosa as a heart valved conduit. J. Thorac. Cardiovasc. Surg. 2020, 160, e201–e215. [Google Scholar] [CrossRef]
- Filippo, N.; Paola, A.; Laura, I. Biocompatibility Evaluation Criteria for Novel Xenograft Materials: Distribution and Quantification of Remnant Nucleic Acid and Alpha-Gal Epitope. J. Stem Cell Res. Ther. 2013, 6, 1–8. [Google Scholar] [CrossRef]
- Brown, B.; Lindberg, K.; Reing, J.; Stolz, D.B.; Badylak, S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006, 12, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Orberg, J.W.; Klein, L.; Hiltner, A. Scanning electron microscopy of collagen fibers in intestine. Connect. Tissue Res. 1982, 9, 187–193. [Google Scholar] [CrossRef]
- Baume, A.S.; Boughton, P.C.; Coleman, N.V.; Ruys, A.J. Sterilization of Tissue Scaffolds. Elsevier Ltd: London, UK, 2016; ISBN 9781782420958. [Google Scholar]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Łopianiak, I.; Butruk-Raszeja, B.A. Evaluation of sterilization/disinfection methods of fibrous polyurethane scaffolds designed for tissue engineering applications. Int. J. Mol. Sci. 2020, 21, 8092. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef]
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Lantz, G.C.; Badylak, S.F.; Coffey, A.C.; Geddes, L.A.; Blevins, W.E. Small intestinal submucosa as a small-diameter arterial graft in the dog. J. Investig. Surg. 1990, 3, 217–227. [Google Scholar] [CrossRef]
- Borile, G.; Sandrin, D.; Filippi, A.; Anderson, K.I.; Romanato, F. Label-Free Multiphoton Microscopy: Much More Than Fancy Images. Int. J. Mol. Sci. 2021, 22, 2657. [Google Scholar] [CrossRef]
- Filippi, A.; Gintoli, M.; Filippi, A.; Sasso, E.D.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; et al. Multimodal label-free ex vivo imaging using a dual-wavelength microscope with axial chromatic aberration compensation. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; Van De Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A comprehensive comparison of bovine and porcine decellularized pericardia: New insights for surgical applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef]
- Oldenburg, K. LoadSpectra. Available online: https://www.mathworks.com/matlabcentral/fileexchange/57904-loadspectra (accessed on 11 February 2022).
- Neuman, R.E.; Logan, M.A. The determination of collagen and elastin in tissues. J. Biol. Chem. 1950, 186, 549–556. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Allingham, P.G.; Jones, A. Hydroxyproline quantification for the estimation of collagen in tissue using multiple reaction monitoring mass spectrometry. J. Chromatogr. A 2008, 1212, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater. 2018, 67, 282–294. [Google Scholar] [CrossRef]
- C.S.A. ISO 10993-5 in vitro cytotoxicity. Int. Organ. 2009, 2007, 1–11. [Google Scholar]
| Sample Name | Turbidity within 14 Days (Yes/NO) |
|---|---|
| Native | YES |
| Only decellularized | YES |
| Decellularized + 70% Ethanol | YES |
| Decellularized + antibiotic/antimycotic/PAA | NO |
| Control (only media) | NO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarin, M.; Fortunato, T.M.; Imran, S.; Todesco, M.; Sandrin, D.; Borile, G.; Toniolo, I.; Marchesan, M.; Gerosa, G.; Bagno, A.; et al. Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. Int. J. Mol. Sci. 2022, 23, 2826. https://doi.org/10.3390/ijms23052826
Casarin M, Fortunato TM, Imran S, Todesco M, Sandrin D, Borile G, Toniolo I, Marchesan M, Gerosa G, Bagno A, et al. Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. International Journal of Molecular Sciences. 2022; 23(5):2826. https://doi.org/10.3390/ijms23052826
Chicago/Turabian StyleCasarin, Martina, Tiago Moderno Fortunato, Saima Imran, Martina Todesco, Deborah Sandrin, Giulia Borile, Ilaria Toniolo, Massimo Marchesan, Gino Gerosa, Andrea Bagno, and et al. 2022. "Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches" International Journal of Molecular Sciences 23, no. 5: 2826. https://doi.org/10.3390/ijms23052826
APA StyleCasarin, M., Fortunato, T. M., Imran, S., Todesco, M., Sandrin, D., Borile, G., Toniolo, I., Marchesan, M., Gerosa, G., Bagno, A., Romanato, F., Carniel, E. L., Morlacco, A., & Dal Moro, F. (2022). Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. International Journal of Molecular Sciences, 23(5), 2826. https://doi.org/10.3390/ijms23052826












